Introduction
Placenta previa is a complication that occurs during
pregnancy in which the placenta is partially or wholly attached to
the lower uterine segment (1).
Placenta previa is a leading cause of antepartum haemorrhage. Due
to the rising incidence of caesarean section births, along side
increasing maternal age, the number of cases of placenta previa,
and its complications, which include placenta accreta and
postpartum haemorrhage, are expected to increase (2). Placenta accreta is an obstetric
complication in which the placental trophoblast invades the
endometrium beyond Nitabuch's layer, as a result of a defect in the
decidua basalis (3). If the
placenta attaches itself even more deeply into the muscle wall of
uterus, it will lead to placenta increta. The major morbidity
associated with this abnormal placentation is caused by the
significant blood loss that occurs during labour, resulting in a
longer maternal hospital stay and blood transfusion treatment
(4). Risk factors for placenta
previa include previous caesarean section delivery, grand
multiparity and recurrent miscarriage (5). Although the risk factors for placenta
previa are well defined, much less is known regarding its aetiology
and underlying molecular mechanisms.
Damage to the endometrium or myometrial uterine
lining during abortion or cesarean delivery may significantly
damage the endometrium and lead to inflammation, which increases
the risk for placenta previa (6).
In addition to the classic theory of inflammatory injury, abnormal
angiogenesis has recently been considered a novel mechanism
underlying placenta previa (7).
Data derived from animal and human studies demonstrate that various
regulatory molecules serve functional roles in controlling
trophoblast invasion and placental angiogenesis (8). As an inflammatory cytokine, the
effects of high mobility group box protein 1 (HMGB1) on
inflammation, tumourigenesis (9)
and pregnancy (10) have been
defined. Throughout gestation growth factors such as vascular
endothelial growth factor (VEGF) are abundantly secreted from
diverse cell types of the fetal-maternal interface and were shown
to promote proliferation, adhesion and/or invasion (11). The present study aimed to
investigate the expression levels of HMGB1 in the placenta, in
order to explore the possible mechanisms underlying placenta
previa.
Materials and methods
Study population
The present study was conducted between November
2014 and January 2015. A total of 37 women without other medical
and surgical diseases were recruited to the present study at the
Shanghai First Maternity and Infant Hospital, Tongji University
School of Medicine (Shanghai, China). A total of 22 women had
normal term pregnancies, whereas 15 women suffered from placenta
previa. In the placenta previa group, 7 women also suffered from
placenta accreta and 9 suffered from postpartum haemorrhage. All of
the 37 women underwent elective caesarean section. There were no
significant differences in age between women in the healthy and
placenta previa groups. Written informed consent was obtained from
all patients. All experiments, including any relevant details, were
approved in advance by the Ethics Committee of the Shanghai First
Maternity and Infant Hospital, Tongji University School of
Medicine, and were performed in accordance with relevant guidelines
and regulations.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
As soon as the placenta peeled from the uterine in
the caesarean section, samples were harvested from the parental
placenta around the umbilical cordunder sterile conditions, rinsed
by sterile saline, wiped with a gauze, treated with liquid nitrogen
and stored in 80°C. Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
cDNA was synthesised from total RNA using the mRNA 1st strand cDNA
synthesis kit (BioTNT, Inc., Shanghai, China), according to the
manufacturer's protocol. Template cDNA was subjected to PCR
amplification using gene-specific sense and antisense primers,
which were all designed by BioTNT via Primer 5.0 software, and
synthetized by Invitrogen; Thermo Fisher Scientific, Inc. (Table I). Reactions contained 1 µl cDNA
template, 2 µl forward primers, 2 µl reverse primer; and 10 µl PCR
Premix (BioTNT, Inc.) in a total volume of 20 µl. RT-qPCR
thermocycling conditions were as follows: Initial denaturation at
95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 5
sec, annealing and extension at 60°C for 30 sec. The expression
levels of each gene were standardised against the housekeeping gene
β-actin. mRNA expression levels were expressed as a ratio, using
the 2−ΔΔCq method for comparing the relative expression
results (12).
 | Table I.Polymerase chain reaction primers used
in the present study. |
Table I.
Polymerase chain reaction primers used
in the present study.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| HMGB1 | CTGGGAGAGATGTGGAAT
A |
GCAGCAATATCCTTTTCGT |
| VEGF-A |
TGTGCCCACTGAGGAGTC |
CATTTGTTGTGCTGTAGGA |
| β-actin | AAGGTGACAGCA GTCGGT
T |
TGTGTGGACTTGGGAGAGG |
Western blot analysis
Frozen samples were homogenized in liquid nitrogen.
Protein extracts were prepared by 1:1 dilution of the initial
homogenate with radioimmunoprecipitation assay buffer (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) in the presence of
protease inhibitors and extracted by ultracentrifugation. The
bicinchoninic acid assay (Pierce; Thermo Fisher Scientific, Inc.)
was used for protein quantitation. A total of 20 µg protein for
each sample was loaded and separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis, and were subsequently
transferred to polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA) in blotting buffer (25 mM Tris, 150 mM glycine
and 20% methanol) for 2 h at room temperature. After blocking with
5% skimmed milk in PBS for 1 h at room temperature, the membranes
were then incubated with anti-human HMGB1 rabbit immunoglobulin
(Ig)G antibody (catalog no. ab79823; 1:1,000; Abcam, Cambridge, MA,
USA) or anti-human (VEGF) rabbit IgG antibody (catalog no. ab46154;
1:1,000; Abcam) at 4°C overnight. Anti-β-actin (catalog no. 4970;
1:3,000; Cell Signaling Technology, Inc., Danvers, MA, USA) was
used as an internal control. The membranes were further incubated
for 1 h at room temperature with peroxidase-labelled secondary
antibodies (catalog no. sc-2004; 1:1,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). The bands were detected using an enhanced
chemiluminescence system (Pierce; Thermo Fisher Scientific, Inc.).
