Introduction
As the most common bacterial-associated infectious
oral disease, early childhood caries remains a serious public
health challenge worldwide (1,2).
Despite advances in dentistry, caries remains problematic,
particularly for people in disadvantaged socioeconomic groups
(3,4). The Third National Oral Health Survey
in China reported that 66% of 5-year-old children had a history of
caries, with an average of 3.5 decayed, missing or filled teeth
(5). Therefore, it is imperative
to address dental caries in early childhood.
Streptococcus mutans is principally the
causative organism of dental caries (6). The cariogenic mechanism undertaken by
S. mutans involves growth and glycolysis at pH <5.0, and
demineralization of the tooth surface (7–9).
Therefore, the ability of S. mutans to tolerate low pH is
crucial for its virulence and pathogenesis in dental caries
(10). To mitigate acid stressors
in the oral cavity, S. mutans has evolved numerous stress
response pathways (8,11), including two-component systems
(TCSs), which are most commonly used as transcription regulators
within bacteria. TCSs have been reported to permit numerous
environmental stimuli including alterations in pH (12–14).
The involvement of numerous TCSs in acid adaption has been observed
in S. mutans (15). TCS-3
(ScnRK-like) is associated with cell survival at acidic pH; TCS-2
(CiaRH) is involved in environmental stress tolerance; TCS-9
affects the response to acidic conditions (12); TCS-1 (vicK) regulates intracellular
pH homeostasis in S. mutans (16); and TCS (LiaSR) modulates acid
tolerance within S. mutans (17). However, the underlying mechanisms
of acid tolerance within S. mutans remain unclear.
Over the past decade, studies have demonstrated that
small non-coding RNAs (sRNAs) can be employed by bacteria to
modulate gene expression, control environmental stress responses,
and contribute to metabolic and virulence pathways (18,19).
Some TCS-associated sRNAs have been identified in various bacterial
species. For example, five similar sRNAs were reported to control
competence development by targeting the CiaRH TCS within S.
pneumoniae (20). Similarly, a
negative feedback circuit was reported to exist between EnvZOmpR
TCS and the OmrA/B sRNAs (21).
However, the physiological function of sRNAs in acid tolerance of
S. mutans and whether sRNAs regulate acid responses of S.
mutans through TCS has yet to be investigated.
Various isolates of S. mutans possess an
assortment of virulence-associated traits; it is therefore
necessary to investigate the functions of sRNA within clinical
isolates of S. mutans. Strains of S. mutans isolated
from children with caries are genetically distinct from those found
in cavity-free children. Within a patient diagnosed with severe
early childhood caries, 49 unique gene segments were identified
from a strain of S. mutans (22). Our previous study revealed that
children with high severity caries were associated with S.
mutans containing thymidine at locus 168 of the srtA
gene (23). A previous study using
distinct strains of S. mutans suggested that expression
patterns of virulence- and regulatory-associated genes were
heterogeneous and strain-specific (24). Studies have previously used
laboratory strains of S. mutans to investigate sRNAs;
however, clinical strains are rarely employed.
In the present study, the association between
sRNA133474 and acid tolerance were investigated in clinical strains
of S. mutans, to explore the underlying mechanisms of acid
tolerance in S. mutans. It was demonstrated that sRNA133474
was more highly expressed at pH 7.5 compared with pH 5.5 in UA159
and in clinical isolates. The expression levels of sRNA133474 were
measured in isolates at different growth phases under different pH
conditions. The pathways likely to involve sRNA133474 were analyzed
via Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
analysis. To the best of our knowledge, the present study is the
first to describe an sRNA associated with acid tolerance in
clinical isolates of S. mutans.
Materials and methods
Isolation of S. mutans strains
The protocol of the present study was approved by
the Ethics Committee of Guanghua School of Stomatology, Sun Yat-sen
University [Guangzhou, China; ERC-(2015)-8]. Clinical strains of
S. mutans were isolated from 5-year old children who
participated in an epidemiological survey in Guangzhou according to
the protocol described by Yu et al (23). Briefly, plaque samples from 20
children were vortexed and sonicated at maximum amplitude using a
sonic oscillator (UR-200; Tomy Seiko Co., Ltd., Tokyo, Japan) in an
ice bath for 30 sec, followed by serial 10−3 dilutions
in PBS. Subsequently, 50 µl diluent was plated onto
Mitis-Salivarius-Bacitracin agar supplemented with 20% sucrose and
0.2 U/ml bacitracin, and incubated anaerobically (85%
N2, 5% CO2 and 10% H2) at 37°C for
2 days. One clone from each sample was randomly selected based on
its ability to ferment mannitol, sorbitol, raffinose, melibiose and
aesculin, and hydrolyze arginine. Clinical isolates were preserved
in 25% glycerol at −80°C prior to use.
Bacterial strains and culture
conditions
S. mutans UA159 (ATCC700610; American Type
Culture Collection, Manassas, VA, USA) and clinical isolates were
cultured in brain heart infusion (BHI) or tryptone, 3%; glucose, 20
mM; yeast extract, 0.3% (TYG) medium. To investigate growth,
strains were incubated in an atmosphere containing 5%
CO2. S. mutans UA159 and clinical isolates were
cultured overnight in BHI broth. The cultures were then diluted
1:50 in fresh BHI broth and grown to the indicated phase [optical
density at 600 nm (OD600=0.2, 0.4 and 0.7)], after which
they were collected by centrifugation (7,585 × g, 4°C, 15 min) for
RNA isolation. For acid shock assays, HCl or NaOH were added to TYG
medium to adjust final pH to 5.5 or 7.5; UA159 and clinical
isolates of S. mutans were cultured overnight in TYG medium
and diluted 1:100 with fresh TYG medium until a mid-logarithmic
phase (OD600=0.4) was achieved. Cells were subsequently
divided into two aliquots, pelleted by centrifugation (7,585 × g,
4°C, 15 min) and resuspended in TYG medium with buffer solution to
maintain the pH at 5.5 or 7.5 for 1.5, 2.5 and 3.5 h for RNA
isolation.
RNA isolation
Cells were collected and centrifuged (7,585 × g) at
4°C for 15 min, and resuspended in 100 ml lysis buffer (20 mg/ml
lysozyme, 60 mAU/ml proteinase K) at 37°C for 45 min. Total RNA
extraction and purification was performed using an miRNeasy Mini
kit (Qiagen, GmbH, Hilden, Germany) according to the manufacturer's
protocol. RNA samples of UA159 and clinical isolates of S.
mutans were prepared in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In our previous study, three libraries of sRNAs were
constructed and exposed to acidic conditions (25). A total of 91 sRNAs with ≥1,000 mean
reads were identified and nine candidates were randomly selected
(Table I). sRNAs with read counts
≥1,000 were selected due to a greater interest in bacterial sRNAs
with many sequencing results (26). The primer sequences for the nine
selected sRNAs and five mRNAs are presented in Tables II and III, respectively. The reverse primer
used for qPCR was a commercialized universal primer supplied with
the Mir-X miRNA qRT-PCR SYBR kit (Takara Bio, Inc., Otsu, Japan)
(27–29). Total RNA was converted into cDNA
using a Mir-X miRNA First-Strand Synthesis kit (Takara Bio, Inc.)
