Introduction
As a first-line antitumor drug, doxorubicin (DOX)
has been widely used to treat cancers such as lymphomas, leukemias,
solid tumors and soft-tissue sarcomas (1). Unfortunately, doxorubicin agent has
severe, irreversible and dose-dependent cardiotoxicity (2,3).
Nowadays, DOX-induced cardiotoxicity has been attributed to
mitochondrial impairment, oxidative stress, apoptosis and calcium
overloading (4,5), with oxidative stress as the main
cause (6,7). Reactive oxygen species (ROS) produced
under oxidative stress finally induce cardiomyocyte apoptosis
(8,9).
p38 mitogen-activated protein kinase (p38MAPK) have
been demonstrated to potentially serve a crucial role in
DOX-induced cardiotoxicity (10,11).
The authors have previously reported this (12). As a member of the MAPK family,
p38MAPK can be activated by chemical and physical stresses that
promote growth and result in oxidative stress, apoptosis and
vasoconstriction (13,14).
Given that DOX-induced cardiotoxicity is mediated by
oxidative stress, it may be helpful to use antioxidants for
intervention. Recently, flavonoids have received considerable
attention by being able to effectively scavenge free radicals and
to protect against oxidative stress (15,16).
Besides exerting significant cardioprotective effects and lowering
the risk of cardiovascular disease (17–19),
flavonoids can also protect against DOX-induced cardiomyopathy and
apoptosis of cardiomyocytes (20).
As a citrus flavonoid, naringin
(4,5,7-trihydroxy-flavonone-7-rhamnoglucoside, NRG) is the major
active constituent of tomentose pummelo peel that is a famous
traditional Chinese medicine. Like most flavonoids, NRG has
antioxidative (21),
anti-inflammatory, hypolipidemic (22) and hypoglycemic effects (23). Hence, the authors speculated that
NRG may protect against DOX-induced cardiotoxicity through
antioxidative actions. However, it remains unknown whether NRG
indeed can do so by suppressing the p38MAPK pathway.
In the current study, a chemotherapy-induced
cardiotoxicity model was established by treating H9c2 cells with 5
µM DOX (24). The study aimed to
explore the following: i) The influence of NRG on DOX-induced
cardiotoxicity; ii) the relationship between the p38MAPK signaling
transduction pathway and oxidative stress in cardiotoxicity; iii)
whether such cardiotoxicity can be alleviated by NRG through
inhibiting the p38MAPK pathway.
Materials and methods
Materials
NRG ≥95%, DOX, SB203580, N-acetyl-L-cysteine (NAC),
Hoechst 33258, rhodamine123 (Rh123) and 2′,7′-dichlorofluorescin
diacetate (DCFH-DA) were all bought from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Cell Counting kit-8 (CCK-8) was provided by
Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Caspase-3,
t-p38 and p-p38 were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Fetal bovine serum (FBS) and Dulbecco's
modified Eagle's medium (DMEM)-F12 were supplied by Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). H9c2 cells were
obtained from the Experimental Animal Center of Sun Yat-sen
University (Guangzhou, China).
Cell culture
Embryonic rat cardiac cell line H9c2 was provided by
the Experimental Animal Center of Sun Yat-sen University
(Guangzhou, China) and cultured at 37°C in DMEM-F12 that was
supplemented with 10% FBS in an atmosphere comprising 5%
CO2.
Cell viability assay
Following being cultured in 96-well plates
(1×106), the cells received different treatments, with
the viability detected by CCK-8 assay. CCK-8 solution (10 µl,
diluted by 1/10) was added into each well, following which the
cells were further incubated for 2 h at 37°C. The absorbance at 450
nm was read using Multiskan MK3 microplate reader (Thermo Fisher
Scientific, Inc.). According to the following formula, the
percentage of cell viability was calculated based on the average
optical density (OD) of five wells from each group: Cell viability
percentage = ODtreatment group / ODcontrol
group × 100%. The experiments were conducted in
triplicate.
Detection of cell apoptosis by Hoechst
33258 staining
The apoptosis of H9c2 cells was evaluated by Hoechst
33258 staining. Following different treatments, the cells were
fixed for 10 min with 4% paraformaldehyde in phosphate-buffered
saline (PBS), then washed by PBS three times, stained for 5 min by
5 mg/l Hoechst 33258, rinsed again by PBS and observed under
BX50-FLA fluorescence microscope (Olympus Corporation, Tokyo,
Japan). The viable cells emitted uniform blue fluorescence and
presented normal nuclear sizes, but the apoptotic ones had
fractured, condensed or distorted nuclei.
Detection of intracellular ROS
levels
Intracellular ROS levels were detected by the
oxidative conversion of DCFH-DA (cell-permeable) to
dichlorofluorescein (DCF, fluorescent). Following being cultured in
24-well plates (7×106), H9c2 cells were treated
differently and washed with PBS twice, into which serum-free medium
containing 10 µM DCFH-DA solution was thereafter added to incubate
the cells for 60 min at 37°C. They were then washed with PBS three
times, DCF fluorescence in which was detected over the whole visual
field by BX50-FLA fluorescence microscope (Olympus Corporation)
connected with an imaging system. In addition, the mean
fluorescence intensity (MFI) of four randomly selected fields was
determined with ImageJ software (version, 1.41o; National
Institutes of Health, Bethesda, MD, USA). ROS levels were
represented by MFI of DCF.
Detection of mitochondrial membrane
potential (MMP)
MMP was detected by Rh123, a cell-permeable,
fluorescent cationic dye preferentially entering mitochondria on
the basis of a highly negative MMP. MMP depolarization leads to
Rh123 loss from mitochondria, thus decreasing intracellular
fluorescence. H9c2 cells were herein cultured in 24-well plates
(7×106) and treated differently. Following addition of
Rh123 (100 mg/l) into the culture medium, the cells were further
incubated at 37°C for 45 min and observed under BX50-FLA
fluorescence microscope (Olympus Corporation) connected with an
imaging system. The MFI of Rh123 from four randomly selected
fields, which was analyzed using ImageJ software, represented the
level of MMP. The experiments were repeated three times.
