Introduction
Nitric oxide (NO) is synthesized by the NO synthase
(NOS) enzyme. NO is a cytotoxin and a ubiquitous signaling molecule
(1,2). Nitric oxide synthases (NOSs) are
hemoproteins and catalyze the oxidation of arginine to nitric oxide
(NO) and citrulline. Three genetically and functionally distinct
NOS isoforms, endothelial NOS (eNOS), inducible NOS (iNOS) and
neuronal NOS (nNOS) have been identified from endothelial cells,
microphages and neurons, respectively (3,4).
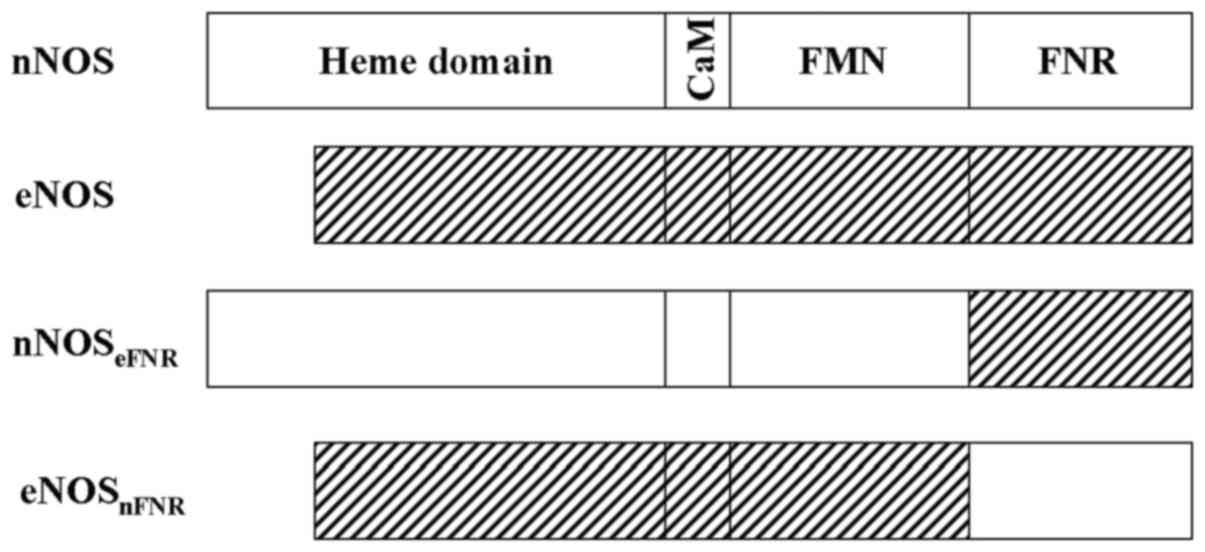
NOSs are incorporate three domains in a single polypeptide: i) The
N-terminal P450-like oxygenase domain that binds heme,
tetrahydrobiopterin (H4B), and L-arginine, is the site
of oxidation of arginine; ii) a C-terminal cytochrome P450
reductase (CPR)-related reductase domain that contains binding
sites for FAD, FMN and NADPH, is responsible for providing
electrons to the oxygenase domain; iii) a connecting peptide
between the two domains binds calmodulin in a
Ca2+-dependent manner for constitutive NOS (cNOS), nNOS
and eNOS and essentially Ca2+-independent manner in iNOS
(1,3,5–9).
Regulation of NOS activity by CaM has been shown to occur via the
control of electron transfer chain in NOS
(NADPH®FAD®FMN®Heme) across the
domain-domain interaction (10,11).
It was shown that CaM can activate NADPH-dependent flavin reduction
by suppressing the repressors within reductase domain, such as a
C-terminal tail (CT) (12–14) and an autoinhibitory insert (AI)
(15–17) within the middle of FMN sub-domain
of cNOS.
NOS reductase domain can be dissected into two
domains: The N-terminal FMN binding domain is homologous to
bacterial flavodoxin, and the C-terminal fragment, containing
binding sites for NADPH and FAD is related to the
ferredoxin-NADP+ reductase (FNR) (18). Each flavin nucleotide in NOS plays
an obvious role in the electron flow sequence: FAD can transfer
NADPH-divided electrons to linked FMN or exogenous electron
acceptors such as ferricyanide and 3-acetylpypyridine adenine
dinucleotide phosphate (AcPyADP+), and the FMN can
transfer the electrons to attached heme or non-physiological
acceptors such as cytochrome c (19,20).
It has been demonstrated that each of the recombinant
flavin-binding domains can be expressed separately and retains the
chemical and catalytic properties of their native counterparts
(19–21). Recent reported crystal structures
of rat nNOS reductase domain and mutation studies suggested that a
movement of the FMN module from FNR may be required for its optimal
position to transfer electron out of FMN (18). Crystal structure studies of the
sulfite reductase indicated that the interaction between the FNR
and FMN modules displays lower affinity than in the case of CPR
(22). FMN-free mutant studies
suggested that removing FMN relieved suppression of FAD reduction
within the FNR module and increase ferricyanide reduction (23). These data established the
importance of domain-domain interaction and domain movement in
flavoprotein catalysis.
The two constitutive NOS, eNOS and nNOS share
similarities both in their structure and biochemical behaviors.
However, nNOS exhibits much higher overall enzymatic activities
than eNOS (24,25). Investigations of chimera enzymes
that possess exchanged oxygenase and reductase domain indicated
that heme reduction rate in cNOS is controlled mainly by the
reductase domains (25). The heme
reduction rate is almost independent of oxygenase domain (25). Previous results showed that the
lower intrinsic activity of eNOS is caused by a lower ability of
its electron transfer rate to the catalytic heme domain or external
acceptors (26), but the
NADPH-dependent flavin reduction rates of both CaM-bound cNOS are
similar (27). Results showed that
the electron transfer from full-reduced FMN (FMNH2) to
heme or cytochrome c in cNOS is primarily influenced through
a mechanism that does not involve changing the rate of flavins
reduction. The details are not clear. it was suspected that the
interaction of the FNR and FMN modules may differ between nNOS and
eNOS. In this work, we will investigate how electron transfer and
catalysis is related to the domain-domain interaction.