according to the manufacturer's protocol. The qPCR reaction was
performed on a LightCycler 480 Real-time PCR system (Roche
Diagnostics GmbH, Mannheim, Germany) with a SYBR Premix Ex Taq II
kit (Takara Bio, Inc.). The reaction conditions were as follows:
95°C for 30 sec, followed by 40 cycles of 95°C for 15 sec and 63°C
for 30 sec. Melting curve analysis was subsequently performed from
60–95°C. RT-qPCR was used to measure sRNA133474 expression and
predicted mRNA expression at different growth phases and under
different pH conditions. The 16S rRNA gene was used as an internal
control. Each assay was performed in triplicate, and fold-changes
in expression were calculated using the 2−∆∆Cq method
(30).
 | Table I.sRNAs selected for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
sRNAs selected for reverse
transcription-quantitative polymerase chain reaction.
| sRNA | Start location | Length | Sequence
(5′-3′) | Strands | Mismatch |
|---|
| sRNA133474 |
293191 | 33 |
CAGCCCTAAGCGATGTAAGCTGTGTGCTCTATT | + | 0 |
| sRNA133473 |
293190 | 34 |
GCAGCCCTAAGCGATGTAAGCTGTGTGCTCTATT | + | 0 |
| sRNA884831 | 1930106 | 35 |
TGGGTCGCTCTGTATCTCTG | + | 0 |
|
|
|
|
CGGTGGCTGTGAGTATGAAG |
|
|
| sRNA133477 |
293194 | 30 |
TGGATTTTCATGCCTGCTG | + | 0 |
|
|
|
|
GGCTGCATTACCAGAAAGGT |
|
|
| sRNA628417 |
1327899 | 37 |
CAACACAGCTCTAAAACTGTGGCAAGTCATGTCCGAA | – | 0 |
| sRNA628420 | 1327902 | 34 |
CACAGCTCTAAAACTGTGGCAAGTCATGTCCGAA | – | 0 |
| sRNA133484 |
293201 | 23 |
CGATGTAAGCTGTGTGCTCTATT | + | 0 |
| sRNA628658 | 1328100 | 34 |
CACAGCTCTAAAACAGAGCACTAACTGCGCTAGC | – | 0 |
| sRNA628415 | 1327897 | 39 |
AACAACACAGCTCTAAAACTGTGGCAAGTCATGTCCGAA | – | 0 |
 | Table II.Primers for selected sRNAs. |
Table II.
Primers for selected sRNAs.
| sRNA | Sequence
(5′-3′) | Primer sequence
(5′-3′) |
|---|
| sRNA133474 |
CAGCCCTAAGCGATGTAAGCTGTGTGCTCTATT |
ATGTAAGCTGTGTGCTCTATTA |
| sRNA133473 |
GCAGCCCTAAGCGATGTAAGCTGTGTGCTCTATT |
GCAGCCCTAAGCGATGTAAG |
| sRNA884831 |
TACAAAACGTGAATCATCGGTGCCAATACAGCATT |
TCATCGGTGCCAATACAGCAT |
| sRNA133477 |
CCCTAAGCGATGTAAGCTGTGTGCTCTATT |
GATGTAAGCTGTGTGCTCTATT |
| sRNA628417 |
CAACACAGCTCTAAAACTGTGGCAAGTCATGTCCGAA |
TGTGGCAAGTCATGTCCGAAA |
| sRNA628420 |
CACAGCTCTAAAACTGTGGCAAGTCATGTCCGAA |
TGTGGCAAGTCATGTCCGAA |
| sRNA133484 |
CGATGTAAGCTGTGTGCTCTATT |
CGATGTAAGCTGTGTGCTCTA |
| sRNA628658 |
CACAGCTCTAAAACAGAGCACTAACTGCGCTAGC |
TAAAACAGAGCACTAACTGCG |
| sRNA628415 |
AACAACACAGCTCTAAAACTGTGGCAAGTCATGTCCGAA |
GTGGCAAGTCATGTCCGAAA |
 | Table III.Primers for mRNAs. |
Table III.
Primers for mRNAs.
| miRNA | Primer sequence
(5′-3′) |
|---|
| 16s-F |
5′-CCTACGGGAGGCAGCAGTAG-3′ |
| 16s-R |
5′-CAACAGAGCTTTACGATCCGAAA-3′ |
| comE-F |
5′-AGCCCATAAGCTCTGCCTTT-3′ |
| comE-R |
5′-AGCGATGGCACTGAAAAAGT-3′ |
| covR-F |
5′-GCTCTTTTGCAGCAAATCAAATCGT-3′ |
| covR-R |
5′-GCCAATAATCGGAAAATAAAGGCGC-3′ |
| ciaR-F |
5′-CAGGTGTCGTTATCCCTTTTTCAC-3′ |
| ciaR-R |
5′-GCAGAGAGTGGCGTTTATGATTTG-3′ |
| liaS-F |
5′-CGAGATTTGAGTTACGGCTTG-3′ |
| liaS-R |
5′-GCATCCCCTTTCATTATTGG-3′ |
| liaR-F |
5′-ACCAAACGATTGCTGATGAG-3′ |
| liaR-R |
5′-CCTGTGGCACTAAATGATGC-3′ |
Acid killing assay
An acid killing assay was conducted to assess acid
tolerance according to previously published methods (6). Briefly, stationary-phase cells of the
overnight culture were harvested and immediately subjected to acid
stress by incubation in 1 ml pH 2.8 glycine solution for 0, 30 and
60 min at 37°C. Cells were washed with 1 ml PBS at pH 7.5 to
terminate the process and were then diluted with PBS for plating.
Plates were incubated at 37°C and colonies were counted after 2
days. Bacterial survival was assessed after exposure to lethal pH
to measure acid tolerance of S. mutans isolates.
Bioinformatics analysis
RNApredator (http://rna.tbi.univie.ac.at/RNApredator) was used to
predict target mRNAs of sRNA133474 (31,32).
KEGG pathway analyses were performed using the Database for
Annotation, Visualization and Integrated Discovery (DAVID) gene
annotation tool (http://david.abcc.ncifcrf.gov/) (33). A Fisher's exact test P-value was
used to denote significance.
