Introduction
Hepatitis C virus (HCV) is one of the common factors
associated with development of chronic liver inflammation and liver
disease, a major problem in global health. HCV causes not only
hepatitis, but also a variety of diseases including liver fibrosis,
cirrhosis and hepatocellular carcinoma (HCC) (1). HCV is a single-stranded RNA virus
that encodes several polyproteins; three structural proteins (core,
E1, and E2) and seven non-structural proteins (p7, NS2, NS3, NS4A,
NS4B, NS5A and NS5B) (2). It has
been reported that these structural proteins constitute the HCV
viral particle, and the non-structural proteins serve a variety of
roles in the HCV life cycle (2).
However, the exact function and mechanism of HCV polyproteins are
not fully understood.
One of the non-structural proteins, p7, has been
reported to be a small hydrophobic protein that not only interacts
with a variety of biological membranes (3), but also has ion channel activity as a
viroporin (4). p7 is localized to
a variety of cellular organelles, including the endoplasmic
reticulum and plasma membrane (3,5). A
previous study demonstrated that p7 is also localized in
mitochondria (4). This variety of
subcellular localizations suggests that p7 has diverse
physiological roles in hepatocytes. However, to date, the function
of p7 has been mainly discussed with regard to the viral life
cycle; for example, the assembly or release of viral particles
(6,7). In particular, the correlation between
p7 and mitochondrial function has not yet been fully
elucidated.
Mitochondria are the central organelles of cells.
Since mitochondria supply the majority of the cellular energy by
oxidative phosphorylation, they serve a key role in energy
metabolism in cells. For this reason, mitochondria are often called
the ‘powerhouses’ of cells (8).
Therefore, mitochondrial function is one of the most important
factors for cellular function, and the mitochondrial membrane
potential (∆Ψm) is a major determinant of the fate of mitochondria
and cells (9). Several studies
have suggested that viral infection affects mitochondrial
functions, and in particular is correlated with mitochondrial
depolarization (10–14). It has been reported that certain
viroporins, including human T-cell leukemia virus type 1 (HTLV-1)
p13 and picornavirus 2B, can be localized on mitochondria and cause
mitochondrial depolarization (15,16).
However, the exact molecular mechanisms of viral infection-induced
mitochondrial dysfunction and changes in cell metabolism remain to
be elucidated.
A number of studies into HCV infection have
previously reported that HCV infection is associated with
mitochondrial dysfunction in hepatocytes, and it has been
documented that the HCV core protein alters mitochondrial functions
in Huh7 and Huh7.5 cells (10,13,17).
However, to the best of the authors' knowledge, there is no direct
evidence of the association between p7 and liver mitochondria.
Therefore, the purpose of the present study was to investigate the
exact role of p7 in mitochondrial function. To this end, isolated
mouse liver mitochondria and synthesized p7 protein were used in
order to investigate the effects of p7.
Materials and methods
Chemicals
Dimethyl sulfoxide, adenosine 5′-diphosphate (ADP),
D-mannitol, sucrose, rotenone, ethylene glycol-bis
(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA),
4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid, magnesium
chloride (MgCl2), n-(2-hydroxyethyl)
piperazine-n'-2-ethanesulfonic acid) (HEPES), trifluoroethanol
(TFE), 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein,
acetoxymethyl ester (BCECF-AM), carbonyl cyanide
3-chlorophenylhydrazone (CCCP) and bovine serum albumin (BSA) were
all purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Anhydrous monobasic potassium phosphate,
(KH2PO4), Triton X-100, 5-,
5′-,6-,6′-tetrachloro-1,1′, 3,3′ tetraethyl benzimidazolyl
carbocyanine iodide/chloride (JC-1; M34152), MitoTracker Green FM
(M7514), and an ATP determination kit (A22066) were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The HCV p7 amino
acid sequence (genotype 1a, H77 strain) is ALENLVI
LNA10ASLAGTHGLV20SFLVFFCFAW30YLKGRWVPGA40VYAFYGMWPL50LLLLLALPQR60AYA.
5-carboxy-tetramethylrhodamine (TAMRA)-p7 (HCV genotype 1a) was
chemically synthesized by GL Biochem (Shanghai, China) and
dissolved in TFE.
Animals
The experimental procedures were approved by the
Institutional Animal Care and Use Committee of the Korea University
College of Medicine (KOREA-2016-0174). Adult male C57 BL/6 N mice
(6 weeks old; weight, 16–21 g) were purchased from Orient Bio Inc.
