Introduction
Staphylococcus aureus (SA or S. aureus) is a common
pathogen that causes local and systemic infections in patients in
the community and hospitalised patients, due to its large array of
virulence factors. It is the most common germ found in the pharynx
and nasal cavities in screening samples. Although the nasal
cavities are considered the primary carriage site for SA, data
suggest that the pharynx can equally contribute to carrier status
(1,2).
In many cases of hospitalised patients,
Staphylococcus colonizing nasal or pharyngeal sites can become
virulent and can cause severe and even fatal infections in cases
of: endocarditis, meningitis, blood stream infections, surgical
site infections (3), allogenic
transplant (4), acquired vitamin K
coagulopathies (5), parapneumonic
pleurisy (6). In the hospital
environment, SA strains initially sensitive to methicillin
[methicillin-susceptible strains (MSSA)] can transform into
methicillin-resistant SA (MRSA). Fundamental differences have been
found between community-acquired MRSA (CA-MRSA) and
hospital-acquired MRSA (HA-MRSA) (2), which exhibits an increased drug
resistance due to antibiotic selective pressure. The increase in
the resistance of MRSA strains has a significant impact on patient
care and also influences all the components of the infection
control system (7).
Mult-iresistant MRSA strains are defined as strains resistant to
three or more non-β lactam drugs. These strains are designated as
methicillin-oxacillin resistant SA (MORSA) and are associated with
treatment failure. From these reasons, it is clear that there is a
need to monitor the incidence and antibiotic resistance of MRSA
strains on a regular basis (8).
There is also a need for the discovery of novel molecules that may
have antibacterial activity against SA strains. Some progress has
been made with testing the essential oil of propolis from the
Cerrado biome, as well as anhydrofusarubin and methyl ether of
fusarubin extracted from the endorphytic fungus, Cladosporium sp.,
isolated from the leaves of Rauwolfia serpentina (L.) Benth. ex
Kurz. (family, Apocyanaceae); these tests have yielded promising
results against SA strains (9,10);
however, further studies are required to confirm these
findings.
The current study aimed to evaluate the prevalence
of colonisation with SA in a hospital environment and in the
Oltenia province in Romania where our hospital is located, and to
compare the risk factors for colonisation with multi-resistant
strains of SA. We also aimed to characterise the antibiotic
resistance phenotypes of SA strains circulating in the Oltenia
province in order to orient the preventive antibiotic therapy.
Materials and methods
This cross-sectional study was conducted between
January-December 2016 and included a total of 329 patients (167
males and 162 females) aged between 6 months and 94 years; 210
patients were hospitalised in the County Clinical Emergency
Hospital of Craiova (Craiova, Romania) and 119 were outpatients. We
collected 322 pharyngeal exudates and 142 nasal exudates for
screening purposes [active surveillance cultures (ASC)]. In total,
2 pharyngeal exudates were collected from 19 patients, and 2 nasal
exudates were collected from 12 patients. The reason for the
collection of 2 exudates was the fact that the first exudate
culture was negative, despite the clinical symptoms, and the
physician ordered the collection of a second sample.
This study was carried out in accordance with the
Helsinki Declaration of 1975, and was approved by the Review Ethics
Board of the University Medicine and Pharmacy of Craiova and of the
County Clinical Emergency Hospital of Craiova, Romania. All
patients involved in this study signed a full informed consent
prior to obtaining the samples. We collected both pharyngeal and
nasal exudates from 135 patients, only pharyngeal exudates from 187
patients and only nasal exudates from 7 patients. One swab was
taken from the nostrils which was rotated gently in both nostrils,
and one swab was taken from the pharynx by sweeping both tonsils.
We used rayon-tipped swab with Amies charcoal transport medium
(Copan Diagnostics Inc., Brescia, Italy).
The germs were identified by classical
microbiological diagnosis, as previously described (11). We plated both swabs directly on
selective media for SA (ChromID S. aureus) and MRSA (ChromID MRSA;
both from Biomerieux, Marcy-l’Étoile, France). Antibiotic
susceptibility testing was performed according to the Clinical
Laboratory Standards Institute (CLSI) guidelines released in 2015
(12), using the Kirby-Bauer
method. From isolated colonies on selective medium for SA (ChromID
S.aureus, Biomerieux) and MRSA (ChromID MRSA, Biomerieux), we
performed an inoculum in liquid broth (Biomerieux) which we
adjusted to 0.5 McFarland turbidity with a Densimat instrument
(Biomerieux). The inoculum was poured into Muller Hinton agar
plates (Biomerieux). After drying the plates for 3 min at 37°C, we
placed the antibiotic disks (Oxoid Ltd., Basingstoke, UK) in an
equally spaced fashion, using a maximum of 6 disks per plate. The
plates were then incubated at 37°C for 18 h and the following day
the inhibition zone diameters were measured using an electronic
caliper for maximum precision of the measurement. For the quality
control of the Muller Hinton agar plates and antibiotic disks, we
used the Kirby-Bauer method with the SA control strains, ATCC 25923
and ATCC 43300 (Liofilchem s.r.l, Teramo, Italy).
Statistical analysis
Consecutive samples collected from the same patient
after an interval of <7 days were excluded from the analysis.
For data entry and all statistical calculations, we used Microsoft
Excel (Microsoft Corp., Redmond, WA, USA) and Stata (StataCorp LLC,
College Station, TX, USA). Numerical variables are expressed as the
means ± standard deviation. We divided the patients into categories
[adults (age, >18 years) and children (age, ≤18 years)].
Categorical variables were expressed as proportions. For
differences between resistance indexes of different patient groups,
we used the Student's t-test when the values distribution was
normal (as assessed by the Kruskal-Wallis rank test when the values
distribution was not normal (normality distribution was tested by
the Shapiro-Walk method). For differences between proportions of
SA, MRSA and MORSA in the various groups, we used the Chi-square
test the test on the equality of proportions with Normal
distribution. A value of P<0.05 was considered to indicate a
statistically significant difference.
The statistical method hierarchical clustering was
used in order to construct an inheritance tree of the isolates
based on the antibiotic resistance pattern. As the strains that
transmit from a patient to another will probably suffer mutations
in the genes of antibiotic resistance according to the administered
antibiotic treatment, the relatedness by the antibiotic resistance
pattern can be used as an indication of the genetic relatedness of
the SA strains. We measured the diameters of inhibition areas
around antibiotic disks on a Petri dish and used them to perform
hierarchical clustering analysis in STATA software with the option
of Ward's minimum variance clustering. The assignment of isolates
to clusters was based upon inhibition zone diameters.
Results
From the 322 pharyngeal exudates, 104 (32.30%) were
positive, whereas from the 142 nasal exudates, 48 (33.80%) were
positive. The species isolated consisted mostly of S. aureus
(67.21% in pharyngeal swabs and 75.41% in nasal swabs), coagulase
negative staphylococci (0.82% in pharyngeal swabs and 4.92% in
nasal swabs), Klebsiella spp. (21.31% in pharyngeal swabs
and 9.84% in nasal swabs) and in smaller percentages,
Escherichia coli, Proteus spp., Enterobacter
spp., Pseudomonas spp. and glucose non-fermenters
Gram-negative rods (Table I and
Fig. 1). The prevalence in the two
types of swabs differed only for Klebsiella (Chi-square
test, P=0.0540) and for coagulase-negative staphylococci, without
reaching statistical significance (P=0.0739). The prevalence in the
nasal cavity of coagulase negative staphylococci was greater in
females compared with males (12.00 vs. 0.00%, P=0.0330). The
prevalence of S. aureus was significantly (P<0.0001)
greater in outpatients (91.84%) that in inpatients (50.68%)
(Table I). In addition, 3 strains
of Candida albicans were isolated only from inpatients from
two pharyngeal swabs and one nasal swab (0.91% of patients) (data
not shown).
 | Table I.The bacterial species isolated from
pharyngeal and nasal swabs, broken down by patient sex, age group
(adults/children) and hospitalisation status
(inpatient/outpatient). |
Table I.