Western blot analysis
Differently treated H9c2 cells were collected and
lysed with ice-cold lysis solution (10 mM Tris-HCl (pH 7.4), 0.15 M
NaCl, 5 mM EDTA (pH 8.0), 1% Triton X100), and the resulting
homogenate was centrifuged for 10 min at 9,500 × g and 4°C.
Subsequently, total protein in the supernatant was quantified by
using bicinchoninic acid assay protein assay kit (Sigma-Aldrich),
resolved by 12% SDS-PAGE and transferred to a polyvinylidene
difluoride membrane that was then blocked by 5% fat-free milk in
TBS-0.05% Tween 20 at room temperature for 1 h, incubated overnight
with t-p38MAPK (Sigma-Aldrich; cat no. M8177; dilution, 1:1,000),
p-p38MAPK (Sigma-Aldrich; cat no. CS0430; dilution, 1:1,000) and
caspase-3 antibodies (Sigma-Aldrich; cat no. C9598; dilution,
1:1,000) or horseradish peroxidase (HRP)-conjugated GAPDH antibody
(Sigma-Aldrich; cat no. SAB2108668; dilution, 1:5,000) at 4°C under
gentle agitation, and further incubated with a HRP-conjugated
secondary antibody (Sigma-Aldrich; cat no. A9542; dilution,
1:3,000) at room temperature for 1.5 h. Following being washed with
TBS-0.05% Tween three times, the membrane was developed by enhanced
chemiluminescence (Sigma-Aldrich) and thereafter exposed to X-ray
films that were scanned and determined by ImageJ software to
quantify protein expression.
Statistical analysis
All experimental data were expressed as mean ±
standard error of the mean. Inter-group differences were analyzed
with one-way analysis of variance and Fisher's Least Significant
Difference by using SPSS software (version, 13.0; Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
NRG inhibited DOX-induced cytotoxicity
against H9c2 cells
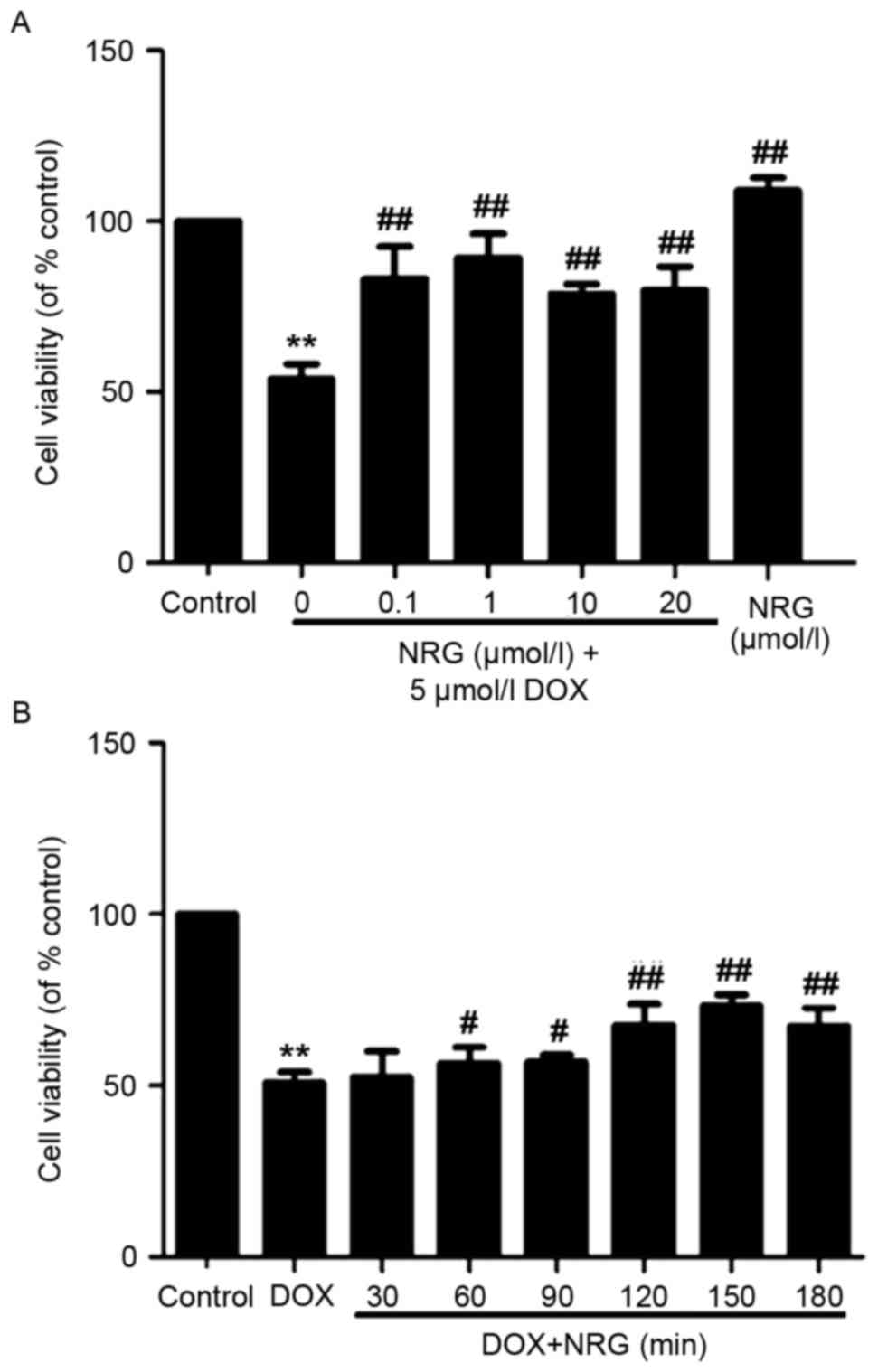
Fig. 1A
demonstrated that 24 h of exposure of H9c2 cells to 5 µmol/l DOX
induces obvious cytotoxicity (P<0.01) that reduces their
viability, compared with the control group. The NRG concentration
required to protect against DOX-induced cytotoxicity was calculated
by performing a dose-response study in the presence of 0.1, 1, 10
and 20 µmol/l NRG (Fig. 1A). The
cytotoxic effects of DOX were significantly attenuated after 2 h of
pretreatment by using 0.1–20 µmol/l NRG. Although 1 µmol/l NRG
exerted the maximum effect, it alone hardly affected cell viability
(P>0.05).