The protein bands for HMGB1 or VEGF were semi-quantified and
normalised to the control band using ImageQuant LAS 4000 (GE
Healthcare Life Sciences, Chalfont, UK).
Immunohistochemistry
Placental tissues were fixed with 10% formalin for
24 h, embedded with paraffin and sliced continuously to sections of
4 mm. Slides were preheated at 70°Cfor 1 h prior to
deparaffinization and rehydration with ethanol. Antigen retrieval
was performed in citrate buffer at 95°C for 15 min. For detection
of HMGB1 expression, the slides were incubated with anti-HMGB1
antibody-ChIP Grade (catalog no. ab18256, 1:100; Abcam) in 5%
bovine serum albumin (Sigma-Aldrich, Merck KGaA, Darmstadt,
Germany) for 45 min at room temperature, and were then incubated
with horseradish peroxidase-coupled to streptavidin-conjugated
secondary antibody (catalog no. sc-2004; 1:1,000; Santa Cruz
Biotechnology, Inc.) for 30 min at room temperature. Slides stained
without the primary antibody were used as negative controls. The
resulting signal was developed with diaminobenzidine
(Sigma-Aldrich, Merck KGaA), according to the manufacturer's
protocol, and the sections were counter stained with Mayer's
haematoxylin. Staining for VEGF (catalog no. ab46154; 1:1,000;
Abcam) was performed using the some staining protocol.
Evaluation of
immunohistochemistry
The evaluated sections spanned the whole placental
tissue, from the fetal membranes to the decidual plate.
Trophoblasts, mesenchymal cells, and villous vascular endothelial
cells from the stem villous, mature intermediate villous and
terminal villous, were evaluated.
A semi-quantitative scale was used to evaluate HMGB1
and VEGF staining, as follows: Stain intensity was scored between 0
and 3 (0, no staining; 1, weak but detectable; 2, moderate or
distinct; 3, intense) and was multiplied by the average percentage
of positive staining, which was scored between 0 and 4 (0, no
positive cells; 1, 0–25% positive cells; 2, 26–50% positive cells;
3, 51–75% positive cells; 4, 76–100% positive cells) (13,14).
In each slide, five areas were evaluated under a microscope (×400
original magnification). Evaluation of immunohistochemistry was
performed blindly by three independent investigators and the
average score was used. The results were assessed by a pathologist
under a light microscope. The agreement between the different
investigators was >90%.
Statistical analysis
Each experiment was repeated three times
independently. Results from each independent experiment were