(Gyenggi-do, South Korea). Mice were housed in regulated conditions
(21±2°C; 50±5% humidity; 12 h/12 h light/dark cycle with light on
from 8 AM) with free access to food and water.
Isolation of mitochondria
All isolation steps were performed on ice or using
ice-cold isolation buffers (IB: 225 mM mannitol, 75 mM sucrose and
30 mM Tris-HCl, pH 7.4; IB-1: 0.5% BSA and 0.5 mM EGTA in IB; IB-2:
0.5% BSA in IB) and the mitochondrial sample was prepared as
described in (18). Briefly,
following the decapitation of the mice, the livers (0.8 g ± 5%)
were washed with IB buffer and cut into small pieces. Liver tissue
was homogenized with a glass-tissue grinder (Wheaton Industries
Inc., Millville, NJ, USA) in IB-1 buffer, and crude mitochondrial
fractions were isolated from suspensions by density-gradient
centrifugation (10,000 × g at 4°C) for 10 min. Mitochondrial
pellets were resuspended in mitochondrial resuspension buffer [MRB:
250 mM mannitol, 5 mM HEPES (pH 7.4) and 0.5 mM EGTA]. All the
following experiments were performed within 9 h from the time of
mitochondria isolation.
Flow cytometric analysis
Flow cytometric assays to measure ∆Ψm and
mitochondrial matrix acidification were performed using the
FACSCalibur (BD Biosciences, San Jose, CA, USA) and data were
analyzed with FlowJo software version 10.0.7 (Tree Star, Inc.,
Ashland, OR, USA). Mitochondrial samples were gated according to
side scatter and forward scatter to optimize mitochondrial
selection. For each sample, ~300 events/sec were counted, up to
20,000 counts. Isolated mitochondrial samples were incubated in a
buffer composed of 250 mM mannitol, 5 mM HEPES (pH 7.4), 1 mM
KH2PO4, 10 µM EGTA and 2 µM rotenone at room
temperature.
Confocal microscopy of isolated mouse
liver mitochondria
Isolated mitochondria were incubated with 0.1 µM
TAMRA-p7 for 5 min to target mitochondria and stained with 0.1 µM
MitoTracker Green FM for 5 min at room temperature. The samples
were loaded onto a cover glass with a confocal dish, and confocal
microscopy was performed in a dedicated room at a constant
temperature (24±2°C) and humidity (~40%). The data were acquired
using a Zeiss LSM 700 microscope (Zeiss GmbH, Jena, Germany).
Spectrophotometric assay of ∆Ψm
Mitochondrial samples were treated with p7 protein
(1 µg/ml) for 5 min and then incubated with JC-1 (1 µM) for 10 min
and transferred to black 96-well plates (Optiplate-96F;
PerkinElmer, Inc., Waltham, MA, USA) for the measurement of
fluorescence at 488 nm excitation. The fluorescence was detected at
emission wavelengths of 525 nm (monomers) and 594 nm (aggregates).
The mitochondrial membrane potentials were measured using a
microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
ATP assay
For the ATP assay, 10 µM ADP and 5 mM succinate were
added to the mitochondrial samples (8 µg/ml) for 10 min at room
temperature. The samples were subsequently incubated with ATP assay
mix (Thermo Fisher Scientific, Inc.) for 15 min, and ATP was
measured using a GloMax 20/20 luminometer (Promega Corporation,
Madison, WI, USA). ATP amounts were calculated from an ATP standard
curve.
