Introduction
Quercetin is the most abundant dietary flavonoid
(1). It was previously
demonstrated that quercetin exerts an antiplatelet effect that may
be utilized to prevent and alleviate cardiovascular diseases
(2–8). However, quercetin exhibits low
hydrophilicity and low water solubility. In particular, the low
water solubility is associated with the limited bioavailability of
quercetin, and remains as a major obstacle to its therapeutic
applications (9,10). The strong intermolecular packing of
planar phenyl and hereto rings among quercetin molecules is
presumably attributed to the low solubility of quercetin in water.
Acylation of the hydroxyl groups of quercetin by an acyl donor
containing a short aliphatic chain is a feasible way for destroying
this intermolecular packing, hence improving the solvation of
quercetin. However, non-selective acylation of hydroxyl groups may
lead to loss of the biological activities of quercetin. The two
adjacent hydroxyl groups at C3 and C4 in ring B, and the double
bond between C2-C3 and the carbonyl group at C4 in ring C, are the
most critical elements for quercetin to be biologically active
(11,12). Therefore, to improve quercetin
water solubility without compromising its biological activities,
these functional groups must be kept intact.
Regioselective acylation of the hydroxyl group at C3
offers a reasonable solution (13,14).
Chemo-enzymatic synthesis of regioselectively acylated quercetin
has been previously investigated (13–17).
However, synthesis methods without using enzymes are desirable, as
they may reduce the reaction time and eliminate the need for costly
enzymes. Only few studies using enzyme-free synthesis methods for
the synthesis of quercetin analogues have been reported to date
(18). A major challenge is that
quercetin's hydroxyl groups may be randomly acylated, yielding a
mixture of products. The aim of the present study was to develop a
complete synthetic method
(benzylation-hydrolysis-acylation-hydrogenation) for the synthesis
of quercetin analogues using low-cost rutin as a starting material.
With this method, regioselective acylation of C3 becomes possible
as the hydroxyl of C3 is naturally protected with glycoside via a
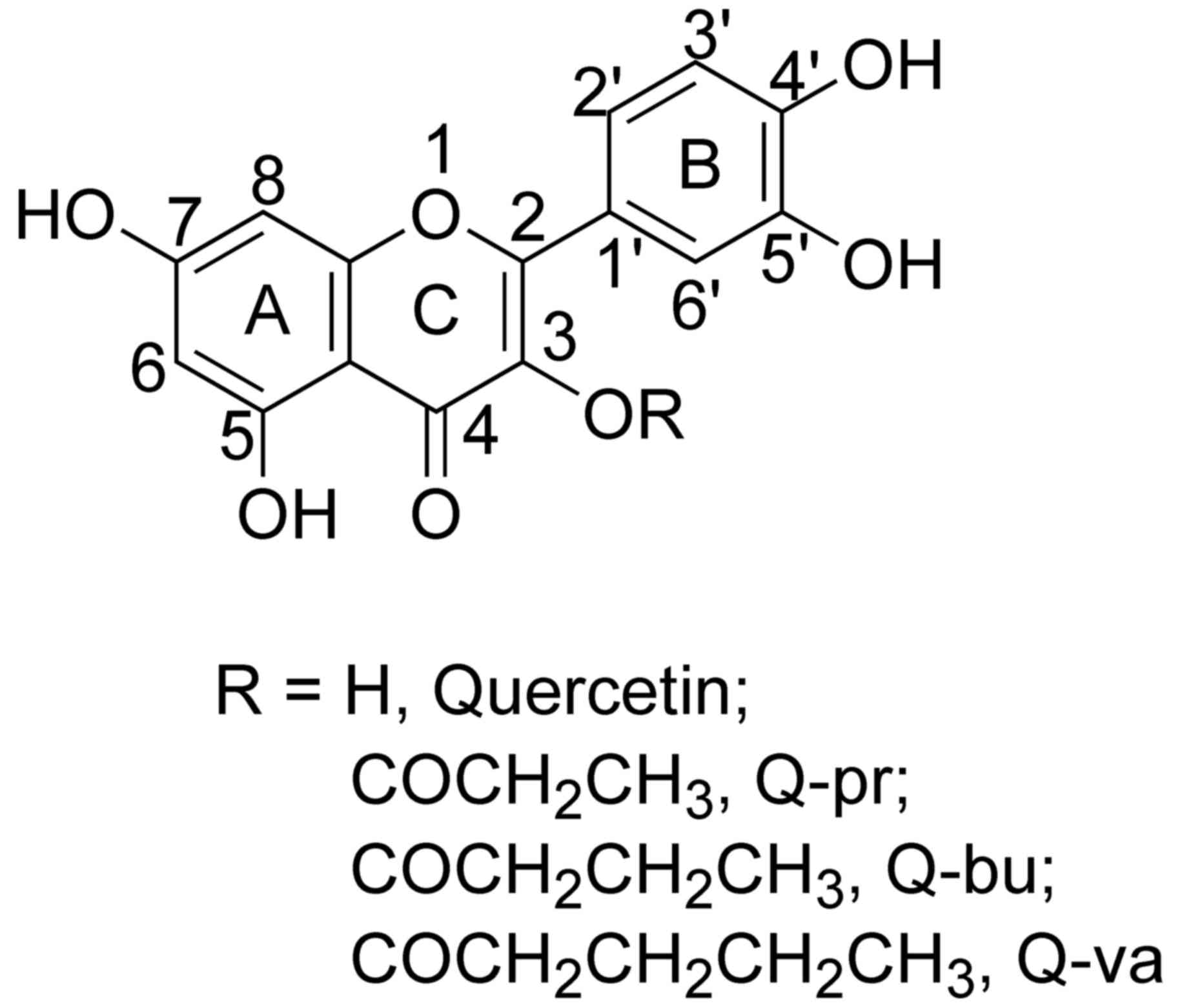
glycosidic bond. Three quercetin analogues were synthesized,
quercetin-3-O-propionate (Q-pr),
quercetin-3-O-butyrate (Q-bu) and
quercetin-3-O-valerate (Q-va), with high yields (Fig. 1), and their water solubility,
lipophilicity and antiplatelet activity were examined against
quercetin.
