Introduction
Epilepsy has developed into a serious worldwide
health concern, accounting for ~1% of the global disease burden
(1). Epilepsy is a chronic
condition, or a group of chronic conditions, in which abnormal
neuronal discharge leads to transient disturbance of cerebral
function and recurrent seizures. It has been reported that
one-third of all epilepsy patients exhibit medically intractable
epilepsy (IE), which accounts for ~80% of the overall cost of
epilepsy (2,3). Patients with IE exhibit an increased
risk of premature death, psychosocial dysfunction and poor quality
of life (4). A previous study
concluded that a number of etiological, pharmacological,
demographic and genetic factors may contribute to the development
of IE (5). Temporal lobe epilepsy
(TLE) is the most common type of focal epilepsy, and ~35% of
patients with TLE experience chronic seizures that are not
responsive to antiepileptic-drugs (AEDs) (5,6).
Therefore, the identification of potential drug targets for IE is
urgently required in order to develop improved clinical
solutions.
At present, an increasing number of studies have
been conducted to investigate the molecular mechanisms of IE, and
the identification of abnormally-expressed molecules may aid the
development of novel targets for the treatment of patients with IE.
It has been reported that the expression of the microRNAs
miR-199a-5p and miR-199b-5p was markedly decreased, whereas the
levels of HIF-1α were upregulated, in epileptic brain tissues
compared with those from control subjects (7). A deficiency of glutathione peroxidase
was previously identified in patients with IE (8). Mitochondrially-encoded cytochrome b,
ubiquinol-cytochrome c reductase binding protein,
ubiquinol-cytochrome c reductase core protein I and
ubiquinol-cytochrome c reductase hinge protein were identified to
be potential drug targets for IE through analysis of
protein-protein interactions and drug-target relationships
(9). However, the mechanisms
underlying the pathogenesis of IE remain unclear.
Rho family small GTPases serve important roles in
the regulation of the cytoskeleton, phagocytosis, membrane
trafficking, cell migration and subsequent morphological changes in
neurons (10–13). Rho family GTPases have been
reported to be associated with neuronal network formation (14). Rho7 (additionally termed Rnd2 and
RhoN), a Rho family small GTPase, is specifically expressed in
brain neurons (15). Rho7 has been
observed to act as a regulator of certain processes in neurons that
are associated with IE (16,17).
Considering the roles of Rho7 in neurons, it may be hypothesized
that Rho7 may be associated with IE. However, few studies have
investigated the alterations in the expression of Rho7 in patients
with IE.
In the present study, the expression level and
location of Rho7 protein was assessed in temporal lobe tissues
obtained from patients with IE and healthy controls, using
immunohistochemistry, double-label immunofluorescence staining and
western blotting.
Materials and methods
Brain sample collection
The present study was performed in accordance with
the Declaration of Helsinki (18).
Brain tissue samples were collected from the temporal lobes of 33
patients with IE who had received neurosurgery between January 2003
and December 2008 at the Newbridge Hospital of the Third Military
Medical University (Chongqing, China), 999 Brain Hospital of
Guangdong (Guangzhou, China) and The First Affiliated Hospital of
Chongqing University of Medical Science (Chongqing, China). All
patients exhibited clinical TLE, which was independently evaluated
by two neuropathologists. The resected temporal lobe tissues were
obtained from the suspected area of seizure onset. Seizure types
were classified according to the 1981 International Classification
of Epileptic Seizures of the International League against Epilepsy
(19). The inclusion criteria for
the patients were as follows: i) All patients exhibited the typical
electroencephalogram and clinical manifestation of epilepsy; ii)
AED therapy had failed in all patients with maximum tolerable doses
of at least three AEDs (including phenytoin, valproic acid,
carbamazepine, phenobarbital, topiramate, oxcarbazepine,
clonazepam, gabapentin and lamotrigine); iii) the patients
exhibited no apparent underlying cause of epilepsy; iv) physical
inspection of the nervous system indicated no focal physical signs,
and pathology findings in the resected tissues demonstrated no
direct focus responsible for the epilepsy; v) chemotherapy or
magnetic resonance imaging exhibited no abnormalities, except for
hippocampal sclerosis; vi) no patients had contraindications of
surgery according to the preoperative evaluation; and vii) written
consent was obtained from a family member.
For comparison, 10 healthy temporal lobe tissue
samples were used as controls, eight of which were histologically
normal temporal tissues from autopsy specimens with craniocerebral
trauma, and the other two specimens were from two persons who had
succumbed to an accident. All of the samples matched the conditions
listed below: i) The subjects suffered no epilepsy or any other
neurology disease; ii) temporal tissue structure was integral as
confirmed by pathological analysis; and iii) written informed
consent was obtained from a family member.
All the experiments in the present study were
approved by the ethics committee of the Newbridge Hospital of the
Third Military Medical University.
Immunohistochemistry
The temporal tissue was extracted from each
participant, and then fixed with 4% paraformaldehyde at 4°C
overnight. Next, the tissue was dehydrated with gradient ethanol,
vitrified with xylene and embedded in paraffin wax. The slices were
cut into slices of 3–5-µm thickness using a microtome. The sections
were prepared for the further studies.