Results
The p7 protein targets isolated mouse
liver mitochondria
Previous studies have demonstrated that p7 localizes
to mitochondria (5); however, the
exact role of p7 in mitochondria is difficult to clarify due to the
mixed activity of various HCV-encoded proteins. Therefore, an
experimental system was designed using isolated mouse liver
mitochondria and synthesized p7 protein to clarify its function in
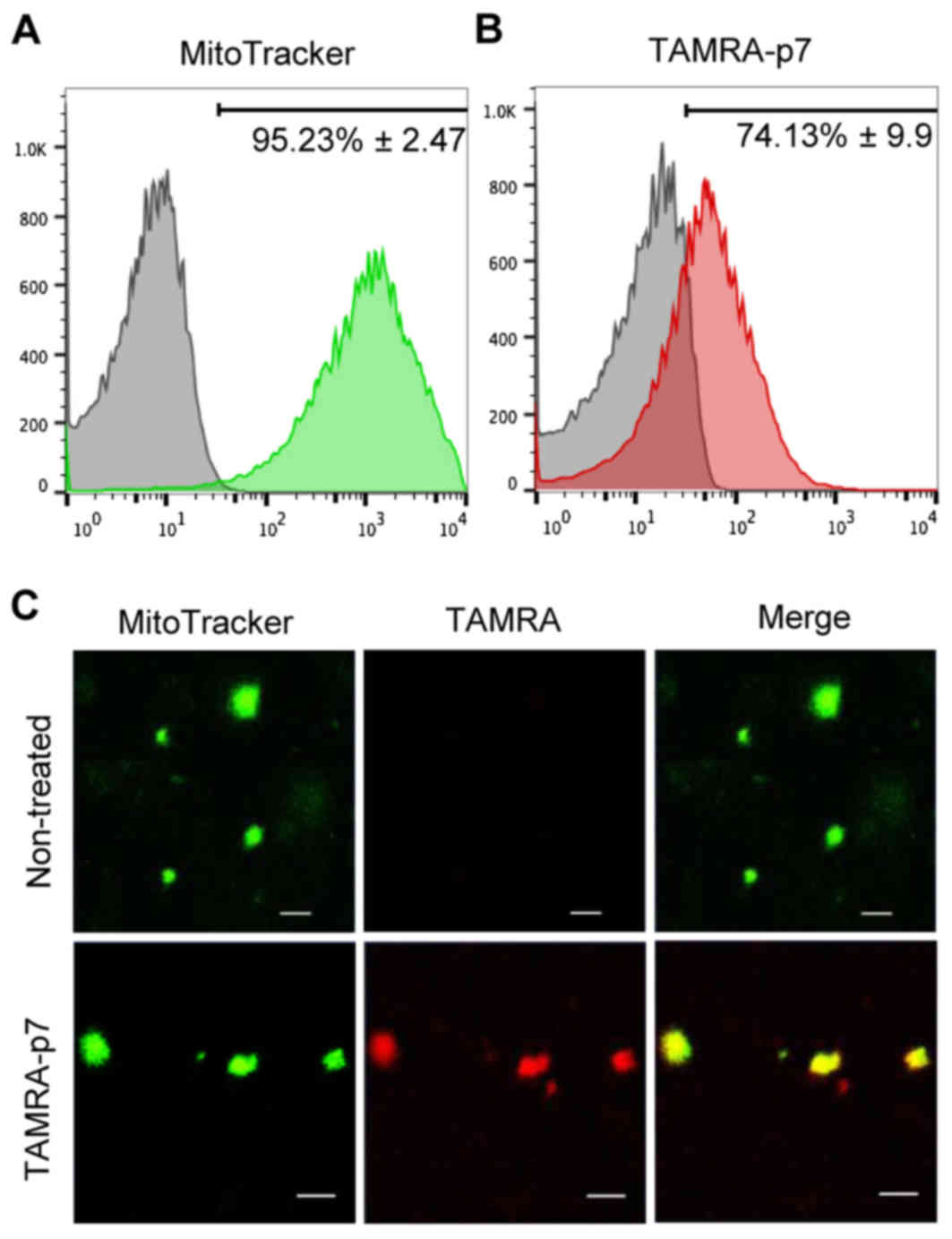
mitochondria. First, a flow cytometric assay was performed with a
mitochondria-sensitive probe (MitoTracker Green FM) to check the
quality of isolated mitochondria. As demonstrated in Fig. 1A, the proportion of mitochondria in
the prepared sample was >90%. Next, the mitochondria were
treated with the synthesized p7 protein. To visualize the p7
protein, p7 labeled with TAMRA was used and the mitochondrial
targeting of p7 examined by flow cytometry. As expected, p7 was
detected in mitochondria and the targeting efficiency was ~75%
(Fig. 1B). p7 targeting to
mitochondria was also confirmed using confocal laser microscopy
(Fig. 1C).
p7 induces mitochondrial
depolarization
The ∆Ψm is an important factor determining the fate
of the mitochondria, and therefore of the cells. It has previously
been demonstrated that HCV infection of hepatoma cells reduces ∆Ψm
(10,13,17).
However, the role of p7 in mitochondrial dysfunction in cells
infected by HCV has not been reported. To address this, whether p7
affected the ∆Ψm in isolated mitochondria from normal hepatocytes
was tested. To investigate the direct effect of p7 in mitochondria,
the mitochondria were treated with p7 protein, and the ΔΨm change
was examined using a mitochondria-sensitive fluorescent probe
(JC-1), which is an indicator of mitochondrial membrane potential.
