Introduction
Osteoporosis is a very common disease in
postmenopausal women. It is frequently associated with bone
fractures, which are a major health problem worldwide (1). Osteoporosis is characterized by a
reduction in bone mass caused by a disturbance in the ratio between
bone formation and bone resorption. This imbalance may be
associated with low blood serum estrogen levels in postmenopausal
women (2). Estrogen deficiency is
recognized as an important risk factor in the development of
postmenopausal osteoporosis (3).
Although prolonged exposure to relatively high doses of estrogen
may result in sustained stimulation of osteoblastic activity in
postmenopausal women (4), the
precise mechanism by which estrogen enhances bone formation is not
clear.
Osteoblast precursors are considered to be derived
from marrow mesenchymal stem cells (MSCs) (5). It has been hypothesized that estrogen
increases bone formation by enhancing the proliferation and
differentiation of MSCs. Primary bone marrow stromal cells (BMSCs)
are multipotent and capable of differentiating along the
osteoblastic cell lineage (6). Rat
BMSCs (rBMSCs) have been used in previous tissue engineering
studies because they are strongly adherent, highly proliferative
and readily available (7,8). Therefore, rBMSCs were used in the
present study to examine the contribution of estrogen to osteogenic
differentiation.
Mitogen-activated protein kinases (MAPKs) are
involved in the regulation of gene expression, mitosis, metabolism,
motility, survival, apoptosis and differentiation of stem cells.
MAPK subfamilies mediate various biological effects: Extracellular
signal-regulated kinase 1 (ERK1) and ERK2 regulate cell
differentiation and proliferation; c-Jun N-terminal kinases (JNKs)
have an important role in cell cycle regulation, apoptosis and
cellular stress; and p38 MAPKs affect numerous biological
processes, such as proliferation, differentiation and survival, and
responses to stress and inflammation in certain cell types
(9). A number of previous studies
have focused on the functions of ERK1/2 and p38 MAPKs in other
contexts, such as tumor and diabetes studies (10,11).
MAPKs may also be activated during osteogenesis. The JNK signaling
pathway has previously been demonstrated to serve a role in the
regulation of osteogenic differentiation of MSCs (12). The present study hypothesized that
estradiol may enhance the induction of osteogenesis by upregulating
the JNK signaling pathway. To validate this hypothesis the
underlying mechanisms of estradiol-induced osteogenesis in rBMSCs
were investigated.
Materials and methods
Ethics statement
A total of 5 male Sprague-Dawley rats (age, 6 weeks;
weight 200±10 g) were purchased from Vital River Laboratories Co.,
Ltd. (Beijing, China). Rats were housed in a climate-controlled
environment (temperature, 22±1°C; humidity, 50±5%) with a 12/12 h
light/dark cycle and ad libitum access to food and water.
All procedures were performed under 10% chloral hydrate anesthesia
when necessary. The rats were sacrificed by asphyxiation with
CO2. All experimental procedures were approved by
(permit no. 2011-167) and followed the guidelines of the Animal
Ethics Committee of Liaoning University of Traditional Chinese
Medicine (Shenyang, China).
Primary culture of rBMSCs
rBMSCs were isolated from the bone marrow of the
posterior tibias and femurs of 8-week-old Sprague-Dawley rats. Bone
marrow cells were flushed out with α-minimum essential medium
(α-MEM; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and
centrifuged at 300 × g for 3 min. rBMSCs adhering to the plastic
surface were selected for further experimentation. Primary cells
(1×107 cells/ml) were cultured with α-MEM containing 10%
FBS (HyClone; GE Healthcare Life Sciences) for 5 days (37°C, 5%
CO2), changing the medium every 3 days. At 80%
confluence, the cells were passaged at a ratio of 1:2. All
experiments were performed using cells cultured to the fourth
passage.
Flow cytometric analysis
Cells were grown to passage 4, washed twice with PBS
and incubated with 0.25% trypsin for ~1 min; the reaction was
stopped by adding PBS. Trypsinized cells were transferred to tubes
at a density of 1×106 cells/ml. The cells were then
stained with antibodies (1:200) against cell surface markers CD29
(cat. no. 102215), CD90.1 (cat. no. 202515) and CD45 (cat. no.
202207) (BioLegend, Inc., San Diego, CA, USA) in the dark for 30
min at 4°C, followed by centrifugation at 300 × g for 5 min.
Following centrifugation, cells were washed with PBS (1 ml),
centrifuged at 300 × g for 5 min and resuspend in 300 µl PBS. Cells
were analyzed using a BD FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA), and the FlowJo software (FlowJo,
LLC, Ashland, OR, USA).
Alkaline phosphatase (ALP) activity
assay
A colorimetric p-nitrophenyl phosphate (pNPP) assay
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to
evaluate ALP activity and to quantify the osteogenesis of rBMSCs
cultured in either induction medium (IND group; 10−7 mol
dexamethasone, 50 µmol ascorbic acid and 10 mmol/l sodium
β-glycerophosphate) or induction medium supplemented with estradiol
(E2 group) at concentrations of 10−6, 10−7,
10−8 and 10−9 mol. ALP activity was assayed
at days 5, 7 and 9. Briefly, rBMSCs (5×104 cells) were
diluted in 0.2 ml of control (α-MEM supplemented with 10% FBS), IND
or E2 medium and transferred to a 96-well plate (8
wells/experimental group). Triton X-100 (100 µl; 0.1% Triton X-100)
was then added and the cells were incubated at −20°C overnight.