In the present study, we have constructed chimeric
NOS enzymes by interchanging the FNR module between the two cNOS,
and have studied the consequence of FNR module on catalytic
activities, electron transfer properties by spectral methods. Our
results clearly indicated that FNR module is important in
controlling electron flow through the reductase domain to attached
heme and outside from FMN module.
Materials and methods
Materials
His-binding resin and CaM-sepharose were products of
Amersham Biosciences (Uppsala, Sweden). Cytochrome c was
purchased from Sigma (St. Louis, MO, USA). Restriction enzymes were
purchased from New England Bio-lab (Ipswich, MA, USA). All other
chemicals were purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Generation of chimera DNAs
The original vector coding was a gift to R.W. from
Professor Philip Marsden (University of Toronto, Canada). Briefly,
a unique restriction site Nru I was generated at S866 and
R877 for nNOS, and S656 and R657 for eNOS by Site-directed
mutagenesis using Quick Change polymerase chain reaction (PCR)
in vitro mutagenesis kit from Stratagene (Agilent
Technologies, Inc., Santa Clara, CA, USA).
Expression and purification of chimera
and wild-type enzymes
Like wild-type rat nNOS and bovine eNOS, the chimera
enzymes containing a His-6 tag attached to their N-termini were
over-expressed in E. coli BL21 and purified by sequential
chromatograph on Ni2+-NTA and Calmodulin-sepharose
resins (28,29). The ferrous-CO adduct absorbing at
444 nm was used to quantitative heme protein content using an
extinction coefficient of 74 mM/cm. The contents of FAD
and FMN were measured by HPLC with fluorometric detection.
Spectrophotometric methods
The assays of steady-state enzymatic activities for
ferricyanide and cytochrome c reduction, NO synthesis, and
NADPH oxidation were carried out spectrophotometrically. The rates
of ferricyanide reduction were obtained by determining the
absorbance decrease at 420 nm using an extinction coefficient of
1.02 mM/cm. The concentration of ferricyanide and NADPH
was 1.0 mM and 0.3 mM, respectively. Cytochrome c reduction
was determined by monitoring the absorbance increasement at 550 nm
using a difference extinction coefficient of 21 mM/cm
between reduced and oxidized forms, with 0.3 mM NADPH and 0.1 mM
cytochrome c. The rate of NADPH oxidation was measured by
monitoring the absorbance decrease at 340 nm using an extinction
coefficient of 6.22 mM/cm with 0.1 µM NADPH and in the
presence or absence of 5 µM CaM, 300 µM Ca2+, 10 µM
H4B, 250 µM Arginine or Agatine. NO synthesis rate was
monitored from the conversion of oxyhemoglobin to methemoglobin
mediated by NO. The rate was obtained by using a difference
extinction coefficient of 38 mM/cm between oxyhemoglobin
and methemoglobin at 401 nm, with 0.3 mM NADPH, 4 µM
H4B, 10 µM oxyhemoglobin and 1 mM L-arginine. All
results were obtained at 25°C in EPPS buffer (40 mM, pH 7.6)
containing 0.25 M NaCI, 10% glycerol, 10 units/ml superoxide
dismutase (SOD), 0.5 µM FAD/FMN, 100 units/ml catalase, and 50 µM
EDTA (for minus CaM assays) using a U-3110 spectrophotometer
(HITACHI Ltd, Tokyo, Japan). For assays of calmodulin activation, 5
µM CaM and 0.5 mM Ca2+ were added to the reaction
mixture, respectively.
Kinetics of NADPH-dependent flavin and
heme reduction
Rapid mixing stopped-flow reaction were performed
using a stopped-flow spectrophotometer. The stopped-flow apparatus
had a dead time of 2 ms and was equipped with an anaerobic workbox.
Stopped-flow experiments were obtained in 0.25 M NaCl, 40 mM pH 7.6
EPPS buffer. The constant temperature is 10°C. Flavin reduction was
measured by monitoring the decrease of absorbance (485 nm) after
mixing 50 µM NADPH with 2 µM enzyme in the presence and absence of
Ca2+/CaM under anaerobic conditions. Anaerobic heme
reduction rate was obtained by monitoring the increase of
absorbance at 444 nm, and this rate represents the formation of
ferrous-CO complex. Reaction was initiated by rapid mixing 50 µM
NADPH with 2 µM chimera or wild-type in 40 µM EPPS buffer, pH 7.6,
10 µM H4B, 1 mM L-arginine, 10 µM CaM, and 1 mM
Ca2+. In these experiments, NADPH, chimera and wild-type
were prepared in an anaerobic CO-saturated solutions.
Results
Expression and purification of chimera
enzymes
FNR swapped chimera enzymes, nNOSeFNR and
eNOSnFNR were expressed in E. coli BL21. Their
yields were similar to those of wild-type nNOS and eNOS,
respectively. The chimera enzymes were purified in the same way as
wild-type enzymes as described in experiment procedures. Both the
chimera enzymes showed correct molecular mass and over 90% purity
as judged by SDS-PAGE. The chimera enzymes (Fig. 1) contained 1 FMN and 1 FAD per NOS
heme measured by HPLC.
Optical spectra properties of chimera
enzymes
Spectroscopic analysis showed that the chimera
enzymes were consistent with the flavins-bound wild-type proteins
as shown in Fig. 2 spectrum a.
Dithionite reduction of the chimera enzymes produced the expected
absorbance peak at 444 nm for the ferrous-CO complex in all cases
in the presence of Arg, H4B and CO. Results indicated
that swapped FNR sub-domain between nNOS and eNOS did not alter
proteins folding, expression and physical properties.