Materials and methods
Chemicals
Rutin (≥98%) was obtained from Nanjing TCM Institute
of Chinese Materia Medica (Nanjing, China) and kept at 110°C under
1.3 kPa for 12 h to remove bound water. Propionyl chloride, butyryl
chloride and valeroyl chloride (analytical grade) were purchased
from Aladdin Industrial Corporation (Shanghai, China). Benzyl
bromide was obtained from Sinopharm Chemical Reagent (Shanghai,
China) and distilled prior to use. N,N-dimethylformamide
(DMF), triethylamine (TEA), and dichloromethane (DCM) were
purchased form Tianjin Kemiou Chemical Reagent (Tianjin, China) and
dehydrated prior to use. Adenosine diphosphate (ADP), arachidonic
acid (AA) and platelet-activating factor (PAF) were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Palladium on carbon
(Pd/C) (10%) was obtained from Sinopharm Chemical Reagent.
Animals
Male New Zealand rabbits (license no. 0017075) and
male Wistar rats (250–300 g; license no. 0010061) were obtained
from the Experimental Animal Center of Shandong Lukang
Pharmaceutical (Jinan, China). The experimental procedures were
approved by the Animal Experimentation Ethics Committee of Weifang
Medical College and conducted in accordance with the guidelines of
the National Health and Medical Research Council of China for the
care and use of animals.
Synthesis of acylated quercetin
analogues
The synthesis followed a complete chemical synthetic
procedure, namely benzylation-hydrolysis-acylation-hydrogenation,
using rutin as the starting material.
Step 1
Rutin (24.42 g, 40 mmol) and
K2CO3 (18.35 g, 133 mmol) were sequentially
added to 160 ml of DMF under nitrogen and stirred for 1 h at room
temperature (19). Subsequently,
benzyl bromide (16 ml, 133 mmol) was added dropwise into the
reaction mixture. The reaction mixture was stirred at 40°C for 3 h
under nitrogen, and then adjusted to pH 6.0 with 10% (v/v) acetic
acid in ice bath. Deionized water (300 ml) was then added to this
mixture and the suspension was filtered.
Step 2
The filtered residue was dissolved in 600 ml of 95%
(v/v) ethanol at 70°C. Hydrochloric acid [90 ml, 36% (w/w)] was
added to the solution and the hydrolysis continued at 70°C for 2 h.
The suspension was cooled down to room temperature and then
filtered to yield hydrolyzate. The hydrolyzate was washed with ice
water until its pH became neutral.
Step 3
The hydrolyzate was dissolved in 250 ml of DCM
followed by addition of chloride (propionyl chloride, butyryl
chloride or valeroyl chloride) (44 mmol) and TEA (44 mmol). The
mixture was stirred at room temperature until thin-layer
chromatography (TLC) analysis showed the completeness of the
reaction. The reaction mixture was then extracted with 1 mol/l
hydrochloric acid, and the organic layer was washed with a
saturated aqueous solution of NaHCO3 and deionized
water. The pooled extracts were dried over
Na2SO4 and then taken to dryness under vacuum
to retrieve the acylated compound.
Step 4
The acylated compound was dissolved in 5,000 ml of
ethanol/dioxane (1:1, v/v). Pd/C (2 g) was added to the solution.
The reaction mixture was stirred at room temperature under hydrogen
at atmospheric pressure for 2 h. Pd/C was filtered off, and the
filtrate was evaporated in vacuum to retrieve the crude product of
acylated quercetin analogues.