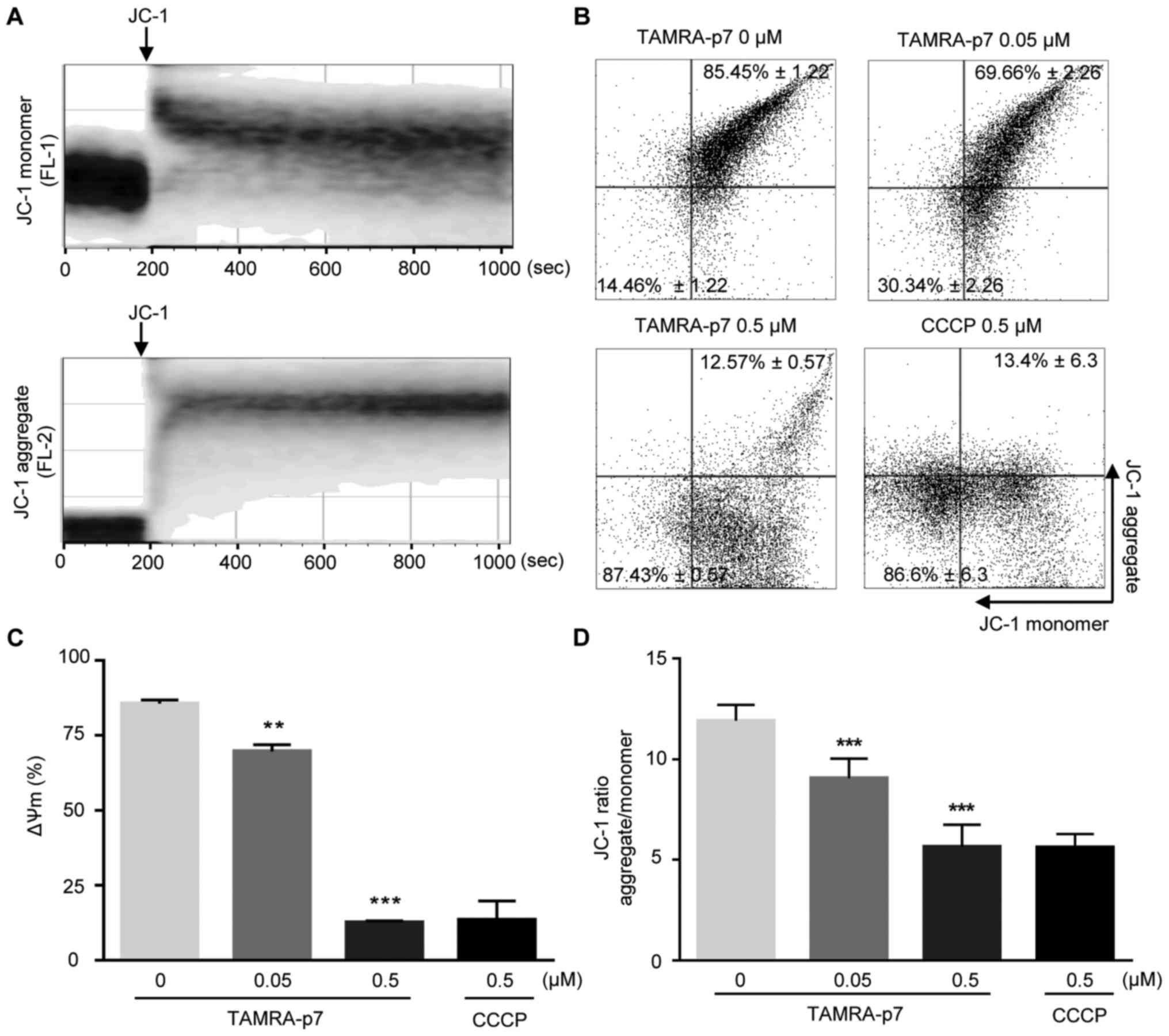
First, the time for JC-1 staining to measure the mitochondrial
membrane potential was optimized. As demonstrated in Fig. 2A, the intensity ratio of red/green
fluorescence reached a maximum level within 10 min. p7 treatment
reduced the ΔΨm in a concentration-dependent manner (Fig. 2B). Fig. 2C shows the quantified data of
Fig. 2B by representing the ratio
of the signal of high ΔΨm to the signal of low ΔΨm. To confirm this
result, ΔΨm was also examined using a spectrophotometric assay. As
demonstrated in Fig. 2D, p7
noticeably induced mitochondrial depolarization. CCCP was used as a
positive control (Fig. 2B-D).