To find out the optimum treatment time of NRG for
DOX-induced cytotoxicity, H9c2 cells were pretreated by 1 µmol/l
NRG for 30, 60, 90, 120, 150 and 180 min respectively prior to DOX
exposure (Fig. 1B). Within 30–180
min after NRG pretreatment, the cell viability continuously
increased and peaked at 150 min. Therefore, 150 min of pretreatment
with 1 µmol/l NRG was selected for subsequent experiments.
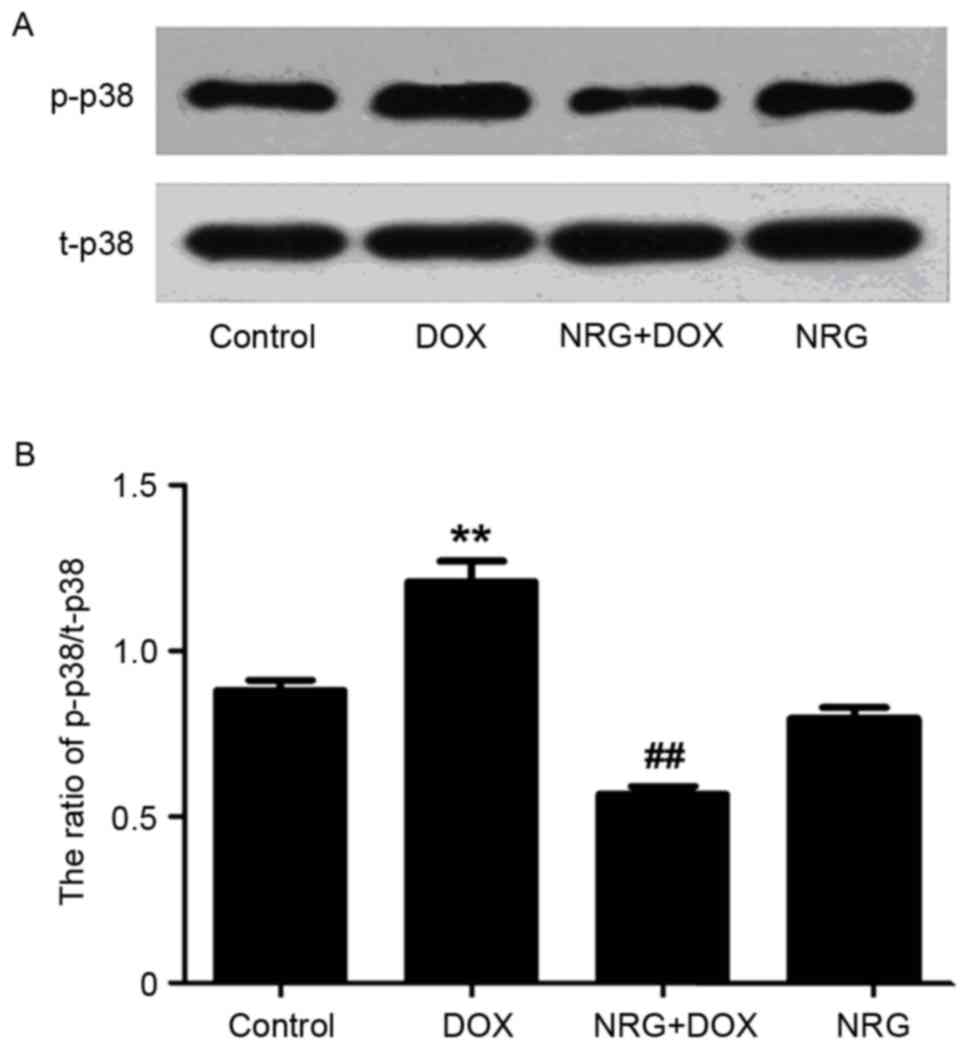
NRG inhibited DOX-induced increase in
phosphorylated p38MAPK (p-p38MAPK) expression
The expression of p-p38MAPK was detected by western
blot analysis. As presented in Fig.
2, the expression level of p-p38MAPK, which is significantly
elevated by 60 min of exposure of H9c2 cells to DOX at 5 µM
(P<0.01) compared with the control group, can be reduced by
pretreatment with 1 µM NRG for 150 min (P<0.01 compared with the
control group). However, 1 µM NRG alone barely affected the basal
expression of p-p38MAPK (P>0.05).