Statistical analysis
All experiments were performed in triplicate. Data
were analyzed using IBM SPSS 19.0 for Windows (SPSS; IBM Corp.,
Armonk, NY, USA). Data are expressed as the mean ± standard
deviation. The Shapiro-Wilk test was first used to check whether
the data were parametric or not. Two-group comparisons were
performed using Student's t-test. Multiple-group comparisons were
analyzed using a one-way analysis of variance or Kruskal-Wallis,
followed by Bonferroni multiple comparison tests. Correlations were
analyzed with a Spearman rank correlation coefficient. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression levels of sRNA133474 in
clinical strains of S. mutans and S. mutans UA159
RT-qPCR was conducted to determine the expression
levels of the nine selected sRNAs in UA159 and three clinical
isolates. In total, six sRNAs with low Cq (Cq<35) and three
sRNAs with high Cq (Cq≥35; representing low expression; data not
shown) were reported. Expression levels of the six sRNAs were
determined in UA159 and three clinical isolates using RT-qPCR and
were subsequently measured under different pH conditions. At pH 7.5
and 5.5, the differentially expressed sRNAs included sRNA133474,
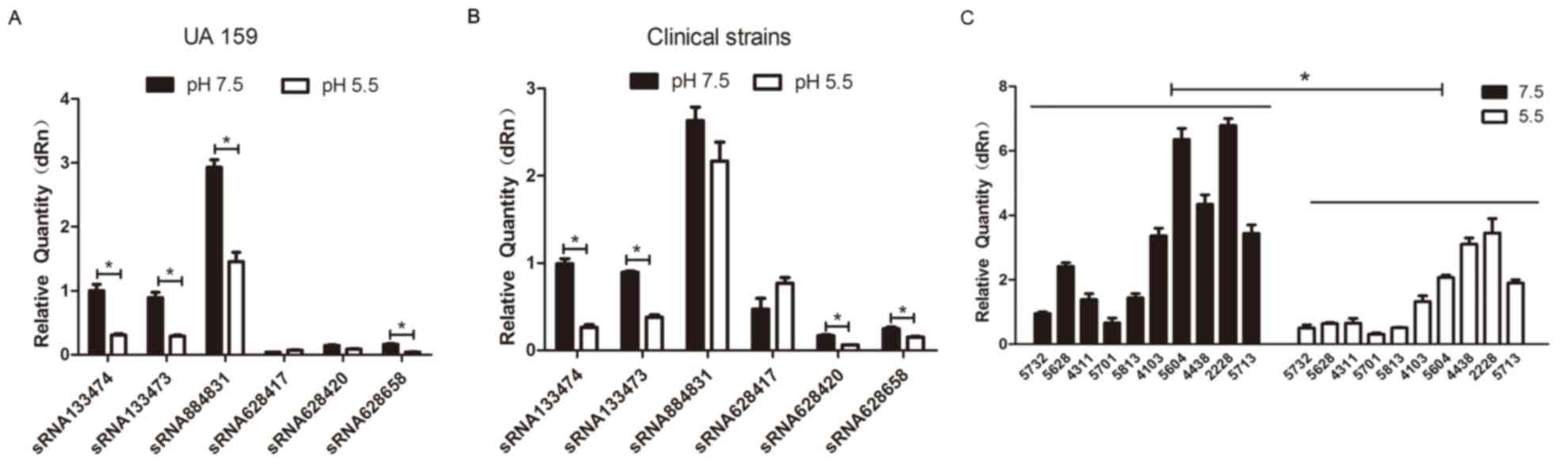
sRNA133473 and sRNA628658 in UA159 and clinical strains (Fig. 1A and B) among which sRNA133474 was
the most significantly downregulated at pH 5.5 compared with at pH
7.5. Therefore, sRNA133474 was selected for subsequent experiments.
sRNA133474 expression levels within 10 clinical isolates at pH 7.5
and 5.5 were subsequently measured; regardless of strain, the
expression levels of sRNA133474 were consistently elevated at pH
7.5 compared with at pH 5.5 (Fig.
1C) when cells were grown to a mid-logarithmic phase
(OD600=0.4).
Expression patterns of sRNA133474 in
different growth phases of S. mutans clinical strains
Characteristics associated with sRNA133474 in
clinical isolates of S. mutans were analyzed by measuring
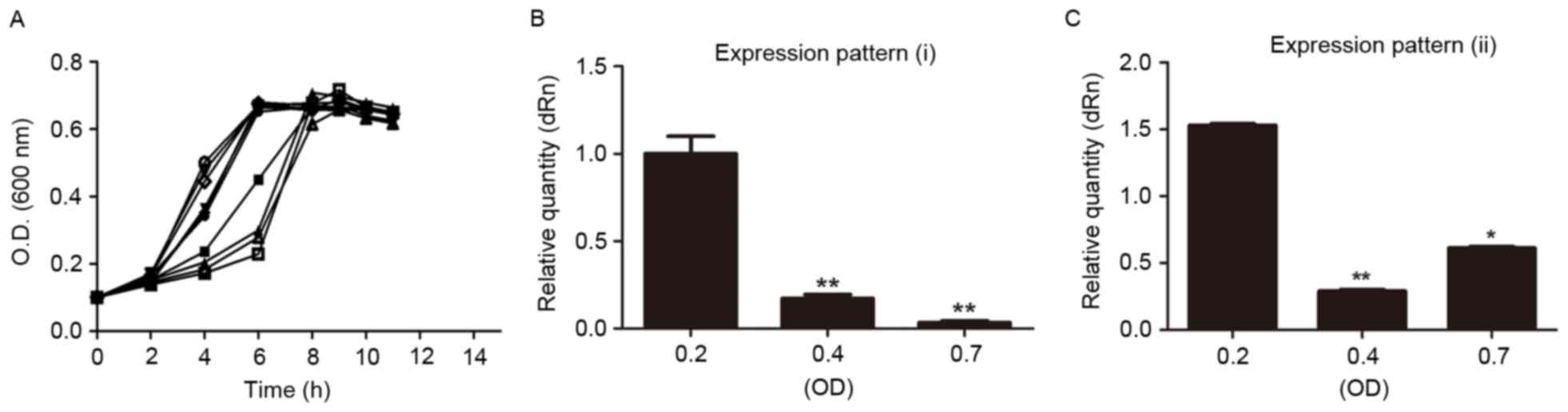
growth curves of 10 S. mutans clinical isolates cultured in
pH 7.5 TYG medium at different growth phases (Fig. 2A). S. mutans strains of the
early-logarithmic growth phase (OD600=0.2),
mid-logarithmic growth phase (OD600=0.4), and stationary
growth phase (OD600=0.7) were cultured; subsequently,
total RNA was extracted. Among the 10 strains analyzed, two
distinct expression patterns of sRNA133474 were identified:
Expression pattern (i), expression levels of sRNA133474 during
different growth phases were downregulated from an
early-logarithmic growth phase to an early stationary growth phase
(Fig. 2B); expression pattern
(ii), expression levels of sRNA133474 were downregulated from an
early-logarithmic growth phase to a mid-logarithmic growth phase,
and were then slightly upregulated in the early stationary phase
(Fig. 2C). The two expression
patterns were almost equally prevalent amongst the clinical
strains; they were present in six and four of the strains,
respectively.