Following overnight incubation, cells were lysed in 100 µl
pNPP/diethanolamine buffer (1:1) at 37°C for 30 min. The reaction
was terminated by adding 80 µl NaOH buffer (0.5 mol). All wells
were analyzed in a microplate reader at an optical density of 415
nm.
ALP staining assay
The ALP staining assay comprised 3 experimental
groups: i) A control group, which consisted of rBMSCs cultured in
growth medium (10% FBS in α-MEM); ii) an IND group; and iii) an E2
group cultured in IND + estradiol (10−8 mol). Each group
was further divided into 2 treatment groups in which the medium was
supplemented with or without the JNK-specific inhibitor SP600125
(10 µmol, 37°C, 30 min) (Merck KGaA). The 6 experimental groups
were subjected to ALP staining assays using the Gomori method.
Briefly, cells were seeded at a density of 1×105
cells/well in 24-well plates with 3 wells/group. Following
incubation for 14 days at 37°C, the cells were stained with fresh
ALP incubation buffer (2% barbitone sodium, 2% anhydrous calcium
chloride, 3% β-sodium glycerol-phosphate, 2% magnesium sulfate and
5 ml distilled water, pH 9.4) for 4 h at 37°C. The cells were
rinsed with ultrapure water for 10 min, treated with 2% cobalt
nitrate for 5 min, followed by 1% ammonium sulfide solution for 1
min. After drying, the stained cells were imaged under a light
microscope and quantitative analysis was conducted using the
BI-2000 digital image analysis system (Chengdu TME Technology Co.,
Ltd., Chengdu, China).
Alizarin red S staining
Alizarin red S staining was used to examine the
mineralized nodule formation of rBMSCs. Cells were prepared as
described for the ALP staining assay and cultured for 21 days. The
cells were stained with alizarin red S [0.1% (w/v) in Tris-HCl] for
2 h at 37°C, then washed three times with PBS. Finally, mineralized
nodules were imaged under an inverted light microscope and
quantitative analysis was conducted using the BI-2000 digital image
analysis system (Chengdu TME Technology Co., Ltd.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR analysis was performed on the 6 experimental
groups described in the aforementioned ALP staining assay. Cells
(3×105) were seeded in flasks and cultured with the
relevant incubation medium (control, IND or E2) with or without 10
µmol SP600125 for 7 days. Total RNA was extracted with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), and quantified with a spectrophotometer at 260/280 nm.
Following the removal of genomic DNA using gDNA Eraser (Takara
Biotechnology Co., Ltd., Dalian, China), total RNA was reverse
transcribed using a PrimeScript First Strand cDNA Synthesis kit
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
instructions.
PCR was performed using a SYBR Green kit (Takara
Biotechnology Co., Ltd.) and an Mx3000P qPCR system (Agilent
Technologies, Inc., Santa Clara, CA, USA) using the following
cycling conditions: 1 cycle of 95°C for 2 min, followed by 40
cycles of 95°C for 30 sec and 60°C for 30 sec. Primers were
designed using Primer Premier 5.0 (Premier Biosoft International,
Palo Alto, CA, USA) and were custom-made by Takara Biotechnology
Co., Ltd. Gene expression was normalized relative to that of the
housekeeping gene GAPDH. The following rat primers were used: GAPDH
forward, 5′-TGTGTCCGTCGTGGATCTGA-3′ and reverse,
5′-TTGCTGTTGAAGTCGCAGGAG-3′; core-binding factor α1 (Cbfα1)
forward, 5′-CACTGGCGGTGCAACAAGA-3′ and reverse,
5′-ATGACGGTAACCACAGTCCCATC-3′; and transforming growth factor-β1
(TGF-β1) forward, 5′-GTGTGGAGCAACATGTGGAACTCTA-3′ and reverse,
5′-CGCTGAATCGAAAGCCCTGTA-3′. Relative mRNA expression levels were
quantified using the 2−ΔΔCq method (13).
Western blot analysis
rBMSCs were prepared as described in the ALP
staining assay and proteins were extracted using a Mammal Tissue
Protein Extraction kit (Boster Biological Technology, Pleasanton,
CA, USA) with 1 mmol phenylmethanesulfonyl fluoride. Protein
quantification was performed with a bicinchoninic acid protein
assay. Protein samples (50 µg) were separated by 12% SDS-PAGE,
transferred to a polyvinylidene difluoride membrane, and incubated
with primary and secondary antibodies (37°C; 1 h). The primary
antibodies were against GAPDH (cat. no. 5174), total JNK (cat. no.
9252), phosphorylated (p)-JNK (cat. no. 9251) (Cell Signaling
Technology, Inc., Danvers, MA, USA), TGF-β1 (cat. no. sc-146; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and Cbfα1 (cat. no.
ab76956; Abcam, Cambridge, UK). Protein bands were visualized using
an enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.)
and exposed to X-ray films (Kodak, Rochester, NY, USA).
Densitometric analysis was performed with AlphaView SA Software
(ProteinSimple; Bio-Techne, Minneapolis, MN, USA) to analyze
protein quantification. And the results were expressed as a ratio
of the level of expression of the target protein to GAPDH
expression. The level of JNK phosphorylation was represented by the
ratio of p-JNK to JNK protein expression levels.
Statistical analysis
Results are presented as the mean ± standard
deviation. Multiple comparisons were performed by one-way analysis
of variance followed by Fisher's least significant difference test
using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference. Each experiment was repeated at least 3
times.