p7 induces mitochondrial matrix
acidification
The proton motive force between the mitochondrial
matrix and intermembrane space is determined predominantly by the
ΔΨm in mitochondria, and to a lesser extent, by the hydrogen ion
(H+) concentration gradient (9). These two components contribute to the
electrochemical proton gradient and are important for mitochondrial
function. Therefore, changes in mitochondrial matrix pH were
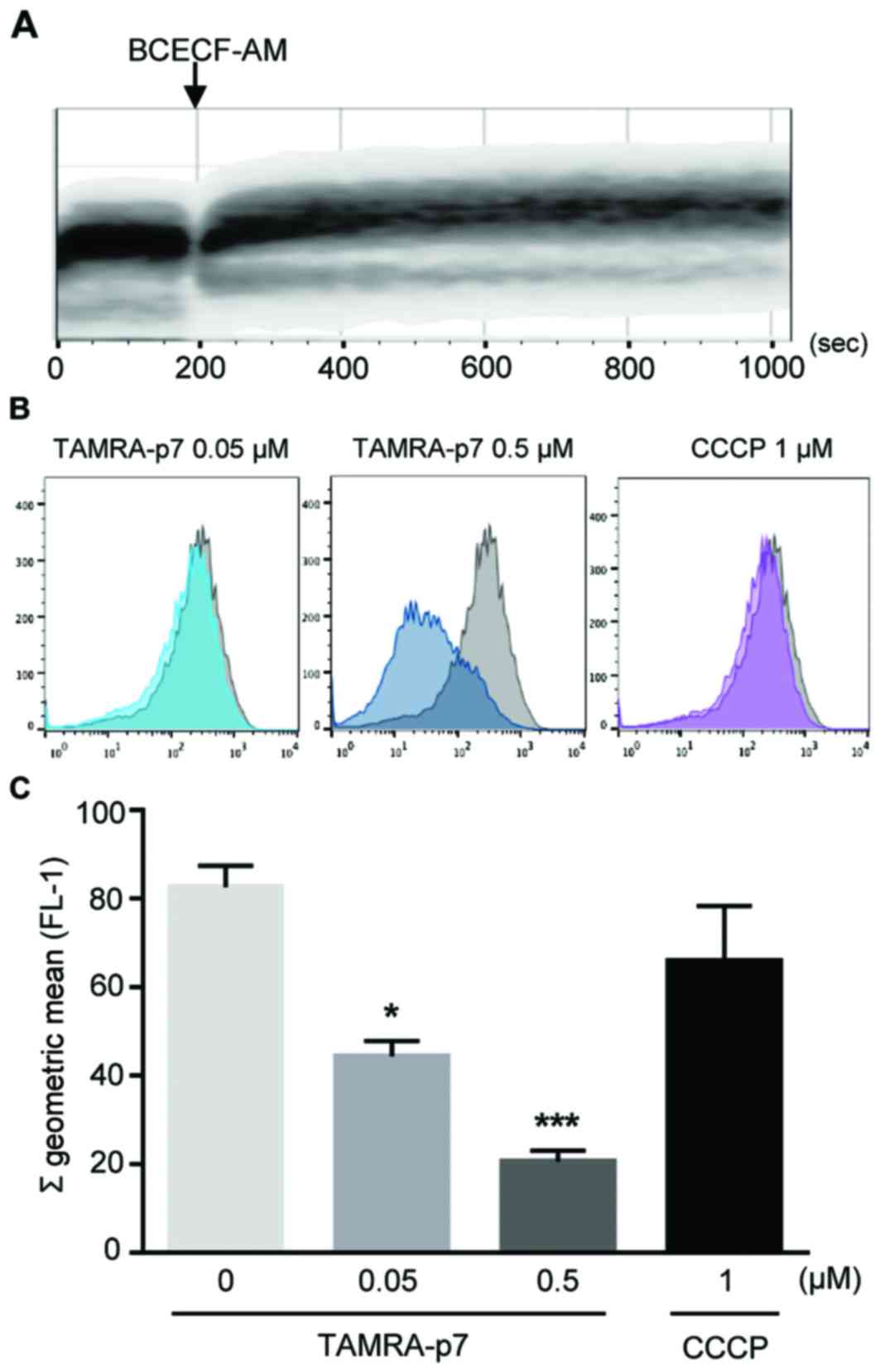
examined using the fluorescence indicator, BCECF-AM (19,20).
To determine the optimal staining duration for BCECF-AM in isolated
mitochondria, flow cytometric assays with BCECF-AM were performed.