Purification of acylated quercetin
analogues
Acylated derivatives were purified by using
semi-preparative high-performance liquid chromatography (HPLC). An
Agilent 1200 infinity preparative chromatography system performed
chromatographic separation; this system was equipped with an
Agilent Prep LC controller, an ultraviolet (UV) detector
(VWD-G1314B), a preparative column (ZORBAX-SB C18, 250×9.4 mm, 5
µm), an injector (3725i) and an auto-fraction collector (G1364C)
(all from Agilent Technologies, Inc., Santa Clara, USA). Q-pr and
Q-bu were purified using the linear elutions of acetonitrile/water
(40:60, v/v and 30:70, v/v, respectively). Q-va was purified using
a linear gradient of acetonitrile and water for 0 min (30:70, v/v),
7 min (40:60, v/v), 15 min (50:50, v/v), and 25 min (30:70, v/v).
The flow rate was 5 ml/min, and the elution was performed at room
temperature at 254 nm with the UV detector.
TLC
The acylation process was monitored using TLC on
silica gel 60-GF254 (Merck KGaA), with a solvent mixture
of ethyl acetate, methanol and acetic acid (at a ratio of 6:4:0.1,
v/v/v). The plate was observed and detected under UV light (254
nm).
Nuclear magnetic resonance (NMR)
spectroscopy and mass spectrometry (MS)
Quercetin and three acylated derivatives, Q-pr, Q-bu
and Q-va, were characterized with 1H NMR (500 MHz) and
13C NMR (100 MHz) in DMSO-d6 using a
Bruker AV500 NMR spectrometer (Bruker Corporation, Billerica, MA,
USA) with tetramethylsilane as an internal reference, and with MS
without breaking into fragment ions using an Agilent 6410 liquid
chromatography-mass spectrometer (Agilent Technologies, Inc.) with
electrospray ionization.
Water solubility measurements
The water solubility of quercetin and the three
acylated derivatives was determined according to the method
described by Bonina et al (20). An excess amount of the compound was
weighed into a glass tube containing 2 ml of water, and the tube
was sealed with a Teflon-lined cap. The mixture was stirred by a
magnetic stirrer for 24 h at room temperature and was then filtered
using a Millex HV13 filter unit (0.22 µm; Merck KGaA). The drug
concentration in the saturated solution at 254 nm was determined
using a Shimadzu chromatography system (LC-20AT), equipped with a
UV detector (SPD-M10Avp) and a column (Shim-pack VP-ODS C18,
250×4.60 mm, 5 µm) (all from Shimadzu, Kyoto, Japan). The mobile
phase was water/methanol (70:30, v/v) at a flow rate of 1
ml/min.
Lipophilicity measurements
Lipophilicity may be estimated using reverse-phase
chromatographic retention time due to the good association between
the logarithm of the n-octanol/water partition coefficient
(log P) and the logarithm of the capacity factor eluting
with 100% water (log kw) determined using
octadecyl silica columns. The value of log kw was
obtained according to the method described by Braumann (21). Each compound was dissolved in
methanol to a final concentration of 10 µg/ml. The sample
(n=3) was filtered prior to injection using a filter (Millex HV13,
0.22 µm; Merck KGaA) and the aliquot (20 µl) was injected
into the HPLC. The value of logarithm of the capacity factor (log
k') was calculated using the following equation:
logk′=tr–t0t0
where tr is the retention time of
the flavonoid peak and t0 denotes the retention
time of the non-retained solvent peak. The HPLC condition was
maintained the same as in the water solubility test. Only the
volume fraction of methanol in mobile phase was changed for each
measurement. A series of values of retention times and log
k' were obtained. At a plot of log k' vs. the volume
fraction of methanol in mobile phase, φ, extrapolated the
data to 100% water to obtain the intersection point with the y
axis, that is log kw value. A formula (21), which exhibited an excellent
correlation between log kw and log P for
the compound containing conjugated aromatic, was selected to
calculated the value of log P:
logkw=0.988xlogP+0.020
The lipophilicity of quercetin and the three
acylated derivatives was also calculated using the ACD/ChemSketch
(Advanced Chemistry Development Inc., Toronto, Canada) and the
Property Explorer Applet (www.openmolecules.org).
The association between log k' and φ,
the values of log kw and log P of
quercetin and the three acylated derivatives are presented in
Table I.
 | Table I.Association between log k' and
φ, and values of log kw, log P and
water solubility of quercetin, Q-pr, Q-bu and Q-va. |
Table I.