The bacterial species isolated from
pharyngeal and nasal swabs, broken down by patient sex, age group
(adults/children) and hospitalisation status
(inpatient/outpatient).
|
|
|
|
| Pharyngeal swabs | Nasal swabs | Pharyngeal swabs | Nasal swabs | Pharyngeal swabs | Nasal swabs |
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Species | Pharyngeal swabs
(n=122) | Nasal swabs
(n=61) | P-value | Males (n=68 | Females (n=54) | P-value | Males (n=36) | Females (n=25) | P-value | Adults (n=112) | Children (n=10) | P-value | Adults (n=61) | Children (n=0) | P-value | Inpatients
(n=73) | Outpatients
(n=49) | P-value | Inpatients
(n=58) | Outpatients
(n=3) |
|---|
| S.
aureus | 82 | 46 | 0.2543 | 44 | 38 | 0.5083 | 29 | 17 | 0.2627 | 75 | 7 | 0.8447 | 46 | – | – | 37 | 45 |
<0.001a | 43 | 3 |
0.3104 |
|
| (67.21%) | (75.41%) |
| (64.71%) | (70.37%) |
| (80.56%) | (68.00%) |
| (66.96%) | (70.00%) |
| (75.41%) |
|
| (50.68%) | (91.84%) |
| (74.14%) | (100.00%) |
|
| Coagulase-negative
staphylococci | 1 | 3 | 0.0739 | 0 | 1 | 0.2601 | 0 | 3 | 0.0330a | 1 | 0 | 0.7641 | 3 | – | – | 1 | 0 | 0.4107 | 3 | 0 | 0.6862 |
|
| (0.82%) | (4.92%) |
| (0.00%) | (1.85%) |
| (0.00%) | (12.00%) |
| (0.89%) | (0.00%) |
| (4.92%) |
|
| (1.37%) | (0.00%) |
| (5.17%) | (0.00%) |
|
| E. coli | 3 | 1 | 0.7207 | 2 | 1 | 0.6993 | 1 | 0 | 0.4008 | 3 | 0 | 0.6003 | 1 | – | – | 1 | 2 | 0.3441 | 1 | 0 | 0.8186 |
|
| (2.46%) | (1.64%) |
| (2.94%) | (1.85%) |
| (2.78%) | (0.00%) |
| (2.68%) | (0.00%) |
| (1.64%) |
|
| (1.37%) | (4.08%) |
| (1.72%) | (0.00%) |
|
| Klebsiella
sp. | 26 | 6 | 0.0540a | 16 | 10 | 0.5021 | 3 | 3 | 0.6363 | 24 | 2 | 0.9158 | 6 | – | – | 25 | 1 |
<0.001a | 6 | 0 | 0.5574 |
|
| (21.31%) | (9.84%) |
| (23.53%) | (18.52%) |
| (8.33%) | (12.00%) |
| (21.43%) | (20.00%) |
| (9.84%) |
|
| (34.25%) | (2.04%) |
| (10.34%) | (0.00%) |
|
| Proteus
sp. | 1 | 1 | 0.6151 | 1 | 0 | 0.3710 | 0 | 1 | 0.2263 | 1 | 0 | 0.7641 | 1 | – | – | 1 | 0 | 0.4107 | 1 | 0 | 0.8186 |
|
| (0.82%) | (1.64%) |
| (1.47%) | (0.00%) |
| (0.00%) | (4.00%) |
| (0.89%) | (0.00%) |
| (1.64%) |
|
| (1.37%) | (0.00%) |
| (1.72%) | (0.00%) |
|
| Enterobacter
sp. | 1 | 1 | 0.6151 | 1 | 0 | 0.3710 | 1 | 0 | 0.4008 | 1 | 0 | 0.7641 | 1 | – | – | 1 | 0 | 0.4107 | 1 | 0 | 0.8186 |
|
| (0.82%) | (1.64%) |
| (1.47%) | (0.00%) |
| (2.78%) | (0.00%) |
| (0.89%) | (0.00%) |
| (1.64%) |
|
| (1.37%) | (0.00%) |
| (1.72%) | (0.00%) |
|
|
| Pseudomonas
sp. | 5 | 2 | 0.7852 | 3 | 2 | 0.4294 | 2 | 0 | 0.2308 | 4 | 1 | 0.3259 | 2 | – | – | 4 | 1 | 0.3476 | 2 | 0 | 0.7436 |
|
| (4.10%) | (3.28%) |
| (4.41%) | (3.70%) |
| (5.56%) | (0.00%) |
| (3.57%) | (10.00%) |
| (3.28%) |
|
| (5.48%) | (2.04%) |
| (3.45%) | (0.00%) |
|
|
| Glucose
non-fermenters Gram-negative rods | 3 | 1 | 0.7207 | 1 | 2 | 0.4294 | 0 | 1 | 0.2263 | 0 | 0 | 1.0000 | 1 | – | – | 3 | 0 | 0.1508 | 1 | 0 | 0.8186 |
|
| (2.46%) | (1.64%) |
| (1.47%) | (3.70%) |
| (0.00%) | (4.00%) |
| (0.00%) | (0.00%) |
| (1.64%) |
|
| (4.11%) | (0.00%) |
| (1.72%) | (0.00%) |
|
|
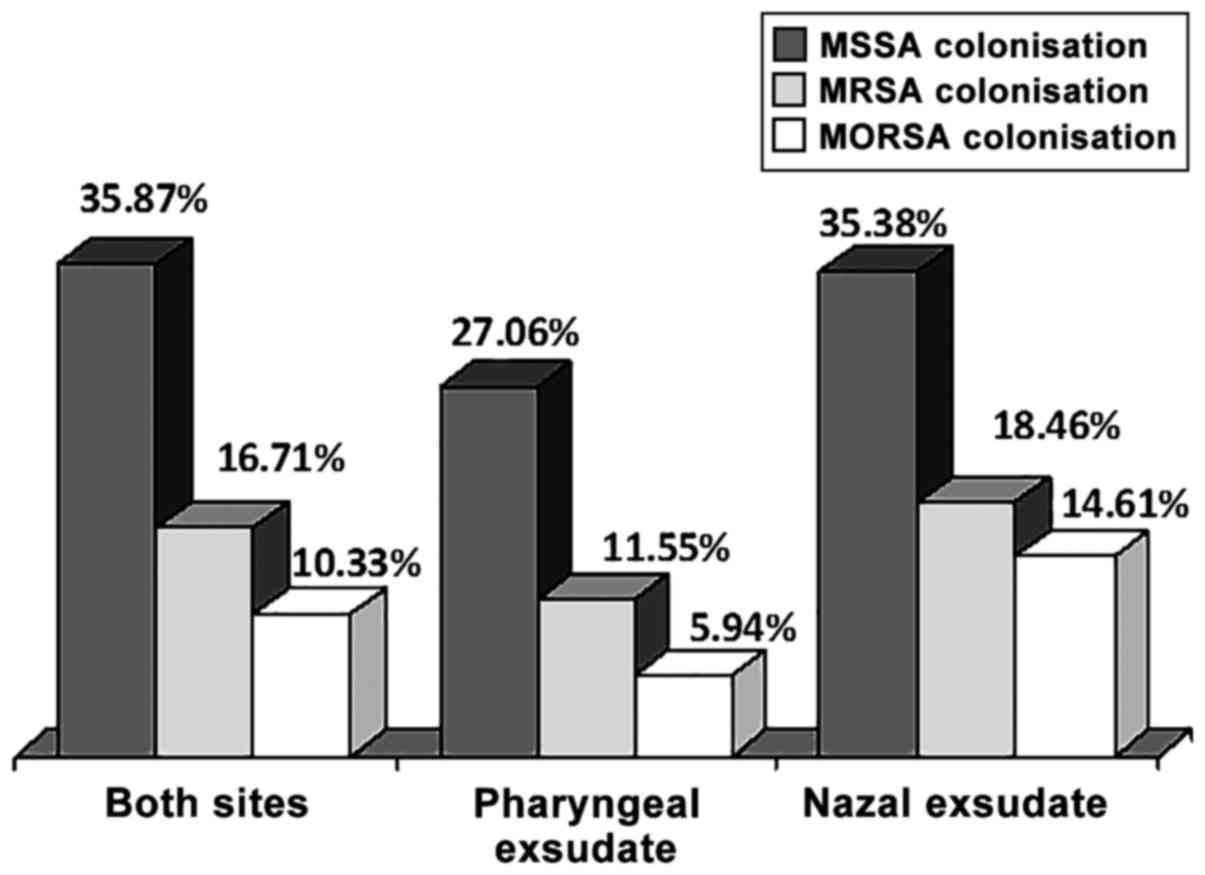
The absolute S. aureus carriage was 35.87%, as 118
out of the 329 patients had S. aureus either in the pharynx or in
the nose. In total, 82 patients (27.06%) out of the 303 patients
with screened pharyngeal swabs had S. aureus in the throat and 46
patients (35.38%) out of the 130 patients with screened nasal swabs
had SA in the nose (Table II). A
total of 10 patients had SA both in the throat and nose. Thus, the
nasal carrier rate was marginally significantly higher than that in
the pharynx (proportion's test, P=0.0820). The absolute MRSA
prevalence was 16.72% (55 out of the 329 patients). MRSA was
present in the pharyngeal exudates in 35 patients out of the 303
screened patients (11.55%) and in the nasal exudates in 24 screened
patients, out of 130 (18.46%). In total, 4 patients (3.85%) had
MRSA carriage both in the nose and pharynx. When the MRSA
prevalence was expressed as the proportion of staphylococcal
isolates, the global rate was then 46.61%, the rate in pharyngeal
exudate was 42.68% and that in the nasal exudate was 52.17%