As demonstrated in Fig. 3A, the
mitochondrial samples were saturated by BCECF-AM fluorescence
within 10 min; this indicated a stable pH level of the isolated
mitochondria. Matrix acidification triggered by p7 was examined by
flow cytometric analysis in mitochondrial samples, and this
revealed that the pH of the mitochondrial matrix was decreased by
p7 treatment in a concentration-dependent manner (Fig. 3B and C). These results demonstrated
that p7 caused acidification of the mitochondrial matrix.
p7 decreases mitochondrial ATP
production
The findings above demonstrated that p7 induced
mitochondrial depolarization and mitochondrial matrix
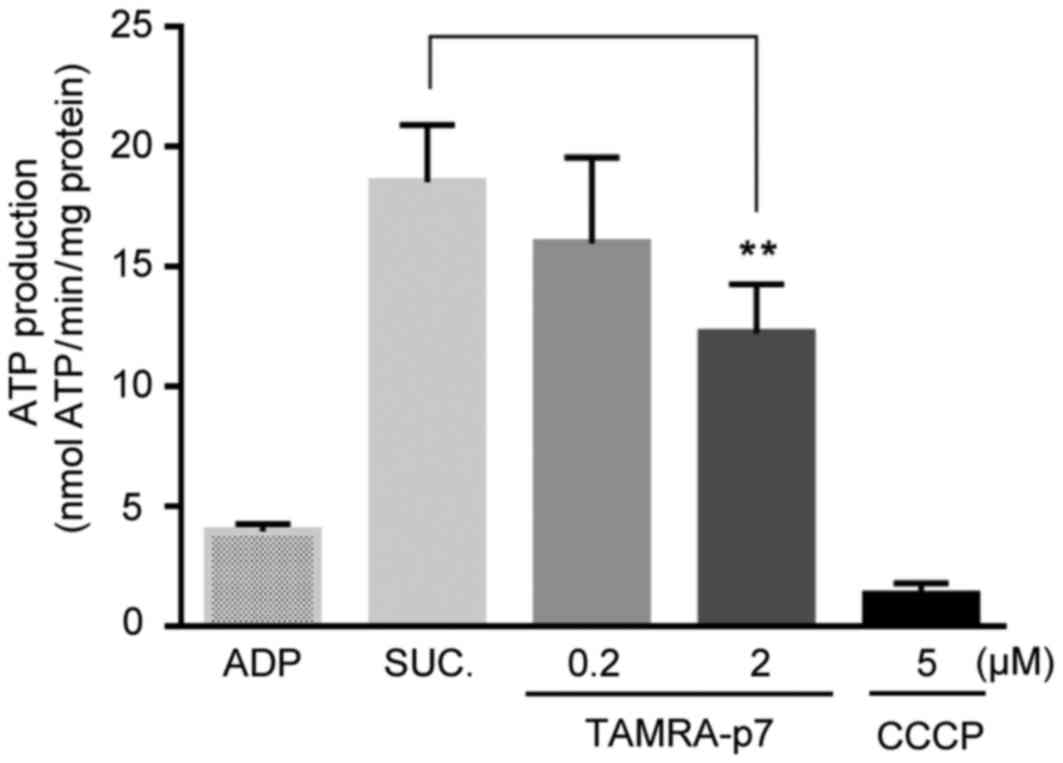
acidification. Subsequently, the effect of p7 on mitochondrial ATP
production was measured, since ΔΨm is known to be the driving force
for ATP synthesis, and an electrochemical hydrogen ion gradient
also contributes to the ATP synthesis process (9). As expected, ATP production was
decreased by p7 treatment in a concentration-dependent manner
(Fig. 4). On the basis of results
obtained in the present study, it is suggested that the p7 protein
directly targets mitochondria to induce mitochondrial
dysfunction.
Discussion
Previous studies have reported that disruption of
ΔΨm was induced by HCV infection in Huh7.5 cells, and that HCV
infection affected mitochondrial functions, including the redox
system, calcium signaling and ATP generation (12,14,21).
To date, the involvement of HCV p7 in mitochondrial dysfunction has
been not reported, although p7 is known to target to mitochondria
(5). Therefore, the aim of the
present study was to investigate the direct effects of p7 in
mitochondria. To minimize the contribution of indirect effects of
p7 in mitochondria, purified mitochondria from mouse liver and
synthesized p7 protein were used. It was identified that p7 induced
mitochondrial depolarization (Fig.
2), matrix acidification (Fig.