Association between log k' and
φ, and values of log kw, log P and
water solubility of quercetin, Q-pr, Q-bu and Q-va.
|
|
|
| log
Pb |
|
|---|
|
|
|
|
|
|
|---|
| Compound | log
k'-φ (Ra) | log
kw | A | B | C | Water solubility
(µg/ml) |
|---|
| Quercetin | log
k'=1.67–2.16φ (R=-0.98221) | 1.67 | 1.67 | 2.07 | 1.49 |
1.98 |
| Q-pr | log
k'=3.04–4.21φ (R=-0.9837) | 3.04 | 3.06 | 2.77 | 2.43 | 16.27 |
| Q-bu | log
k'=3.47–4.41φ (R=-0.9943) | 3.47 | 3.48 | 3.30 | 2.88 |
9.36 |
| Q-va | log
k'=3.72–4.77φ (R=-0.9995) | 3.72 | 3.75 | 3.83 | 3.34 |
1.07 |
In vitro antiplatelet activity
test
Fresh whole blood was collected from healthy male
New Zealand rabbits with 3.8% (w/v) sodium citrate at a volume
ratio of 9:1. Platelet-rich plasma (PRP) was obtained by
centrifugation at 328.7 × g for 8 min at room temperature with no
brake. PRP (300 µl) was incubated for 3 min at 37°C in the
presence of various final concentrations (0–350 µM) of
quercetin or acylated analogue (n=8–10), predissolved in DMSO. The
final concentration of DMSO was <1.0% (v/v) to eliminate false
positive results. Subsequently, platelet aggregation was stimulated
by ADP (7 µM), AA (350 µM), or PAF (7.2 nM). The
aggregation was monitored for 5 min using a platelet aggregometer
(LBY-NJ4A, Precill, Beijing, China) with constant stirring at 241.5
× g, and the aggregation rates were recorded to determine the
percentage of aggregation.
In vivo antiplatelet activity
test
Administration of investigational agents and sample
preparation for platelet aggregation were performed according to
the method described by Mosawy et al (22). In brief, the rats (8–10 per group)
were treated with 0.03 mmol/kg quercetin, Q-pr, Q-bu, Q-va, or
control consisting of 0.5% DMSO with 2.2 mM polyethylene glycol
(PEG) in saline. DMSO and PEG were used to improve the solubility
of the investigational agents in the blood. The investigational
agents were administered via a single intravenous (IV) bolus via
the tail vein. Experimental procedures and blood sample collection
were performed 30 min after the IV bolus treatment. In each rat, 5
ml of fresh whole blood was collected into tubes containing 600
µl of 3.8% (w/v) sodium citrate via cardiac puncture. PRP
was obtained by centrifugation at 377.3 × g for 8 min at room
temperature with no brake. PRP was incubated for 3 min at 37°C, and
platelet aggregation was then stimulated by 7 µM ADP, 350 µM
AA, or 7.2 nM PAF (final concentration). The aggregation was
monitored for 5 min by using an aggregometer (LBY-NJ4A, Precill) at
a constant stirring of 241.5 × g, and the aggregation rates were
recorded to determine the percent of aggregation. The value of the
aggregation inhibitory rate (AIR) was calculated as follows:
AIR%=(1–AB)x100%
where A is the platelet aggregation rate of
the sample and B is the platelet aggregation rate of the no
compound treated group (control).
Statistical analysis
All values are expressed as mean ± standard error of
the mean. Comparisons between test samples and blank were performed