expressed as the mean ± standard deviation. Analysis of enumeration
data was performed using Pearson χ2 test. Measurement
data were assessed by independent-samples t-test. Statistical
analysis of immunohistochemistry results was performed with the
Mann-Whitney U-test. Statistical analyses were conducted using SPSS
19.0 statistical software (IBM SPSS, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinical characteristics of the
enrolled women
The demographic, clinical and pregnancy outcome
characteristics of 37 women are presented in Table II. Women with placenta previa had
a significantly increased history of abortion and caesarean section
births, both of which are known risk factors for placenta previa.
Compared with the control group, women in the placenta previa group
delivered at an earlier gestational age and gave birth to babies of
a lower birth weight. Women with placenta previa had a higher
frequency of placental accrete, thus resulting in more cases of
postpartum haemorrhage and blood transfusion.
 | Table II.Demographic characteristics and
outcome data. |
Table II.
Demographic characteristics and
outcome data.
| Variable | Placenta previa
(n=15) | Controls (n=22) | P-value |
|---|
| Characteristics at
enrolment |
|
|
|
| Maternal
age, years | 30.87±2.16 | 30.77±1.99 | 0.9340 |
|
Gestational age, weeks |
37±1.0 |
39±0.6 | <0.0001 |
| Abortion
history, n (%) | 10 (66.7) | 5 (22.7) | 0.0160 |
| Prior
caesarean section, n (%) | 2
(13.3) | 0 | 0.0780 |
| Outcome
characteristics |
|
|
|
| Placenta
accreta, n (%) | 7
(46.7) | 0 (0) | 0.0010 |
| Birth
weight, g | 3,030±388 | 3,413±260 | 0.0100 |
|
Postpartum haemorrhage,
ml |
902±1,194 |
302±11 | 0.0230 |
| Blood
transfusion, n (%) | 3
(12.5) | 0 (0) | 0.0290 |
|
Caesarean hysterectomy, n
(%) | 0 (0) | 0 (0) | N/A |
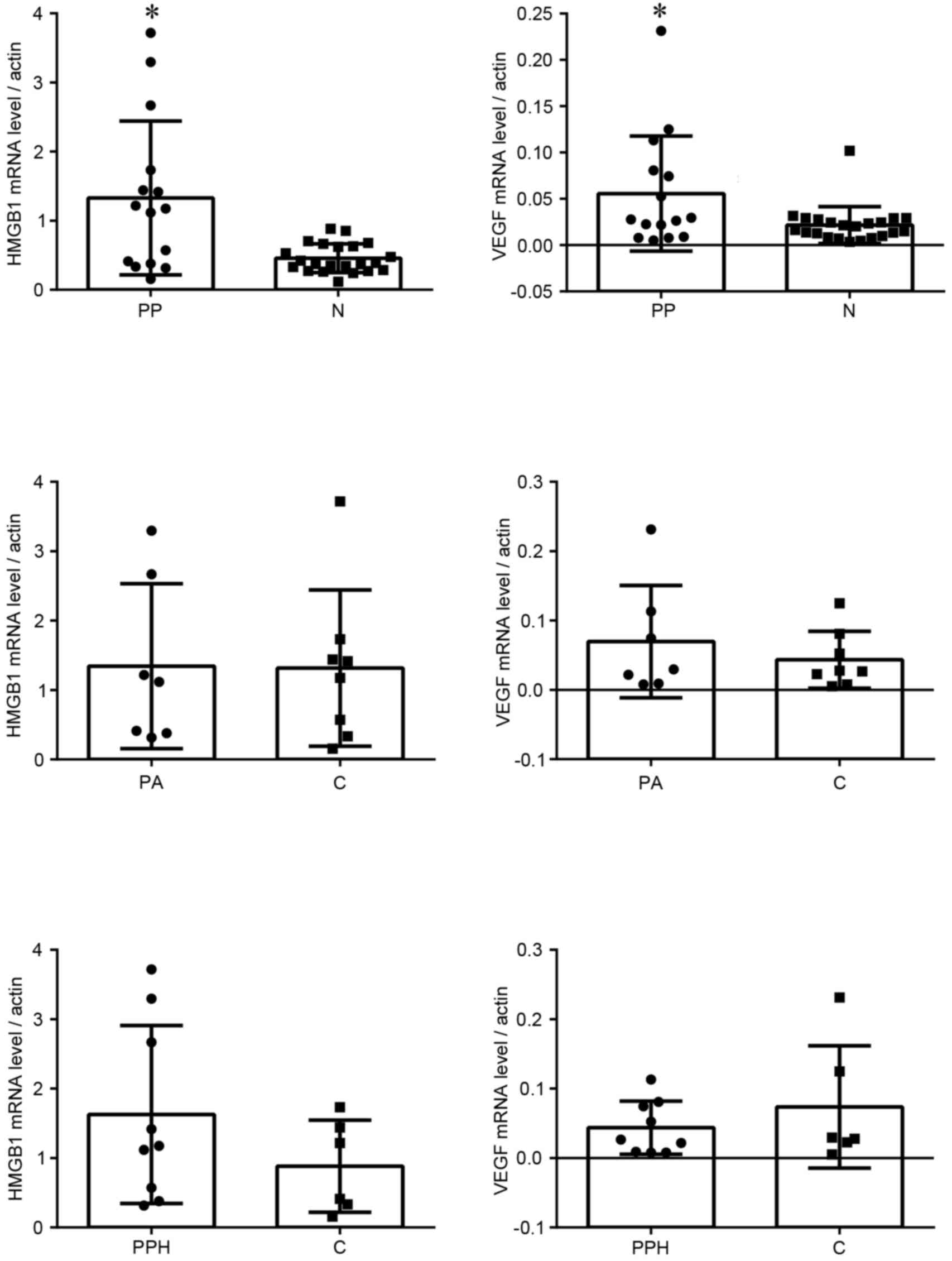
mRNA expression levels of HMGB1 and
VEGF
The mRNA expression levels of HMGB1 and VEGF were
analysed by RT-qPCR. The mRNA expression levels of HMGB1 and VEGF
in the placenta previa group were significantly higher compared
with in the normal group (Fig. 1).
In the placenta previa group, there were no significant differences
in HMGB1 and VEGF mRNA expression between groups with or without
placenta accreta, or between groups with or without postpartum
haemorrhage.
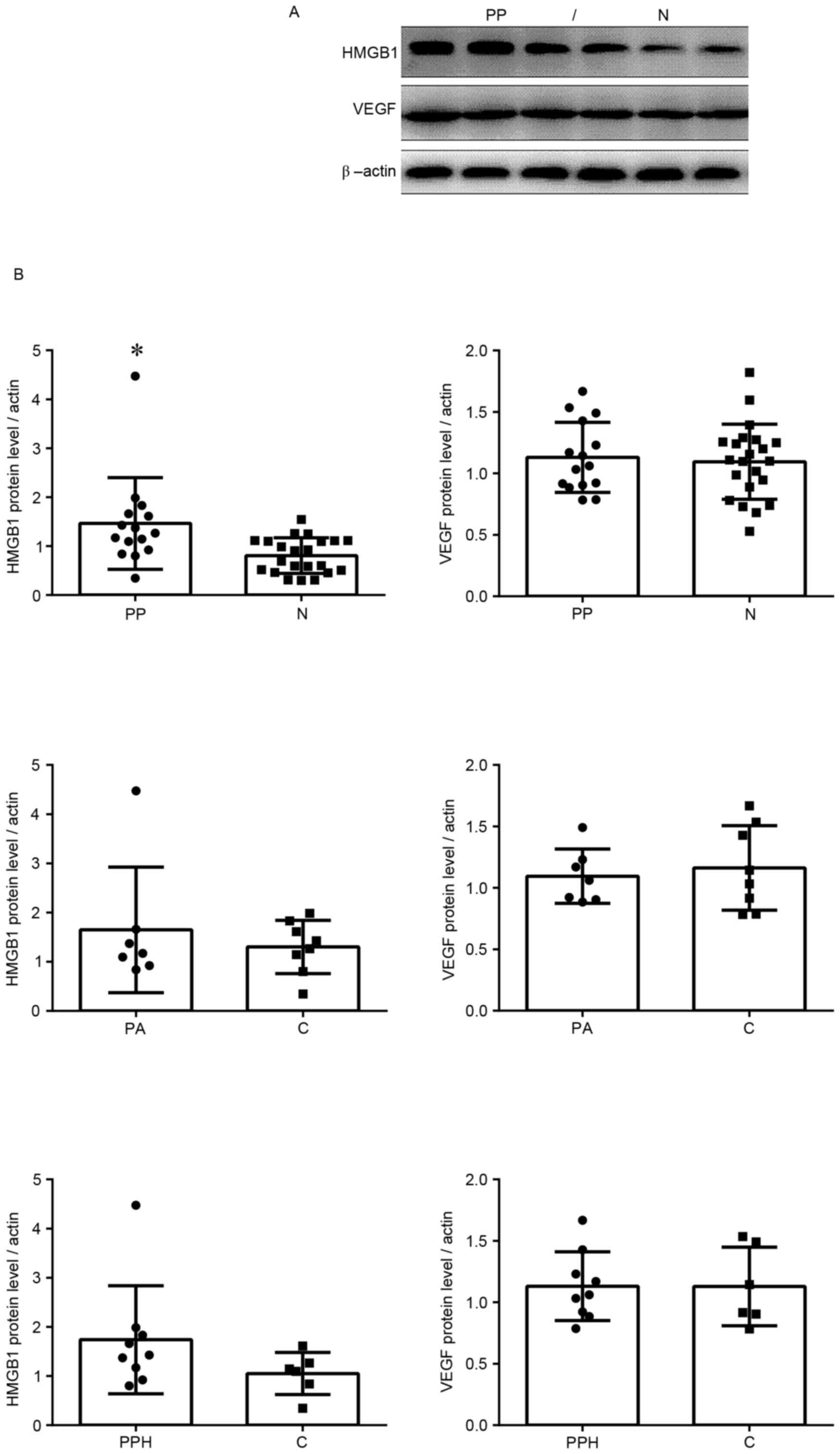
Protein expression levels of HMGB1 and
VEGF
Protein expression was measured by western blotting.