The Rho7 expression in patients with IE and controls
was evaluated using an immunohistochemistry assay. All
paraffin-embedded sections were deparaffinized in xylene and
rehydrated with graded ethanol. Antigens were retrieved using 0.01
M citric acid (pH 6.0) for 15 min at 92–97°C. Following treatment
with 3% H2O2 for 15 min to quench endogenous
peroxidase activity, the sections were blocked in 5% normal rabbit
serum (cat. no. ZLI-9025; OriGene Technologies, Inc., Beijing,
China) at room temperature for 30 min to reduce non-specific
binding, followed by incubation with the primary antibody (rabbit
anti-human Rho7; cat. no. sc-28565; 1:50; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at 4°C overnight. Following washing three
times with PBS, the sections were incubated with biotinylated
secondary goat anti-rabbit antibody (1:500; cat. no. ZB-2010;
Zhongshan Golden Bridge Biotechnology Co., Ltd.) at room
temperature for 30 min and reacted with avidin-biotin peroxidase
complex solution (cat. no. PK-4001; OriGene Technologies, Inc.) at
30°C for 30 min. 3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used as the final chromogen, and
hematoxylin was utilized as the nuclear counterstain at room
temperature for 5 min. For negative controls, the primary or
secondary antibody was omitted and replaced with PBS or non-immune
immunoglobulin (Ig)G to evaluate the experimental procedure and
reagent quality. The Olympus PM20 automatic microscope (Olympus
Corporation, Tokyo, Japan) and the TCFY-2050 pathology system
(Yuancheng, Inc., Beijing, China) were utilized for image
acquisition. Images for each sample were automatically analyzed and
semi-quantitated using the Motic Med 6.0 CMIAS pathology image
analysis system (Motic China Group Co., Ltd., Xiamen, China). A
total of 10 visual fields were randomly selected for each section
under light microscopy (magnification, ×400). A total of 100 cells
in each field were selected and the number of positive cells was
calculated. The positive cell proportion was calculated as follows:
Positive cell proportion=(number of positive cells/100)x100. All
the experiments were performed three times.
Double-label immunofluorescence
Double-label immunofluorescence was performed to
detect the cellular colocalization of Rho7 and neuron-specific
enolase (NSE), an enolase isoform expressed in the central nervous
system, which has been widely used as a neuronal marker (20). Following dewaxing, rehydration and
antigen retrieval (as described above), tissue sections (3–5 µm)
were incubated in calf serum (OriGene Technologies, Inc.) at room
temperature for 1 h, and subsequently in normal goat serum (OriGene
Technologies, Inc.) at room temperature for 5 h. The sections were
incubated with rabbit anti-human Rho7 antibody (1:100; cat. no.
sc-28565; Santa Cruz Biotechnology, Inc.) or mouse monoclonal
anti-human NSE antibody (1:200; cat. no. ZM-0203; OriGene
Technologies, Inc.) at 4°C overnight, followed by incubation with
goat anti-rabbit tetramethylrhodamine isothiocyanate-conjugated
secondary antibody (1:100; cat. no. ZF-0311; OriGene Technologies,
Inc.) or goat anti-mouse fluorescein isothiocyanate-conjugated
secondary antibody (1:100; cat. no. ZF-0312; OriGene Technologies,
Inc.) in the dark at room temperature for 2 h. The sections were
subsequently cover-slipped, sealed and dried overnight.
Fluorescent-stained sections were examined using confocal laser
scanning microscopy (magnification, ×400; Leica Microsystems GmbH,
Wetzlar, Germany). Images were captured and processed using the
Olympus Micro Image analysis software system (version 4.0; Olympus
Corporation).
Western blotting
The samples were randomly selected from the temporal
lobe tissues of patients with IE (n=4) and controls (n=2) to cut
into small pieces. The samples were homogenized in buffer including
protease inhibitors (15 µg/ml aprotinin and 1 mM
phenylmethylsulfonyl fluoride), and centrifuged at 16,000 × g and
4°C for 5 min. The crude nuclear pellet was lysed in a lysis buffer
[20 mM Tris, (pH 7.4), 1% Triton X-100, 10% glycerol, 137 mM NaCl,
2 mM EDTA, 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, 2
mM pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml
leupeptin] for 30 min and centrifuged at 16,000 × g and 4°C for 15
min. The protein concentration was determined using a Coomassie
Blue G-250 kit (Sigma-Aldrich; Merck KGaA). The samples (40 µg)
were resolved using SDS-PAGE on a 10% gel and electrotransferred
onto a polyvinylidene fluoride membrane (DuPont, Wilmington, DE,
USA). Subsequently, the membranes were blocked with 3% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) for 2 h at room temperature.