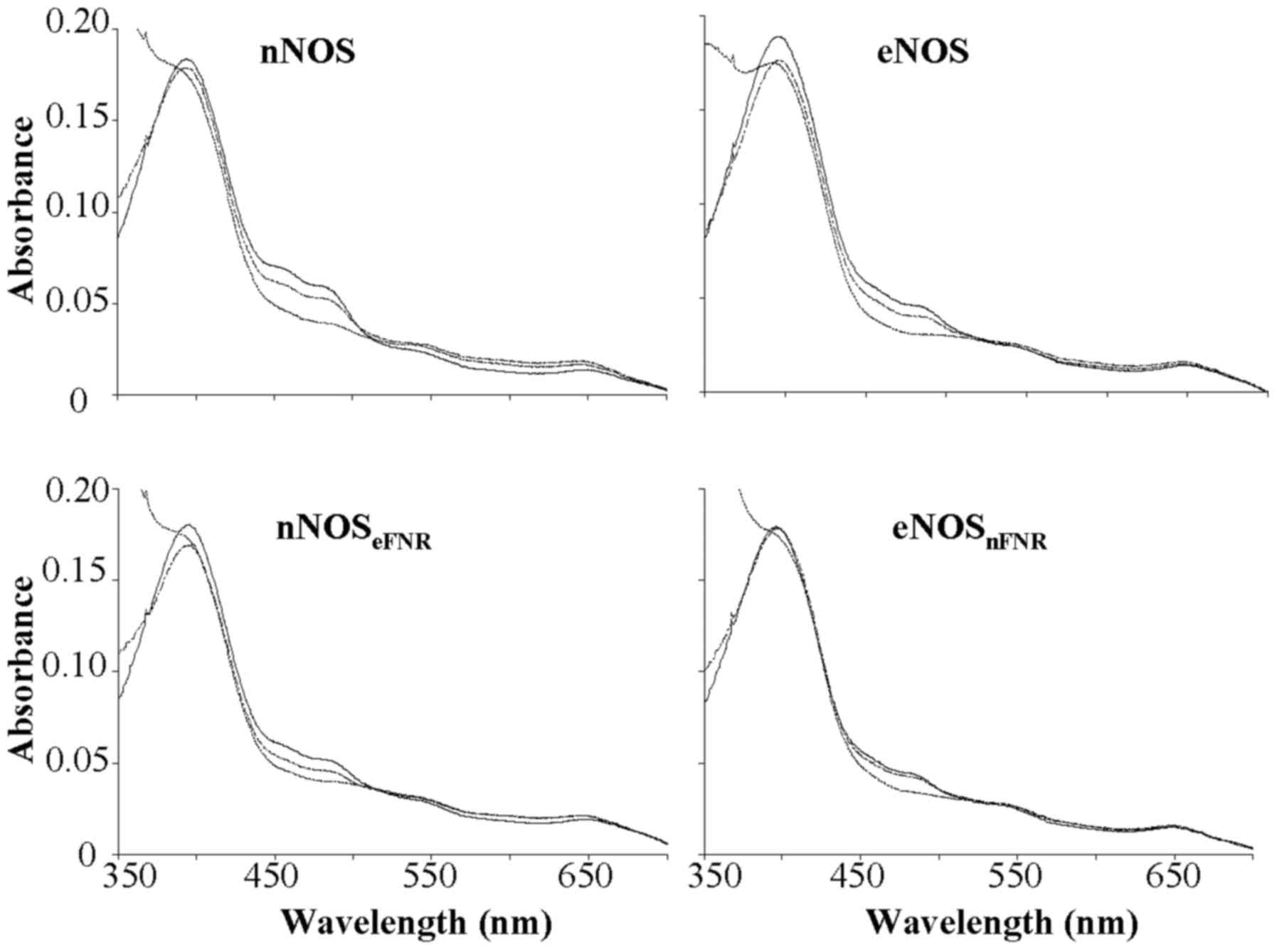 | Figure 2.Absorbance spectra record during
oxidation-reduction reactions of wild-type and chimera enzymes. (−)
The oxidized enzymes. (…..) The spectra were recorded immediately
after mixing excess NADPH with enzyme. (----) The spectra were
recorded at 30 min after addition of excess NADPH to enzyme
solution. Final concentrations: Enzyme, 1.8 µM; NADPH, 100 µM; CaM,
5 µM; Ca2+, 300 µM; H4B, 10 µM; Agatine, 250
µM. Results are representative of three similar individual
experiments. nNOS, neuronal nitric oxide synthase; H4B,
tetrahydrobiopterin; eNOS, endothelial nitric oxide synthase; FNR,
ferredoxin-NADP+ reductase. |
NADPH oxidation
The steady-state oxidation of NADPH was measured in
the presence or absence of CaM, Arginine or Agatine, H4B
as shown in Fig. 3 and Table I. Compared with wild-type nNOS, the
chimera enzyme, nNOSeFNR showed a lower (approximately
12–30 %) NADPH oxygenase activity both in the presence and absence
of CaM. In contrast, the chimera enzyme, eNOSnFNR showed
higher (~4.5-fold) NADPH oxygenase activity than that of wild-type
eNOS without CaM. However, unlike nNOSeFNR, it did not
increase upon CaM.