Alterations of pH during different
growth phases
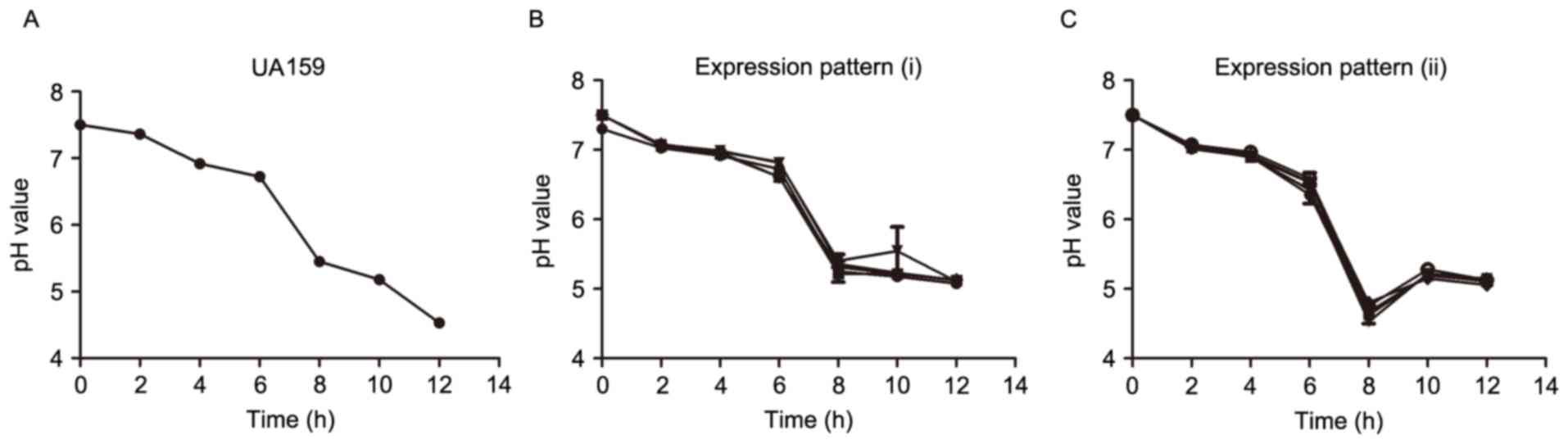
pH was investigated during different growth phases
of the clinical strains in order to understand the importance of
the different expression patterns of sRNA133474. The present study
demonstrated that the pH of the medium decreased during specific
growth phases (Fig. 3A and B). For
clinical strains with sRNA133474 expression pattern (i), pH
gradually declined during the growth phase (Fig. 3B); for clinical strains with
sRNA133474 expression pattern (ii), pH decreased to 4.69 and then
slightly increased at the stationary growth phase (Fig. 3C).
Expression levels of sRNA133474 under
different acid stress conditions
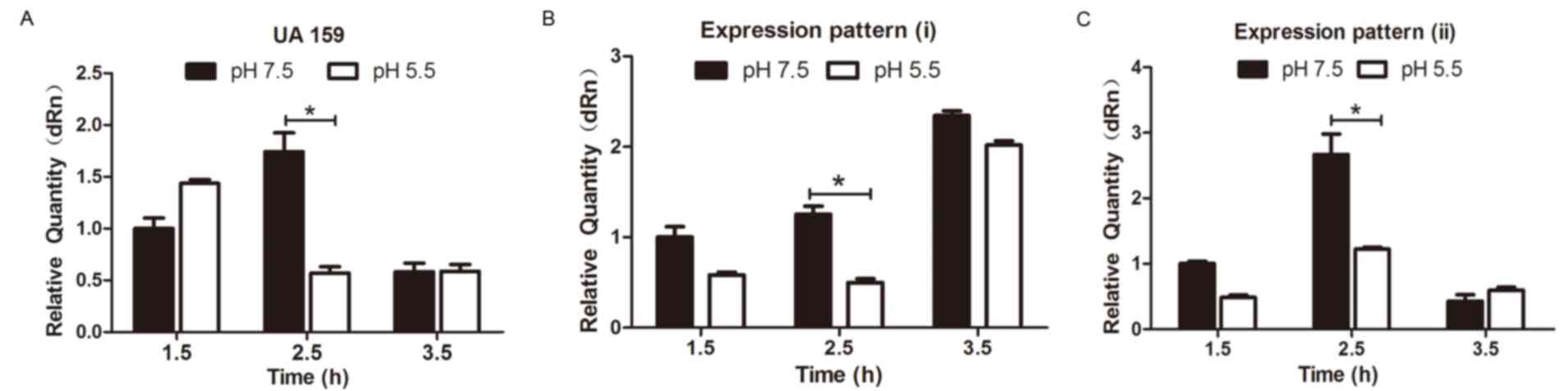
To clarify the association between sRNA133474
expression and acid stress, UA159 and clinical isolates of S.
mutans were cultured at pH 7.5 and 5.5 for 1.5, 2.5 and 3.5 h.
Expression levels of sRNA133474 in UA159 and clinical isolates were
determined by RT-qPCR (Fig. 4A-C).
Regardless of exposure time, expression levels of sRNA133474 were
increased at pH 7.5 compared with at pH 5.5 in clinical isolates
(Fig. 4B and C); whereas lower
expression levels of sRNA133474 were observed in UA159 3.5 h
post-treatment at pH 7.5 (Fig.
4C); however, the difference was not significant (P>0.05).
However, sRNA133474 expression consistently increased at pH 7.5 in
UA159 and clinical strains after 2.5 h compared with in pH 5.5
(P<0.05).
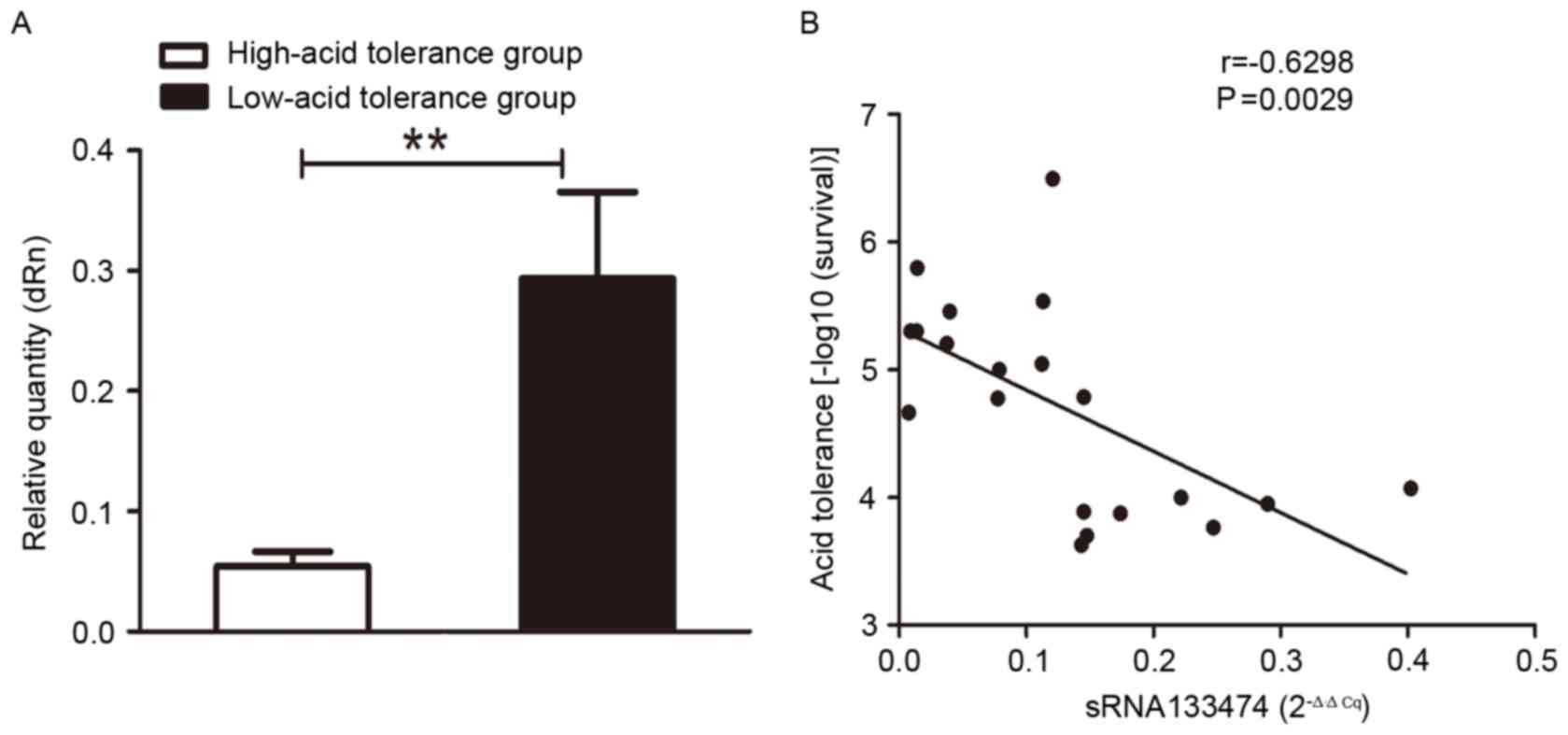
Association between sRNA133474 and
acid tolerance of S. mutans
Based on acid tolerance, 10 clinical isolates were
placed into two groups. Expression levels of sRNA133474 in the
low-acid tolerance group were consistently greater than in the
high-acid tolerance group; the greatest fold-changes occurred at pH
5.5 at 2.5 h (Fig. 4A-C). The
association between sRNA133474 and acid tolerance was investigated
at pH 5.5 at 2.5 h in 20 clinical isolates of S. mutans. The
data of the present study revealed that sRNA133474 expression
levels were lower in clinical isolates with higher acid tolerance
(Fig. 5A). A correlation between
sRNA133474 expression and acid tolerance was observed (r=-0.6298,
P<0.01; Fig. 5B).