The membranes were incubated with rabbit anti-human Rho7 (1:100;
cat. no. sc-28565; Santa Cruz Biotechnology, Inc.) or mouse
anti-human β-actin (1:1,000; cat. no. PR-0255; OriGene
Technologies, Inc.) antibodies at 37°C for 1 h. Following washing
with PBS, the membranes were incubated with goat anti-rabbit
horseradish peroxidase (HRP)-IgG (1:5,000; cat. no. RABHRP1;
Sigma-Aldrich; Merck KGaA) or goat anti-mouse HPR-IgG (1:5,000;
cat. no. RABHRP2; Sigma-Aldrich; Merck KGaA) secondary antibodies
at 37°C for 1 h. The immunoreactive bands were visualized using an
enhanced chemiluminescence (ECL) system (DuPont). Relative
quantification of the ECL signal on X-ray film was analyzed using
Labworks™ Analysis Software (version 4.5; UVP, Inc.,
Upland, CA, USA). Following global background subtraction, gray
densities of Rho7 bands were normalized to the β-actin band for
each lane. All the experiments were performed in triplicate.
Data analysis
Quantitative data are expressed as the mean ±
standard deviation, and qualitative data are expressed as a
percentage of subjects. All statistical analyses were performed
using SPSS (version 19.0; IBM Corp., Armonk, NY, USA). Student's
t-test was used to compare the quantitative data and χ2
test was used to compare the qualitative data between the IE and
control groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Age and gender composition of the IE
group and control group
The treatment history of the included 33 patients is
summarized in Table I. The age and
sex distributions were compared between the IE and control groups,
and the results are presented in Table II. The mean age was 24.4±8.68 and
26.4±6.75 years in the IE and control groups, respectively. There
were more male patients (51.6%) in the IE group, while there were
more women (60%) in the control group. However, there were no
significant differences in age (t-test; P=0.606) or sex
(χ2 test; P=0.523) between the patients with IE and the
control subjects.
 | Table I.Treatment history of the 33 patients
in the epilepsy group. |
Table I.
Treatment history of the 33 patients
in the epilepsy group.
| N | A | G | D | Antiepiletic
drugs | Seizure type | Imaging | Pathology |
|---|
| 1 | 32 | M | 12 | CBZ, PHT, PB,
TCM | SPS, CPS | fcd | g, nl |
| 2 | 38 | M | 16 | CBZ, PHT, PB,
TCM | SPS, CPS | rhs | ac |
| 3 | 25 | M | 8 | CBZ, VPA, PHT,
TPM | SPS, CPS | n | g, nd |
| 4 | 12 | F | 8 | VPA, CBZ, TCM | CPS | fcd | g, nd, nl |
| 5 | 12 | F | 10 | CBZ, PB, VPA | SPS, CPS | n | nl, g |
| 6 | 17 | M | 10 | CBZ, PHT, VPA,
TPM | SPS | n | g |
| 7 | 25 | F | 4 | CBZ, PB, VPA | SPS, CPS | n | g |
| 8 | 23 | F | 15 | CBZ, PHT, VPA,
PB | GTCS | n | g, nl |
| 9 | 28 | M | 5 | CBZ, VPA, PHT,
TPM | GTCS | n | nl |
| 10 | 32 | F | 19 | VPA, CBZ, PB,
TPM | CPS | n | g, nd |
| 11 | 30 | M | 8 | VPA, CBZ, TPM | SGS | fcd | ac |
| 12 | 44 | F | 20 | PHT, VPA, LMT | CPS | rhs | g |
| 13 | 17 | M | 10 | CBZ, PHT, PB,
VPA | SGS | n | g, nl |
| 14 | 56 | F | 10 | PHT, CBZ, GBA | SGS | fcd | nl, nd |
| 15 | 22 | M | 10 | CBZ, VPA, LMT | SGS | rhs | g, na |
| 16 | 28 | M | 6 | TPM, CBZ, VPA | CPS | rhs | g, nl, nd |
| 17 | 27 | F | 20 | PHT, PB, VPA,
LMT | CPS | lhs | g |
| 18 | 33 | F | 10 | VPA, TPM, CBZ | SGS | n | nl, g |
| 19 | 14 | F | 8 | VPA, PB, LMT | SGS | n | ac |
| 20 | 26 | F | 12 | CBZ, PHT, VPA | SGS | n | nl, g |
| 21 | 27 | F | 14 | PHT, VPA, LMT | SGS | n | g |
| 22 | 47 | M | 29 | PB, PHT, VPA, TPM,
LMT6 | SGS | n | ac |
| 23 | 28 | M | 3 | VPA, PB, CBZ | SGS | n | nl, nd, g |
| 24 | 29 | F | 27 | VPA, CBZ, LMT | SGS | n | g, nl |
| 25 | 22 | F | 10 | CBZ, PHT, Pb | SPS, CPS | n | g, nl |
| 26 | 24 | M | 5 | CBZ, PHT, TPM,
TCM | SPS, GTCS | n | g |
| 27 | 31 | M | 10 | CBZ, PB, TPM,
TCM | SPS, CPS | n | g |
| 28 | 20 | M | 12 | CBZ, PHT, TPM,
TCM | SPS, CPS | n | nl, g |
| 29 | 15 | F | 6 | CBZ, PB, TPM,
TCM | SPS, CPS | n | ac |
| 30 | 19 | M | 10 | CBZ, PB, TPM | SPS, CPS | n | ac |
| 31 | 37 | M | 11 | CBZ, VPA, PB | SPS, CPS | n | nl, nd |
| 32 | 28 | M | 10 | CBZ, PB, TPM | SPS, CPS | n | g, nd |
| 33 | 46 | F | 11 | CBZ, PHT, TPM,
TCM | SPS, CPS | n | nl |
 | Table II.Age and sex composition of the IE and
control groups. |
Table II.