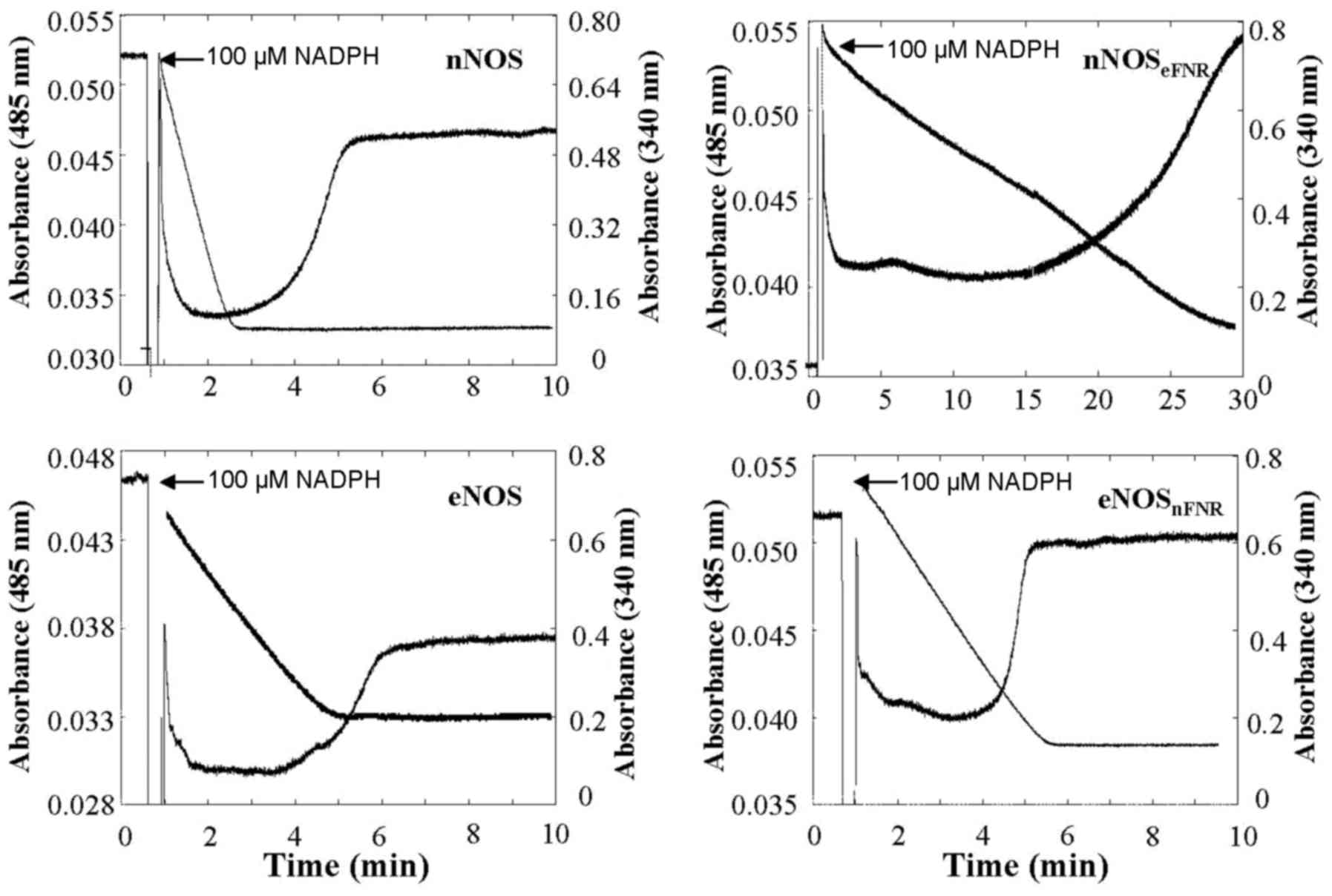 | Figure 3.Time course of absorbance change at
485 nm represent flavin oxidation-reduction, and at 340 nm
represents NADPH oxidation, respectively. The enzyme solution
contain: Enzyme, 1.8 µM; CaM, 5 µM; Ca2+, 300 µM;
H4B, 10 µM; Aratine, 250 µM. The narrows indicate the
point of addition of 100 µM NADPH. Results are representative of
three similar individual experiments. H4B, tetrahydrobiopterin;
eNOS, endothelial nitric oxide synthase; FNR,
ferredoxin-NADP+ reductase. |
 | Table I.NADPH oxygenase activities of native
and chimeric enzymes. |
Table I.
NADPH oxygenase activities of native
and chimeric enzymes.
| CaM/Arg or
Aga/H4B | nNOS | eNOS |
nNOSeFNR |
eNOSnFNR |
|---|
| −/Arg/+ | 8.0±0.5 | 4.8±0.6 | 2.4±0.1 | 22±1.0 |
| +/Aga/+ | 27±3.0 | 11±1.5 | 5.9±0.5 | 27±2.0 |
| +/Arg/+ | 117±10 | 33±2.0 | 15±1.0 | 37±2.0 |
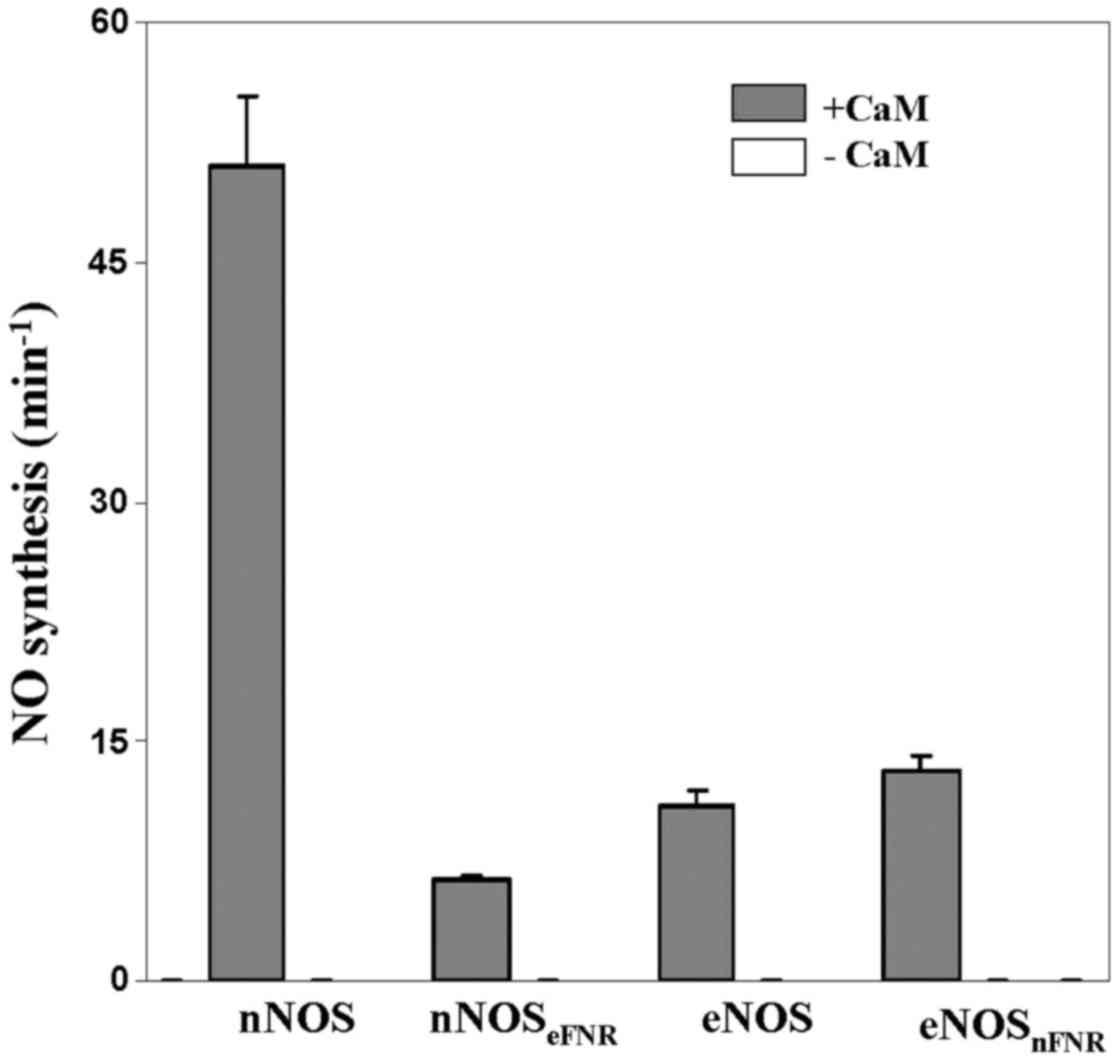
NO synthesis
The binding of CaM is essential for electron
transfer from FMN to heme thereby initiated NO synthesis. Wild-type
nNOS showed higher ability of NO synthesis (~5-fold) than that of
wild-type eNOS. We measured the steady-state NO synthesis of
chimera enzymes as shown in Fig. 4
and Table II. As the wild-type
enzymes, both chimera enzymes are required CaM binding for NO
synthesis activity. The nNOS chimera (nNOSeFNR)
incorporates eNOS FNR module and showed NO synthesis activity that