(proportion's test, P=0.0547). MORSA strains were isolated from 34
patients (10.33%), and the prevalence rates were 5.61% in the
pharyngeal exudates and 13.85% in the nasal exudates (proportion's
test, P=0.040). In total, 1 patient (0.96%) had MORSA present both
in the nose and pharynx (Table
II). Thus, MORSA strains were clearly more prevalent in the
nasal swabs, compared with the pharyngeal swabs. It should be noted
that all the 7 nasal exudates collected from children were negative
(Table I).
 | Table II.Carriage rates in the pharynx and
nose for the strains of S. aureus, MRSA and MORSA. |
Table II.
Carriage rates in the pharynx and
nose for the strains of S. aureus, MRSA and MORSA.
| Strain | Pharyngeal carriage
(303 patients screened) | Nasal carriage (130
patients screened) | P-value | Double carriage
(104 patients screened) | Global carriage
(329 patients screened) |
|---|
| S. aureus
colonisation | 82 (27.06%) | 46 (35.38%) | 0.0820 | 10 (9.62%) | 118 (35.87%) |
| MRSA
colonisation | 35
(11.55/42.68%)a | 24
(18.46/52.17%)a | 0.0547 | 4
(3.85/40.00%)a | 55
(16.72/46.61%) |
| MORSA
colonisation | 17
(5.61/48.57%)b | 18
(13.85/75.00%)b | 0.0040c | 1
(0.96/25.00%)b | 34
(10.33/61.81%) |
| Not infected with
S. aureus | 221 (72.94%) | 84 (64.62%) | 0.0820 | 94 (90.38%) | 211(64.13%) |
The prevalence of S. aureus colonisation was
marginally higher (Chi-square, P=0.1024) in males (40.12%) compared
with females (31.48%), and significantly higher (Chi-square,
P=0.0225) in adults (38.01%) vs. children (18.92%). The S. aureus
colonisation rates did not differ significantly between outpatients
and inpatients (Chi-square, P=0.3015) (Table III).
 | Table III.Prevalence rates of colonisation with
S. aureus, MRSA and MORSA by age, hospitalisation status
(inpatient/outpatient), ward type and sex. |
Table III.
Prevalence rates of colonisation with
S. aureus, MRSA and MORSA by age, hospitalisation status
(inpatient/outpatient), ward type and sex.
| Strain | Adults (292
patients) | Children (37
patients) | P-value | Inpatients (210
patients) | Outpatients (119
patients) | P-value | ICU (99
patients) | Non-ICU (230
patients) | P-value | Males(167
patients) | Females (162
patients) | P-value |
|---|
| S. aureus
colonisation | 111 (38.01%) | 7 (18.92%) | 0.0225 | 71 (33.81%) | 47 (39.50%) | 0.3015 | 35 (35.35%) | 83 (35.93%) | 0.8988 | 67 (40.12%) | 51 (31.48%) | 0.1024 |
| MRSA
colonisation | 54 | 1 | 0.0153c | 38 | 17 | 0.3736 | 20 | 35 | 0.2664 | 37 | 18 | 0.0730 |
|
|
(18.49/48.65%)a |
(2.70/14.29%)a |
|
(18.09/53.52%)a |
(14.29/36.17%)a |
|
(20.20/57.14%)a |
(15.15/42.17%)a |
|
(22.16/55.22%)a |
(11.11/35.29%)a |
|
| MORSA
colonisation | 33 | 1 | 0.1055 | 27 | 7 | 0.0458c | 13 | 21 | 0.2742 | 23 | 11 | 0.0375c |
|
|
(11.30/29.73%)b |
(2.70/14.29%)b |
|
(12.86/38.03%)b |
(5.88/14.89%)b |
|
(13.13/37.14%)b |
(9.09/25.30%)b |
|
(13.77/34.33%)b |
(6.79/21.57%)b |
|
| Not infected with
S. aureus | 181 (61.99%) | 30 (81.08%) | 0.0225c | 139 (66.19%) | 72 (60.50%) | 0.3015 | 64 (64.65%) | 146 (64.07%) | 0.8988 | 100 (59.88%) | 111 (68.52%) | 0.1024 |
A marked difference in MRSA prevalence in adults was
observed, as this was >3-fold higher than that in children, with
a significant difference (P=0.0225). In addition, MRSA was more
frequent in inpatients, compared with outpatients (P=0.0458). No
significant difference was observed in MRSA prevalence between
intensive care unit (ICU) patients and patients in other wards of
the hospital (P=0.2664) (Table
III).
The MORSA prevalence as a proportion of SA isolates
was 61.81% of the isolated S. aureus strains (Table II). A marked difference in MORSA
prevalence was observed in adults (11.30%), which was almost 2-fold
higher than that in children (2.70%), although the differene was
not statistically significant (P=0.1055). In additoin, MORSA
prevalence was significantlymore frequent (P=0.0458) in inpatients
(12.86%), compared with outpatients (5.88%). No significant
difference was observed in MORSA prevalence between ICU patients
and patients in other wards of the hospital (P=0.2742) (Table III).