Age and sex composition of the IE and
control groups.
|
| IE (%), n=33 | Control (%),
n=10 | P-value |
|---|
| Age | 24.4±8.68 | 26.4±6.75 | 0.606 |
| Sex |
|
|
|
|
Female | 16 (48.4) | 6 (60) | 0.523 |
|
Male | 17 (51.6) | 4 (40) |
|
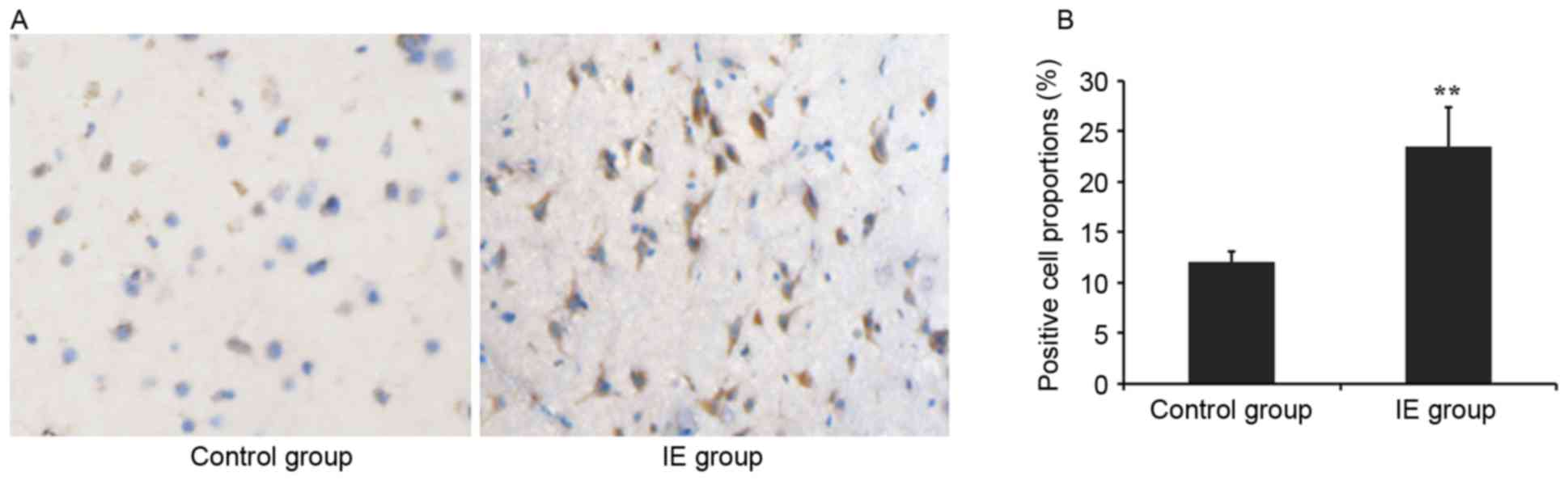
Rho7 is overexpressed in temporal
tissue of IE patients, as demonstrated by immunohistochemistry
Temporal tissue sections from IE and control
subjects were immunohistochemically stained. The results of the
present study demonstrated that marked immunoreactivity for Rho7
was detected in the IE group, while faint and scattered
immunoreactive staining was observed in the control group (Fig. 1A). The positive cell count of Rho7
in the IE patients was significantly increased compared with the
control subjects (23.47±3.9% vs. 12.09±1.05%; P<0.01; Fig. 1B).
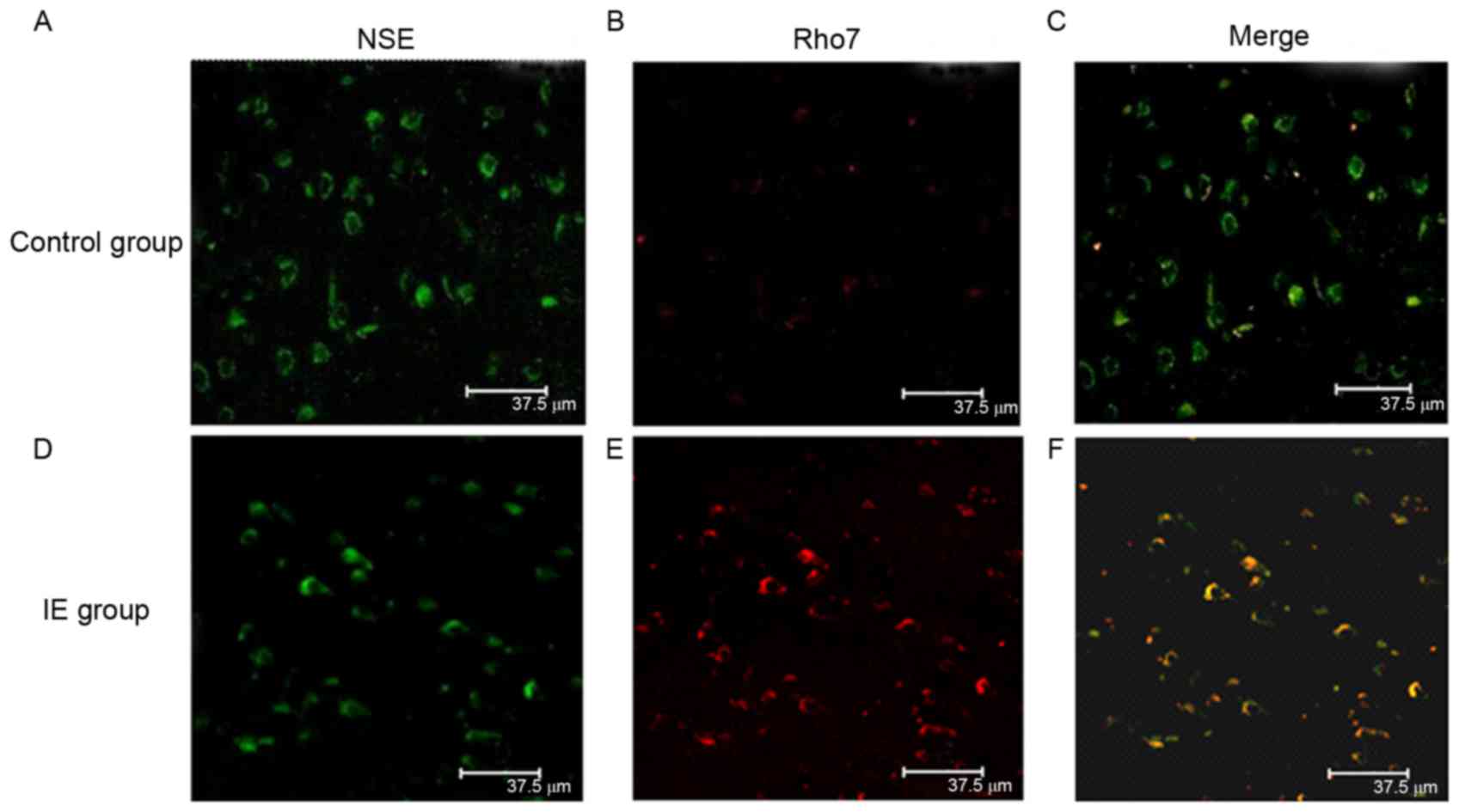
Rho7 is colocalized with NSE in
neurons of the temporal lobe
Double-label immunofluorescence staining was