Results
Morphological features and
characterization of rBMSCs by flow cytometry
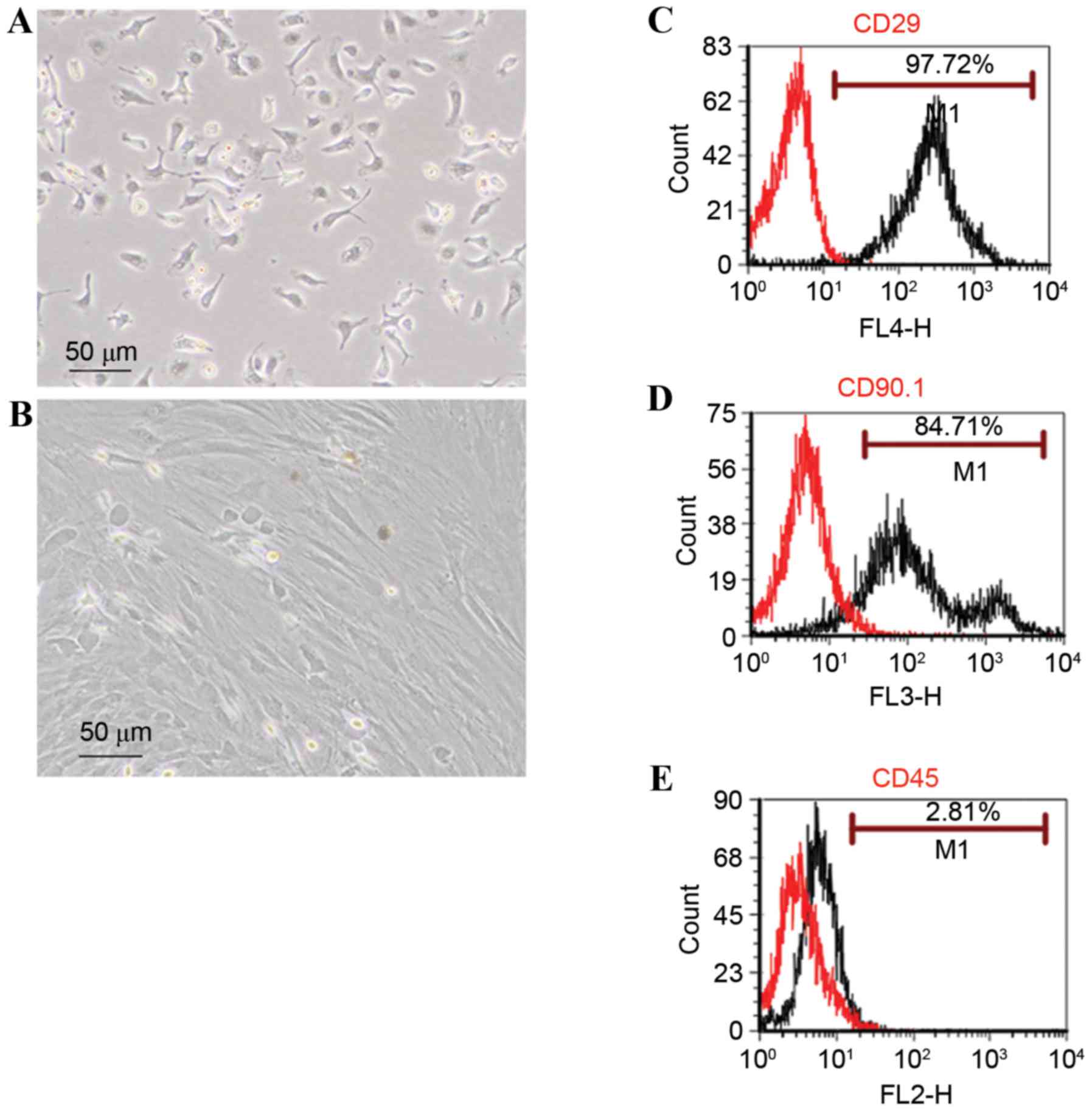
rBMSCs were imaged under an inverted microscope
(Fig. 1A). Primary cells were
circular or oval in shape. Cells were passaged when they reached
80% confluence at 8–10 days incubation. Cells at passage 4 were
larger and spindle-shaped (Fig.
1B). Cells were allowed to grow to 80% confluence in a culture
flask.
Cells were harvested and subjected to flow cytometry
to examine cell surface markers. A total of 97.72 and 84.71% of
cells were positive for the mesenchymal surface markers CD29 and
CD90.1, respectively (Fig. 1C and
D); whereas 2.81% were positive for the hematopoietic stem cell
marker CD45 (Fig. 1E).