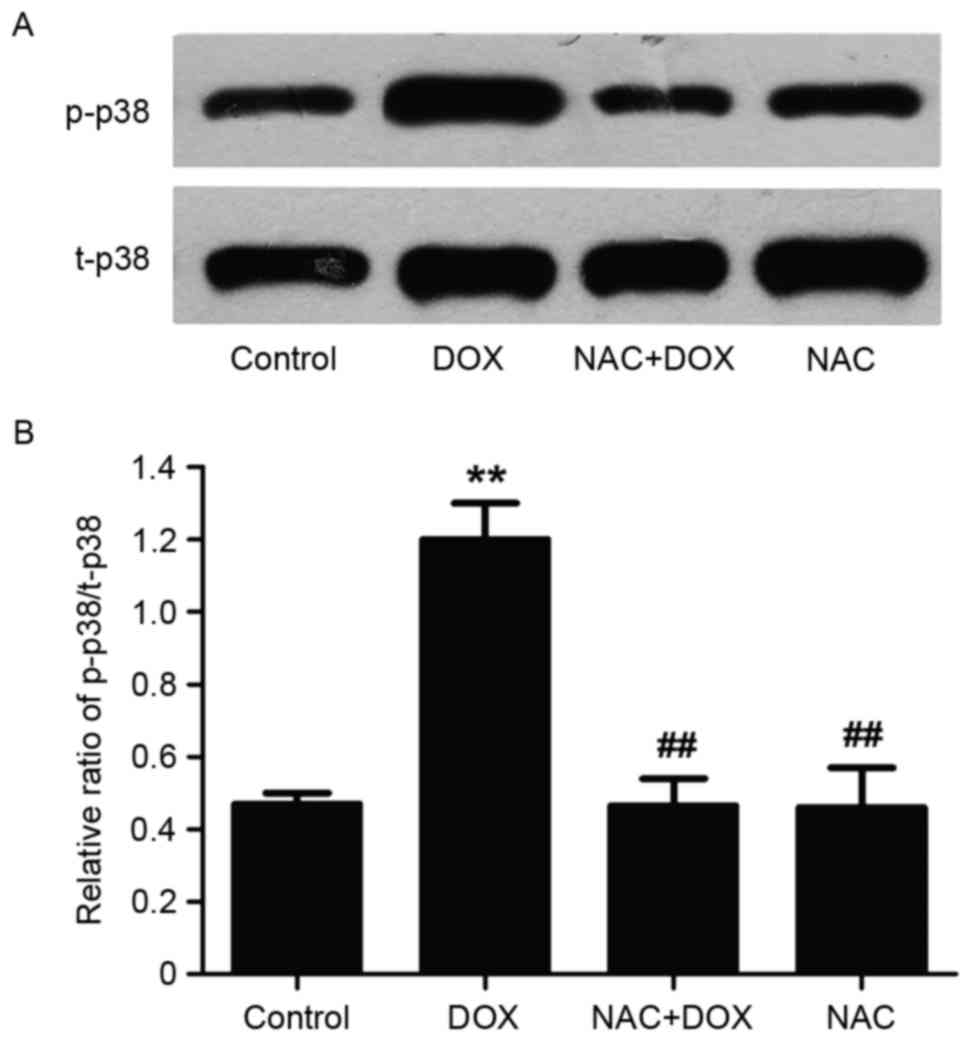
NAC suppressed DOX-induced
upregulation of p38MAPK
To elucidate whether NRG exerted inhibitory effects
on DOX-induced increase in p-p38MAPK expression through
antioxidative action, H9c2 cells were pretreated for 60 min with
NAC (a ROS scavenger) at 1,000 µM prior to 5 µM DOX exposure. As
presented in Fig. 3, pretreating
the cells with NAC, similar to that of using NRG, significantly
depresses DOX-induced upregulation of p-p38MAPK expression
(P<0.01). Nevertheless, NAC alone hardly affected basal
p-p38MAPK expression (P>0.05). Accordingly, NRG inhibited the
DOX-induced increase in p-p38MAPK expression by resisting
oxidation.
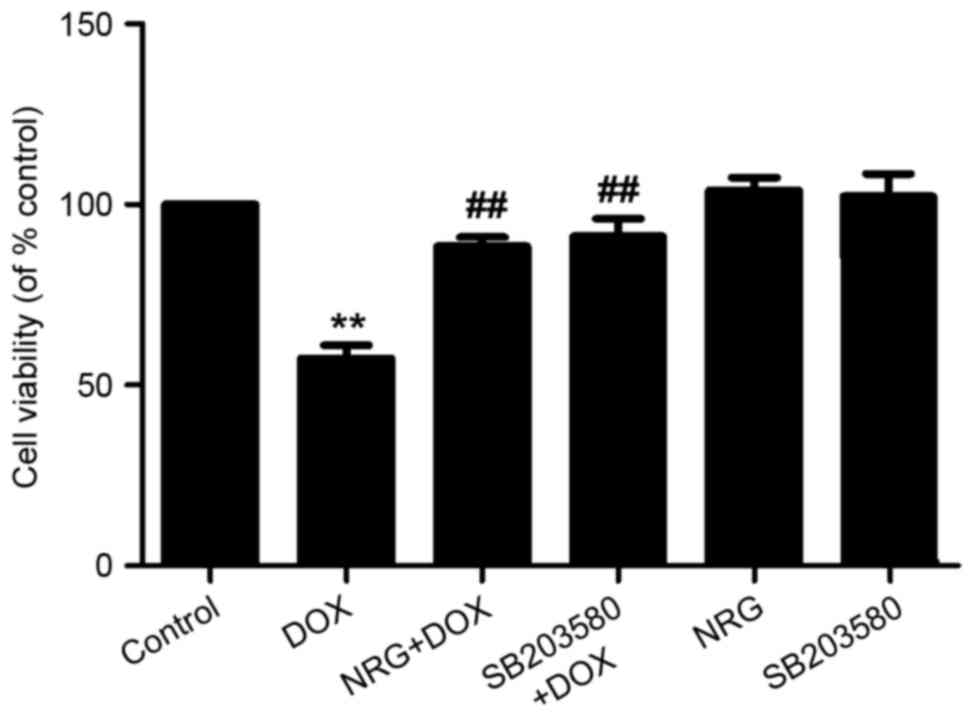
NRG and SB203580 attenuated
DOX-induced cytotoxicity
The viability of H9c2 cells was analyzed by
detecting the reduction in percentage with CCK-8 assay. Such
viability, which was decreased by ~50% following 24 h of exposure
to 5 µM DOX, was significantly increased by preconditioning with 1
µM NRG for 150 min (P<0.01 compared with the control group;
Fig. 4). Moreover, preconditioning
of the cells for 150 min by using SB203580 (a selective inhibitor
of p38MAPK) at 3 µM had similar cytoprotective effects to those of
NRG, as suggested by the increase in cell viability. Either NRG or
SB203580 alone failed to significantly affect the viability of H9c2
cells (P>0.05). Therefore, the activation of p38MAPK was
involved in the cytotoxicity against H9c2 cells induced by DOX.