The protein expression levels of HMGB1 in the placenta previa group
were significantly increased compared with in the normal group
(Fig. 2). These findings were
similar to the results of the RT-qPCR analysis. In addition, VEGF
protein expression levels were higher in the placenta previa group
compared with in the normal group; however, this finding was not
statistically significant. In the placenta previa group, there were
no significant differences in the protein expression levels of
HMGB1 and VEGF between groups with or without placenta accreta, or
between groups with or without postpartum haemorrhage.
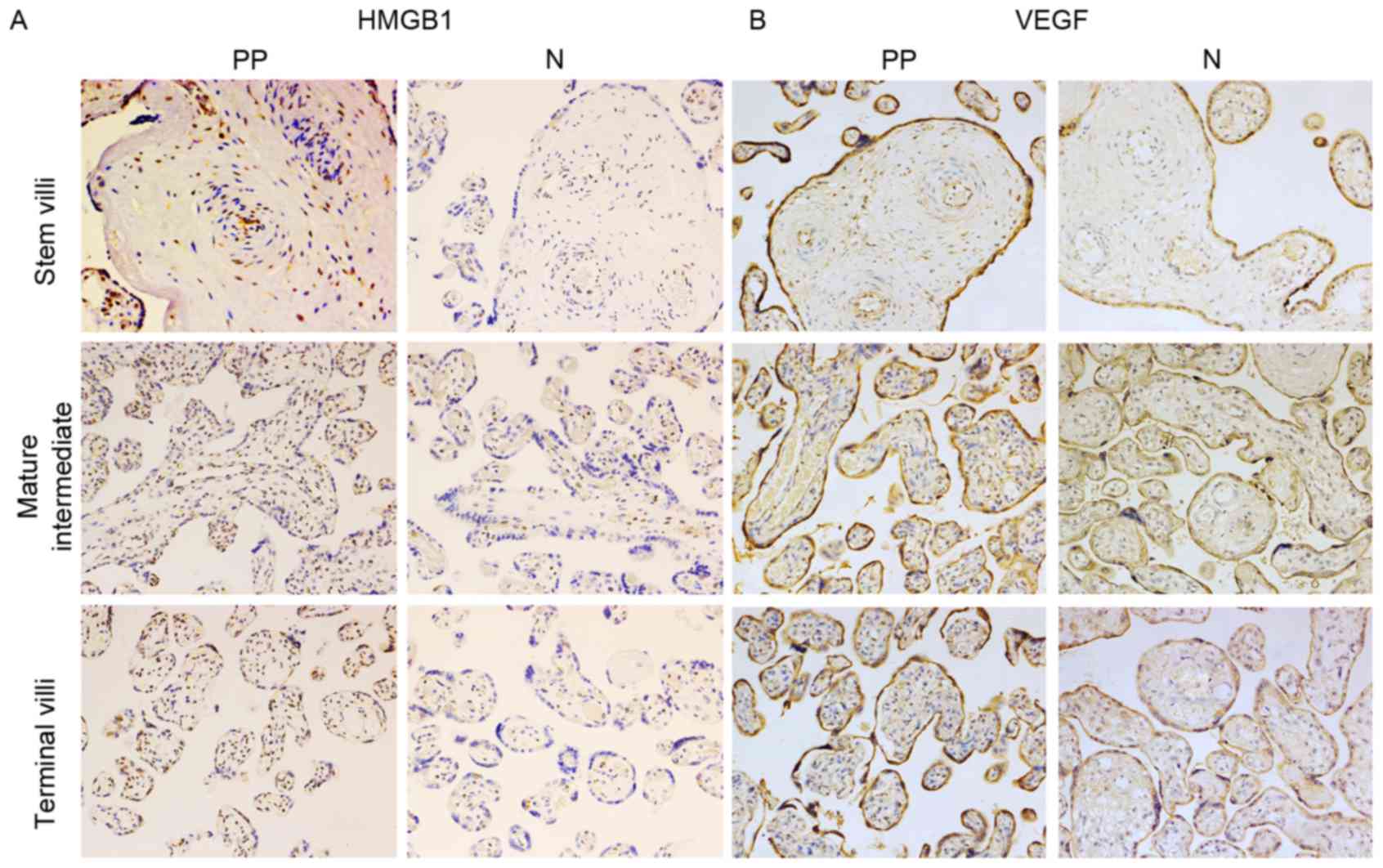
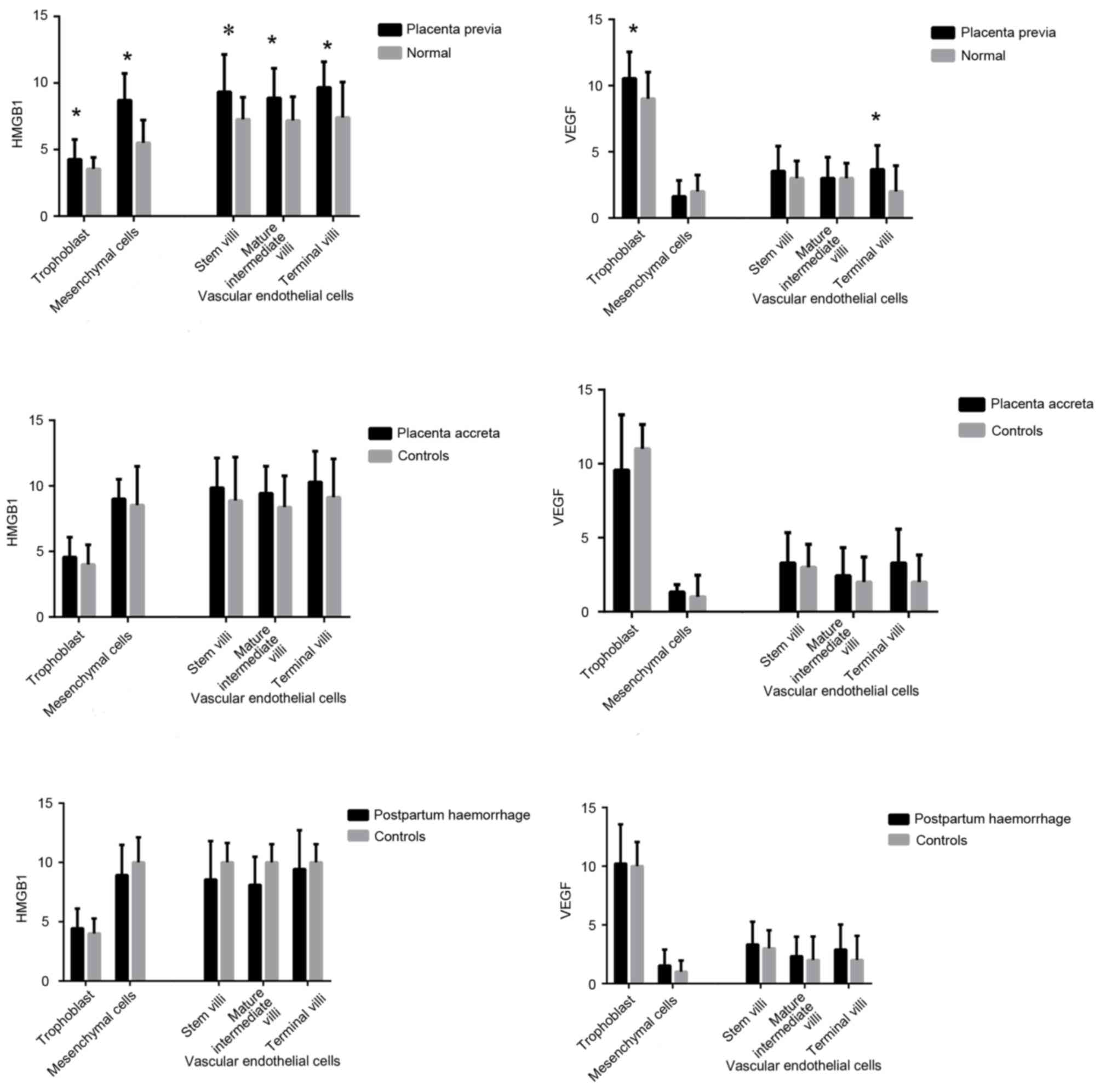
Histological and immunohistochemical
examination of HMGB1 in placental tissue
HMGB1 and VEGF protein expression was detected in
placental tissue using immunohistochemistry (Fig. 3). The present study detected strong
nuclear expression of HMGB1 in vascular endothelial cells and
mesenchymal cells from the placenta of women who had normal
pregnancies. Immunohistochemical staining also revealed that HMGB1
was slightly expressed in the placental syncytiotrophoblast and
cytotrophoblastnuclei. In the placenta previa group homogeneous
cytoplasmic expression of HMGB1 was detected in vascular
endothelial cells and mesenchymal cells (Fig. 3A). Furthermore, in the placenta
previa group increased HMGB1 staining was observed in vascular
endothelial cells covering the peripheral chorionic villi, compared
with in the normal group. In mesenchymal cells, there was a
significant trend toward a higher mean staining intensity in the
placenta previa group. The HMGB1 staining score also differed in
trophoblast cells between the placenta previa and normal groups. To
investigate if HMGB1 is associated with the depth of placental
invasion and postpartum haemorrhage, the present study divided the
placenta previa group into placenta accreta and control groups.
There was no significant difference between the placenta accreta
and control groups. Similarly, the placenta previa group was
divided into the postpartum haemorrhage and control groups; no
statistically significant difference was detected between these two
groups (Fig. 4).
Histological and immunohistochemical
examination of VEGF in placental tissue
VEGF was strongly and consistently expressed in
trophoblasts and vascular endothelial cells covering the peripheral
chorionic villi, predominantly in the cytoplasm of
syncytiotrophoblasts and cytotrophoblasts, in both placenta previa
and normal placental samples (Fig.