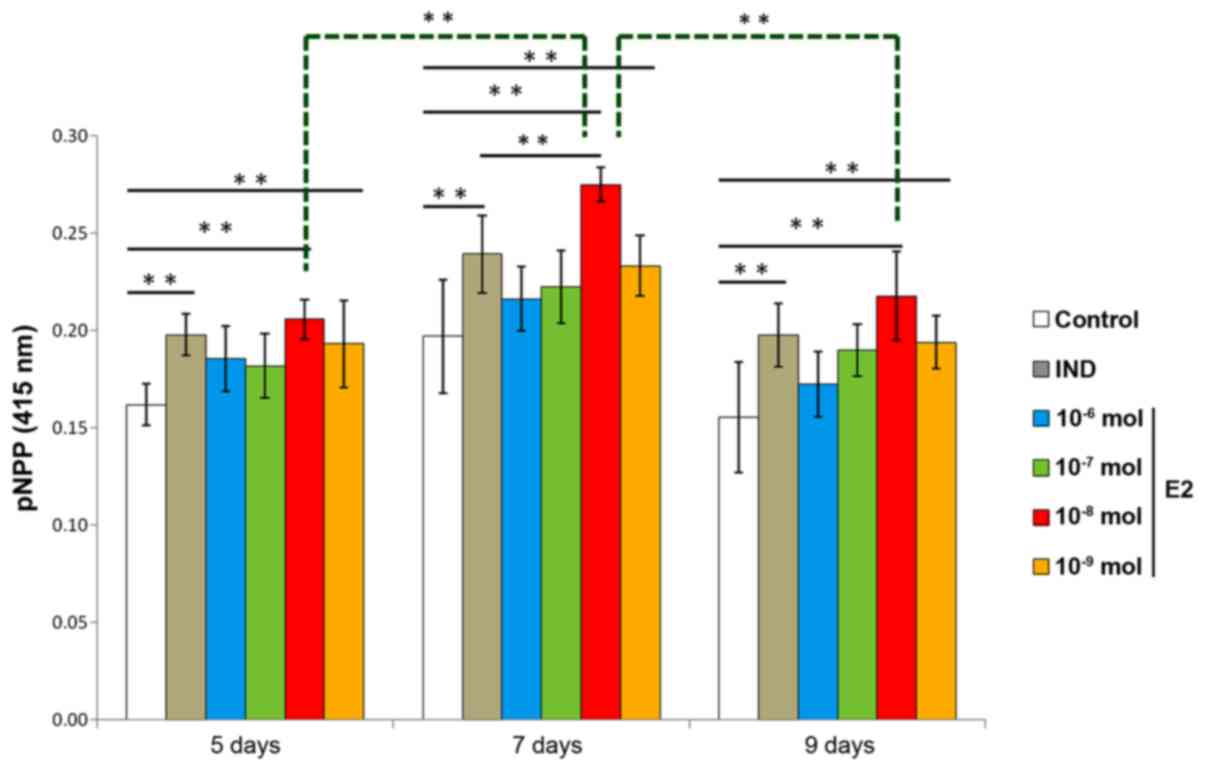
rBMSC ALP activity
ALP is an enzyme that is expressed during rBMSC
osteogenesis and is considered a well-defined and early marker for
their differentiation. ALP activity was examined to evaluate the
optimal concentration and time required for estradiol-induced rBMSC
osteogenesis. Compared with the control group, two of the E2 groups
(10−8 mol and 10−9 mol estradiol) and the IND
group exhibited higher levels of ALP on days 5, 7 and 9. Compared
with the IND group, the 10−8 mol E2 group had
significantly higher levels of ALP on day 7 (P<0.01); rBMSCs
treated with 10−8 mol estradiol also had significantly
higher levels of ALP on day 7 than those cells receiving the same
treatment on days 5 and 9 (P<0.01). Therefore, estradiol
induction of osteogenesis was considered optimal at a concentration
of 10−8 mol estradiol and 7 days incubation (Fig. 2).
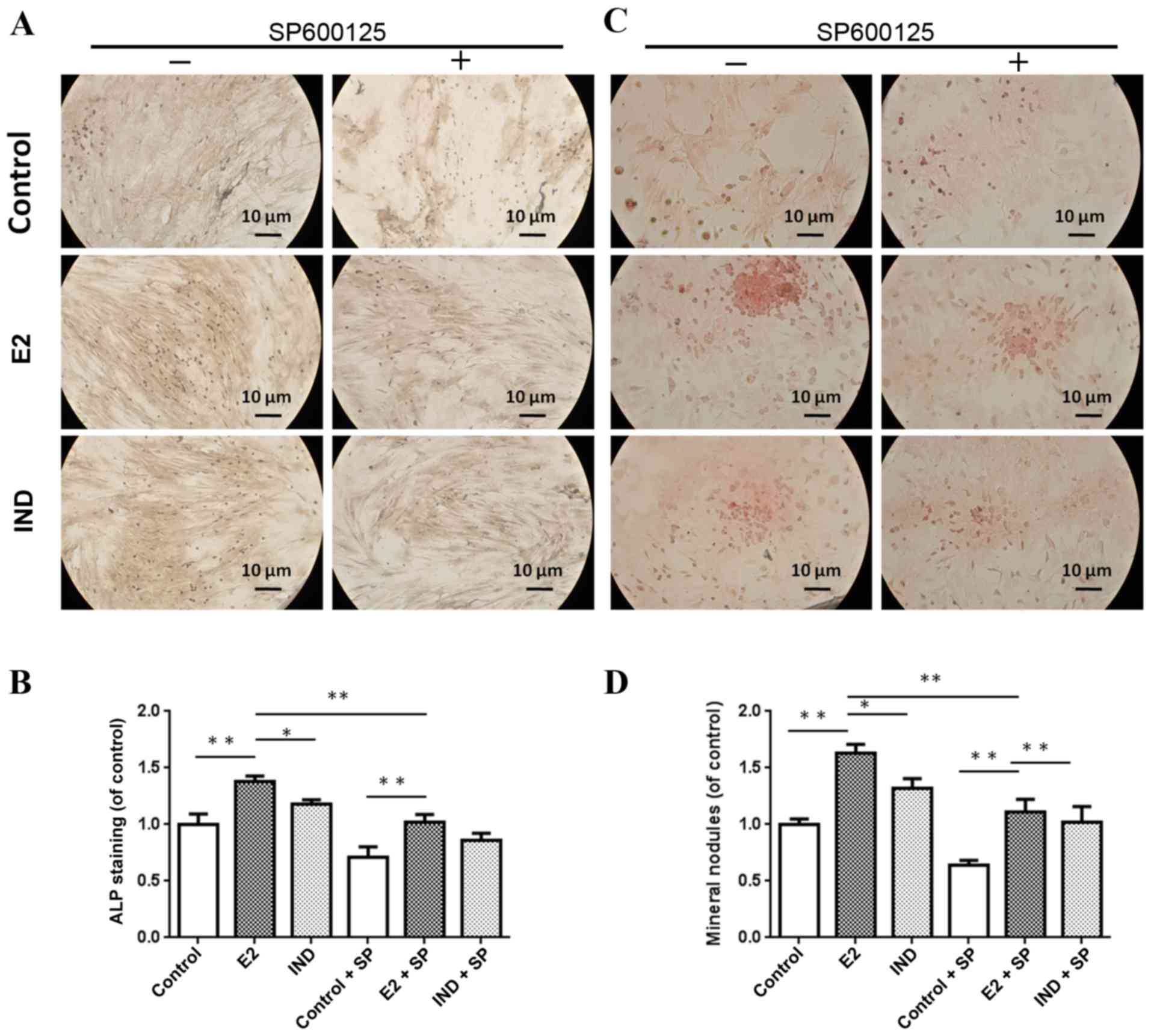
ALP staining and mineral nodule
formation
Cell cultures were imaged with an inverted light
microscope (Fig. 3). The E2 and
IND rBMSC groups had significantly higher levels of ALP compared
with the control group (P<0.01) (Fig. 3A and B). The level of ALP in rBMSCs