performed to examine the cellular localization of Rho7 in the
temporal lobe tissues of patients with IE and control subjects. The
double-label immunofluorescence staining demonstrated that
Rho7-positive cells (red) co-localized with NSE (green) in neurons
in the temporal lobe tissues (Fig.
2). According to the results, Rho7 was primarily expressed in
the neuronal cytoplasm and colocalized with NSE in the temporal
lobe tissues. Compared with control group, the Rho7-positive cells
were notably increased in the IE group, exhibiting stronger green
fluorescence.
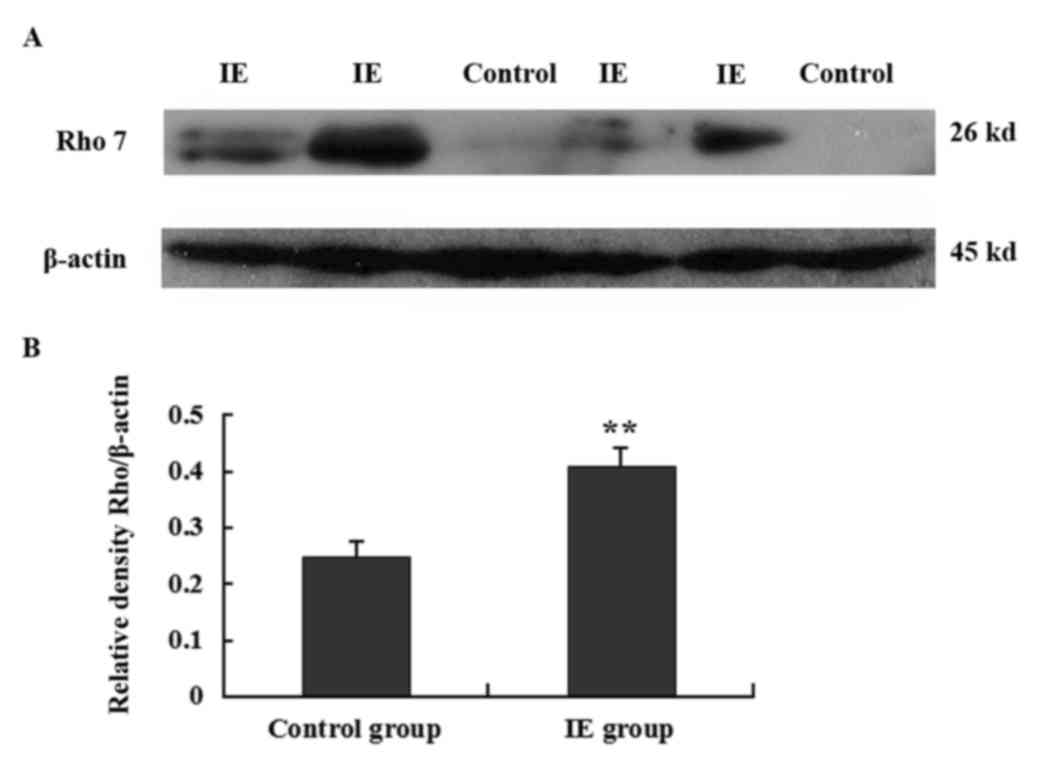
Rho7 is overexpressed in the temporal
tissue of patients with IE, as demonstrated by western
blotting
In order to further confirm the altered expression
of Rho7 in IE temporal tissues, the expression of Rho7 in the
temporal lobe tissues obtained from patients with IE and controls
was examined using western blotting. Rho7 (26 kDa) and β-actin (42
kDa internal control) proteins were detected in the present study.