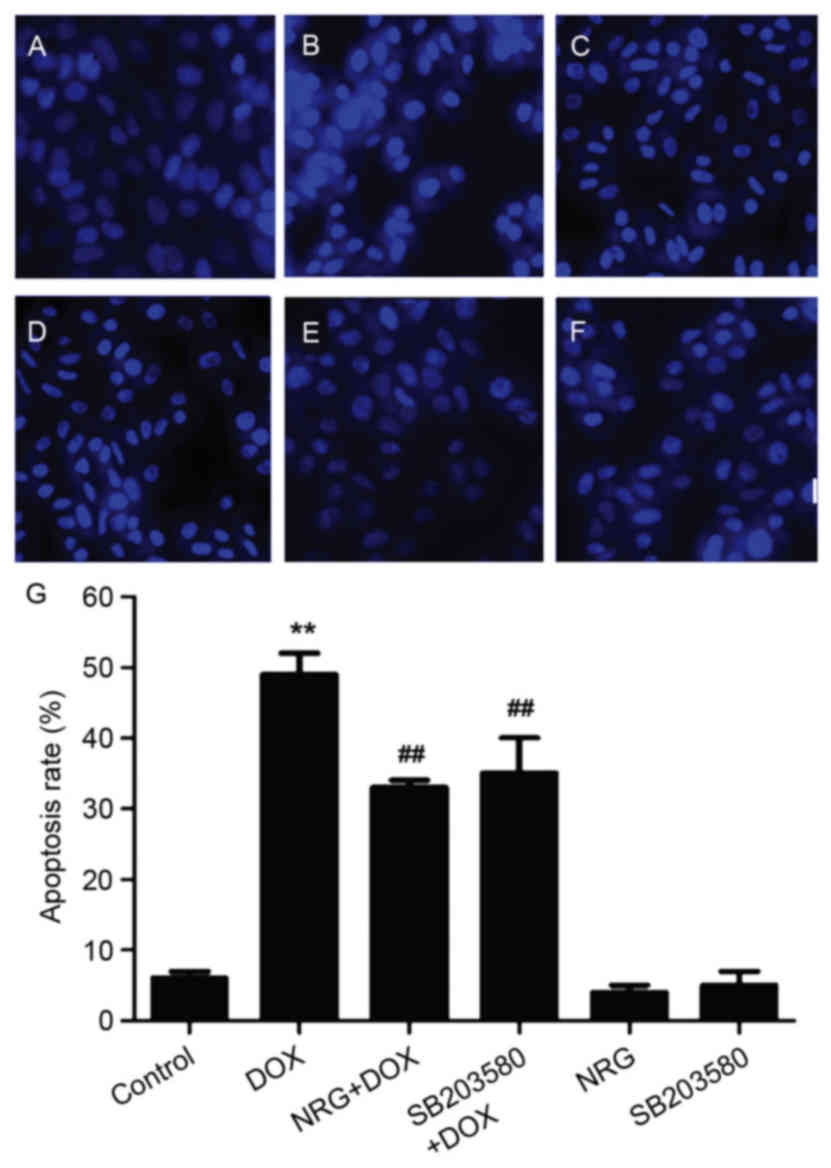
NRG and SB203580 mitigated DOX-induced
cell apoptosis
The morphological changes during H9c2 cell apoptosis
were observed by Hoechst 203580 staining (Fig. 5). To evaluate the effects of NRG
and SB203580 on the apoptosis induced by DOX, H9c2 cells were
pretreated for 150 min with 1 µM NRG or for 60 min with 3 µM
SB203580 prior to DOX exposure. As presented in Fig. 5B, 24 h of preconditioning with 1 µM
DOX induces typical apoptosis characteristics including chromatin
condensation and nuclear shrinkage. The apoptotic cells with these
characteristics decreased by preconditioning with NRG (Fig. 5C). In contrast, NRG alone almost
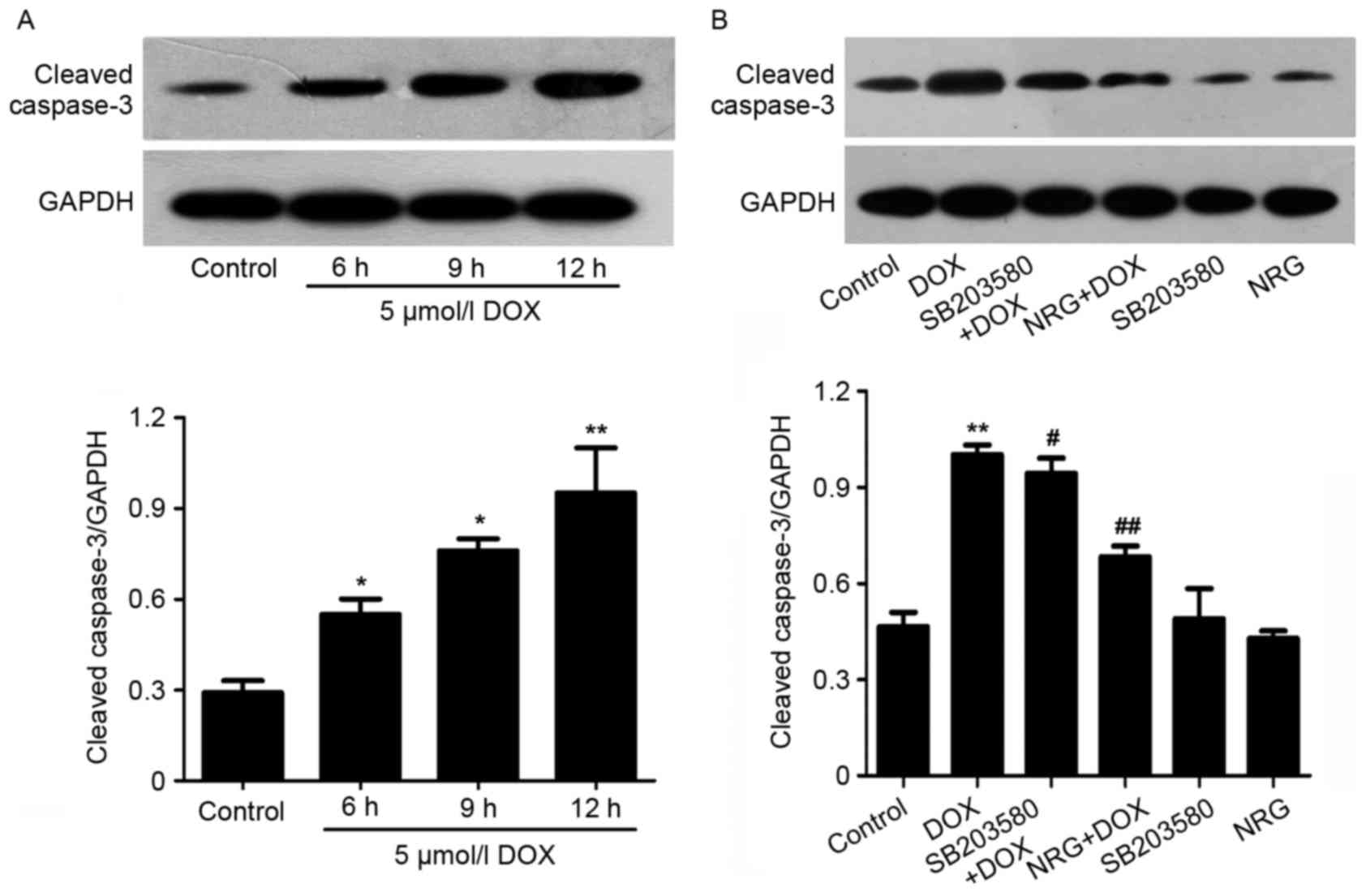
had no visible effect on cell apoptosis (Fig. 5E). Furthermore, western blotting
(Fig. 6A) indicated that 12 h of
treatment by using 5 µM DOX significantly upregulated the
expression of cleaved caspase-3, an effector protease degrading
most cellular targets that result in apoptotic cell death, with
respect to the control group. However, the upregulation was
markedly suppressed by preconditioning with 1 µM NRG for 150 min.
Individual NRG at 1 µM did not affect the expression of cleaved
caspase-3 (P>0.05). These results indicated that H9c2 cells were
protected by NRG against apoptosis induced by DOX.