of the E2 group was significantly higher compared with cells in the
IND group (P<0.05).
Alizarin red S staining was used to examine the
formation of mineralized nodules in the various rBMSC cultures. As
shown in Fig. 3C and D, the number
of nodules forming in the E2 and IND groups were significantly
larger compared with the control group, and those of the E2 group
were significantly larger compared with the IND group at 21 days
(P<0.01 or P<0.05). Treatment with the JNK inhibitor SP600125
reduced the osteogenesis-stimulating effects of estradiol; however,
nodule formation remained significantly higher in the E2 and IND
groups, compared with the control group.
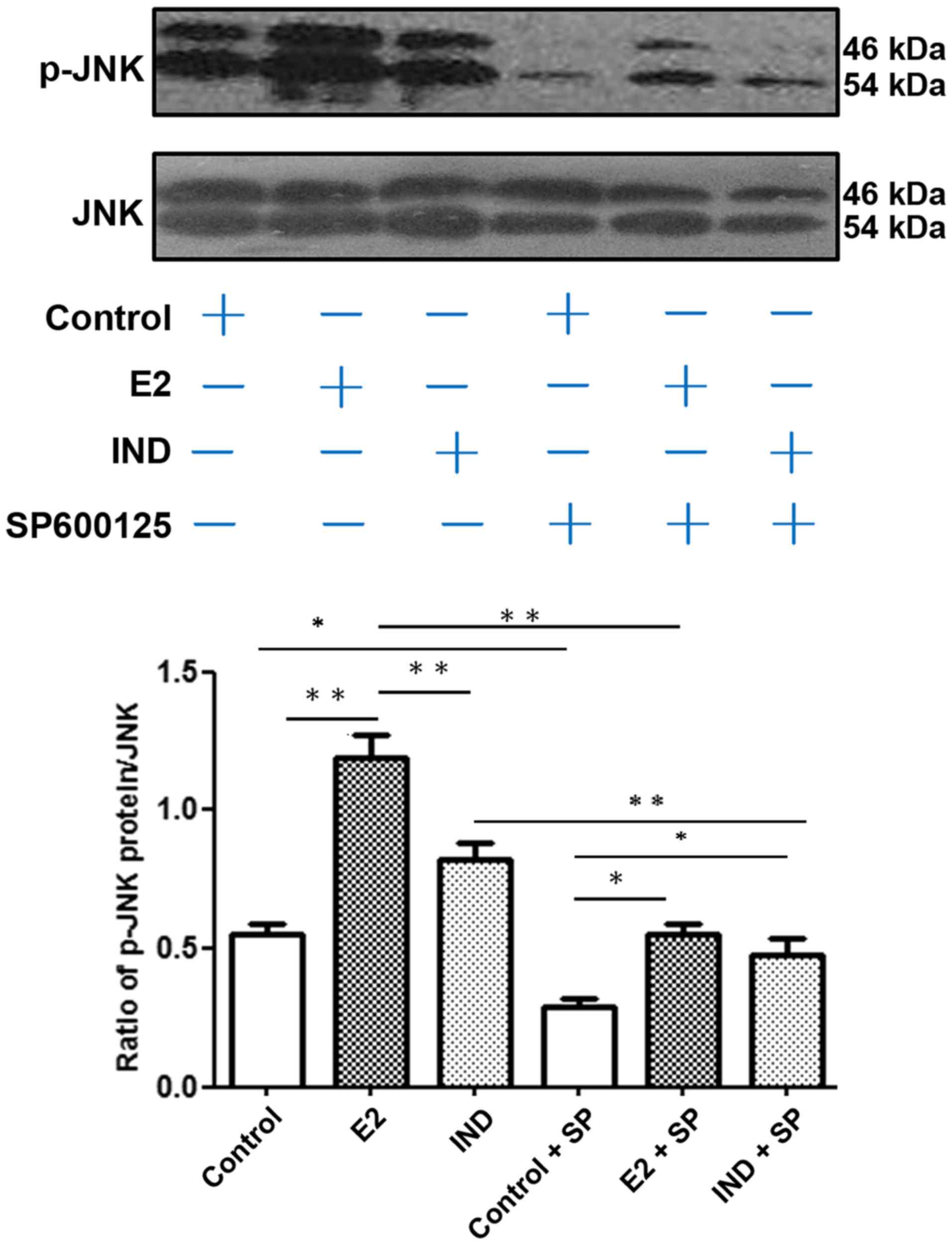
JNK phosphorylation
The relative level of p-JNK compared with the total
JNK protein expression in rBMSCs was assayed by western blotting
(Fig. 4). Compared with the E2
group, the control and IND groups had lower levels of p-JNK
(P<0.01). Addition of the JNK inhibitor SP600125 significantly
reduced the levels of JNK and p-JNK protein expression under all
conditions. However, the expression levels of p-JNK in the E2 and
IND groups co-cultured with SP600125 remained higher compared with
the control group treated with the inhibitor (P<0.05).