was about 12% of native nNOS. However, the eNOS chimera
(eNOSnFNR) incorporates nNOS FNR module and showed
similar NO synthesis activity with native eNOS.
 | Table II.Steaty-state activities for NO
synthesis, cyt. c reduction and ferricyanide reduction. |
Table II.
Steaty-state activities for NO
synthesis, cyt. c reduction and ferricyanide reduction.
|
| NO synthesis | Cyt. c
reduction | Ferricyanide
reduction |
|---|
|
|
|
|
|
|---|
|
| − | + | − | + | − | + |
|---|
| nNOS | ND |
51±4.4 |
554±13 |
4,798±148 |
7,800±508 |
11,983±745 |
| eNOS | ND |
11±0.9 |
63±4 |
340±10 |
6,012±165 |
7,168±248 |
|
nNOSeFNR | ND |
6.3±0.3 |
59±4.0 |
151±14 |
6,349±312 |
6,665±100 |
|
eNOSnFNR | ND |
13±1.0 |
787±42 |
562±32 |
8,366±194 |
8,627±131 |
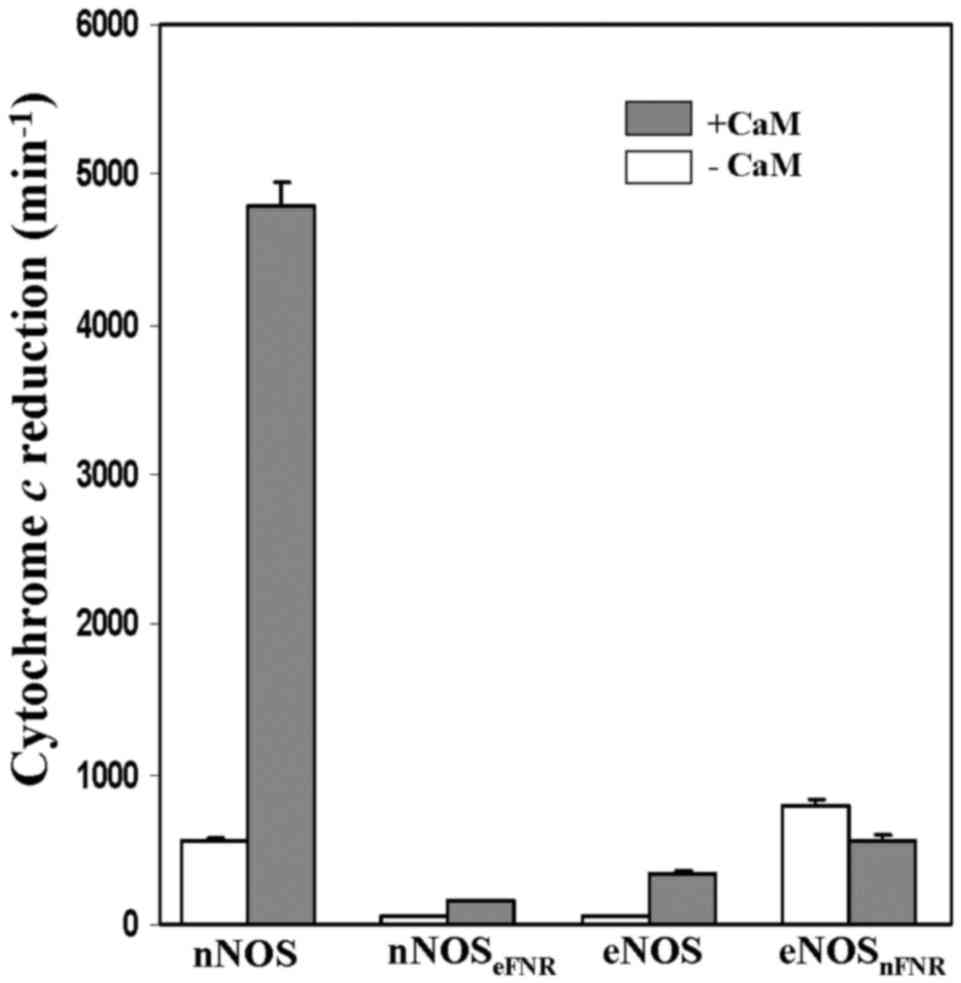
Cytochrome c and ferricyanide
reductions
Both the chimera enzymes did not alter their
ferricynide reducatase activies, however, they dramatically changed
their cytochrome c reductase activities comparing with the
wild-type enzymes (Fig. 5,
Table II). The CaM-free
nNOSeFNR chimera showed a lower (~4%) cytochrome
c reductase activity than that of wild-type nNOS. On the
contrary, cytochrome c reductase activity of
eNOSnFNR chimera was 14-fold higher than that of
wild-type eNOS in the absence of CaM. However, its activity did not
increased up on CaM binding.