Resistance of SA strains
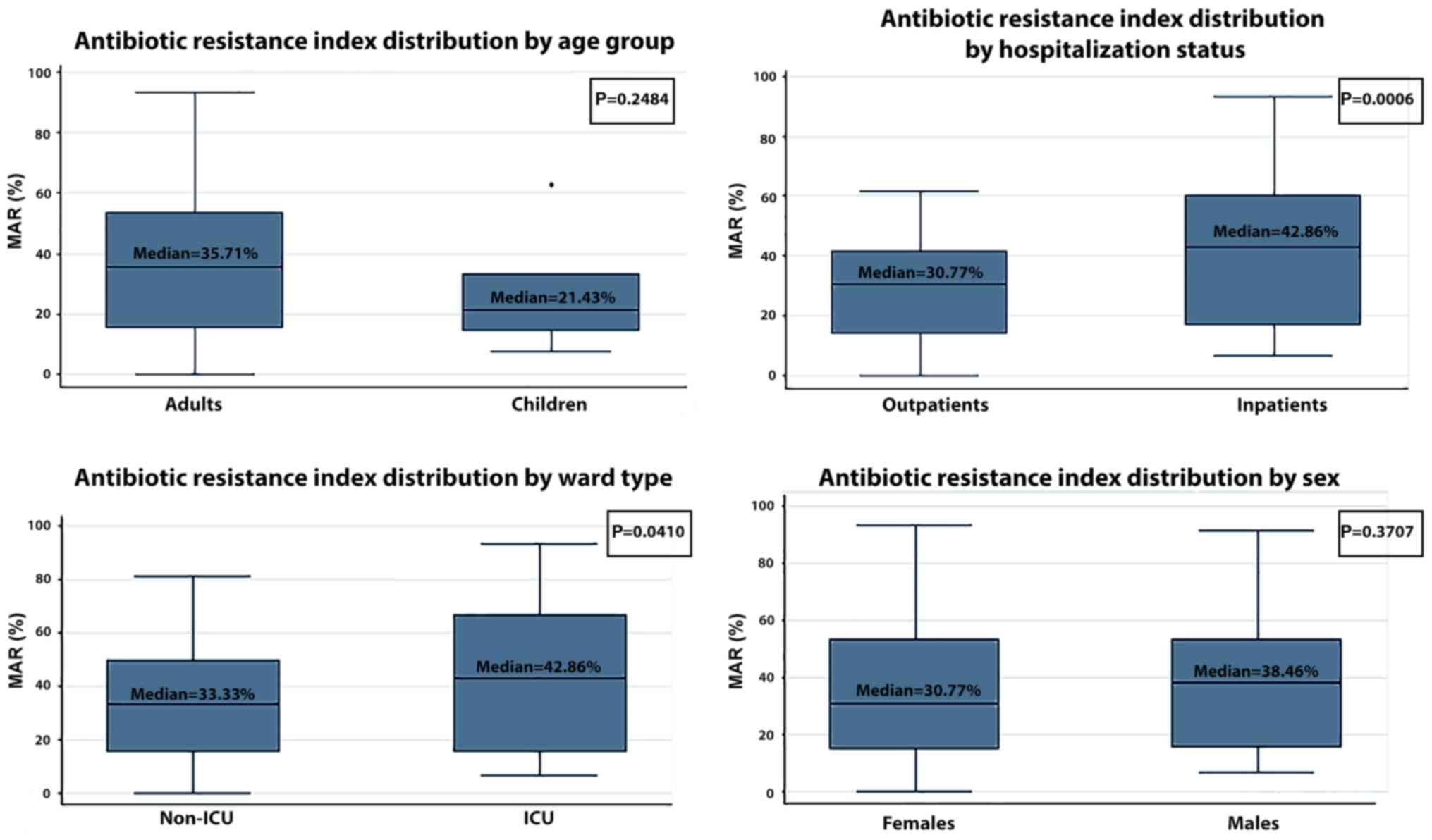
The median multiple antibiotic resistance (MAR)
index of the SA strains was 33.33% (Table IV). As expected, the median MAR of
MRSA was higher than that of MSSA (45.45 vs. 18.75%) and the median
MAR of MORSA was even higher (57.14%), as was expected (Table IV). The median MAR of the
inpatients was clearly higher than the median MAR of the
outpatients (42.86 vs. 30.77%, P=0.0006). In addition, ICU patients
had a higher median MAR that non-ICU patients (42.41 vs. 33.56%,
P=0.0410). No statistically significant differences in the median
MAR were observed between adults and children (35.71 vs. 21.43%,
P=0.2484) or between females and males (30.77 vs. 38.46%, P=0.3707)
(Fig. 2). We observed an increased
MAR in the inpatients compared with the outpatients, both for MRSA
strains (53.33% vs. 30.77%, P=0.0024) and MORSA strains (61.25% vs.
50.00%, P=0.0250) (Table IV).
 | Table IV.The median multiple antibiotics
resistance index of the isolated strains of Staphylococcus aureus,
MRSA and MORSA by age group, hospitalization status, ward type and
sex. |
Table IV.
The median multiple antibiotics
resistance index of the isolated strains of Staphylococcus aureus,
MRSA and MORSA by age group, hospitalization status, ward type and
sex.
|
| All 329
patients | Adults (292
patients) | Children (37
patients) | P-value | Inpatients (210
patients) | Outpatients (119
patients) | P-value | ICU (99
patients) | Non-ICU (230
patients) | P-value | Males (167
patients) | Females (162
patients) | P-value |
|---|
| S.
aureus | 33.33% | 35.71% | 21.43% | 0.2484 | 42.86% | 30.77% | 0.0006a | 42.86% | 33.33% | 0.0410a | 38.46% | 30.77% | 0.3707 |
| MRSA | 45.45% | 44.60% | 62.50% | –b | 53.33% | 30.77% | 0.0024a | 44.16% | 46.15% | 0.1256 | 43.75% | 47.73% | 0.5821 |
| MSSA | 18.75% | 18.75% | 19.05% | 0.3456 | 24.05% | 18.18% | 0.2370 | 15.39% | 24.05% | 0.5662 | 22.42% | 18.75% | 0.4353 |
| MORSA | 57.14% | 55.49% | 62.50% | –b | 61.25% | 50.00% | 0.0250a | 69.05% | 53.33% | 0.1050 | 55.24% | 60.00% | 0.7080 |
The multivariate analysis of MRSA infection
(Table V) revealed a higher risk
for males (OR=2.16, P=0.050) and patients aged >50 years
(OR=3.38, P=0.048). Surprisingly hospitalisation in the ICU ward or
the patient type (ambulatory or inpatient) had no significant
influence on the rate of MRSA colonisation.
 | Table V.Results of the multivariate logistic
regression analysis on the resistance index of MRSA strains, and
risk of acquiring MRSA and MORSA. |
Table V.