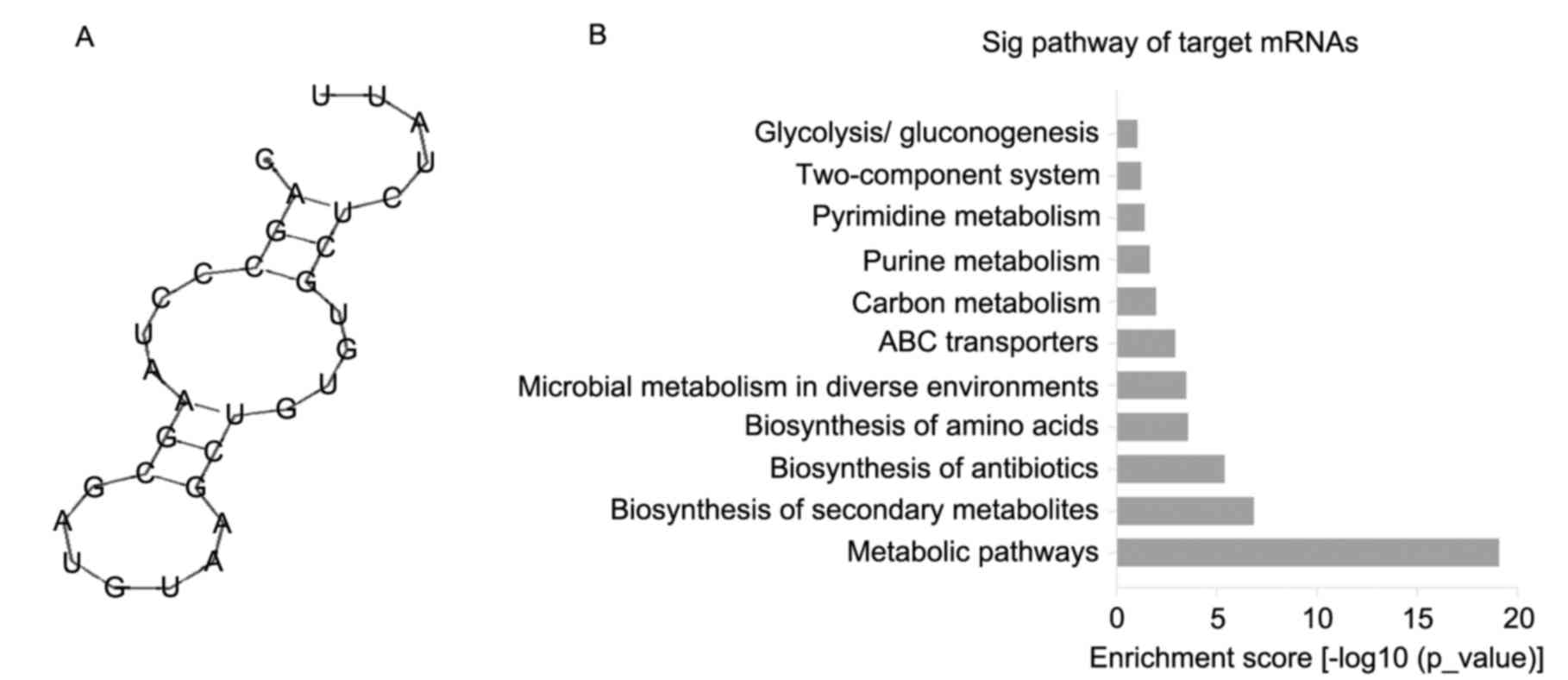
Target genes prediction and functional
annotation analysis
The secondary structure of sRNA133474 was predicted
using the RNAfold webserver (rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi).
It was reported that sRNA133474 possessed a stem loop structure
with the dG value of −5.4 kJ/mol (Fig.
6A). The possible target mRNAs of sRNA133474 were investigated
using RNApredator and 1,960 mRNAs were predicted. To determine the
KEGG pathways associated with the target mRNAs of sRNA133474, the
DAVID gene annotation tool (http://david.abcc.ncifcrf.gov/) was applied, the chief
pathways identified included: i) Metabolic pathways; ii) synthesis
of secondary metabolites; iii) antibiotic synthesis; iv) amino acid
biosynthesis; v) microbial metabolism in different environments;
vi) ATP-binding cassette transporters; vii) carbon metabolism;
viii) purine metabolism; x) pyrimidine metabolism; xi) TCS and xii)
glycolysis/starch production (Fig.
6B). The TCS pathway in S. mutans helps to coordinate
regulatory networks and gene expression in response to acid stress
(15). The TCSs CiaHR, LiaSR and
CovSR are considered to contribute to acid resistance of S.
mutans. Therefore, five putative target mRNAs: comE, covR,
ciaR, liaS and liaR involved in TCSs were chosen for
investigation (12,14,17,34).