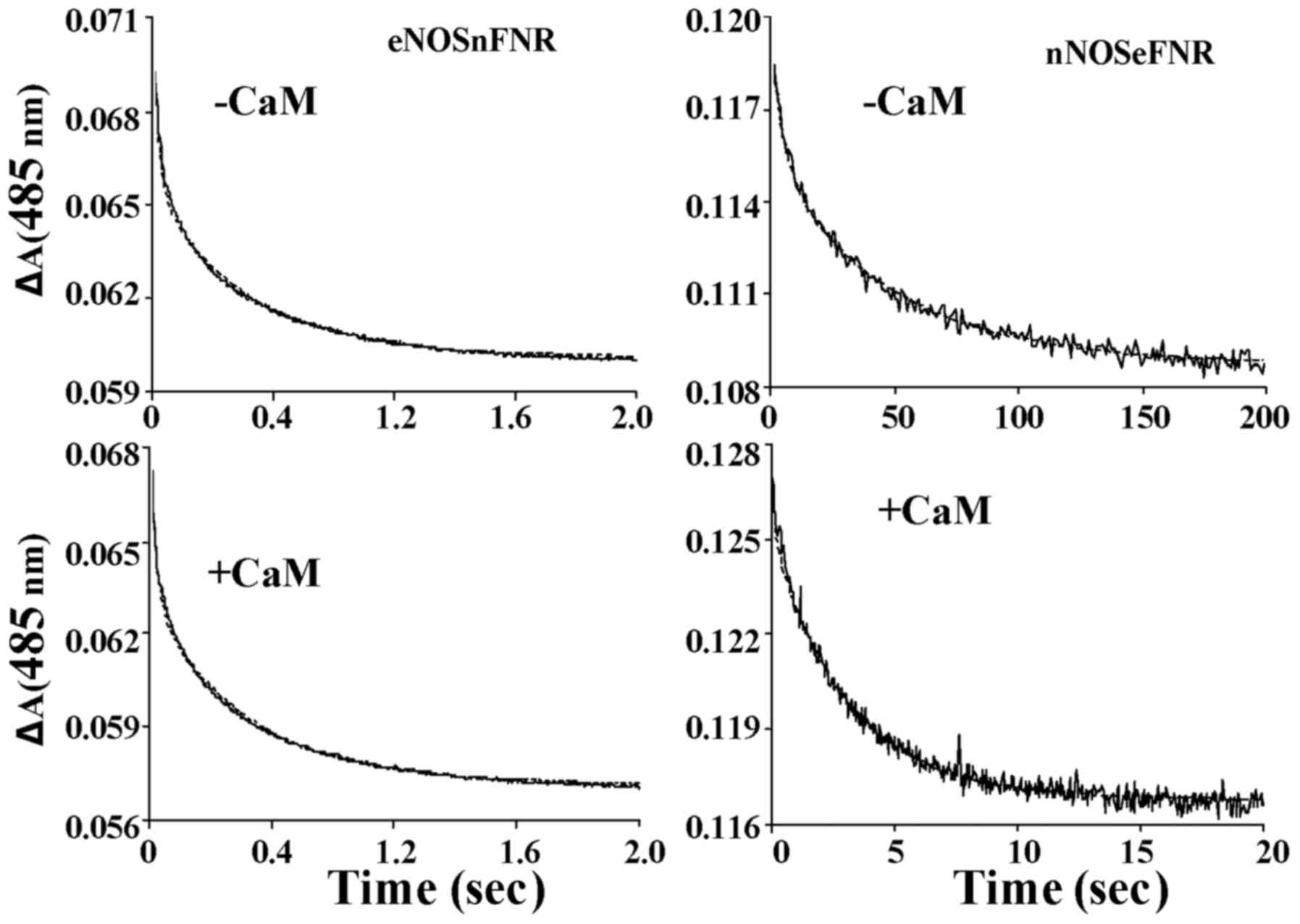
Kinetics of flavin and heme
reduction
Under anaerobic conditions, the rates of
NADPH-dependent flavin and heme reduction were measured by the aid
of stopped-flow spectroscopy. Fig.
6 showed the spectra traces (485 nm) of flavin reduction of
chimera enzymes in the absence and presence CaM as an absorbance
decrease vs. time. The traces of flavin reduction were biphasic for
both chimera enzymes. The flavin reduction rate was slower in
nNOSeFNR chimera than that of native nNOS both in the
presence and absence of CaM/Ca2+. However, in contrast,
eNOSnFNR chimera showed a higher flavin reduction rate
without CaM/Ca2+, and it weakly increased upon CaM
binding (Table III). These
results consistent with the results acquired from steady-state
cytochrome c reduction. Like wild-type enzymes, both
chimeras are required CaM binding for heme reduction. However, the
nNOSeFNR chimera showed a lower heme reduction rate
comparing with that of wild-type nNOS, and no significant change
was found between eNOSnFNR and wild-type eNOS.
 | Table III.Observed rate constants for
NADPH-dependent flavin and heme reduction in the presence and
absence of Ca2+/CaM (10°C). |
Table III.