Results of the multivariate logistic
regression analysis on the resistance index of MRSA strains, and
risk of acquiring MRSA and MORSA.
|
| Risk factor |
|---|
|
|
|
|---|
|
| Resistance index
analysis | Chance to acquire
MRSA | Chance to acquire
MORSA |
|---|
|
|
|
|
|
|---|
|
| Coefficient | P-value | Odds ratio | P-value | Odds ratio | P-value |
|---|
| Sex |
|
|
|
|
|
|
| Males
vs. females | 0.017 | 0.681 | 2.16 | 0.050a | 0.611 | 0.467 |
| Age group |
|
|
|
|
|
|
| <30
years | −0.376 | 0.027a | 3.040 | 0.243 | 1 | – |
| 30–39
years | 0.152 | 0.158 | 2.096 | 0.463 | 0.082 | 0.209 |
| 40–49
years | −0.024 | 0.829 | 1.682 | 0.620 | 1 | – |
| >50
years | −0.072 | 0.255 | 3.382 | 0.048a | 0.323 | 0.368 |
| Patient type |
|
|
|
|
|
|
|
Inpatients vs.
outpatients | 0.292 | 0.008 | 0.746 | 0.622 | 18.92 | 0.025a |
| Ward type |
|
|
|
|
|
|
| ICU vs.
non-ICU | 0.004 | 0.937 | 1.141 | 0.784 | 0.487 | 0.379 |
| Constant | 0.257 | 0 | 0.297 | 0.003 | 1.184 | 0.807 |
Only the state of hospitalised patients greatly
increased the MORSA rate (OR=18.92%, P=0.025) (Table V). The sex and age of the patients
had no influence in this case.
The regression of the resistance index of MRSA
revealed that a young age (<30 years) (beta coefficient=-0.376,
P=0.027) and hospitalisation (beta coefficient=0.292, P=0.008) had
a significant impact on the antibiotic resistance of MRSA (Table V).
The resistances to individual antibiotics presented
significant differences between the categories of patients in a few
cases. When comparing the antibiotic resistances in adults vs.
children, these were increased in adults for clarithromycin (60.87
vs. 28.57%; Chi-square test, P=0.0920) and increased in children
for oxacillin (57.14 vs. 100%, P=0.0250). The antibiotic resistance
was markedly increased in inpatients compared to outpatients for
ciprofloxacin (37.33 vs. 4.35%, P<0.0001), gentamycin (27.63 vs.
4.17%, P=0.0012), rifampin (28.36 vs. 0%, P<0.0001), oxacillin
(75.00 vs. 50.00%, P=0.084 and sulfamethoxazole/trimethoprim (46.88
vs. 21.43%, P=0.0050). The antibiotic resistances of strains
isolated from ICU patients were higher compared with those isolated
from non-ICU patients for gentamycin (31.71 vs. 12.05%;
proportion's test, P=0.0109) and oxacillin (100 vs. 50.00%;
proportion's test, P<0.0001) (Table VI).
 | Table VI.Antibiotic resistance of
Staphylococcus aureus strains. |
Table VI.
Antibiotic resistance of
Staphylococcus aureus strains.
| Antibiotic | Global (128
strains) | Adults (111
strains) | Children (7
strains) | P-value | Inpatients (71
strains) | Outpatients (47
strains) | P-value | ICU (35
strains) | Non-ICU (83
strains) | P-value | Males (67
strains) | Females (51
strains) | P-value |
|---|
| Ciprofloxacin | 24.79% | 25.00% | 14.28% | 0.5216 | 37.33% | 4.35% |
<0.001a | 32.50% | 20.99% | 0.1836 | 25.37% | 24.53% | 0.9169 |
| Clarithromycin | 59.02% | 60.87% | 28.57% | 0.0920 | 59.21% | 58.70% | 0.9560 | 62.50% | 57.32% | 0.6015 | 64.29% | 51.92% | 0.1760 |
| Clindamycin | 56.91% | 57.76% | 42.86% | 0.4401 | 58.44% | 54.35% | 0.6606 | 52.50% | 59.04% | 0.5121 | 58.90% | 54.00% | 0.5945 |
| Erythromycin | 61.34% | 61.61% | 57.14% | 0.8138 | 64.86% | 55.56% | 0.3102 | 62.50% | 60.76% | 0.8593 | 63.77% | 58.00% | 0.5239 |
| Gentamycin | 18.55% | 18.64% | 14.28% | 0.7727 | 27.63% | 4.17% | 0.0012a | 31.71% | 12.05%a | 0.0109a | 19.18% | 17.65% | 0.8321 |
| Oxacillin | 62.50% | 57.14% | 100% | 0.0250a | 74.65% | 51.06% | 0.0084a | 100% | 50.60% |
<0.001a | 74.62% | 50.98% | 0.0079a |
| Penicillin | 91.60% | 92.86% | 71.43% | 0.0476a | 90.67% | 93.18% | 0.6291 | 92.50% | 91.14% | 0.8084 | 95.59% | 86.27% | 0.0712 |
| Rifampin | 19.00% | 20.43% | 0.00% | 0.1833 | 28.36% | 0.00% | 0.0001a | 25.71% | 15.38% | 0.1863 | 22.03% | 14.63% | 0.3084 |
|
Sulfamethoxazole/trimethoprim | 36.79% | 36.00% | 57.14% | 0.2619 | 46.88% | 21.43% | 0.0050a | 43.33% | 34.21% | 0.3484 | 43.33% | 28.26% | 0.0927 |
| Tetracycline | 58.00% | 58.95% | 42.86% | 0.4029 | 64.18% | 45.45% | 0.0444a | 65.79% | 53.23% | 0.2082 | 70.91% | 42.22% | 0.0017a |
We also analysed the resistance phenotypes, based
upon resistance to key antibiotics (Table VII). For MSSA, the most prevalent
phenotype was that resistant only to penicillin, followed by a
phenotype resistant to penicillin, clindamycin, clarithromycin,
doxycycline, erythromycin and tetracycline. For MRSA, the most
prevalent phenotype was that resistant only to penicillin and
cefoxitin, followed by a phenotype with an additional resistance to
clindamycin.
 | Table VII.Resistance phenotypes in MSSA and
MRSA. |
Table VII.
Resistance phenotypes in MSSA and
MRSA.