The results indicated that the expression of Rho7 protein in the IE
group was increased compared with the control group (representative
results are presented in Fig. 3A).
In addition, the differences in relative protein expression were
significant (0.41±0.031 vs. 0.25±0.025; P<0.01; Fig. 3B).
Discussion
Patients with medically-confirmed IE are at an
increased risk of depression and suicide, particularly patients
with more severe and frequent seizures (21). It has been reported that the
prevalence of depression and suicidality in patients with medically
intractable seizures may be 50 and 19%, respectively (22,23).
Therefore, it is necessary to investigate the mechanisms and
abnormally expressed molecules in IE, which may improve the
understanding of IE and provide novel research targets. Rho
proteins are involved in neuronal and non-neuronal cell migration,
axonal outgrowth and guidance, and also dendrite formation
(24). Rho7 is specifically
expressed in brain neurons, and is able to induce synapse growth
and sprouting (25–29). In the present study, it was
demonstrated that Rho7 protein was expressed in the temporal lobe
tissue of patients with IE and control subjects. However, the
expression of Rho7 in the IE group was significantly increased
compared with the control group.
According to the results of the immunohistochemical
analysis and immunofluorescence staining, Rho7 was expressed in the
neuronal membrane and cytoplasm, and co-localized with the neuronal
marker NSE. Neurons migrate extensively to reach their permanent
location in the nervous system (30). The correct positioning of neurons
during development is the basis for proper brain function (31). It has also been reported that
abnormal neuronal migration may lead to abnormal cortical function,
including epilepsy (32,33). Rho7 is able to regulate the
migration and morphological alterations associated with the
development of pyramidal neurons in vivo (16). In addition, excessive hypertrophic
pyramidal neurons have been reported in patients with IE who
underwent brain resections (34).
Heng et al (17) identified
that Rho7 acted as an essential regulator of neuronal migration,
disorders of which are reported to be associated with IE (35). Therefore, the overexpression of
Rho7 may be associated with IE. Ras homolog family member A (RhoA)
activity is associated with neuronal migration, and certain
molecules are able to regulate neuronal migration via RhoA
inhibition (36). Rho7 inhibits
RhoA signaling in cortical neurons and regulates migration by
inhibiting RhoA activity in a variety of cellular compartments
(37). Therefore, it was
hypothesized that Rho7 may regulate neuronal migration in IE by
acting as a RhoA inhibitor. However, the specific mechanism of Rho7
in IE was not investigated in the present study, and future studies
are required to elucidate this.
In addition, the axon of the neuron is associated
with epilepsy. The axon initial segment (AIS) serves a role in
neuronal excitability, as the site of action potential initiation
is located here (38). It has been
hypothesized that AIS plasticity may alter the excitability of a
neuron in response to the environment, enhancing or attenuating
neuronal sensitivity (39,40). A previous study demonstrated that
Rho7 is involved in the molding of neurons by translocating from
the cytoplasm to the cell membrane (41). At the onset of epilepsy, Rho7 may
activate RhoA and promote the detachment of the axon from a target
cell (42), and subsequently
induce neurite branching via rapostlin, an effector of Rho7
(43,44). Pragmin, another effector of Rho7,
is activated to promote the growth of neurite branches, which will
form abnormal synaptic connections (45). These abnormal connections account
for the abnormal neural network that is the pathological basis of
epilepsy (46). In the present
study, it was observed that the expression of Rho7 in the IE group
was significantly increased compared with the control group. Based
on pathological investigations, it was hypothesized that Rho7 may
be involved in the formation of abnormal neuronal networks and be
associated with the pathogenesis of IE. Notably, Rho7 was also
observed to be expressed in astrocytes and deposited in the synapse
(data not shown). As Rho GTPases are associated with the regulation
of the cytoskeleton in a variety of cells, including astrocytes
(47,48), it was hypothesized that Rho7 may be
associated with the growth and development of astrocytes. However,
the role of Rho7 in the astrocytes of patients with IE requires
further investigation.
In conclusion, the results of the present study
demonstrated that Rho7 expression was increased in the neurons of
patients with IE compared with control samples, which indicated
that Rho7 may be associated with the progression of IE or act as a
potential target for IE treatment. However, the localization and
expression levels of Rho7 were only detected in temporal lobe
tissues. Further studies are required to investigate the
association between Rho7 and IE, and to investigate the underlying
mechanism through which this protein is involved in IE.