using SPSS 9.0 software for Windows (SPSS Inc., Chicago, IL, USA)
with Dunnett's test for post hoc comparisons, and P≤0.01 was
considered to indicate statistically significant differences.
Results and Discussion
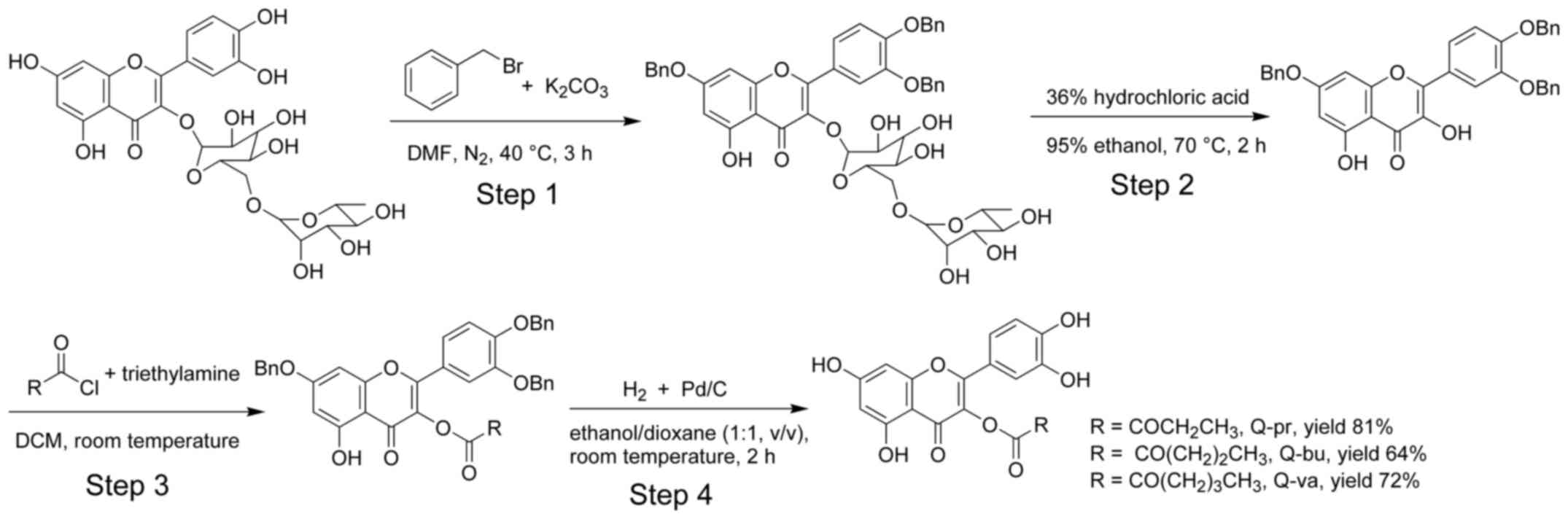
The synthesis of the three quercetin analogues
involves four steps (Fig. 2). The
hydroxyl group at C5 is considered non-reactive, as it forms an
intramolecular hydrogen bond with the carbonyl at C4 (22). Therefore, this hydroxyl group
remained intact throughout the reactions. In step 1, anhydrous
K2CO3 was added to facilitate the formation
of oxygen anions for hydroxyl groups at C3, C4 and C7 of rutin.
Subsequently, benzyl bromide was added to benzylate the forming
oxygen anions. A protic solvent, DMF, was used to avoid the
production of C-alkylated derivatives. The hydroxyl group at C3 was
recovered after hydrolysis of the glycoside (step 2), and became
the only reactive group for subsequent acylation in step 3. In the
final step, the benzyl groups at C3, C4 and C7 were removed by
hydrogenation. The resulting acylated quercetin derivatives were
purified using semi-preparative HPLC. All these derivatives were
yellow powders.
The 1H NMR, 13C NMR and MS
spectra collectively confirmed the structures of regioselectively
acylated quercetin analogues.
Q-pr
1H NMR chemical shifts: δ (ppm) 12.23
(1H, s, 5-OH), 10.67 (1H, br, s, 7-OH), 9.72 (3H, br s, 3, 3′,
4′-OH), 7.33 (1H, d, H-2′), 7.27 (1H, d, H-6′), 6.92 (1H, d, H-5′),
6.48 (1H, d, H-8), 6.25 (1H, d, H-6), 2.68 (2H, t, CH2),
1.15 (3H, t, CH3). 13C NMR chemical shifts: δ
(ppm) 175.39 (C-4), 171.78 (C=O), 165.18 (C-7), 161.54 (C-9),
157.08 (C-5), 156.36 (C-2), 149.78 (C-4′), 145.95 (C-3′), 130.06
(C-3), 120.98 (C-1′), 120.08 (C-6′), 116.45 (C-5′), 115.46 (C-2′),
103.89 (C-10), 99.55 (C-6), 94.56 (C-8), 27.08 (CH2),
9.32 (CH3). MS (m/z) for Q-pr: 381.1
[M+Na]+.
Q-bu
1H NMR chemical shifts: δ (ppm) 12.20
(1H, s, 5-OH), 7.32 (1H, d, H-2′), 7.26 (1H, d, H-6′), 6.91 (1H, d,
H-5′), 6.47 (1H, d, H-8), 6.25 (1H, d, H-6), 2.63 (2H, t,
CH2), 1.66 (2H, m, CH2), 0.96 (3H, t,
CH3). 13C NMR chemical shifts: δ (ppm) 174.95
(C-4), 170.48 (C=O), 164.72 (C-7), 161.08 (C-9), 156.63 (C-5),
156.01 (C-2), 149.31 (C-4′), 145.45 (C-3′), 129.56 (C-3), 120.54
(C-1′), 119.60 (C-6′), 115.95 (C-5′), 115.01 (C-2′), 103.55 (C-10),
99.11 (C-6), 94.12 (C-8), 28.85 (CH2), 17.88
(CH2), 13.33 (CH3). MS (m/z): 395.1
[M+Na]+.
Q-va
1H NMR chemical shifts: δ (ppm) 12.23
(1H, s, 5-OH), 7.32 (1H, d, H-2′), 7.27 (1H, d, H-6′), 6.92 (1H, d,
H-5′), 6.48 (1H, d, H-8), 6.25 (1H, d, H-6), 2.65 (2H, t,
CH2), 1.62 (2H, m, CH2), 1.36 (2H, m,
CH2), 0.90 (3H, m, CH3). 13C NMR
chemical shifts: δ (ppm) 175.40 (C-4), 171.01 (C=O), 165.22 (C-7),
161.55 (C-9), 157.09 (C-5), 156.48 (C-2), 149.77 (C-4′), 145.97
(C-3′), 130.04 (C-3), 122.97 (C-1′), 120.07 (C-6′), 116.38 (C-5′),
115.49 (C-2′), 103.89 (C-10), 99.56 (C-6), 94.56 (C-8), 33.30
(CH2), 26.85 (CH2), 21.93 (CH2),
14.04 (CH3). MS (m/z): 409.1 [M+Na]+.