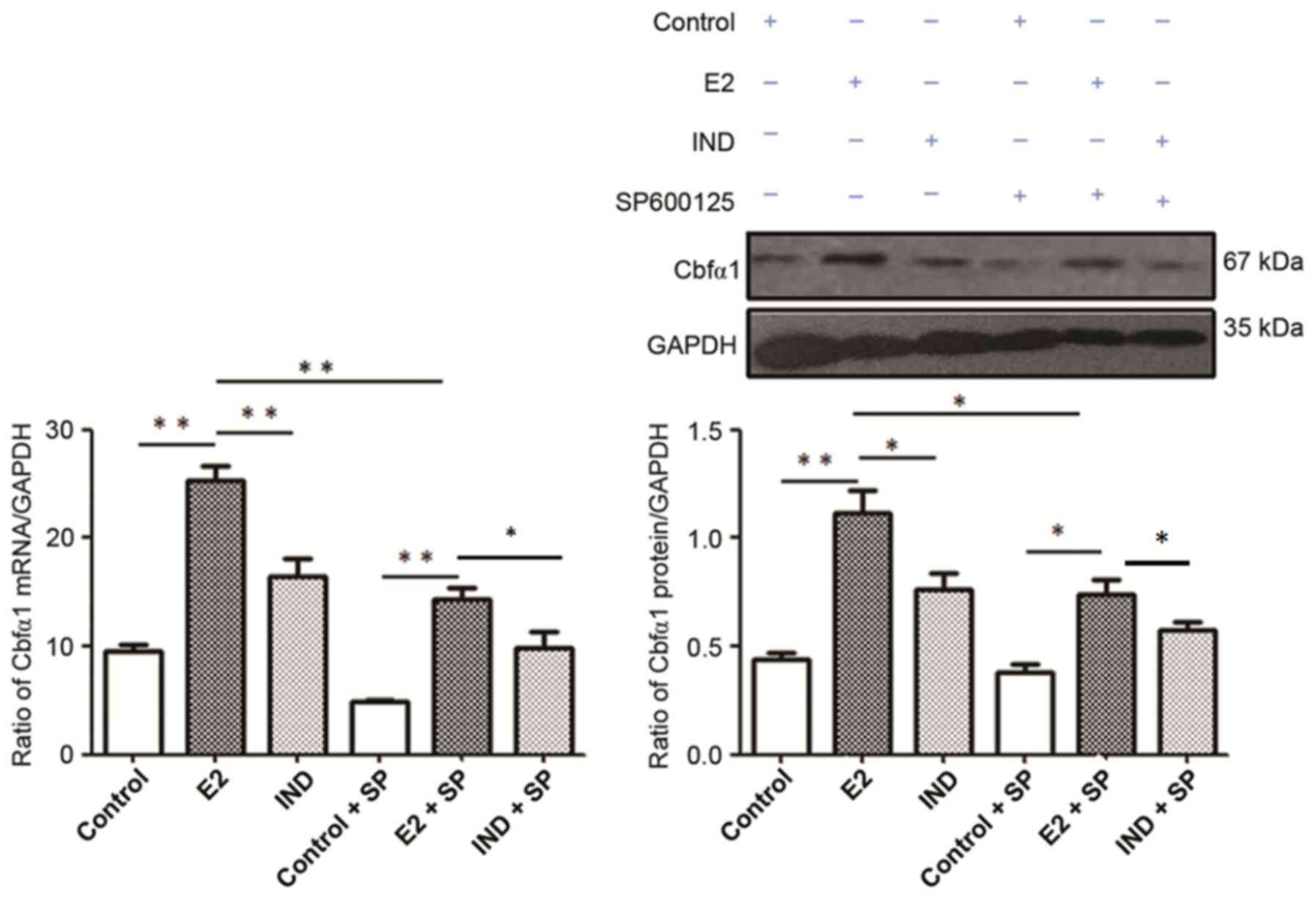
Cbfα1 mRNA and protein expression
Cbfα1 mRNA and protein expression levels were
significantly higher in rBMSCs from the E2 group compared with the
control and IND groups (P<0.01 or P<0.05; Fig. 5). Although the levels of Cbfα1 mRNA
and protein expression were lower in all groups following the
addition of SP600125, they remained higher in the E2 group compared
with the IND and control groups (P<0.01 or P<0.05).