References
|
1
|
Engel J Jr, McDermott MP, Wiebe S,
Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G,
Fried I, et al: Early surgical therapy for drug-resistant temporal
lobe epilepsy: A randomized trial. JAMA. 307:922–930. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berg AT: Understanding the delay before
epilepsy surgery: Who develops intractable focal epilepsy and when?
CNS Spectr. 9:136–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Begley CE, Famulari M, Annegers JF,
Lairson DR, Reynolds TF, Coan S, Dubinsky S, Newmark ME, Leibson C,
So EL and Rocca WA: The cost of epilepsy in the United States: An
estimate from population-based clinical and survey data. Epilepsia.
41:342–351. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hao XT, Wong IS and Kwan P: Interrater
reliability of the international consensus definition of
drug-resistant epilepsy: A pilot study. Epilepsy Behav. 22:388–390.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wassenaar M, Leijten FS, Egberts TC, Moons
KG and Uijl SG: Prognostic factors for medically intractable
epilepsy: A systematic review. Epilepsy Res. 106:301–310. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engel J Jr; International League Against
Epilepsy (ILAE), : A proposed diagnostic scheme for people with
epileptic seizures and with epilepsy: Report of the ILAE task force
on classification and terminology. Epilepsia. 42:796–803. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang G, Zhou R, He X, Shi Z, Huang M, Yu
J and Wang X: Expression levels of microRNA-199 and
hypoxia-inducible factor-1 alpha in brain tissue of patients with
intractable epilepsy. Int J Neurosci. 126:326–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nazıroğlu M and Yürekli VA: Effects of
antiepileptic drugs on antioxidant and oxidant molecular pathways:
Focus on trace elements. Cell Mol Neurobiol. 33:589–599. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chu H, Zhou X, Liu G, Lv M, Wang Y, Liu L,
Li X, Sun P, Zhu Y, Sun C, et al: Network-based detection of
disease modules and potential drug targets in intractable epilepsy.
Journal. 1–140. 2014.
|
|
10
|
Kawauchi T: Regulation of cell adhesion
and migration in cortical neurons: Not only Rho but also Rab family
small GTPases. Neuron. 2:36–40. 2011.
|
|
11
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moon SY and Zheng Y: Rho GTPase-activating
proteins in cell regulation. Trends Cell Biol. 13:13–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
14
|
Negishi M and Katoh H: Rho family GTPases
as key regulators for neuronal network formation. J Biochem.
132:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Decourt B, Bouleau Y, Dulon D and Hafidi
A: Expression analysis of neuroleukin, calmodulin, cortactin, and
Rho7/Rnd2 in the intact and injured mouse brain. Brain Res Dev
Brain Res. 159:36–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura K, Yamashita Y, Tamamaki N, Katoh
H, Kaneko T and Negishi M: In vivo function of Rnd2 in the
development of neocortical pyramidal neurons. Neurosci Res.
54:149–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heng JI, Nguyen L, Castro DS, Zimmer C,
Wildner H, Armant O, Skowronska-Krawczyk D, Bedogni F, Matter JM,
Hevner R and Guillemot F: Neurogenin 2 controls cortical neuron
migration through regulation of Rnd2. Nature. 455:114–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
World Medical Association, . World Medical
Association Declaration of Helsinki. Ethical principles for medical
research involving human subjects. Bull World Health Organ.
79:373–374. 2001.PubMed/NCBI
|
|
19
|
Dreifuss FE, Bancand J, Henriksen O,
Rubio-Donnadieu F, Seino M and Penry JK: Commission on
classification and terminology of the international league against
epilepsy, proposal for revised clinical and electroencephalographic
classification of epileptic seizures. Epilepsia. 1–501.
1981.PubMed/NCBI
|
|
20
|
Fang M, Liu GW, Pan YM, Shen L, Li CS, Xi
ZQ, Xiao F, Wang L, Chen D and Wang XF: Abnormal expression and
spatiotemporal change of Slit2 in neurons and astrocytes in
temporal lobe epileptic foci: A study of epileptic patients and
experimental animals. Brain Res. 1324:14–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimiskidis VK, Triantafyllou NI, Kararizou
E, Gatzonis SS, Fountoulakis KN, Siatouni A, Loucaidis P,
Pseftogianni D, Vlaikidis N and Kaprinis GS: Depression and anxiety
in epilepsy: The association with demographic and seizure-related
variables. Ann Gen Psychiatry. 6:282007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanner AM, Soto A and Gross-Kanner H:
Prevalence and clinical characteristics of postictal psychiatric
symptoms in partial epilepsy. Neurology. 62:708–713. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boylan LS, Flint LA, Labovitz DL, Jackson
SC, Starner K and Devinsky O: Depression but not seizure frequency
predicts quality of life in treatment-resistant epilepsy.
Neurology. 62:258–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Govek EE, Hatten ME and Van Aelst L: The
role of Rho GTPase proteins in CNS neuronal migration. Dev
Neurobiol. 71:528–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McLoughlin HS, Fineberg SK, Ghosh LL,
Tecedor L and Davidson BL: Dicer is required for proliferation,
viability, migration and differentiation in corticoneurogenesis.