In the 13C NMR spectrum of quercetin, the
chemical shift of C3 appears at 136.18 ppm. However, the chemical
shift of C3 in the synthesized analogues moved upfield (130.06 ppm
for Q-pr, 129.56 ppm for Q-bu, and130.04 ppm for Q-va).
Furthermore, the chemical shifts attributed to the ester carbonyl
carbons of Q-pr, Q-bu and Q-va were identified at 171.78, 170.48
and 171.01 ppm, respectively. The ions at m/z 381.1, m/z 395.1 and
m/z 409.1 in the MS spectra correspond to sodiated adducts
[M+Na]+ for Q-pr, Q-bu and Q-va, respectively,
consistent with the C3-substituted structures. This further
confirmed the successful substitution of aliphatic acyls at C3.
Q-pr and Q-bu exhibited significantly improved water
solubility (Table I). In
particular, the water solubility of Q-pr was 8.2-fold higher
compared with that of quercetin, while Q-bu exhibited a 4.7-fold
higher water solubility. However, Q-va did not exhibit improved
water solubility, as its water solubility was only 54% that of
quercetin. These results suggested that the water solubility of
quercetin analogues is affected by the carbon chain length at C3.
Propionyl group has the shortest carbon chain, possessing a total
of three carbons. It was shown to be the most effective in
increasing water solubility. Butyryl group has one more methylene
compared with propionyl group and a total of four carbons. It was
still effective in increasing the water solubility of quercetin.
However, the five-carbon valeroyl group failed to render the
quercetin analogue more water soluble. This was probably because 3-
and 4-carbon short aliphatic acyl chains were efficient in
disrupting intermolecular packing of quercetin molecules, thus
enabling solvent molecules to enter the intermolecular gap to
solvate quercetin molecules more easily. A further increase in
chain length to 5 carbons made the hydrophobic effect dominant,
making quercetin substituted with valeroyl group more hydrophobic.
Their n-octanol/water partition coefficients (Ps) were
tested and three different methods were used to calculate log
P (Table I). The
lipophilicity of a compound is reflected by log P. Acyl
chain length increase resulted in stronger lipophilicity. The
results indicate that the log P values of the acylated
derivatives were higher than compared with of quercetin, and it
increased with aliphatic acyl chain length with log P of
Q-va being the highest.
Given that the acylation regioselectively occurred
at C3 of quercetin and the bioactive hydroxyl groups remained
unchanged, the biological activities of quercetin were expected to
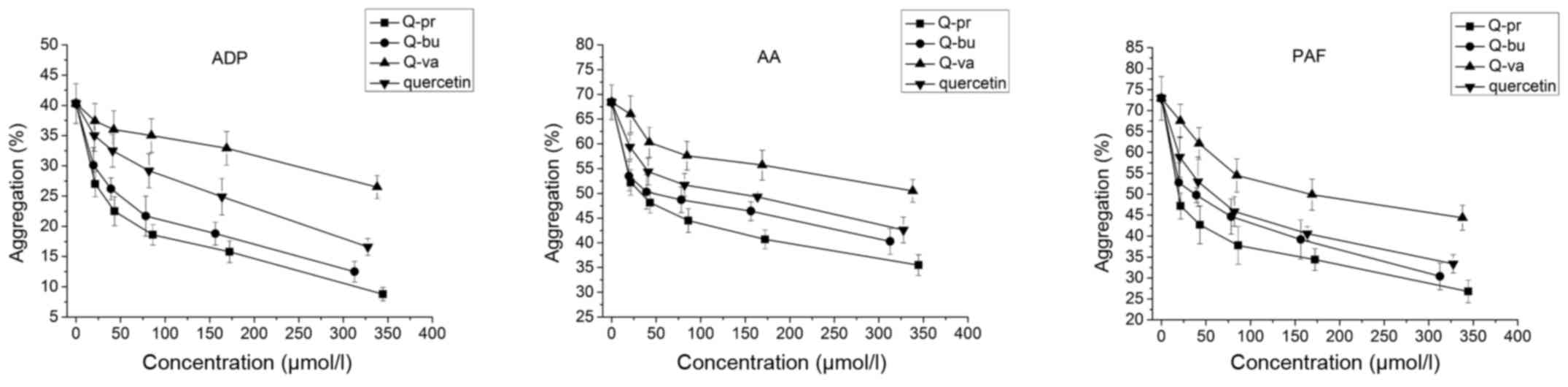
be maintained. The in vitro assay demonstrated that the
three acylated derivatives were able to inhibit platelet
aggregation induced by ADP, AA and PAF in a dose-dependent manner
(Fig. 3). Q-pr and Q-bu were found
to be more potent compared with quercetin.