Observed rate constants for
NADPH-dependent flavin and heme reduction in the presence and
absence of Ca2+/CaM (10°C).
|
| Flavin
reduction | Heme reduction |
|---|
|
|
|
|
|---|
|
| k1 (%) | k2 | k |
|---|
| nNOS | −4.3±0.2 (63) | 0.4±0.02 | ND |
|
| +48±2.1 (61) | 5.1±0.004 | 3.6±0.2 |
| eNOS | −0.3±0.03 (59) | 0.02±0.002 | ND |
|
| +59±4.4 (77) | 4.3±0.9 | 0.005±0.0004 |
|
nNOSeFNR | −0.17±0.003
(40) | 0.019±0.0007 | ND |
|
| +19±3.5 (43) | 0.19±0.006 | 0.0018±0.0002 |
|
eNOSnFNR | −18±1.0 (62) | 1.0±0.02 | ND |
|
| +32±1.7 (64) | 1.1±0.03 | 0.0024±0.0002 |
Clinical implication
Experimental and clinical results show that NO is
involved in the genesis of depression as well as in antidepressant
drug effects (30). NOS catalyze a
two-step oxidation of L-arginine to form NO. NOS is also the
molecular target of anti-inflammatory compounds (30). Inhibition of NOS activity will
exert antidepressant-like effect in animal models (31). NOS chimeras have been successfully
created in this work. Investigations provide a better understanding
of how distinct protein structures or structural features influence
the activities of NOS enzymes. Our results provide general
fundamental understanding for rational design of NOS enzymes in
order to regulate their functions in various biological settings
(32).
The reductase domains of eNOS, nNOS and the two
constitutive nitric oxide synthase (cNOS) share higher sequence
similarity (over 60%). These domains contain same regulatory
peptide (a C-terminal tail and an AI) and same cofactor binding
sites. Nevertheless, they differ observably in the ability for
transferring their electrons to heme or hemeprotein acceptors. In
order to evaluate the role of FNR module in controlling NOS
catalytic activities, chimeras were created by interchanging the
FNR-like module between eNOS and nNOS in this work. The eNOS
chimera (eNOSnFNR) showed a higher cytochrome c
reductase activity without calmodulin (CaM), and the activity was
over 10-fold higher than that of native eNOS. Meanwhile, the
activity did not increase upon CaM binding. The NO synthesis rate
was similar to that of native eNOS. In contrast, the nNOS chimera
(nNOSeFNR) had a ~10% cytochrome c reductase
activity of native nNOS without CaM. The NO synthesis activity for
nNOS chimera (nNOSeFNR) was ~12% activity of native
nNOS. The NOS FNR modules transferred their ferricyanide reductase
character to the chimera enzymes. In conclusion, the FNR module is
important in adjusting electrons flow through the reductase domain
and out of the FMN module. Results indicated that the FNR module
plays a critical role in controlling electron transfer capacities
of the FMN module.
Acknowledgements
The present study was supported by 2016 Key science
and technology plan project of Henan province (162102310198), the
Henan province health department general project (201403046) and
youth innovation fund of the first Affiliated Hospital of Zhengzhou
University.
Glossary
Abbreviations
Abbreviations:
|
NO
|
nitric oxide
|
|
NOS
|
NO synthase
|
|
eNOS
|
endothelial NOS
|
|
nNOS
|
neuronal NOS
|
|
cNOS
|
constitutive NOS (nNOS and eNOS)
|
|
NOSred
|
reductase domain of NOS
|
|
FNR
|
ferredoxin-NADP+
reductase
|
|
nNOSeFNR
|
nNOS incorporates with eNOS FNR
module
|
|
eNOSnFNR
|
eNOS incorporates with nNOS FNR
module
|
|
EPPS
|
4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid
|
|
CaM
|
calmodulin
|
|
EGTA
|
ethylene glycol tetraacetic acil
|
|
IPTG
|
isopropyl-β-D-thiogalactopyranoside
|
|
PMSF
|
phenylmethylsulphonyl fluoride.
|
References
|
1
|
Roman LJ, Martásek P and Masters BS:
Intrinsic and extrinsic modulation of nitric oxide synthase
activity. Chem Rev. 102:1179–1190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stuehr DJ, Kwon NS, Nathan CF, Griffith
OW, Feldman PL and Wiseman J: N omega-hydroxy-L-arginine is an
intermediate in the biosynthesis of nitric oxide from L-arginine. J
Biol Chem. 266:6259–6263. 1991.PubMed/NCBI
|
|
3
|
Bredt DS, Hwang PM, Glatt CE, Lowenstein
C, Reed RR and Snyder SH: Cloned and expressed nitric oxide
synthase structurally resembles cytochrome P-450 reductase. Nature.
351:714–718. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamas S, Marsden PA, Li GK, Tempst P and
Michel T: Endothelial nitric oxide synthase: Molecular cloning and
characterization of a distinct constitutive enzyme isoform. Proc
Natl Acad Sci USA. 89:pp. 6348–6352. 1992; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klatt P, Schmidt K, Uray G and Mayer B:
Multiple catalytic functions of brain nitric oxide synthase.
Biochemical characterization, cofactor-requirement and the role of
N omega-hydroxy-L-arginine as an intermediate. J Biol Chem.
268:14781–14787. 1993.PubMed/NCBI
|
|
6
|
Moncada S and Higgs EA: The discovery of
nitric oxide and its role in vascular biology. Br J Pharmacol. 147
Suppl 1:S193–S201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt HH, Smith RM, Nakane M and Murad
F: Ca2+/calmodulin-dependent NO synthase type I: A
biopteroflavoprotein with Ca2+/calmodulin-independent diaphorase
and reductase activities. Biochemistry. 31:3243–3249. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McMillan K, Bredt DS, Hirsch DJ, Snyder
SH, Clark JE and Masters BS: Cloned, expressed rat cerebellar
nitric oxide synthase contains stoichiometric amounts of heme,
which binds carbon monoxide. Proc Natl Acad Sci USA. 89:pp.