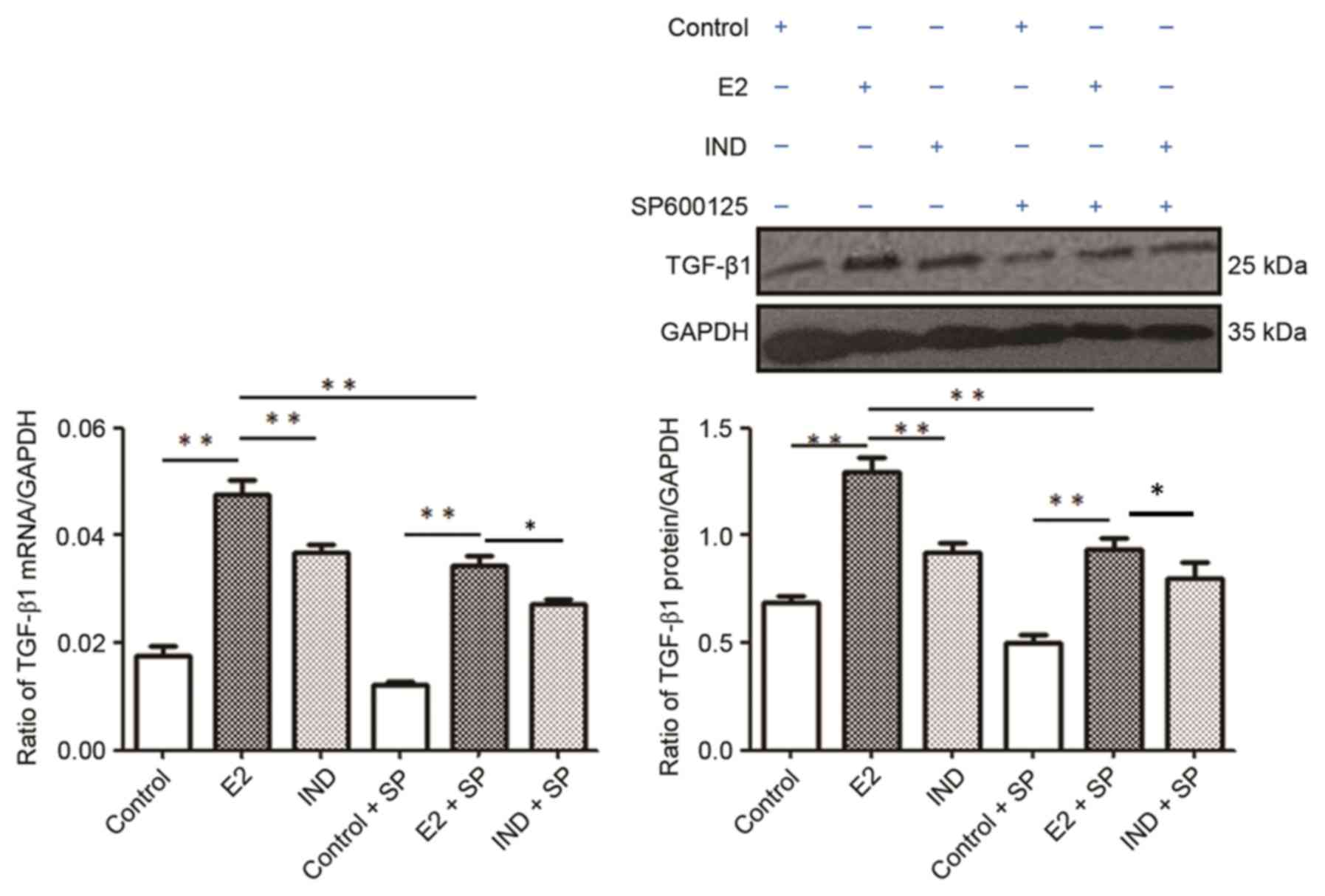
TGF-β1 mRNA and protein
expression
The E2 group had significantly higher levels of
TGF-β1 mRNA and protein expression compared with the control and
IND groups (P<0.01; Fig. 6).
The expression levels of TGF-β1 mRNA and protein were subsequently
lower in each group when co-treated with SP600125; in addition, the
levels were significantly higher in the E2 group compared with the
control or IND groups (P<0.01).
Discussion
The present study investigated the effects of the
estrogen steroid sex hormone estradiol on the osteogenic
differentiation of rBMSCs. Estradiol treatment was associated with
an increase in osteogenic induction, as determined by examining
markers of osteogenesis, such as ALP activity and expression, and
the formation of mineralized nodules. ALP is an early stage marker,
whereas the formation of mineralized nodules is an indicator of
middle- and late-stage osteogenesis (14,15).
The present study determined the optimal concentration of estradiol
treatment in rBMSC culture was 10−8 mol and the optimal
incubation time was 7 days. Results indicated that osteogenesis in
rBMSCs treated with estradiol was more robust compared with those
treated with IND media, as indicated by higher levels of ALP and
stronger formation of mineralized nodules.
Animal models of osteoporosis, such as
ovariectomized mice, are widely used to study the signaling
cascades that control rBMSC osteogenesis, including research on
postmenopausal osteoporosis (16).
However, it remains unclear how estrogen contributes to the
osteogenic differentiation of stem cells. A previous study
demonstrated that the JNK signaling pathway is involved in the
regulation of osteogenesis (12).
Guicheux et al (17)
reported that bone morphogenetic protein 2 regulated osteoblastic
differentiation of stem cells through activation of the JNK and p38
MAPK signaling pathways. Therefore, the present study explored the
role of JNK signaling in the estrogen-mediated regulation of
osteogenic differentiation of rBMSCs. The results demonstrated that
estradiol is associated with higher mRNA and protein expression
levels of p-JNK and Cbfα1 in rBMSC cultures. Cbfα1 is a
transcription factor that regulates the expression of numerous
genes relevant to osteogenesis (18,19).