3) and decreased ATP synthesis (Fig. 4), clearly demonstrating that p7
serves a direct role in mitochondrial dysfunction. Other
HCV-encoded proteins, including the core protein and NS5A, are also
associated with mitochondria and affect mitochondrial dysfunction
(10,13). It was difficult to demonstrate that
p7 was a major factor in HCV infection-induced mitochondrial
dysfunction. Nonetheless, it has been demonstrated that p7
specifically targeted the purified mitochondria, and had a clear
effect on mitochondrial function.
The results of the present study demonstrated that
p7 treatment caused mitochondrial matrix acidification (Fig. 3). HCV p7 has been reported to
function as a proton channel (22), and thus could affect mitochondrial
matrix acidification. However, it was assumed that p7-induced
mitochondrial matrix acidification was a small aspect of p7-induced
mitochondrial depolarization, since p7 may also conduct other
cations, including sodium and potassium ions. It is suggested that
p7-induced mitochondrial depolarization is the sum of ion gradient
dissipation of protons and a potassium gradient.
It was also demonstrated that p7 affected ATP
synthesis in mitochondria (Fig.
4). The results of the present study are in accord with those
reported in earlier experimental studies in hepatocytes (14,23,24).
To the best of the authors' knowledge, however, this is the first
demonstration of a direct association between HCV p7 and
mitochondria. Nevertheless, the phenomenon of HCV infection-induced
ATP depletion remains controversial. Normally, the energy
requirement increases in virus-infected cells for synthesis of the
viral proteins, whereas p7-induced mitochondrial depolarization and
the proton gradient dissipation lead to a mitochondrial energy
crisis. It has been reported that certain viral infections induce a
significant decrease in ATP concentration in hepatoma cell lines
(16). In addition, these viral
proteins induce mitochondrial disruptions and, in certain cases,
cellular death. Since p7 serves an important role in HCV maturation
and budding, but not RNA replication, this phenomenon could be
explained by the concept of a ‘viral budding strategy’ to induce
mitochondria-mediated apoptosis at late stages of HCV infection,
including the viral budding process.
In conclusion, the present study suggested that HCV
p7 serves an important role in mitochondrial dysfunction. The
findings have enhanced our understanding of the function of p7 in
mitochondria. Although further studies are required to determine
the exact mechanism and topology of p7 in the mitochondrial
membrane, the findings of the present study may aid the development
of future HCV therapies.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea grant funded by the Korean government
(grant no. NRF-2013R1A1A2063171), by Basic Science Research Program
through the National Research Foundation funded by the Ministry of
Education, Science and Technology (grant no. NRF-2013R1A1A2009792),
by Basic Science Research Program through the National Research
Foundation (NRF) funded by the Ministry of Education (grant no.
NRF-2017R1D1A1B03032322), and by a grant from the Korea Health
Technology R&D Project (grant no. HI15C2796) of the Korea
Health Industry Development Institute, which is funded by the
Ministry of Health and Welfare, Republic of Korea.
Glossary
Abbreviations
Abbreviations:
|
ΔΨm
|
mitochondrial membrane potential
|
|
ADP
|
adenosine 5′-diphosphate
|
|
BCECF-AM
|
2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein,
acetoxymethyl ester
|
|
BSA
|
bovine serum albumin
|
|
CCCP
|
carbonyl cyanide
3-chlorophenylhydrazone
|
|
EGTA
|
ethylene glycol-bis
(2-aminoethylether)-N,N,N',N'-tetraacetic acid
|
|
FSC
|
forward scatter
|
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
SSC
|
side scatter
|
|
TFE
|
trifluoroethanol
|
References
|
1
|
National Institutes of Health, . National
Institutes of Health Consensus Development Conference Statement:
Management of hepatitis C: 2002-June 10–12, 2002. Hepatology. 36(5
Suppl 1): S3–S20. 2002.PubMed/NCBI
|
|
2
|
Suzuki T, Ishii K, Aizaki H and Wakita T:
Hepatitis C viral life cycle. Adv Drug Deliv Rev. 59:1200–1212.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carrere-Kremer S, Montpellier-Pala C,
Cocquerel L, Wychowski C, Penin F and Dubuisson J: Subcellular
localization and topology of the p7 polypeptide of hepatitis C
virus. J Virol. 76:3720–3730. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Griffin SD, Harvey R, Clarke DS, Barclay
WS, Harris M and Rowlands DJ: A conserved basic loop in hepatitis C
virus p7 protein is required for amantadine-sensitive ion channel
activity in mammalian cells but is dispensable for localization to
mitochondria. J Gen Virol. 85:451–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Griffin S, Clarke D, McCormick C, Rowlands
D and Harris M: Signal peptide cleavage and internal targeting
signals direct the hepatitis C virus p7 protein to distinct
intracellular membranes. J Virol. 79:15525–15536. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakai A, Claire MS, Faulk K, Govindarajan
S, Emerson SU, Purcell RH and Bukh J: The p7 polypeptide of
hepatitis C virus is critical for infectivity and contains
functionally important genotype-specific sequences. Proc Natl Acad
Sci USA. 100:pp. 11646–11651. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steinmann E, Penin F, Kallis S, Patel AH,
Bartenschlager R and Pietschmann T: Hepatitis C virus p7 protein is
crucial for assembly and release of infectious virions. PLoS
Pathog. 3:e1032007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McBride HM, Neuspiel M and Wasiak S:
Mitochondria: More than just a powerhouse. Curr Biol. 16:R551–R560.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dzbek J and Korzeniewski B: Control over
the contribution of the mitochondrial membrane potential (DeltaPsi)
and proton gradient (DeltapH) to the protonmotive force (Deltap).