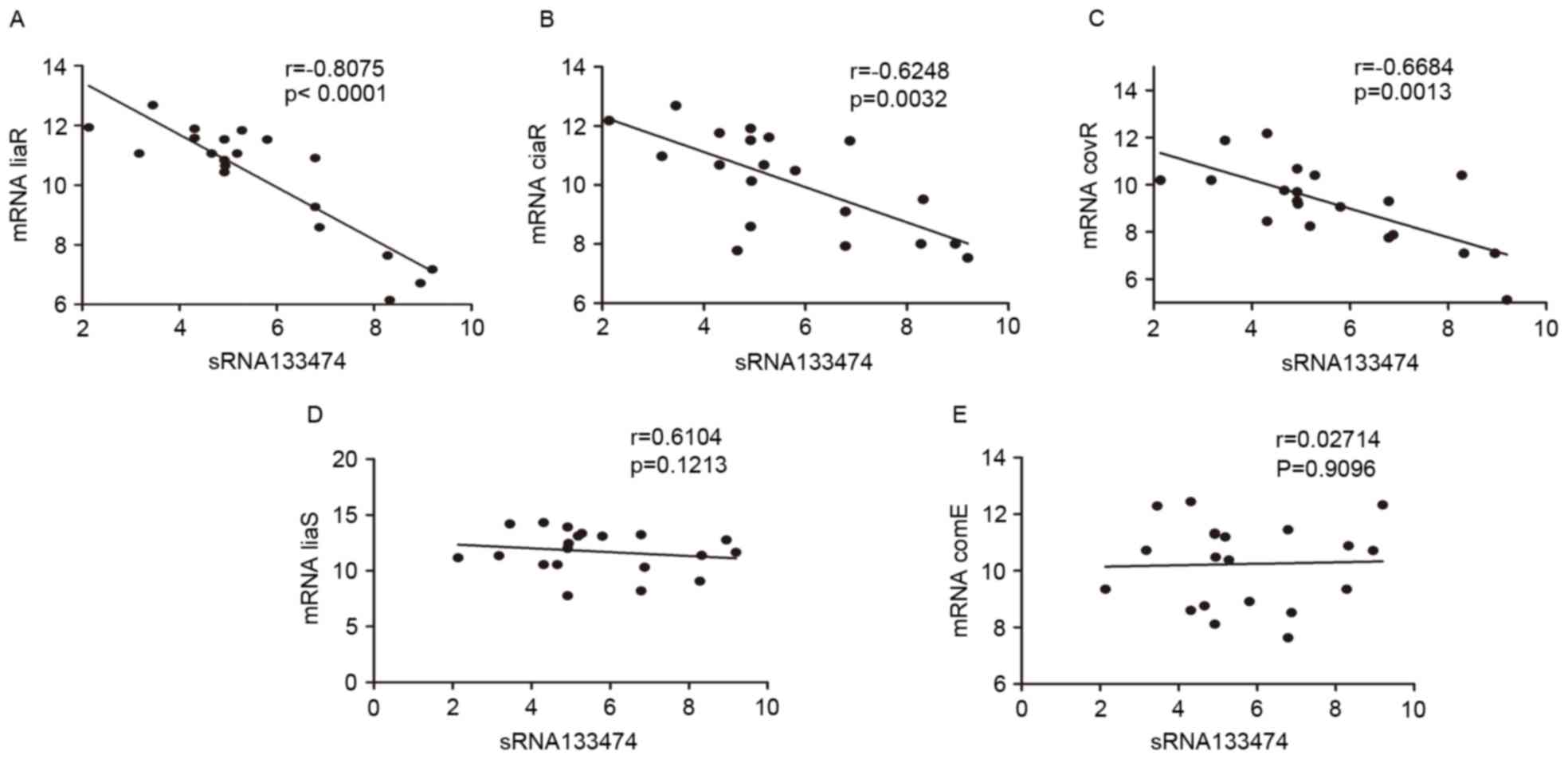
Negative correlation between
sRNA133474 and target mRNAs
To explore the possible mechanisms underlying
sRNA133474-associated acid tolerance within S. mutans, the
expression levels of sRNA133474 and corresponding target mRNAs were
analyzed using RT-qPCR in 20 clinical isolates with different acid
resistance. Data indicated a negative correlation between
sRNA133474 and mRNA liaR (r=-0.8075, P<0.0001),
ciaR (r=-0.6248, P=0.0032) and covR (r=-0.6684,
P=0.0013; Fig. 7). In addition, a
negative association between sRNA133474 and target mRNA during
different growth phases was observed (Fig. 8). These results indicate that
sRNA133474 may modulate acid tolerance of S. mutans, by
inhibiting the expression of acid tolerance related mRNAs
(liaR, ciaR, covR).
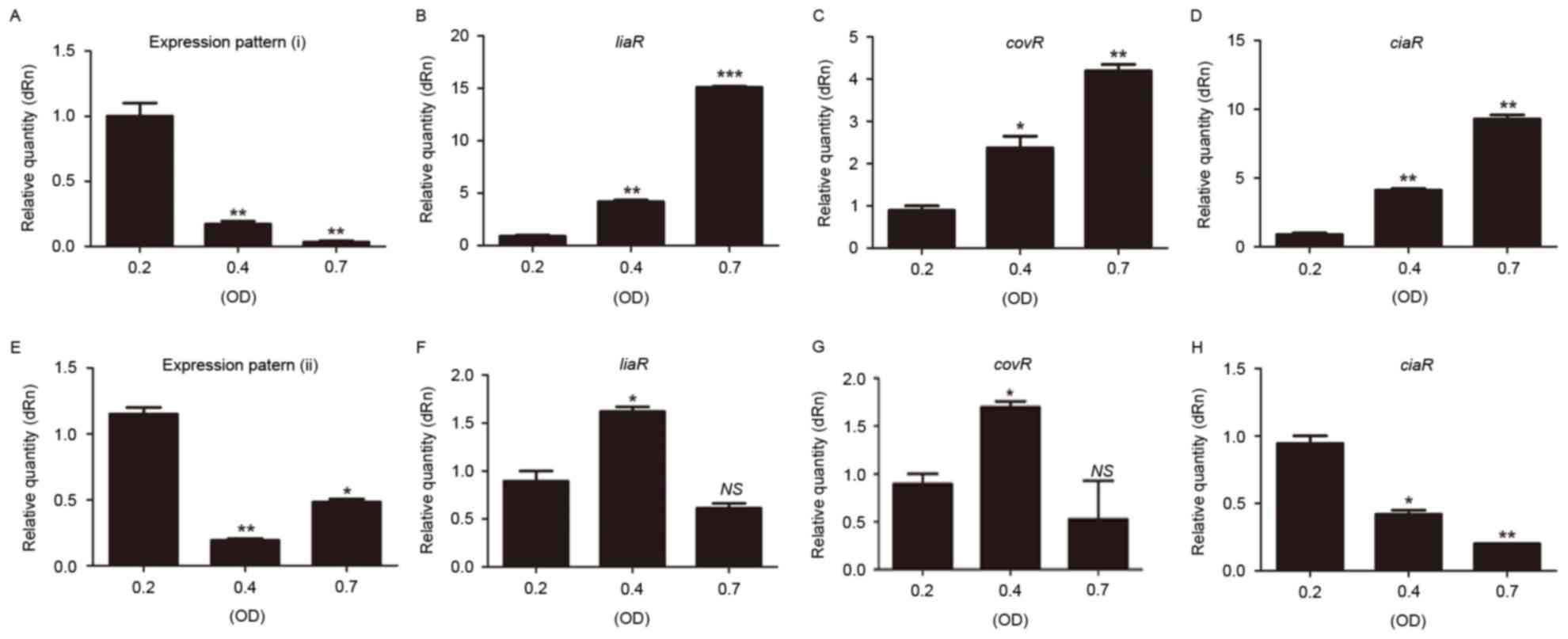 | Figure 8.Expression of sRNA133474 and target
mRNAs (liaR, covR and ciaR) in clinical strains with (A-D)
expression pattern (i) and (E-H) expression pattern (ii). Data are
presented as the mean ± standard deviation. *P<0.05,
**P<0.01, ***P<0.001, NSP>0.05 compared with
the relative quantity at OD600=0.2. (A, B and E) Data
were analyzed using Kruskal-Wallis test and Bonferroni multiple
comparisons test; (C, D, F, G and H) data were analyzed by analysis
of variance and Bonferroni multiple comparisons test. NS, not
significant; OD, optical density; sRNA, small noncoding RNA; dRn,
delta Rn: The magnitude of the fluorescence signal generated during
the PCR at each time point. |
Discussion
As an important factor of virulence, acid tolerance
permits S. mutans to rapidly adapt to acidic environments by
modulating regulatory factors. Numerous acid tolerance-associated
genes have been described; however, the molecular basis for S.