| A, MSSA resistance
patterns |
|---|
|
|---|
| MSSA resistance
profile | No. (%) |
|---|
| PEN | 16 (23.53) |
| CLI CLR DOX ERY PEN
TCY | 8 (11.76) |
| ERY PEN | 3 (4.41) |
| CLR ERY | 2 (2.94) |
| CIP CLI CSL DOX MFX
PEN SXT TCY | 2 (2.94) |
| CLR TCY | 2 (2.94) |
| CLI CLR ERY
PEN | 2 (2.94) |
| PEN SXT | 2 (2.94) |
| Wild-type | 2 (2.94) |
| CLR ERY SXT
TCY | 1 (1.47) |
| CLI PEN | 1 (1.47) |
| CHL CLI CLR DOX ERY
PEN SXT | 1 (1.47) |
| CLI CLR ERY PEN
RIF | 1 (1.47) |
| CIP CLI CLR DOX ERY
MFX PEN RIF TCY | 1 (1.47) |
| CIP CLI CLR DOX ERY
PEN SXT TCY | 1 (1.47) |
| CIP CLI CLR DOX ERY
SXT TCY | 1 (1.47) |
| PEN RIF SXT | 1 (1.47) |
| CIP CLI CLR DOX PEN
TCY | 1 (1.47) |
| CLI CLR PEN | 1 (1.47) |
| CLR CSL ERY PEN
SXT | 1 (1.47) |
| CHL CIP CLI CLR DOX
ERY MFX PEN TCY | 1 (1.47) |
| CIP CLI MFX
SXT | 1 (1.47) |
| DOX ERY PEN SXT
TCY | 1 (1.47) |
| DOX TCY | 1 (1.47) |
| CIP CLR ERY MFX PEN
SXT | 1 (1.47) |
| PEN TCY | 1 (1.47) |
| CHL CLI | 1 (1.47) |
| CIP PEN RIF SXT
TCY | 1 (1.47) |
| CLI CLR ERY PEN
SXT | 1 (1.47) |
| TCY | 1 (1.47) |
| CHL CLI CLR DOX ERY
PEN RIF TCY | 1 (1.47) |
| DOX ERY SXT | 1 (1.47) |
| CHL CIP CLI DOX ERY
MFX PEN TCY | 1 (1.47) |
| CHL CLI CLR ERY PEN
TCY | 1 (1.47) |
| CLI CLR DOX ERY
PEN | 1 (1.47) |
| PEN RIF | 1 (1.47) |
| CLI CLR DOX ERY PEN
SXT TCY | 1 (1.47) |
| SXT | 1 (1.47) |
| Total | 68 (100) |
|
| B, MRSA resistance
patterns |
|
| MRSA resistance
profile | No. (%) |
|
| FOX PEN | 5 (8.33) |
| CLI FOX PEN | 4 (6.67) |
| CLI CLR DOX ERY FOX
PEN TCY | 3 (5.) |
| CLI CLR DOX ERY FOX
PEN SXT TCY | 3 (5.) |
| CLI CLR CSL DOX ERY
FOX PEN TCY | 2 (3.33) |
| CLR ERY FOX
PEN | 2 (3.33) |
| CIP CLI CLR CSL DOX
ERY FOX PEN RIF SXT TCY | 2 (3.33) |
| CIP CLI CLR DOX ERY
FOX PEN SXT TCY | 2 (3.33) |
| CLI CLR ERY FOX
PEN | 2 (3.33) |
| CIP CLI CLR CSL DOX
ERY FOX PEN SXT TCY | 1 (1.67) |
| CIP CLI CLR CSL DOX
FOX MFX PEN RIF TCY | 1 (1.67) |
| CHL CIP CLI CLR DOX
ERY FOX PEN SXT | 1 (1.67) |
| CLR DOX FOX
PEN | 1 (1.67) |
| DOX FOX PEN | 1 (1.67) |
| CHL CIP CSL ERY FOX
MFX PEN RIF SXT | 1 (1.67) |
| CIP CLI CLR ERY FOX
MFX PEN SXT | 1 (1.67) |
| CIP CLI CLR ERY FOX
PEN | 1 (1.67) |
| CHL CLI CLR DOX ERY
FOX SXT TCY | 1 (1.67) |
| CIP CLR CSL DOX ERY
FOX MFX PEN RIF SXT TCY | 1 (1.67) |
| CIP CLR DOX ERY FOX
MFX PEN TCY | 1 (1.67) |
| CIP CLI CLR CSL DOX
ERY FOX MFX PEN SXT TCY | 1 (1.67) |
| CIP CSL ERY FOX
PEN | 1 (1.67) |
| CLI CLR ERY
FOX | 1 (1.67) |
| CIP DOX FOX PEN
SXT | 1 (1.67) |
| CLI CLR CSL DOX ERY
FOX PEN | 1 (1.67) |
| CHL CLI CLR CSL DOX
ERY FOX MFX RIF SXT TCY | 1 (1.67) |
| CLI CLR CSL DOX ERY
FOX PEN RIF TCY | 1 (1.67) |
| CLR CSL ERY FOX
PEN | 1 (1.67) |
| CLI CLR CSL DOX ERY
FOX PEN SXT TCY | 1 (1.67) |
| CLR DOX ERY FOX PEN
TCY | 1 (1.67) |
| CHL CIP CLI CSL DOX
ERY FOX MFX PEN RIF SXT TCY | 1 (1.67) |
| CHL CLI CLR CSL DOX
ERY FOX PEN RIF | 1 (1.67) |
| CLI CLR CSL DOX ERY
FOX RIF SXT TCY | 1 (1.67) |
| CLR ERY FOX PEN
TCY | 1 (1.67) |
| CLI CLR CSL ERY FOX
PEN | 1 (1.67) |
| CLI CLR DOX ERY
FOX | 1 (1.67) |
| DOX FOX PEN
TCY | 1 (1.67) |
| ERY FOX PEN
TCY | 1 (1.67) |
| CHL CIP CLI DOX FOX
PEN | 1 (1.67) |
| CHL CIP CLI CLR CSL
DOX ERY FOX MFX PEN RIF | 1 (1.67) |
| SXT TCY |
| CHL CIP CLI CSL DOX
FOX MFX PEN RIF SXT | 1 (1.67) |
| CLI CLR DOX FOX PEN
RIF TCY | 1 (1.67) |
| CLI CLR DOX FOX PEN
TCY | 1 (1.67) |
| CLI CLR CSL DOX FOX
PEN TCY | 1 (1.67) |
| Total | 60 (100) |
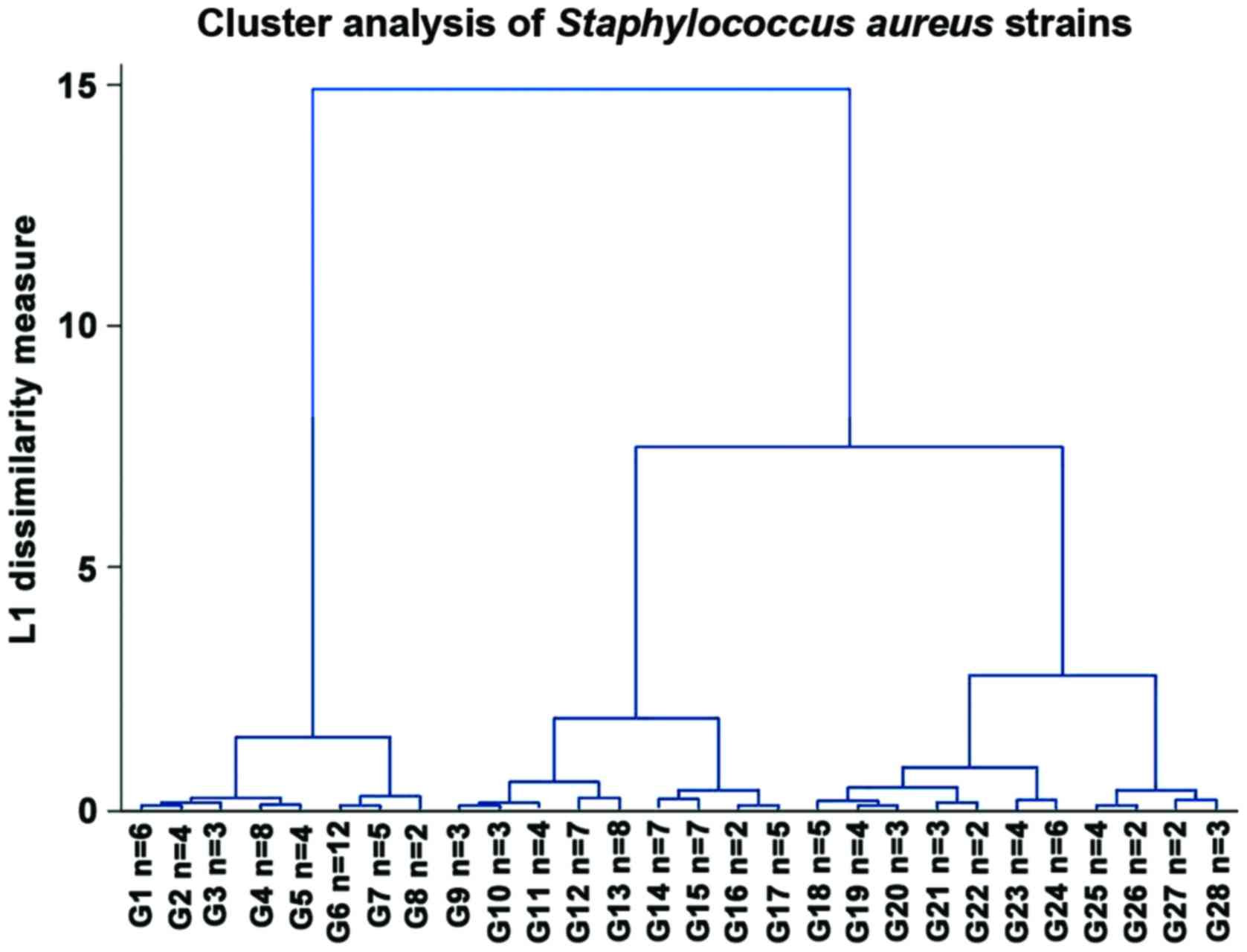
We also performed a hierarchical clustering analysis
of the strains based upon the diameters of inhibition zones in the
Kirby-Bauer antibiotic susceptibility testing method (Fig. 3). We observed 3 main groups: One
very sensitive that was hypothesised to be the MSSA strains, one
with intermediate resistance could be the ‘sensitive MRSA’ strains
that are generally community-acquired, which was the largest group,
and the third group with the greatest resistance that could be
regarded as HA-MRSA.