In silico studies. J Biol Chem. 283:33232–33239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okuda M, Li K, Beard MR, Showalter LA,
Scholle F, Lemon SM and Weinman SA: Mitochondrial injury, oxidative
stress and antioxidant gene expression are induced by hepatitis C
virus core protein. Gastroenterology. 122:366–375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boya P, Pauleau AL, Poncet D,
Gonzalez-Polo RA, Zamzami N and Kroemer G: Viral proteins targeting
mitochondria: Controlling cell death. Biochim Biophys Acta.
1659:178–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piccoli C, Scrima R, D'Aprile A, Ripoli M,
Lecce L, Boffoli D and Capitanio N: Mitochondrial dysfunction in
hepatitis C virus infection. Biochim Biophys Acta. 1757:1429–1437.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang T, Campbell RV, Yi MK, Lemon SM and
Weinman SA: Role of hepatitis C virus core protein in viral-induced
mitochondrial dysfunction. J Viral Hepat. 17:784–793. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brault C, Levy PL and Bartosch B:
Hepatitis C virus-induced mitochondrial dysfunctions. Viruses.
5:954–980. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Silic-Benussi M, Marin O, Biasiotto R,
D'Agostino DM and Ciminale V: Effects of human T-cell leukemia
virus type 1 (HTLV-1) p13 on mitochondrial K+ permeability: A new
member of the viroporin family? FEBS Lett. 584:2070–2075. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su YC and Hong JR: Betanodavirus B2 causes
ATP depletion-induced cell death via mitochondrial targeting and
complex II inhibition in vitro and in vivo. J Biol Chem.
285:39801–39810. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng L, Adachi T, Kitayama K, Bungyoku Y,
Kitazawa S, Ishido S, Shoji I and Hotta H: Hepatitis C virus
infection induces apoptosis through a Bax-triggered,
mitochondrion-mediated, caspase 3-dependent pathway. J Virol.
82:10375–10385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wieckowski MR, Giorgi C, Lebiedzinska M,
Duszynski J and Pinton P: Isolation of mitochondria-associated
membranes and mitochondria from animal tissues and cells. Nat
Protoc. 4:1582–1590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brierley GP, Davis MH, Cragoe EJ and Jung
DW: Kinetic properties of the sodium/hydrogen ion antiport of heart
mitochondria. Biochemistry. 28:4347–4354. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung DW, Davis MH and Brierley GP:
Estimation of matrix pH in isolated heart mitochondria using a
fluorescent probe. Anal Biochem. 178:348–354. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tardif KD, Waris G and Siddiqui A:
Hepatitis C virus, ER stress, and oxidative stress. Trends
Microbiol. 13:159–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
StGelais C, Foster TL, Verow M, Atkins E,
Fishwick CW, Rowlands D, Harris M and Griffin S: Determinants of
hepatitis C virus p7 ion channel function and drug sensitivity
identified in vitro. J Virol. 83:7970–7981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piccoli C, Scrima R, Quarato G, D'Aprile
A, Ripoli M, Lecce L, Boffoli D, Moradpour D and Capitanio N:
Hepatitis C virus protein expression causes calcium-mediated
mitochondrial bioenergetic dysfunction and nitro-oxidative stress.
Hepatology. 46:58–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ando T, Imamura H, Suzuki R, Aizaki H,
Watanabe T, Wakita T and Suzuki T: Visualization and measurement of
ATP levels in living cells replicating hepatitis C virus genome
RNA. PLoS Pathog. 8:e10025612012. View Article : Google Scholar : PubMed/NCBI
|