mutans to sense and integrate pH signals for acid adaptation is
unknown. Previously, sRNAs were identified as crucial regulators of
various physiological responses at the post-transcriptional level
in several bacterial species (34). Thousands of sRNAs have been
confirmed in S. mutans UA159; however, identification and
function analyses of acid tolerance-associated sRNAs within
clinical isolates are yet to be determined. In the present study,
acid tolerance-associated sRNA133474 within clinical isolates of
S. mutans was identified and functional mechanisms were
explored.
sRNA133474 expression patterns at specific growth
phases in UA159 and clinical isolates were analyzed. These data
revealed that the sequence of sRNA133474 was conserved among S.
mutans strains and sRNA133474 abundance was growth
phase-dependent, consistent with previous studies (35–37).
Several sRNAs have been reported to be expressed in a manner
dependent on Staphylococcus aureus growth phases (37). Similarly, sRNA BTH_s1 and sRNA s39
expression levels were altered at different growth phases in
Burkholderia thailandensis (35) and expression levels of three sRNAs
in Listeria monocytogenes were also growth phase-dependent
(36). Bacteria are homogenous
during different growth stages and regulate factors to adapt their
needs according to alterations in population density, cell cycles
and environmental stressors, including pH fluctuations and nutrient
availability (38,39). S. mutans may employ sRNAs to
regulate the responses to adverse environments and stress factors
during different growth phases. Alterations in sRNA133474
expression levels were consistent with decreasing pH during
different growth phases. Therefore, acid tolerance of S.
mutans may be associated with sRNA133474 expression.
The ability of S. mutans to respond to
environmental stress conditions, such as acidic pH, is essential
for survival and cariogenicity. Expression levels of sRNA133474 in
high- and low-acid tolerance groups were measured in the present
study; sRNA133474 may serve a key role in regulating acid tolerance
of S. mutans. The predicted target mRNAs of sRNA133474
comE, covR, liaS, liaR and ciaR have been
reported to be imperative regulons of TCSs that contribute to acid
tolerance in S. mutans.
Genes encoding CiaHR, LevSR, LiaSR, ScnKR,
Hk/Rr1037/1038 and ComDE TCSs were upregulated during acid
adaptation. The ciaR gene encodes the cognate response
regulator CiaR and contributes to acid tolerance. Strains of UA159
S. mutans with ciaR mutations exhibit a marked
reduction in acid tolerance at pH 5.4 (40). The LiaSR (formerly HK/RR11) TCS in
S. mutans has been reported to positively modulate acid
tolerance. As previously reported, hk11 mutants are growth
defective at low pH and exhibit low acid tolerance; the acid
tolerance-associated phenotype exhibited by S. mutans may be
activated by hk11 (14,34).
In addition, covR is deemed to be responsible for the
virulence of S. mutans and contributes to acid tolerance
(15). The negative correlation
between sRNA133474 and target mRNAs (ciaR, liaR and
covR) in clinical isolates suggested a negative regulatory
role for sRNA133474 in S. mutans acid tolerance.
Studies of bacterial strains revealed that
expression of regulatory genes is strain-specific (23,39);
the transcription patterns of response regulators (RR) vicR,
covR, comE, ciaR and RR1 are reported to be
strain-specific within clinical isolates of S. mutans
(39). Differing expression
profiles of target mRNA and sRNA133474 within clinical strains, as
well as the observed negative association, were observed in the
present study. These findings suggested that different
transcriptional patterns of mRNA may result from heterogeneous
expression patterns of sRNA133474. Altered patterns of sRNA133474
expression in clinical isolates may inhibit the expression of
target mRNAs, contributing to distinct virulence properties
(24). Isolates of S.
aureus with different sRNAs and target expression levels
exhibit varying pathogenesis and infective severity. Therefore,
sRNA133474 may regulate mRNA covR/liaR/ciaR expression in
LiaSR, CiaRH and CovRS TCS pathways responsible for acid tolerance
of S. mutans. Consistent with the present study, Laux et
al (41) reported that the
expression of the response regulator ciaR was regulated by
five similar sRNAs in S. pneumoniae R6.
In summary, this is the first study to explore the
function and diversity of sRNA expression patterns in clinical
isolates of S. mutans. The findings of the present study
suggested that sRNA133474 and its target mRNAs are involved in TCSs
and serve critical roles in the regulation of acid tolerance in
clinical isolates of S. mutans. However, it is worthwhile to
point out that the limitations in this study include the analysis
of a limited number of sRNAs and clinical strains. Furthermore, the
underlying mechanisms remain to be elucidated. The present study
indicated that strain-specific expression patterns with sRNAs are
associated with inter-strain variation and sRNA function.
Our previous study (25) revealed that other sRNAs are
differentially expressed within acidic environments. Future
investigations aim to determine the sRNAs involved in the acid
response exhibited by S. mutans, and to understand the
molecular mechanisms and regulation of these molecules. This
research may be useful in the identification of potential
biomarkers of dental caries to aid in prevention and targeted
therapy.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81570967).
References
|
1
|
Selwitz RH, Ismail AI and Pitts NB: Dental
caries. Lancet. 369:51–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marsh PD: Microbial ecology of dental
plaque and its significance in health and disease. Adv Dent Res.
8:263–271. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dye BA and Thornton-Evans G: Trends in
oral health by poverty status as measured by Healthy People 2010
objectives. Public Health Rep. 125:817–830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Capurro DA, Iafolla T, Kingman A,
Chattopadhyay A and Garcia I: Trends in income-related inequality
in untreated caries among children in the United States: Findings
from NHANES I NHANES III and NHANES 1999–2004. Community Dent Oral
Epidemiol. 43:500–510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi X: Report of the third national oral
health survey in China. People's Med Publ House; Beijing: pp.
60–61. 2008, (In Chinese).
|
|
6
|
Guo L, McLean JS, Lux R, He X and Shi W:
The well-coordinated linkage between acidogenicity and aciduricity
via insoluble glucans on the surface of Streptococcus mutans. Sci
Rep. 5:180152015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quivey RG, Kuhnert WL and Hahn K: Genetics
of acid adaptation in oral streptococci. Crit Rev Oral Biol Med.
12:301–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsui R and Cvitkovitch D: Acid tolerance
mechanisms utilized by Streptococcus mutans. Future Microbiol.
5:403–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loesche WJ: Role of Streptococcus mutans
in human dental decay. Microbiol Rev. 50:353–380. 1986.PubMed/NCBI
|
|
10
|
Gross EL, Beall CJ, Kutsch SR, Firestone
ND, Leys EJ and Griffen AL: Beyond Streptococcus mutans: Dental
caries onset linked to multiple species by 16S rRNA community
analysis. PLoS One. 7:e477222012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lemos JA, Quivey RG Jr, Koo H and
Abranches J: Streptococcus mutans: A new Gram-positive paradigm?