Discussion
Due to the high prevalence rate of SA colonisation
in the pharynx and nasal cavity in the general population, the
ratio between the number of multidrug-resistant strains of SA over
the total number of SA strains is used in the literature as a more
accurate measure of colonisation with resistant staphylococci. In
patients with facial acne, these can become infected with the
Staphylococci from the pharynx and nasal cavity and this could lead
to a form resistant to treatment (13). In some patients, these cases of
resistance strains may be associated with non-alcoholic fatty liver
disease (14).
It should be noted that although the SA carriage
rates did not differ significantly between the pharyngeal and nasal
cavities, the MRSA and MORSA rates were significantly higher in the
nasal cavity. The MORSA carriage rate in the nasal cavity was
13.85%, almost 3-fold higher than the carriage rate in the pharynx
(5.61%). Our results revealed that the MRSA nasal carriage rate
(18.46%) was higher than the pharyngeal carriage rate (11.55%).
This ratio is similar with rates recorded in hospitals from the
United States (15). A
surprisingly low number of patients (10; 9.62%) had SA carriage in
both sites, which in our opinion, can partly be explained by the
lower number of nasal swabs collected and by the application of
decolonisation procedures to patients admitted to our hospital.
Nevertheless, the failure of nasal decolonization procedures with
clorhexidin and mupirocin has been reported in patients that also
have pharyngeal colonization with SA. A probable explaination for
this is that pharyngeal strains become resistant to agents used for
decolonization (that are detected in low concentrations in the
pharynx after nasal application) (16) and then re-colonise the nasal
cavities (17).
The pharynx also constitutes a SA reservoir.
Pharyngeal colonisation can be cleared only by oropharyngeal
decolonisation applied concomitantly with nasal decolonisation or
systemic antibiotherapy. Recolonisation has been reported with the
same SA strain after decolonisation (17). Probably, the sources for
recolonisation are other carriage sites, such as the throat, or the
patient's environment. The elimination of S. aureus from extranasal
sites has been proposed in order to increase the efficiency of
future treatment regimens. Repeated treatments have as a
consequence the development of resistance to mupirocin (18).
There are growing concerns about the routine use of
antibiotherapy in hospitalised patients. In this study, 3
inpatients had fungal infections with Candida spp., 2 in the
pharynx and one in the nasal cavity. The prevalence of fungal
infections obtained by us (0.91%) (data not shown) was surprisingly
low compared with a previous study (19). This may be explained by the fact
that screening samples were used, and the majority of the patients
did not suffer from major conditions that can lower the immunity in
order to favorise fungal infections.
The acquired resistance of S. aureus has been the
focus of several publications, especially after penicillin began to
be used in the middle of the past century, regarding MRSA
epidemiology and its resistance to penicillin. Transmission mainly
occurs in hospitals (20–22). The excessive use of antibiotics in
hospitals is considered a major risk in the guidelines of the
Society for Healthcare Epidemiology of America (SHEA) (23). A revised infection control
guideline from 2013 (24) to
prevent MRSA expansion includes the limited use of glycopeptides,
cephalosporins and fluoroquinolones.
As regards surveilance, a complex aspect is the fact
that, as regards MRSA, it has been demonstrated that hospitals are
the main place of occurrence for multi-resistant S. aureus, which
is now known as MRSA (25).
International studies over the past 20 years have shown the rising
prevalence of MRSA (26 and refs therein). The theory that the
highest occurrence occurs in patients that are drug abusers or
persons that undergo hemodialysis has been refuted. Initially, the
first reports of MRSA were in large hospitals (>500 beds) in
1980 (27). However, MRSA was also
later found in smaller ones.
Future studies are warranted in order to determine
the factors that lead to the transition from MSSA to MRSA. The
shift from MSSA to MRSA occurs very rapidly (within 24–48 h) in
patients that are hospitalised. Thus, both the particulars of the
organism and the onset of the infection contradict the
cross-transmission as the first main cause for the appearance of
MRSA in the hospital environment. Another factor that argues
against cross-transmission is the large number of different strains
discovered (28). The effect of
specific antibiotics on MRSA strains has been previously analysed
(29). It was shown that the
resistance level of MRSA in patients who received antibiotic
therapy was 2-fold compared to that in those who did not undergo
antibiotic treatment (30). It has
also been shown that the higher risk was associated with the use of
quinolones, seconded by the use of glycopeptides, cephalosporins
and other β-lactams (31).
In conclusion, the present study demonstrates the
pattern of distribution of nasal and pharyngeal colonisation with
SA, MRSA and MORSA in various categories of patients, which can be
used for adjusting the screening and decontamination protocols in
our hospital. The antibiotic resistance pattern of SA strains
demonstrated a high resistance of MRSA and MORSA strains, probably
driven by antibiotic use. Resistance to erythromycin, tetracycline,
clindamycin and clarithromycin was high and consequently, these
drugs are not recommended for the empirical therapy of S. aureus
infections. S. aureus is a pervasive pathogen with constantly
changing trends in resistance and epidemiology, and thus requires
constant monitoring in healthcare facilities.
References
|
1
|
Tong SY, Chen LF and Fowler VG Jr:
Colonization, pathogenicity, host susceptibility, and therapeutics
for Staphylococcus aureus: What is the clinical relevance? Semin
Immunopathol. 34:185–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hidron AI, Kourbatova EV, Halvosa JS,
Terrell BJ, McDougal LK, Tenover FC, Blumberg HM and King MD: Risk
factors for colonization with methicillin-resistant Staphylococcus
aureus (MRSA) in patients admitted to an urban hospital: Emergence
of community-associated MRSA nasal carriage. Clin Infect Dis.
41:159–166. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Călina D, Docea AO, Roşu L, Zlatian O,
Roşu AF, Anghelina F, Rogoveanu O, Arsene AL, Nicolae AC, Drăgoi
CM, et al: Antimicrobial resistance development following surgical
site infections. Mol Med Rep. 15:681–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tănase A, Coliță A, Ianoşi G, Neagoe D,
Brănişteanu DE, Călina D, Docea AO, Tsatsakis A and Ianoşi SL: Rare
case of disseminated fusariosis in a young patient with graft vs.
host disease following an allogeneic transplant. Exp Ther Med.