In order to examine whether p38MAPK activation was
implicated in the apoptosis of H9c2 cells induced by DOX, they were
pretreated for 60 min by 3 µM SB203580 prior to 24 or 12 h of
exposure to DOX at 5 µM (to test the expression of cleaved
caspase-3). As indicated in Figs.
5D and 6B, SB203580
pretreatment decreases both the number of apoptotic cells and the
expression of cleaved caspse-3 induced by DOX, suggesting that the
activation of p38MAPK participated in DOX-induced cytotoxicity.
Individual SB203580 hardly affected cell apoptosis or caspase-3
activation (Figs. 5F and 6B).
All above findings suggested that the p38MAPK
pathway was involved in the apoptosis of H9c2 cells induced by DOX.
Notably, NRG was able to protect H9c2 cells against this kind of
apoptosis by inhibiting the p38MAPK signaling pathway.
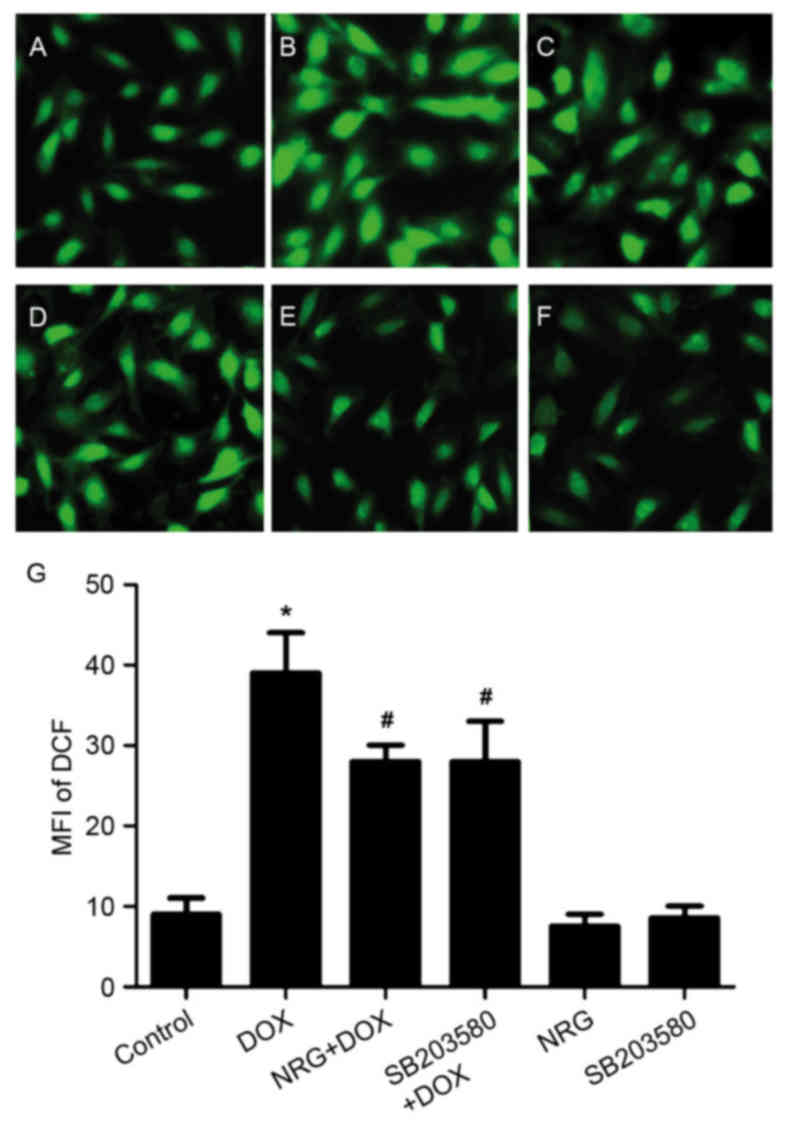
NRG and SB203580 depressed DOX-induced
ROS generation
Following this, the authors assessed the
antioxidative effects of NRG and whether p38MAPK activation
contributed to the ROS overproduction induced by DOX through
detecting the levels of intracellular ROS based on DCFH-DA
staining. Following being treated for 24 h by using 5 µM DOX, the
levels of intracellular ROS in H9c2 cells significantly increased
(P<0.01; Fig. 7).
