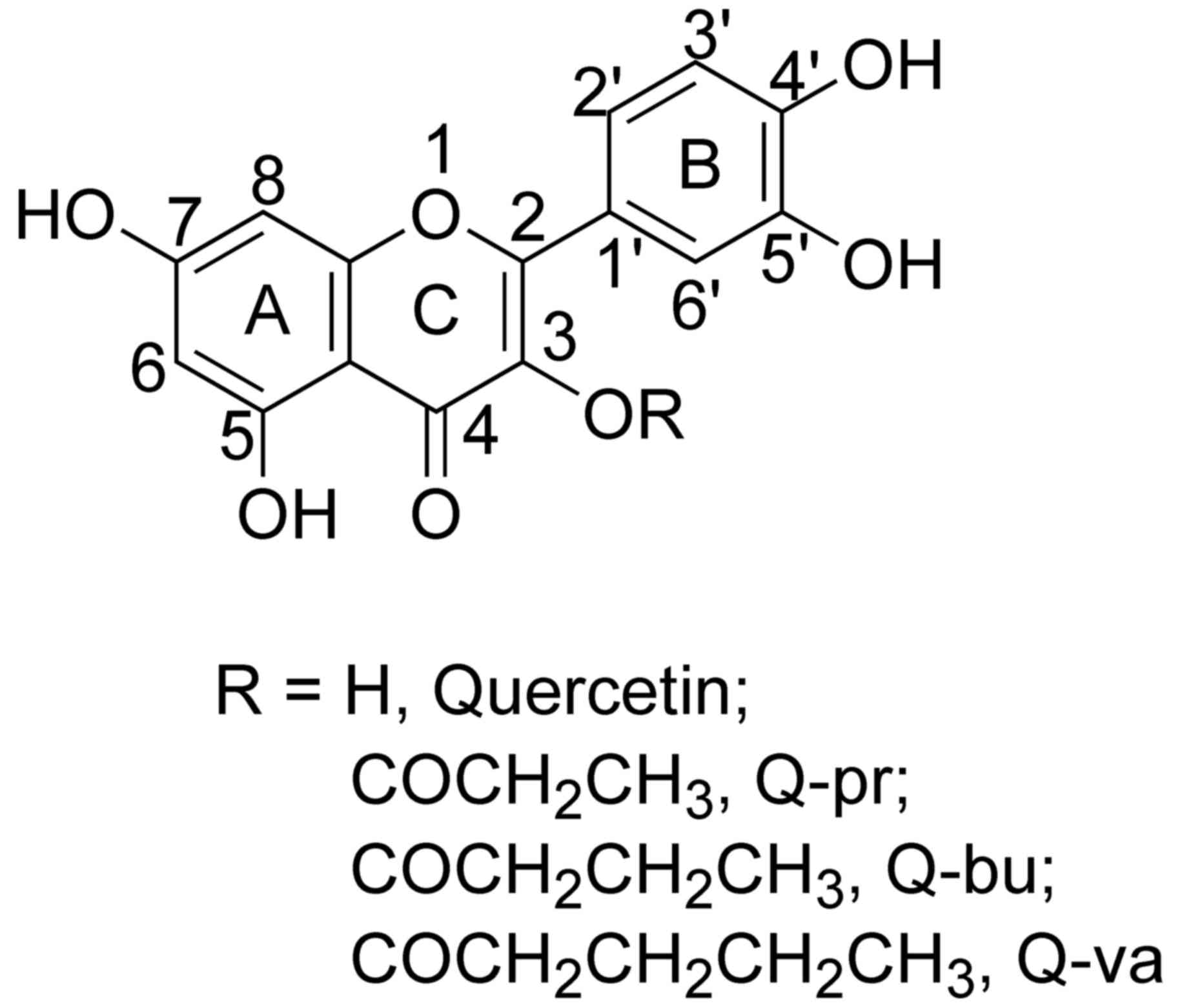
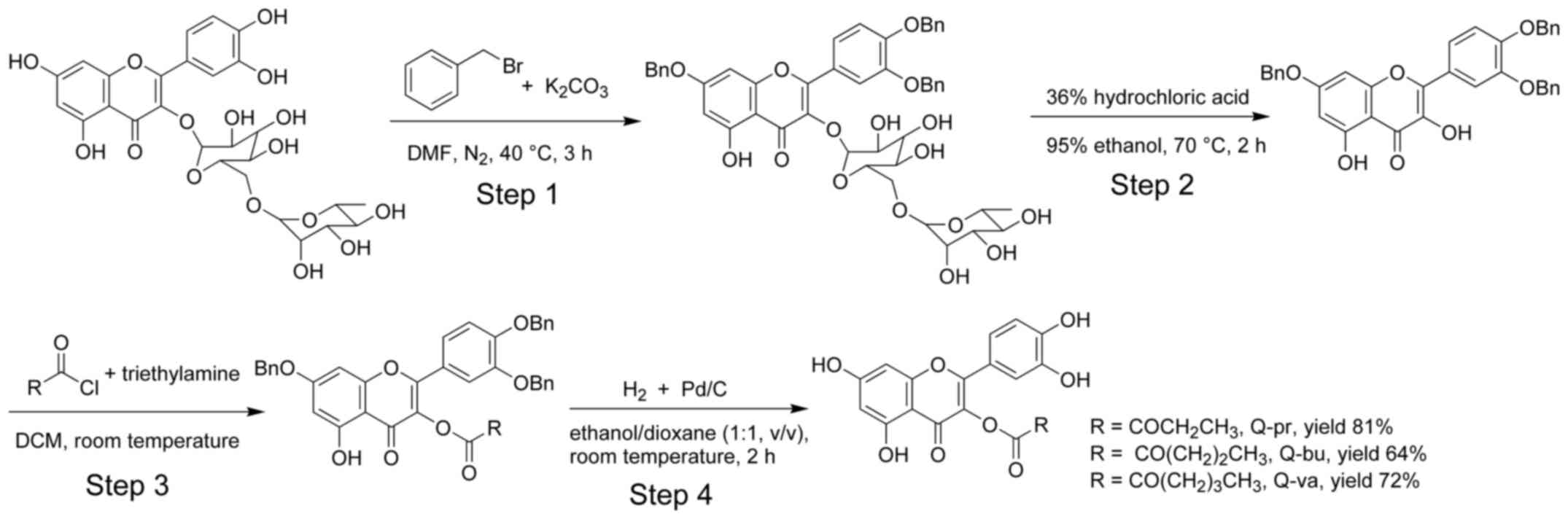
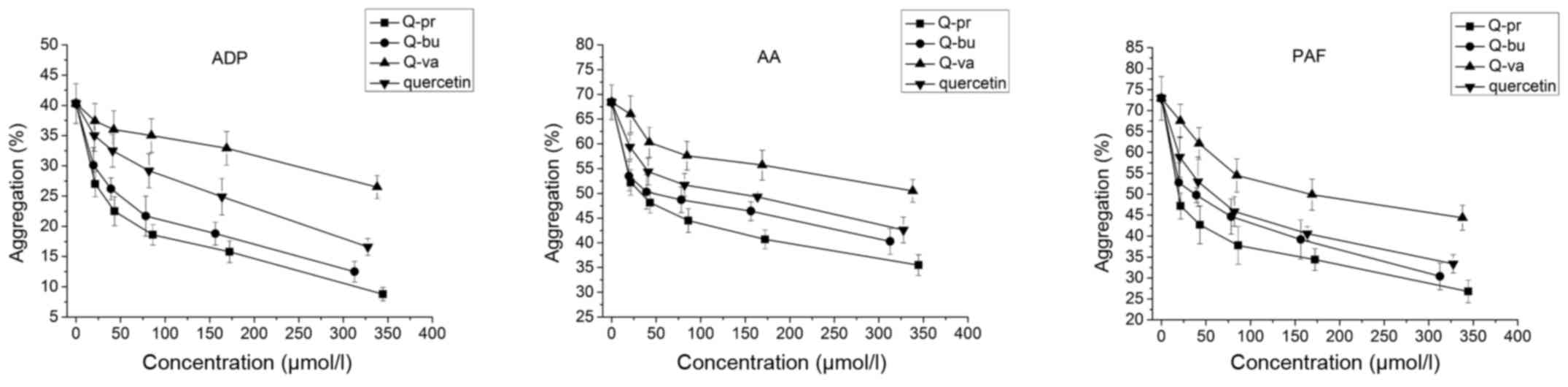
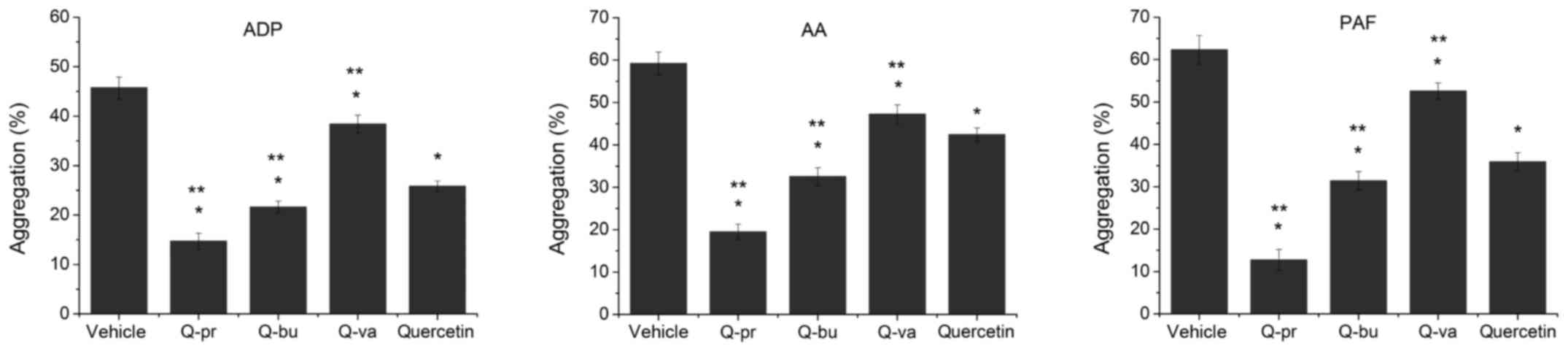
|
1
|
Hertog MG, Hollman PC, Katan MB and
Kromhout D: Intake of potentially anticarcinogenic flavonoids and
their determinants in adults in the Netherlands. Nutr Cancer.
20:21–29. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y and Deuster P: Comparison of
quercetin and dihydroquercetin: Antioxidant-independent actions on
erythrocyte and platelet membrane. Chem Biol Interact. 182:7–12.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuentes E and Palomo I: Relationship
between Platelet PPARs, cAMP levels, and P-Selectin expression:
Antiplatelet activity of natural products. Evid Based Complement
Alternat Med. 2013:8617862013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuentes E and Palomo I: Antiplatelet
effects of natural bioactive compounds by multiple targets: Food
and drug interactions. J Funct Foods. 6:73–81. 2014. View Article : Google Scholar
|
|
5
|
Mosawy S, Jackson DE, Woodman OL and
Linden MD: Treatment with quercetin and 3′,4′-dihydroxyflavonol
inhibits platelet function and reduces thrombus formation in vivo.
J Thromb Thrombolys. 36:50–57. 2013. View Article : Google Scholar
|
|
6
|
Navarro-Núñez L, Lozano ML, Martínez C,
Vicente V and Rivera J: Effect of quercetin on platelet spreading
on collagen and fibrinogen and on multiple platelet kinases.