12:2078–2082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wojciechowski VV, Călina D, Tsarouhas K,
Pivnik AV, Sergievich AA, Kodintsev VV, Filatova EA, Ozcagli E,
Docea AO, Arsene AL, et al: A guide to acquired vitamin K
coagulophathy diagnosis and treatment: The Russian perspective.
Daru. 25:102017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Călina D, Roșu L, Roșu AF, Ianoşi G,
Ianoşi S, Zlatian O, Mitruț R, Docea AO, Rogoveanu O, Mitruț P, et
al: Etiological diagnosis and pharmacotherapeutic management of
parapneumonic pleurisy. Farmacia. 64:946–952. 2016.
|
|
7
|
Joung DK, Mun SH, Choi SH, Kang OH, Kim
SB, Lee YS, Zhou T, Kong R, Choi JG, Shin DW, et al: Antibacterial
activity of oxyresveratrol against methicillin-resistant
Staphylococcus aureus and its mechanism. Exp Ther Med.
12:1579–1584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Li H, Liu Y, Li Q, Bi Y and Fang
G: Upregulated effects of miR-7 in methicillin-resistant
Staphylococcus aureus. Exp Ther Med. 12:3571–3574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernandes FH, Guterres ZR, Violante IMP,
Lopes TFS, Garcez WS and Garcez FR: Evaluation of mutagenic and
antimicrobial properties of brown propolis essential oil from the
Brazilian Cerrado biome. Toxicol Rep. 2:1482–1488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan IH, Sohran H, Rony SR, Tareq FS,
Hasan CM and Mazid A: Cytotoxic and antibacterial naphthoquinones
from an endophytic fungus, Cladosporium sp. Toxicol Rep. 3:861–865.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koneman EW, Allen SD, Janda WM,
Schreckenberger RC and Winn W: Introduction to microbiology. Part
II: Guidelines for collection, transport, processing, analysis, and
reporting of cultures from specific specimen sourcesColor Atlas and
Textbook of Diagnostic Microbiology. 5th edition. Lippincott,
Philadelphia: pp. 121–170. 1997
|
|
12
|
Clinical and Laboratory Standards
Institute (CLSI), . Performance standards for antimicrobial
susceptibility testing16th informational supplement M100-S16. CLSI;
Wayne, PA: 2015
|
|
13
|
Ianoşi S, Ianoşi G, Neagoe D, Ionescu O,
Zlatian O, Docea AO, Badiu C, Sifaki M, Tsoukalas D, Tsatsakis AM,
et al: Age-dependent endocrine disorders involved in the
pathogenesis of refractory acne in women. Mol Med Rep.
14:5501–5506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cioboată R, Găman A, Traşcă D, Ungureanu
A, Docea AO, Tomescu P, Gherghina F, Arsene AL, Badiu C, Tsatsakis
AM, et al: Pharmacological management of non-alcoholic fatty liver
disease: Atorvastatin versus pentoxifylline. Exp Ther Med.
13:2375–2381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davis KA, Stewart JJ, Crouch HK, Florez CE
and Hospenthal DR: Methicillin-resistant Staphylococcus aureus
(MRSA) nares colonization at hospital admission and its effect on
subsequent MRSA infection. Clin Infect Dis. 39:776–782. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Finks J, Wells E, Dyke TL, Husain N,
Plizga L, Heddurshetti R, Wilkins M, Rudrik J, Hageman J, Patel J
and Miller C: Vancomycin-resistant Staphylococcus aureus, Michigan,
USA, 2007. Emerg Infect Dis. 15:943–945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perl TM, Cullen JJ, Wenzel RP, Zimmerman
MB, Pfaller MA, Sheppard D, Twombley J, French PP and Herwaldt LA;
Mupirocin And The Risk Of Staphylococcus aureus Study Team, :
Intranasal mupirocin to prevent postoperative Staphylococcus aureus
infections. N Engl J Med. 346:1871–1877. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muder RR, Brennen C, Wagener MM, Vickers
RM, Rihs JD, Hancock GA, Yee YC, Miller JM and Yu VL:
Methicillin-resistant staphylococcal colonization and infection in
a long-term care facility. Ann Intern Med. 114:107–112. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cristea OM, Zlatian OM, Dinescu SN,
Bălăşoiu T, Avrămescu C, Bălăşoiu M, Niculescu M and Călina D: A
comparative study on antibiotic resistance of Klebsiella strains
from surgical and intensive care wards. Curr Health Sci.
42:169–179. 2016.
|
|
20
|
Eslami G, Salehifar E, Behbudi M and Rezai
MS: Rational use of amikacin in Buali-Sina hospital in Sari 2011. J
Mazandaran Univ Med Sci. 23:2–9. 2013.
|
|
21
|
Cantón R, Novais A, Valverde A, Machado E,
Peixe L, Baquero F and Coque TM: Prevalence and spread of
extended-spectrum beta-lactamase-producing Enterobacteriaceae in
Europe. Clin Microbiol Infect. 14 Suppl 1:144–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rujanavej V, Soudry E, Banaei N, Baron EJ,
Hwang PH and Nayak JV: Trends in incidence and susceptibility among
methicillin-resistant Staphylococcus aureus isolated from
intranasal cultures associated with rhinosinusitis. Am J Rhinol
Allergy. 27:134–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dellit TH, Owens RC, McGowan JE Jr,
Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL,
Fishman NO, Carpenter CF, et al Infectious Diseases Society of
America, ; Society for Healthcare Epidemiology of America, :
Infectious Diseases Society of America and the Society for
Healthcare Epidemiology of America guidelines for developing an
institutional program to enhance antimicrobial stewardship. Clin
Infect Dis. 44:159–177. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bratzler DWI, Dellinger EP, Olsen KM, Perl
TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG,
Slain D, et al American Society of Health-System Pharmacists, ;
Infectious Disease Society of America, ; Surgical Infection
Society, ; Society for Healthcare Epidemiology of America, :
Clinical practice guidelines for antimicrobial prophylaxis in
surgery. Am J Health Syst Pharm. 70:195–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pantosti A and Venditti M: What is MRSA?
Eur Respir J. 34:1190–1196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reddy PN, Srirama K and Dirisala VR: An
update on clinical burden, diagnostic tools, and therapeutic
options of Staphylococcus aureus. Infect Dis (Auckl).
10:11799161177039992017.PubMed/NCBI
|
|
27
|
de Sousa M Aires and de Lencastre H:
Evolution of sporadic isolates of methicillin-resistant
Staphylococcus aureus (MRSA) in hospitals and their similarities to
isolates of community-acquired MRSA. J Clin Microbiol.
41:3806–3815. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Otto M: MRSA virulence and spread. Cell
Microbiol. 14:1513–1521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou T, Li Z, Kang OH, Mun SH, Seo YS,
Kong R, Shin DW, Liu XQ and Kwon DY: Antimicrobial activity and
synergism of ursolic acid 3-O-α-L-arabinopyranoside with oxacillin
against methicillin-resistant Staphylococcus aureus. Int J Mol Med.
40:1285–1293. 2017.PubMed/NCBI
|
|
30
|
Arora S, Devi P, Arora U and Devi B:
Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in
a tertiary care Hospital in Northern India. J Lab Physician.
2:78–81. 2010. View Article : Google Scholar
|
|
31
|
Shrestha B, Pokhrel BM and Mohapatra TM:
Phenotypic characterization of nosocomial isolates of
Staphylococcus aureus with reference to MRSA. J Infect Dev Ctries.
3:554–560. 2009. View
Article : Google Scholar : PubMed/NCBI
|