Interestingly, 150 min of pretreatment by 1 µM NRG or 60 min of
pretreatment by 3 µM SB203580 significantly reduced the levels of
intracellular ROS, revealing the antioxidative effects of NRG and
the involvement of the p38MAPK pathway in the oxidative stress
induced by DOX. Similar to the control group, the cells treated
with 1 µM NRG or 3 µM SB203580 individually emitted weak DCF
fluorescence.
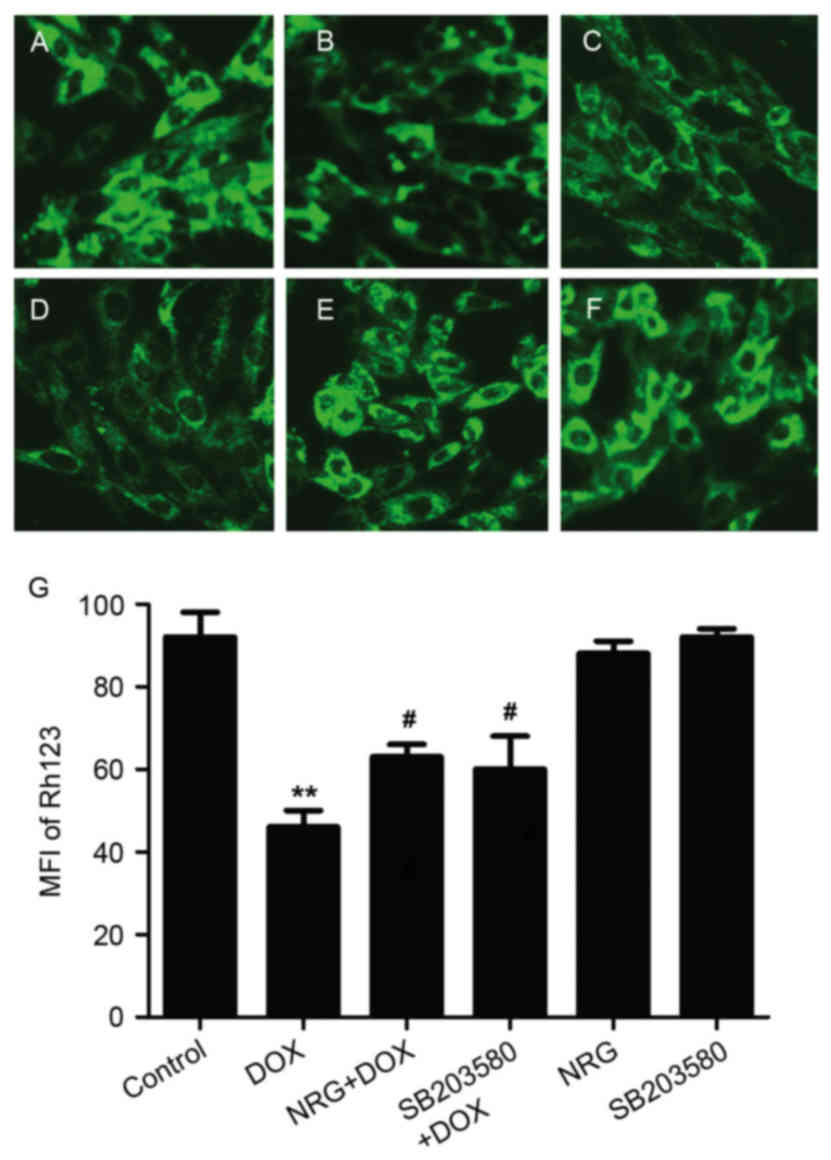
NRG and SB203580 depressed DOX-induced
disruption of MMP
It is well-documented that (8,9,25)
disruption of MMP was involved in the cardiotoxicity induced by
DOX, so the authors examined the effects of NRG and a p38MAPK
inhibitor on DOX-disrupted MMP. As presented in Fig. 8B, following being treated for 24 h
by 5 µM DOX, the mitochondrial number of H9c2 cells is evidently
decreased, which reduced the uptake of Rh123, thereby verifying the
loss of MMP. However, such loss was relieved by 150 h of
preconditioning by 1 µM NRG (Fig.
8C), suggesting that NRG managed to protect the cells against
the mitochondrial damage induced by DOX. Similarly, pretreatment
with SB203580 significantly depressed the loss of MMP induced by
DOX (P<0.05; Fig. 8D),
suggesting that NRG provided mitochondrial protection by inhibiting
p38MAPK expression. Alone, neither NRG nor SB203580 had any effect
on MMP (both P>0.05).