Fitoterapia. 81:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Navarro-Núñez L, Rivera J, Guerrero JA,
Martínez C, Vicente V and Lozano ML: Differential effects of
quercetin, apigenin and genistein on signalling pathways of
protease-activated receptors PAR(1) and PAR(4) in platelets. Brit J
Pharmacol. 158:1548–1556. 2009. View Article : Google Scholar
|
|
8
|
Oh WJ, Endale M, Park SC, Cho JY and Rhee
MH: Dual roles of quercetin in platelets: Phosphoinositide-3-kinase
and MAP kinases inhibition, and cAMP-dependent
vasodilator-stimulated phosphoprotein stimulation. Evid Based
Complement Alternat Med. 2012:4852622012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferry DR, Smith A, Malkhandi J, Fyfe DW,
deTakats PG, Anderson D, Baker J and Kerr DJ: Phase I clinical
trial of the flavonoid quercetin: Pharmacokinetics and evidence for
in vivo tyrosine kinase inhibition. Clin Cancer Res. 2:659–668.
1996.PubMed/NCBI
|
|
10
|
Leonarduzzi G, Testa G, Sottero B, Gamba P
and Poli G: Design and development of nanovehicle-based delivery
systems for preventive or therapeutic supplementation with
flavonoids. Curr Med Chem. 17:74–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Justino GC, Santos MR, Canário S, Borges
C, Florêncio MH and Mira L: Plasma quercetin metabolites:
Structure-antioxidant activity relationships. Arch Biochem Biophys.
432:109–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H and Joseph JA: Structure-activity
relationships of quercetin in antagonizing hydrogen
peroxide-induced calcium dysregulation in PC12 cells. Free Radic
Biol Med. 27:683–694. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Montenegro L, Carbone C, Maniscalco C,
Lambusta D, Nicolosi G, Ventura CA and Puglisi G: In vitro
evaluation of quercetin-3-O-acyl esters as topical prodrugs. Int J
Pharm. 336:257–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saija A, Tomaino A, Trombetta D,
Pellegrino ML, Tita B, Messina C, Bonina FP, Rocco C, Nicolosi G
and Castelli F: ‘In vitro’ antioxidant and photoprotective
properties and interaction with model membranes of three new
quercetin esters. Eur J Pharm Biopharm. 56:167–174. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gatto MT, Falcocchio S, Grippa E, Mazzanti
G, Battinelli L, Nicolosi G, Lambusta D and Saso L: Antimicrobial
and anti-lipase activity of quercetin and its
C2-C163-O-acyl-esters. Bioorg Med Chem. 10:269–272. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar V, Jahan F, Mahajan RV and Saxena
RK: Efficient regioselective acylation of quercetin using Rhizopus
oryzae lipase and its potential as antioxidant. Bioresour Technol.
218:1246–1248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sardone L, Pignataro B, Castelli F,
Sarpietro MG, Nicolosi G and Marletta G: Temperature and pressure
dependence of quercetin-3-O-palmitate interaction with a model
phospholipid membrane: Film balance and scanning probe microscopy
study. J Colloid Interface Sci. 271:329–335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamauchi K, Mitsunaga T, Inagaki M and
Suzuki T: Synthesized quercetin derivatives stimulate melanogenesis
in B16 melanoma cells by influencing the expression of melanin
biosynthesis proteins MITF and p38 MAPK. Bioorg Med Chem.
22:3331–3340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wuts PGM and Greene TW: Protection for
Phenols and CatecholsGreene's Protective Groups in Organic
Synthesis. John Wiley & Sons; New York: pp. 367–430. 2006,
View Article : Google Scholar
|
|
20
|
Bonina FP, Montenegro L, De Capraris P,
Bousquet E and Tirendi S: 1-Alkylazacycloalkan-2-one esters as
prodrugs of indomethacin for improved delivery through human skin.
Int J Pharm. 77:21–29. 1991. View Article : Google Scholar
|
|
21
|
Braumann T: Determination of hydrophobic
parameters by reversed-phase liquid chromatography: Theory,
experimental-techniques, and application in studies on quantitative
structure-activity-relationships. J Chromatogr. 373:191–225. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mosawy S, Jackson DE, Woodman OL and
Linden MD: Treatment with quercetin and 3′,4′-dihydroxyflavonol
inhibits platelet function and reduces thrombus formation in vivo.
J Thromb Thrombolys. 36:50–57. 2013. View Article : Google Scholar
|