It is a major factor in the initiation and regulation of early
osteogenesis, and serves a crucial role in the late mineralization
stages (20). Notably, the present
study revealed that the E2 group had relatively high levels of
p-JNK and Cbfα1 mRNA and protein following treatment with the
JNK-specific inhibitor SP600125, compared with cells in the control
and IND groups. These data indicated that JNK signaling my serve a
crucial role during estrogen-induced osteogenesis of rBMSCs.
Furthermore, results from the present study
demonstrated that the mRNA and protein expression levels of TGF-β1
were higher in the E2 group compared with the IND and control
groups, and were decreased in the presence of SP600125. TGF-β1 may
stimulate the phosphorylation of JNK, which enables the signal to
be transferred into the nucleus to regulate the osteogenic
differentiation of rBMSCs. In conclusion, the present study
provides evidence to suggest that estradiol may promote rBMSC
osteogenesis by activating the JNK signaling pathway. These data
support the hypothesis that estradiol-induced JNK signaling
contributes to osteogenesis and the prevention of postmenopausal
osteoporosis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81373527 and
81774185).
References
|
1
|
Siris ES, Miller PD, Barrett-Connor E,
Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC and
Sherwood LM: Identification and fracture outcomes of undiagnosed
low bone mineral density in postmenopausal women: Results from the
National Osteoporosis Risk Assessment. JAMA. 286:2815–2822. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Rahbi B, Zakaria R, Othman Z, Hassan A
and Ahmad AH: Enhancement of BDNF concentration and restoration of
the hypothalamic-pituitary-adrenal axis accompany reduced
depressive-like behaviour in stressed ovariectomised rats treated
with either Tualang honey or estrogen. ScientificWorldJournal.
2014:3108212014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen FP, Hu CH and Wang KC: Estrogen
modulates osteogenic activity and estrogen receptor mRNA in
mesenchymal stem cells of women. Climacteric. 16:154–160. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan SL, Ahmad TS, Selvaratnam L and
Kamarul T: Isolation, characterization and the multi-lineage
differentiation potential of rabbit bone marrow-derived mesenchymal
stem cells. J Anat. 222:437–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen M, Feng W, Cao H, Zou L, Chen C,
Baatrup A, Nielsen AB, Li H, Kassem M, Zou X and Bünger C: A
traditional Chinese medicine formula extracts stimulate
proliferation and inhibit mineralization of human mesenchymal stem
cells in vitro. J Ethnopharmacol. 125:75–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou DA, Zheng HX, Wang CW, Shi D and Li
JJ: Influence of glucocorticoids on the osteogenic differentiation
of rat bone marrow-derived mesenchymal stem cells. BMC
Musculoskelet Disord. 15:2392014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HK, Kim MG and Leem KH: Osteogenic
activity of collagen peptide via ERK/MAPK pathway mediated boosting
of collagen synthesis and its therapeutic efficacy in osteoporotic
bone by back-scattered electron imaging and microarchitecture
analysis. Molecules. 18:15474–15489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sonowal H, Kumar A, Bhattacharyya J, Gogoi
PK and Jaganathan BG: Inhibition of actin polymerization decreases
osteogeneic differentiation of mesenchymal stem cells through p38
MAPK pathway. J Biomed Sci. 20:712013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwon HS, Johnson TV and Tomarev SI:
Myocilin stimulates osteogenic differentiation of mesenchymal stem
cells through mitogen-activated protein kinase signaling. J Biol
Chem. 288:16882–16894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marom R, Shur I, Solomon R and Benayahu D:
Characterization of adhesion and differentiation markers of
osteogenic marrow stromal cells. J Cell Physiol. 202:41–48. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XD, Wang JS, Chang B, Chen B, Guo C,
Hou GQ, Huang DY and Du SX: Panax notoginseng saponins promotes
proliferation and osteogenic differentiation of rat bone marrow
stromal cells. J Ethnopharmacol. 134:268–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Komori T: Animal models for osteoporosis.
Eur J Pharmacol. 759:287–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guicheux J, Lemonnier J, Ghayor C, Suzuki
A, Palmer G and Caverzasio J: Activation of p38 mitogen-activated
protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their
implication in the stimulation of osteoblastic cell
differentiation. J Bone Miner Res. 18:2060–2068. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Byers BA, Pavlath GK, Murphy TJ, Karsenty
G and Garcia AJ: Cell-type-dependent up-regulation of in vitro
mineralization after overexpression of the osteoblast-specific
transcription factor Runx2/Cbfal. J Bone Miner Res. 17:1931–1944.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teplyuk NM, Haupt LM, Ling L, Dombrowski
C, Mun FK, Nathan SS, Lian JB, Stein JL, Stein GS, Cool SM and van
Wijnen AJ: The osteogenic transcription factor Runx2 regulates
components of the fibroblast growth factor/proteoglycan signaling
axis in osteoblasts. J Cell Biochem. 107:144–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ngueguim FT, Khan MP, Donfack JH, Siddiqui
JA, Tewari D, Nagar GK, Tiwari SC, Theophile D, Maurya R and
Chattopadhyay N: Evaluation of cameroonian plants towards
experimental bone regeneration. J Ethnopharmacol. 141:331–337.
2012. View Article : Google Scholar : PubMed/NCBI
|