3B). According to the VEGF staining scores there were
significant differences in VEGF expression in the villous
trophoblasts and vascular endothelial cells of the terminal villi
between the placenta previa and normal groups. As aforementioned,
the placenta previa group was divided into placenta accreta and
control groups; there were no significant differences in VEGF
expression between these two groups. In addition, VEGF expression
was not associated with postpartum haemorrhage (Fig. 4).
Discussion
Placenta previa is often associated with preterm
delivery, reduced birth weight, a higher frequency of placental
accreta and postpartum haemorrhage, and an increased likelihood of
blood transfusion treatment. Considerable rates of maternal and
fetal morbidity and mortality are associated with placenta previa;
therefore, placenta previa is associated with a high demand for
health care resources. A previous study regarding placenta previa
focused on clinical epidemiology, ultrasonic prediction and
vascular characteristics of the maternal-fetal interface, using
digital technology (3). Research
regarding the underlying molecular mechanisms of placenta previa is
relatively rare. In recent years, studies into the molecular
regulation of trophoblast cell infiltration and placental
angiogenesis have garnered attention.
HMGB1 is constitutively and abundantly expressed in
almost every eukaryotic cell type (15). HMGB1 is able to promote tumour cell
migration (16) and has been
hypothesised to be a potent cytokine mediating the late response to
infection, injury and inflammation (17–20).
Since labour is an inflammatory-like process, HMGB1
may also be involved in embryogenesis and the process of pregnancy
(21). Therefore, it may be
hypothesised that there is a difference in the placental expression
of HMGB1 between normal pregnancies and pregnancies complicated by
intrauterine infection (22). Wang
et al (10) investigated
HMGB1 was expressed highly in preeclamptic placental tissue, which
is a pregnancy-related complication characterised by poor
placentation resulting in hypoxic placental conditions and an
increased inflammatory response.
Based on the pathogenesis of inflammatory injury and
angiogenesis of placenta previa, the present study hypothesised
that HMGB1 may promote the occurrence of placenta previa.
Therefore, the study aimed to determine the expression levels
ofHMGB1 in placental tissues from women with or without placenta
previa. The results confirmed that the mRNA expression levels of
HMGB1 in the placenta were markedly higher in the placenta previa
group compared with in the control group, as determined by RT-qPCR.
In addition, the protein expression levels of HMGB1 were detected
by western blotting; similarly, HMGB1 protein levels were increased
in the placenta previa group. These findings suggested that HMGB1
may be associated with the emergence and progression of placenta
previa.
PCR and western blotting were used to determine the
mRNA and protein expression levels of HMGB1 in whole placental
tissue samples. To determine if there were differences among the
various cell types, immunohistochemistry was conducted.
Understanding the location of HMGB1 expression may provide the
basis for the selection of cell types in further cell function
studies. As expected, in addition to strong nuclear HMGB1
expression in almost all cells in the studied placentas, an
individual variation in cytoplasmic HMGB1 expression was detected
in trophoblasts and vascular endothelial cells. Specifically, a
higher cytoplasmic expression of HMGB1 was detected in trophoblasts
and vascular endothelial cells from placenta previa placentas
compared with in placentas from women with healthy pregnancies. The
intracellular abundance of HMGB1 and its proinflammatory activities
suggested that its release/secretion at tissue damage sites may
serve an important role during inflammatory and immune responses
(23). A previous study (24) demonstrated notable cytokine-like
roles for extracellular HMGB1. Extracellular HMGB1 regulates
cytokine expression and induces inflammatory cell recruitment.
Furthermore, HMGB1 may stimulate migration of adherent cells, such
as fibroblasts and smooth muscle cells (25). Therefore, extracellular HMGB1 may
be regarded as a tissue injury signal and an inflammatory mediator.
These findings suggested that, as a proinflammatory cytokine, HMGB1
may be secreted to the extracellular matrix, participate in the
inflammatory response and prompt the progression of placenta
previa.
The immunohistochemistry results demonstrated that
HMGB1 expression was markedly increased in villous vascular
endothelial cells and mesenchymal cells in the placenta previa
group. These results indicated that HMGB1 may have a relevant role
in angiogenesis. According to the theory of embryonic development,
villous vascular endothelial cells and mesenchymal cells originate
from the mesenchymal cells of embryonic mesoderm, and then
differentiate into vascular and connective tissues, alongside
development of the placental villus. Therefore, protein expression
is always relatively consistent in these cells. This was confirmed
in the results of the present study, which demonstrated that HMGB1
expression was higher in villous vascular endothelial cells and
mesenchymal cells than in trophoblast, whereas the opposite was
observed for expression of VEGF. Due to the importance of
neovascularization at the site of injured tissue, where blood flow
restoration is often required for the initiation of an immune
response to pathogens and for subsequent successful wound repair
(26), the capacity of
extracellular HMGB1 to exert a potent angiogenic activity
strengthens the importance of HMGB1 as a cytokine. The
immunohistochemistry results may also indicate that HMGB1 exerts a
prominent role in numerous processes of specific interest for the
placenta, such as angiogenesis, in addition to its potent
proinflammatory capacities.
Given its cytokine features, a previous study
investigated the capacity of HMGB1 to modulate the various steps of
angiogenesis in vitro, and examined its proangiogenic
activity in vivo (27). In
the ischemic muscle of diabetic mice, HMGB1 administration restored
blood flow recovery and capillary density; this process was
associated with the increased expression of VEGF, whereas
HMGB1-induced angiogenesis was significantly reduced following
suppression of VEGF activity (28). In addition, patients with non-small
cell lung cancer have been reported to possess a higher serum
concentration of HMGB1 and VEGF (29,30).