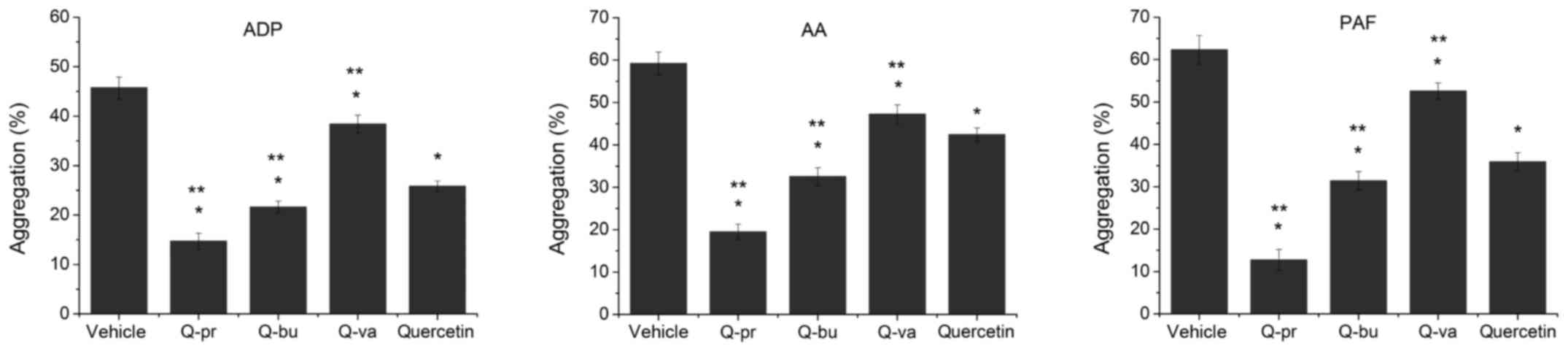
Q-pr and Q-bu also displayed higher antiplatelet
aggregation activities compared with quercetin in vivo
(Fig. 4). In the ADP group, the
AIRs of Q-pr and Q-bu were 67.8 and 52.7%, respectively, which were
significantly higher compared with that of quercetin (43.5%).
Although Q-va successfully inhibited platelet aggregation to a
certain extent with an AIR of 16.0%, it was significantly less
potent compared with quercetin. The same order of drug activity of
the four compounds was also observed in the AA and PAF groups.
In summary, regioselectively acylated quercetin
analogues were successfully synthesized using a complete synthetic
approach. The water solubility and lipophilicity of the resulting
analogues were affected by the length of the aliphatic chains for
acylation of the hydroxyl group located at C3 of quercetin.
Aliphatic acyl donors containing three and four carbons were able
to improve both the water solubility and the lipophilicity of the
acylated analogues. As compared to quercetin, Q-pr and Q-bu
exhibited a higher antiplatelet activity due to the higher water
solubility and enhanced lipophilicity, whereas Q-va was less
effective than quercetin due to its reduced water solubility,
although its lipophilicity was the highest among the three
analogues. Thus, an optimal acyl chain length is crucial for the
quercetin analogues synthesized following this complete synthetic
approach to be more effective.
Acknowledgements
The authors would like to thank Jian Guo and Weijie
Guo for providing assistance with the experiments. The present
study was supported by the Shandong Natural Science Foundation
(grant no. ZR2010HQ052), the Medical and Health Science and
Technology Development Project of Shandong Province (grant nos.
2011QZ025 and 2014WSB27002), the Pharmaceutical Technology
Development Project of Shandong Province (grant no. 2013-238) and
the Chinese Key Technology Program (grant no. 2013GA740103).
References
|
1
|
Hertog MG, Hollman PC, Katan MB and
Kromhout D: Intake of potentially anticarcinogenic flavonoids and
their determinants in adults in the Netherlands. Nutr Cancer.
20:21–29. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y and Deuster P: Comparison of
quercetin and dihydroquercetin: Antioxidant-independent actions on
erythrocyte and platelet membrane. Chem Biol Interact. 182:7–12.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuentes E and Palomo I: Relationship
between Platelet PPARs, cAMP levels, and P-Selectin expression:
Antiplatelet activity of natural products. Evid Based Complement
Alternat Med. 2013:8617862013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuentes E and Palomo I: Antiplatelet
effects of natural bioactive compounds by multiple targets: Food
and drug interactions. J Funct Foods. 6:73–81. 2014. View Article : Google Scholar
|
|
5
|
Mosawy S, Jackson DE, Woodman OL and
Linden MD: Treatment with quercetin and 3′,4′-dihydroxyflavonol
inhibits platelet function and reduces thrombus formation in vivo.