11141–11145. 1992; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng C, Taiakina V, Ghosh DK, Guillemette
JG and Tollin G: Intraprotein electron transfer between the FMN and
heme domains in endothelial nitric oxide synthase holoenzyme.
Biochim Biophys Acta. 1814:1997–2002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rozhkova EA, Fujimoto N, Sagami I, Daff SN
and Shimizu T: Interactions between the isolated oxygenase and
reductase domains of neuronal nitric-oxide synthase: Assessing the
role of calmodulin. J Biol Chem. 277:16888–16894. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daff S, Noble MA, Craig DH, Rivers SL,
Chapman SK, Munro AW, Fujiwara S, Rozhkova E, Sagami I and Shimizu
T: Control of electron transfer in neuronal NO synthase. Biochem
Soc Trans. 29:147–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roman LJ, Miller RT, de La Garza MA, Kim
JJ and Masters BS Siler: The C terminus of mouse macrophage
inducible nitric-oxide synthase attenuates electron flow through
the flavin domain. J Biol Chem. 275:21914–21919. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roman LJ, Martásek P, Miller RT, Harris
DE, de La Garza MA, Shea TM, Kim JJ and Masters BS Siler: The C
termini of constitutive nitric-oxide synthases control electron
flow through the flavin and heme domains and affect modulation by
calmodulin. J Biol Chem. 275:29225–29232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alderton WK, Cooper CE and Knowles RG:
Nitric oxide synthases: Structure, function and inhibition. Biochem
J. 357:593–615. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salerno JC, Harris DE, Irizarry K, Patel
B, Morales AJ, Smith SM, Martasek P, Roman LJ, Masters BS, Jones
CL, et al: An autoinhibitory control element defines
calcium-regulated isoforms of nitric oxide synthase. J Biol Chem.
272:29769–29777. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishida CR and de Montellano PR: Control
of electron transfer in nitric-oxide synthases. Swapping of
autoinhibitory elements among nitric-oxide synthase isoforms. J
Biol Chem. 276:20116–20124. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Daff S, Sagami I and Shimizu T: The
42-amino acid insert in the FMN domain of neuronal nitric-oxide
synthase exerts control over Ca(2+)/calmodulin-dependent electron
transfer. J Biol Chem. 274:30589–30595. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Martàsek P, Paschke R, Shea T,
Masters BS Siler and Kim JJ: Crystal structure of the
FAD/NADPH-binding domain of rat neuronal nitric-oxide synthase.
Comparisons with NADPH-cytochrome P450 oxidoreductase. J Biol Chem.
276:37506–37513. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuda H and Iyanagi T: Calmodulin
activates intramolecular electron transfer between the two flavins
of neuronal nitric oxide synthase flavin domain. Biochim Biophys
Acta. 1473:345–355. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guan ZW and Iyanagi T: Electron transfer
is activated by calmodulin in the flavin domain of human neuronal
nitric oxide synthase. Arch Biochem Biophys. 412:65–76. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guan ZW, Kamatani D, Kimura S and Iyanagi
T: Mechanistic studies on the intramolecular one-electron transfer
between the two flavins in the human neuronal nitric-oxide synthase
and inducible nitric-oxide synthase flavin domains. J Biol Chem.
278:30859–30868. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gruez A, Pignol D, Zeghouf M, Covès J,
Fontecave M, Ferrer JL and Fontecilla-Camps JC: Four crystal
structures of the 60 kDa flavoprotein monomer of the sulfite
reductase indicate a disordered flavodoxin-like module. J Mol Biol.
299:199–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adak S, Ghosh S, Abu-Soud HM and Stuehr
DJ: Role of reductase domain cluster 1 acidic residues in neuronal
nitric-oxide synthase. Characterization of the FMN-FREE enzyme. J
Biol Chem. 274:22313–22320. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andrew PJ and Mayer B: Enzymatic function
of nitric oxide synthases. Cardiovasc Res. 43:521–531. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adak S, Aulak KS and Stuehr DJ: Chimeras
of nitric-oxide synthase types I and III establish fundamental
correlates between heme reduction, heme-NO complex formation, and
catalytic activity. J Biol Chem. 276:23246–23252. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishida CR and de Montellano PR Ortiz:
Electron transfer and catalytic activity of nitric oxide synthases.
Chimeric constructs of the neuronal, inducible, and endothelial
isoforms. J Biol Chem. 273:5566–5571. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abu-Soud HM, Ichimori K, Presta A and
Stuehr DJ: Electron transfer, oxygen binding, and nitric oxide
feedback inhibition in endothelial nitric-oxide synthase. J Biol
Chem. 275:17349–17357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Newman E, Spratt DE, Mosher J, Cheyne B,
Montgomery HJ, Wilson DL, Weinberg JB, Smith SM, Salerno JC, Ghosh
DK and Guillemette JG: Differential activation of nitric-oxide
synthase isozymes by calmodulin-troponin C chimeras. J Biol Chem.
279:33547–33557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Montgomery HJ, Perdicakis B, Fishlock D,
Lajoie GA, Jervis E and Guy Guillemette J: Photo-control of nitric
oxide synthase activity using a caged isoform specific inhibitor.
Bioorg Med Chem. 10:1919–1927. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dvorakova M and Landa P: Anti-inflammatory
activity of natural stilbenoids: A review. Pharmacol Res.
124:126–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
da Silva, Leal VM, Bonassoli VT, Soares
LM, Milani H and de Oliveira RMW: Depletion of 5 hydroxy-triptamine
(5-HT) affects the antidepressant-like effect of neuronal nitric
oxide synthase inhibitor in mice. Neurosci Lett. 656:131–137. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang ZQ, Haque MM, Binder K, Sharma M, Wei
CC and Stuehr DJ: Engineering nitric oxide synthase chimeras to
function as NO dioxygenases. J Inorg Biochem. 158:122–130. 2016.
View Article : Google Scholar : PubMed/NCBI
|