In oesophageal squamous cell carcinoma, HMGB1 was highly expressed
and affected the prognosis of patients via regulation of VEGF-C
expression, which promoted lymph angiogenesis and lymph node
metastasis (30). In a previous
study regarding preeclampsia, it was suggested that the tendency
towards a higher expression of HMGB1 in preeclamptic placentas may
be a result of hypoxia, and may be considered a compensatory
mechanism for the placenta to attempt to increase vascularization
(31). Due to the findings of
these previous studies and the similarities between placental
angiogenesis and tumour growth, HMGB1 may be considered to serve an
important role in placentation; however, this remains to be
investigated.
Although the cause of placenta previa remains
unknown, growing evidence has suggested that an imbalance between
pro- and anti-angiogenic factors may have a fundamental role in its
pathogenesis. Therefore, it was hypothesised that HMGB1 may
regulate placental angiogenesis in placenta previa through the
expression of VEGF. In the present study, the expression levels of
VEGF were detected in placental samples from women with placenta
previa by RT-qPCR and western blot analysis. The results indicated
that the mRNA expression levels of VEGF were significantly higher
in placental tissue from the placenta previa group, whereas there
was no significant difference in the protein expression of VEGF
between the placenta previa and normal groups. This may be due to
the limited sample numbers in the present study. Subsequently,
immunohistochemical staining was used to locate VEGF protein
expression. A strong cytoplasmic VEGF expression was detected in
villous trophoblasts and vascular endothelial cells, particularly
in trophoblasts. Furthermore, VEGF expression was significantly
increased in the placenta previa group. This finding is in
accordance with the theory that VEGF regulates trophoblast invasion
through autocrine modes of action, and promotes placental
vascularization in a paracrine manner (32).
Placenta accreta is associated with a highly
regulated inflammatory-like response and vascularization. Size and
spatial organization of the placenta-increta vascular architecture
at the placental-maternal interface differs from normal and may
partially explain the severe haemorrhage observed during delivery
of placenta-increta (7). Since
HMGB1 is a potent inflammatory and proangiogenic cytokine, the
present study aimed to investigate placental HMGB1 expression in
relation to placenta accreta and postpartum haemorrhage. The
placenta previa group was divided into placenta accrete and
postpartum haemorrhage groups. However, there were no differences
in the expression of HMGB1 and VEGF in the placenta
accreta/postpartum haemorrhage groups compared with in the control
placenta previa group. These results suggested that placental
angiogenesis may have a role in placenta increta, where the
placenta attaches itself even more deeply into the muscle wall of
uterus, rather than in placenta accreta. However, these negative
findings may be associated with the limited sample numbers.
In conclusion, the present study demonstrated that
HMGB1 may participate in the progression of placenta previa, not
only through its role as a proinflammatory cytokine but also as a
proangiogenic cytokine. However, the precise role of HMGB1 in
placenta previa and the underlying molecular mechanisms remain to
be elucidated. In addition, the signalling pathways through which
HMGB1 mediates VEGF expression in placenta previa require further
study.
Acknowledgements
The present study was funded by the Health and
Family Planning commission of Pudong District, Shanghai (grant no.
PW2012D-10); the Key Program of Health and Family Planning
commission of Shanghai (grant no. 20124037); the Shanghai Hospital
Development Center Grant (grant no. SHDC12012116); the Natural
Science Foundation of Shanghai (grant no. 13ZR1432900); the
Shanghai Science and Technology Committee (grant no. 134119a0800);
the Industrial, Teaching and Research Cooperative Program in
Medical Field, Science and Technology Commission of Shanghai
Municipality (grant no. 13DZ1931002); and the National Natural
Science Foundation of China (NSFC) (grant no. 81200443).
References
|
1
|
Royal College of Obstetricians and
Gynaecologists (RCOG), . Placenta Praevia, Placenta Praevia Accreta
and Vasa Praevia: Diagnosis and Management. RCOG Green-top
Guideline No. 27. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_27.pdfJanuary.
2011
|
|
2
|
Oya A, Nakai A, Miyake H, Kawabata I and
Takeshita T: Risk factors for peripartum blood transfusion in women
with placenta previa: A retrospective analysis. J Nippon Med Sch.
75:146–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Publications Committee, Society for
Maternal-Fetal Medicine and Belfort MA Placenta accreta. Am J
Obstet Gynecol. 203:430–439. 2010.PubMed/NCBI
|
|
4
|
Usta IM, Hobeika EM, Musa AA, Gabriel GE
and Nassar AH: Placenta previa-accreta: Risk factors and
complications. Am J Obstet Gynecol. 193:1045–1049. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gielchinsky Y, Rojansky N, Fasouliotis SJ
and Ezra Y: Placenta accreta-summary of 10 years: A survey of 310
cases. Placenta. 23:210–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ananth CV, Smulian JC and Vintzileos AM:
The association of placenta previa with history of cesarean
delivery and abortion: A meta analysis. Am J Obstet Gynecol.