J Thromb Thrombolys. 36:50–57. 2013. View Article : Google Scholar
|
|
6
|
Navarro-Núñez L, Lozano ML, Martínez C,
Vicente V and Rivera J: Effect of quercetin on platelet spreading
on collagen and fibrinogen and on multiple platelet kinases.
Fitoterapia. 81:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Navarro-Núñez L, Rivera J, Guerrero JA,
Martínez C, Vicente V and Lozano ML: Differential effects of
quercetin, apigenin and genistein on signalling pathways of
protease-activated receptors PAR(1) and PAR(4) in platelets. Brit J
Pharmacol. 158:1548–1556. 2009. View Article : Google Scholar
|
|
8
|
Oh WJ, Endale M, Park SC, Cho JY and Rhee
MH: Dual roles of quercetin in platelets: Phosphoinositide-3-kinase
and MAP kinases inhibition, and cAMP-dependent
vasodilator-stimulated phosphoprotein stimulation. Evid Based
Complement Alternat Med. 2012:4852622012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferry DR, Smith A, Malkhandi J, Fyfe DW,
deTakats PG, Anderson D, Baker J and Kerr DJ: Phase I clinical
trial of the flavonoid quercetin: Pharmacokinetics and evidence for
in vivo tyrosine kinase inhibition. Clin Cancer Res. 2:659–668.
1996.PubMed/NCBI
|
|
10
|
Leonarduzzi G, Testa G, Sottero B, Gamba P
and Poli G: Design and development of nanovehicle-based delivery
systems for preventive or therapeutic supplementation with
flavonoids. Curr Med Chem. 17:74–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Justino GC, Santos MR, Canário S, Borges
C, Florêncio MH and Mira L: Plasma quercetin metabolites:
Structure-antioxidant activity relationships. Arch Biochem Biophys.
432:109–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H and Joseph JA: Structure-activity
relationships of quercetin in antagonizing hydrogen
peroxide-induced calcium dysregulation in PC12 cells. Free Radic
Biol Med. 27:683–694. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Montenegro L, Carbone C, Maniscalco C,
Lambusta D, Nicolosi G, Ventura CA and Puglisi G: In vitro
evaluation of quercetin-3-O-acyl esters as topical prodrugs. Int J
Pharm. 336:257–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saija A, Tomaino A, Trombetta D,
Pellegrino ML, Tita B, Messina C, Bonina FP, Rocco C, Nicolosi G
and Castelli F: ‘In vitro’ antioxidant and photoprotective
properties and interaction with model membranes of three new
quercetin esters. Eur J Pharm Biopharm. 56:167–174. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gatto MT, Falcocchio S, Grippa E, Mazzanti
G, Battinelli L, Nicolosi G, Lambusta D and Saso L: Antimicrobial
and anti-lipase activity of quercetin and its
C2-C163-O-acyl-esters. Bioorg Med Chem. 10:269–272. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar V, Jahan F, Mahajan RV and Saxena
RK: Efficient regioselective acylation of quercetin using Rhizopus
oryzae lipase and its potential as antioxidant. Bioresour Technol.
218:1246–1248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sardone L, Pignataro B, Castelli F,
Sarpietro MG, Nicolosi G and Marletta G: Temperature and pressure
dependence of quercetin-3-O-palmitate interaction with a model
phospholipid membrane: Film balance and scanning probe microscopy
study. J Colloid Interface Sci. 271:329–335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamauchi K, Mitsunaga T, Inagaki M and
Suzuki T: Synthesized quercetin derivatives stimulate melanogenesis
in B16 melanoma cells by influencing the expression of melanin
biosynthesis proteins MITF and p38 MAPK. Bioorg Med Chem.
22:3331–3340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wuts PGM and Greene TW: Protection for
Phenols and CatecholsGreene's Protective Groups in Organic
Synthesis. John Wiley & Sons; New York: pp. 367–430. 2006,
View Article : Google Scholar
|
|
20
|
Bonina FP, Montenegro L, De Capraris P,
Bousquet E and Tirendi S: 1-Alkylazacycloalkan-2-one esters as
prodrugs of indomethacin for improved delivery through human skin.
Int J Pharm. 77:21–29. 1991. View Article : Google Scholar
|
|
21
|
Braumann T: Determination of hydrophobic
parameters by reversed-phase liquid chromatography: Theory,
experimental-techniques, and application in studies on quantitative
structure-activity-relationships. J Chromatogr. 373:191–225. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mosawy S, Jackson DE, Woodman OL and
Linden MD: Treatment with quercetin and 3′,4′-dihydroxyflavonol
inhibits platelet function and reduces thrombus formation in vivo.
J Thromb Thrombolys. 36:50–57. 2013. View Article : Google Scholar
|