Microbiology. 159:436–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lévesque CM, Mair RW, Perry JA, Lau PC, Li
YH and Cvitkovitch DG: Systemic inactivation and phenotypic
characterization of two-component systems in expression of
Streptococcus mutans virulence properties. Lett Appl Microbiol.
45:398–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song L, Sudhakar P, Wang W, Conrads G,
Brock A, Sun J, Wagner-Döbler I and Zeng AP: A genome-wide study of
two-component signal transduction systems in eight newly sequenced
mutans streptococci strains. BMC Genomics. 13:1282012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li YH, Lau PC, Tang N, Svensäter G, Ellen
RP and Cvitkovitch DG: Novel two-component regulatory system
involved in biofilm formation and acid resistance in Streptococcus
mutans. J Bacteriol. 184:6333–6342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong Y, Tian XL, Sutherland T, Sisson G,
Mai J, Ling J and Li YH: Global transcriptional analysis of
acid-inducible genes in Streptococcus mutans: Multiple
two-component systems involved in acid adaptation. Microbiology.
155:3322–3332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Senadheera D, Krastel K, Mair R,
Persadmehr A, Abranches J, Burne RA and Cvitkovitch DG:
Inactivation of VicK affects acid production and acid survival of
Streptococcus mutans. J Bacteriol. 191:6415–6424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chong P, Drake L and Biswas I: LiaS
regulates virulence factor expression in Streptococcus mutans.
Infect Immun. 76:3093–3099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ortega AD, Quereda JJ, Pucciarelli MG and
Garcia-del Portillo F: Non-coding RNA regulation in pathogenic
bacteria located inside eukaryotic cells. Front Cell Infect
Microbiol. 4:1622014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gottesman S and Storz G: Bacterial small
RNA regulators: versatile roles and rapidly evolving variations.
Cold Spring Harb Perspect Biol. 3:pii: a0037982011. View Article : Google Scholar
|
|
20
|
Marx P, Nuhn M, Kovács M, Hakenbeck R and
Brückner R: Identification of genes for small non-coding RNAs that
belong to the regulon of the two-component regulatory system CiaRH
in Streptococcus. BMC Genomics. 11:6612010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brosse A, Korobeinikova A, Gottesman S and
Guillier M: Unexpected properties of sRNA promoters allow feedback
control via regulation of a two-component system. Nucleic Acids
Res. 44:9650–9666. 2016.PubMed/NCBI
|
|
22
|
Saxena D, Li Y and Caufield PW:
Identification of unique bacterial gene segments from Streptococcus
mutans with potential relevance to dental caries by subtraction DNA
hybridization. J Clin Microbiol. 43:3508–3511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu LX, Tao Y, Qiu RM, Zhou Y, Zhi QH and
Lin HC: Genetic polymorphisms of the sortase A gene and
social-behavioural factors associated with caries in children: A
case-control study. BMC Oral Health. 15:542015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stipp RN, Goncalves RB, Hofling JF, Smith
DJ and Mattos-Graner RO: Transcriptional analysis of gtfB, gtfC,
and gbpB and their putative response regulators in several isolates
of Streptococcus mutans. Oral Microbiol Immunol. 23:466–473. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu S, Tao Y, Yu L, Zhuang P, Zhi Q, Zhou
Y and Lin H: Analysis of Small RNAs in Streptococcus mutans under
acid stress-A new insight for caries research. Int J Mol Sci.
17:pii: E15292016. View Article : Google Scholar
|
|
26
|
Cho SH, Lei R, Henninger TD and Contreras
LM: Discovery of ethanol-responsive small RNAs in Zymomonas
mobilis. Appl Environ Microbiol. 80:4189–4198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin H, Hu M, Zhang R, Shen Z, Flatow L and
You M: MicroRNA-217 promotes ethanol-induced fat accumulation in
hepatocytes by down-regulating SIRT1. J Biol Chem. 287:9817–9826.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yen YC, Shiah SG, Chu HC, Hsu YM, Hsiao
JR, Chang JY, Hung WC, Liao CT, Cheng AJ, Lu YC and Chen YW:
Reciprocal regulation of microRNA-99a and insulin-like growth
factor I receptor signaling in oral squamous cell carcinoma cells.
Mol Cancer. 13:62014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogata Y, Matsui S, Kato A, Zhou L,
Nakayama Y and Takai H: MicroRNA expression in inflamed and
noninflamed gingival tissues from Japanese patients. J Oral Sci.
56:253–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eggenhofer F, Tafer H, Stadler PF and
Hofacker IL: RNApredator: Fast accessibility-based prediction of
sRNA targets. Nucleic Acids Res. 39:W149–W154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia L, Xia W, Li S, Li W, Liu J, Ding H,
Li J, Li H, Chen Y, Su X, et al: Identification and expression of
small non-coding RNA L10-Leader, in different growth phases of
Streptococcus mutans. Nucleic Acid Ther. 22:177–186.
2012.PubMed/NCBI
|
|
33
|
Lian C, Sun B, Niu S, Yang R, Liu B, Lu C,
Meng J, Qiu Z, Zhang L and Zhao Z: A comparative profile of the
microRNA transcriptome in immature and mature porcine testes using
Solexa deep sequencing. FEBS J. 279:964–975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suntharalingam P, Senadheera MD, Mair RW,
Lévesque CM and Cvitkovitch DG: The LiaFSR system regulates the
cell envelope stress response in Streptococcus mutans. J Bacteriol.
191:2973–2984. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stubben CJ, Micheva-Viteva SN, Shou Y,
Buddenborg SK, Dunbar JM and Hong-Geller E: Differential expression
of small RNAs from Burkholderia thailandensis in response to
varying environmental and stress conditions. BMC Genomics.
15:3852014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Christiansen JK, Larsen MH, Ingmer H,
Søgaard-Andersen L and Kallipolitis BH: The RNA-binding protein Hfq
of Listeria monocytogenes: Role in stress tolerance and virulence.
J Bacteriol. 186:3355–3362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bronsard J, Pascreau G, Sassi M, Mauro T,
Augagneur Y and Felden B: sRNA and cis-antisense sRNA
identification in Staphylococcus aureus highlights an unusual sRNA
gene cluster with one encoding a secreted peptide. Sci Rep.
7:45652017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahn SJ, Lemos JA and Burne RA: Role of
HtrA in growth and competence of Streptococcus mutans UA159. J
Bacteriol. 187:3028–3038. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tremblay YD, Lo H, Li YH, Halperin SA and
Lee SF: Expression of the Streptococcus mutans essential
two-component regulatory system VicRK is pH and growth-phase
dependent and controlled by the LiaFSR three-component regulatory
system. Microbiology. 155:2856–2865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ahn SJ, Wen ZT and Burne RA: Multilevel
control of competence development and stress tolerance in
Streptococcus mutans UA159. Infect Immun. 74:1631–1642. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laux A, Sexauer A, Sivaselvarajah D,
Kaysen A and Brückner R: Control of competence by related
non-coding csRNAs in Streptococcus pneumoniae R6. Front Genet.
6:2462015. View Article : Google Scholar : PubMed/NCBI
|