177:1071–1078. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chantraine F, Blacher S, Berndt S,
Palacios-Jaraquemada J, Sarioglu N, Nisolle M, Braun T, Munaut C
and Foidart JM: Abnormal vascular architecture at the
placental-maternal interface in placenta increta. Am J Obstet
Gynecol. 207:188.e1–189.e1. 2012. View Article : Google Scholar
|
|
8
|
Knofler M: Critical growth factors and
signalling pathways controlling human trophoblast invasion. Int J
Dev Biol. 54:269–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abe A, Kuwata T, Yamauchi C, Higuchi Y and
Ochiai A: High mobility group box1 (HMGB1) released from cancer
cells induces the expression of pro-inflammatory cytokines in
peritoneal fibroblasts. Pathol Int. 64:267–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang B, Koga K, Osuga Y, Hirata T, Saito
A, Yoshino O, Hirota Y, Harada M, Takemura Y, Fujii T, et al: High
mobility group box 1 (HMGB1) levels in the placenta and in serum in
preeclampsia. Am J Reprod Immunol. 66:143–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knofler M: Critical growth factors and
signalling pathways controlling human trophoblast invasion. Int J
Dev Biol. 54:269–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siu MK, Chan HY, Kong DS, Wong ES, Wong
OG, Ngan HY, Tam KF, Zhang H, Li Z, Chan QK, et al: p21-activated
kinase 4 regulates ovarian cancer cell proliferation, migration,
and invasion and contributes to poor prognosis in patients. Proc
Natl Acad Sci USA. 107:pp. 18622–18627. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu W, Xia YH, Qu JJ, He YY, Li BL, Lu C,
Luo X and Wan XP: p21-activated kinase 4 regulation of endometrial
cancer cell migration and invasion involves the ERK1/2 pathway
mediated MMP-2 secretion. Neoplasma. 60:493–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vitali R, Stronati L, Negroni A, Di Nardo
G, Pierdomenico M, del Giudice E, Rossi P and Cucchiara S: Fecal
HMGB1 is a novel marker of intestinal mucosal inflammation in
pediatric inflammatory bowel disease. Am J Gastroenterol.
106:2029–2040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nehil M, Paquette J, Tokuyasu T and
McCormick F: High mobility group box 1 promotes tumor cell
migration through epigenetic silencing of semaphorin 3A. Oncogene.
33:5151–5162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang P, Pan HC, Lin SL, Zhang WQ, Rauvala
H, Schachner M and Shen YQ: HMGB1 contributes to regeneration after
spinal cord injury in adult zebrafish. Mol Neurobiol. 49:472–483.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palone F, Vitali R, Cucchiara S,
Pierdomenico M, Negroni A, Aloi M, Nuti F, Felice C, Armuzzi A and
Stronati L: Role of HMGB1 as a suitable biomarker of subclinical
intestinal inflammation and mucosal healing in patients with
inflammatory bowel disease. Inflamm Bowel Dis. 20:1448–1457. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang PS, Kim DH, Lee YJ, Lee SE, Kang WJ,
Chang HJ and Shin JS: Glycyrrhizin, inhibitor of high mobility
group box-1, attenuates monocrotaline-induced pulmonary
hypertension and vascular remodeling in rats. Respir Res.
15:1482014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Campana L, Santarella F, Esposito A,
Maugeri N, Rigamonti E, Monno A, Canu T, Del Maschio A, Bianchi ME,
Manfredi AA, et al: Leukocyte HMGB1 is required for vessel
remodeling in regenerating muscles. J Immunol. 192:5257–5264. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhutada S, Basak T, Savardekar L, Katkam
RR, Jadhav G, Metkari SM, Chaudhari UK, Kumari D, Kholkute SD,
Sengupta S, et al: High mobility group box 1 (HMGB1) protein in
human uterine fluid and its relevance in implantation. Hum Reprod.
29:763–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Girard S, Heazell AE, Derricott H, Allan
SM, Sibley CP, Abrahams VM and Jones RL: Circulating cytokines and
alarmins associated with placental inflammation in high-risk
pregnancies. Am J Reprod Immunol. 72:422–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitola S, Belleri M, Urbinati C, Coltrini
D, Sparatore B, Pedrazzi M, Melloni E and Presta M: Cutting edge:
Extracellular high mobility group box-1 protein is a proangiogenic
cytokine. J Immunol. 176:12–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee SA, Kwak MS, Kim S and Shin JS: The
role of high mobility group box 1 in innate immunity. Yonsei Med J.
55:1165–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Degryse B, Bonaldi T, Scaffidi P, Muller
S, Resnati M, Sanvito F, Arrigoni G and Bianchi ME: The high
mobility group (HMG) boxes of the nuclear protein HMG1 induce
chemotaxis and cytoskeleton reorganization in rat smooth muscle
cells. J Cell Biol. 152:1197–1206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frantz S, Vincent KA, Feron O and Kelly
RA: Innate immunity and angiogenesis. Circ Res. 96:15–26. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schlueter C, Weber H, Meyer B, Rogalla P,
Roser K, Hauke S and Bullerdiek J: Angiogenetic signaling through
hypoxia: HMGB1: An angiogenetic switch molecule. Am J Pathol.
166:1259–1263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Biscetti F, Straface G, De Cristofaro R,
Lancellotti S, Rizzo P, Arena V, Stigliano E, Pecorini G, Egashira
K, De Angelis G, et al: High-mobility group box-1 protein promotes
angiogenesis after peripheral ischemia in diabetic mice through a
VEGF-dependent mechanism. Diabetes. 59:1496–1505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Naumnik W, Nilklinska W, Ossolinska M and
Chyczewska E: Serum levels of HMGB1, survivin, and VEGF in patients
with advanced non-small cell lung cancer during chemotherapy. Folia
Histochem Cytobiol. 47:703–709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chuangui C, Peng T and Zhentao Y: The
expression of high mobility group box 1 is associated with lymph
node metastasis and poor prognosis in esophageal squamous cell
carcinoma. Pathol Oncol Res. 18:1021–1027. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holmlund U, Wahamaa H, Bachmayer N, Bremme
K, Sverremark-Ekstrom E and Palmblad K: The novel inflammatory
cytokine high mobility group box protein 1 (HMGB1) is expressed by
human term placenta. Immunology. 122:430–437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tseng JJ, Chou MM, Hsieh YT, Wen MC, Ho ES
and Hsu SL: Differential expression of vascular endothelial growth
factor, placenta growth factor and their receptors in placentae
from pregnancies complicated by placentaaccreta. Placenta.
27:70–78. 2006. View Article : Google Scholar : PubMed/NCBI
|