Neuroscience. 223:285–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujita H, Katoh H, Ishikawa Y, Mori K and
Negishi M: Rapostlin is a novel effector of Rnd2 GTPase inducing
neurite branching. J Biol Chem. 277:45428–45434. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kakimoto T, Katoh H and Negishi M:
Identification of splicing variants of Rapostlin, a novel RND2
effector that interacts with neural Wiskott-Aldrich syndrome
protein and induces neurite branching. J Biol Chem.
279:14104–14110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuchiya D, Kitamura Y, Takata K, Sugisaki
T, Taniguchi T, Uemura K, Miki H, Takenawa T and Shimohama S:
Developmental expression of neural Wiskott-Aldrich syndrome protein
(N-WASP) and WASP family verprolin-homologous protein
(WAVE)-related proteins in postnatal rat cerebral cortex and
hippocampus. Neurosci Res. 56:459–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J and Anton E: Rnding up RhoA activity
to link neurogenesis with steps in neuronal migration. Dev Cell.
20:409–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marin O and Rubenstein JL: Cell migration
in the forebrain. Annu Rev Neurosci. 26:441–483. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ayala R, Shu T and Tsai LH: Trekking
across the brain: The journey of neuronal migration. Cell.
128:29–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guerrini R and Parrini E: Neuronal
migration disorders. Neurobiol Dis. 38:154–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chai X, Münzner G, Zhao S, Tinnes S,
Kowalski J, Häussler U, Young C, Haas CA and Frotscher M:
Epilepsy-induced motility of differentiated neurons. Cerebral
Cortex. 24:2130–2140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang DD, Blümcke I, Coras R, Zhou WJ, Lu
DH, Gui QP, Hu JX, Zuo HC, Chen SY and Piao YS: Sturge-weber
syndrome is associated with cortical dysplasia ILAE Type IIIc and
excessive hypertrophic pyramidal neurons in brain resections for
intractable epilepsy. Brain Pathol. 25:248–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roper SN, Gilmore RL and Houser CR:
Experimentally induced disorders of neuronal migration produce an
increased propensity for electrographic seizures in rats. Epilepsy
Res. 21:205–219. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang J, Ip JP, Ye T, Ng YP, Yung WH, Wu Z,
Fang W, Fu AK and Ip NY: Cdk5-dependent Mst3 phosphorylation and
activity regulate neuronal migration through RhoA inhibition. J
Neurosci. 34:7425–7436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pacary E, Heng J, Azzarelli R, Riou P,
Castro D, Lebel-Potter M, Parras C, Bell DM, Ridley AJ, Parsons M
and Guillemot F: Proneural transcription factors regulate different
steps of cortical neuron migration through Rnd-mediated inhibition
of RhoA signaling. Neuron. 69:1069–1084. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wimmer VC, Reid CA, So EY, Berkovic SF and
Petrou S: Axon initial segment dysfunction in epilepsy. J Physiol.
588:1829–1840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshimura T and Rasband MN: Axon initial
segments: Diverse and dynamic neuronal compartments. Curr Opin
Neurobiol. 27:96–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuba H, Oichi Y and Ohmori H: Presynaptic
activity regulates Na(+) channel distribution at the axon initial
segment. Nature. 465:1075–1078. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Katoh H, Harada A, Mori K and Negishi M:
Socius is a novel Rnd GTPase-interacting protein involved in
disassembly of actin stress fibers. Mol Cell Biol. 22:2952–2964.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wennerberg K, Forget MA, Ellerbroek SM,
Arthur WT, Burridge K, Settleman J, Der CJ and Hansen SH: Rnd
proteins function as RhoA antagonists by activating p190 RhoGAP.
Current Biol. 13:1106–1115. 2003. View Article : Google Scholar
|
|
43
|
Goh LL and Manser E: The GTPase-deficient
Rnd proteins are stabilized by their effectors. J Biol Chem.
287:31311–31320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wakita Y, Kakimoto T, Katoh H and Negishi
M: The F-BAR protein Rapostlin regulates dendritic spine formation
in hippocampal neurons. J Biol Chem. 286:32672–32683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tanaka H, Katoh H and Negishi M: Pragmin,
a novel effector of Rnd2 GTPase, stimulates RhoA activity. J Biol
Chem. 281:10355–10364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vaessen MJ, Braakman HM, Heerink JS,
Jansen JF, Debeij-van Hall MH, Hofman PA, Aldenkamp AP and Backes
WH: Abnormal modular organization of functional networks in
cognitively impaired children with frontal lobe epilepsy. Cerebral
Cortex. 23:1997–2006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guasch RM, Blanco AM, Pérez-Aragó A,
Miñambres R, Talens-Visconti R, Peris B and Guerri C: RhoE
participates in the stimulation of the inflammatory response
induced by ethanol in astrocytes. Exp Cell Res. 313:3779–3788.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Höltje M, Hoffmann A, Hofmann F, Mucke C,
Grosse G, Van Rooijen N, Kettenmann H, Just I and Ahnert-Hilger G:
Role of Rho GTPase in astrocyte morphology and migratory response
during in vitro wound healing. J Neurochem. 95:1237–1248. 2005.
View Article : Google Scholar : PubMed/NCBI
|