Discussion
Although NRG has antioxidative (26), anti-inflammatory (27), antimicrobial (28) and anticancer activities (29), whether it can protect against the
cardiotoxicity induced by DOX remains unclear. Thus, the authors
herein first assessed the effects of 0.1, 1, 10 and 20 µmol/l NRG
on DOX-induced cardiotoxicity after 15, 30, 60, 90, 120 and 180 min
of treatment, respectively. In a previous study of the authors, it
was recently established that a DOX-induced cardiomyocyte injury
model, made by exposing H9c2 cells to DOX at 5 µmol/l (30), manifested as decreased cell
viability, increased apoptosis, p-p38MAPK expression, ROS
production and MMP loss. Likewise, in the present study,
preconditioning with different concentrations (0.1, 1, 10 and 20
µmol/l) of NRG decreased the loss of cell viability (Fig. 1A). Above all, 1 µmol/l NRG
significantly increased cell viability by attenuating DOX-induced
cytotoxicity, and the protective effects reached optimum at 150
min. The findings indicated that NRG significantly alleviated
DOX-induced cytotoxicity against H9C2 cells.
The cardioprotective effects of NRG and relevant
mechanisms have attracted increasing attention in recent years.
Rajadurai et al (31)
reported that pretreating isoproterenol-induced rats with NRG
significantly enhanced the activities of NADH, tricarboxylic acid
cycle enzymes and cytochrome c oxidase. Subsequently, they found
that, in the heart of ISO-induced rats, NRG significantly augmented
the activities of catalase, mitochondrial SOD, GST and GPx together
with the mitochondrial level of GSH. Hence, the protective effects
of NRG contributed to antioxidative, membrane-stabilizing and free
radical-scavenging properties. In addition, the authors have
demonstrated that NRG exerted protective effects on diabetic
cardiomyopathy through inhibition of NF-κB (32). Therefore, it is of great
significance to clarify the mechanisms by which NRG protects
against DOX-induced injuries in cardiomyocytes. Guo et al
(12) reported that p38MAPK
participated in the cardiotoxicity induced by DOX. Presumably,
inhibition of p38MAPK contributes to the protective effects of NRG
on this cardiotoxicity, which was supported by the findings in the
present study. Pretreating H9c2 cells with NRG before DOX exposure
significantly reduced DOX-induced elevation in p-p38MAPK
expression. Notably, pretreatment with NRG allowed cardioprotection
similarly to the specific p38MAPK inhibitor SB203580, manifesting
as a decreased number of apoptotic cells, increased cell viability,
ROS accumulation and MMP dissipation. Thus, p38MAPK activation
predominantly controlled the cardioprotective action of NRG.
Moreover, pretreatment with the ROS scavenger NAC inhibited the
expression and activity of p38MAPK like NRG did. This novel finding
suggested that NRG suppressed the activation of p38MAPK probably by
resisting oxidation. Kanno et al (33) indicated that NRG attenuated the
oxidative stress induced by cytosine arabinoside by enhancing the
activities of antioxidant enzymes and inhibiting ROS generation
simultaneously. Kang et al (11) indicated that metallothionein has an
antioxidative capacity, which suppresses p38MAPK by inhibiting
cardiomyocyte apoptosis induced by DOX (11). Clearly, the current results are
well supported by the previous literature.
The activation of caspase-3 is a vital step in
DOX-induced apoptosis (1,34) Maejima et al (35) reported that cardiomyocytes
underwent apoptosis typified by caspase-regulated proteolytic
degradation, activation of caspase and cleavage of internucleosomal
DNA, leading to the progression of myocardial dysfunction upon
heart failure. Accordingly, inhibiting caspase-3 expression may
pave the way for preventing and treating the cardiomyopathy induced
by DOX (36,37). To this end, the authors explored
the relationship between p38MAPK and caspase-3 in the
cardioprotective effect of NRG. Similar to NRG, SB203580
significantly inhibited cleaved caspase-3 expression (Fig. 6B), implying that NRG protected
against DOX-induced cell apoptosis through inhibiting the
activation of p38MAPK.
To the best of the authors' knowledge, the present
study is the first time that NRG was indicated to protect H9c2
cells against the cardiotoxicity induced by DOX through inhibiting
the expression and activity of p38MAPK. Particularly, the
antioxidative property of NRG may contribute to suppressing the
expression of p38MAPK induced by DOX. In addition, the authors
provide novel evidence for indicating that p38MAPK participates in
cell apoptosis, ROS generation and loss of MMP in DOX-induced
injuries. In conclusion, NRG is potentially eligible for treating
or preventing DOX-associated cardiotoxicity.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 61427807), the
Natural Science Foundation of Guangdong Province in China (grant
nos. 2014A030310035 and 2016A030313602), and the Fujian Province
Introduction of Major Research and Development Institution Funding
Project (grant no. 2012I2004).
References
|
1
|
Bai J, Ma M, Cai M, Xu F, Chen J, Wang G,
Shuai X and Tao K: Inhibition enhancer of zeste homologue 2
promotes senescence and apoptosis induced by doxorubicin in p53
mutant gastric cancer cells. Cell Prolif. 47:211–218. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hrdina R, Gersl V, Klimtová I, Simůnek T,
Machácková J and Adamcová M: Anthracycline-induced cardiotoxicity.
Acta Medica (Hradec Kralove). 43:75–82. 2000.PubMed/NCBI
|
|
3
|
Scully RE and Lipshultz SE: Anthracycline
cardiotoxicity in long-term survivors of childhood cancer.
Cardiovasc Toxicol. 7:122–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ludke AR, Al-Shudiefat AA, Dhingra S,
Jassal DS and Singal PK: A concise description of cardioprotective
strategies in doxorubicin-induced cardiotoxicity. Can J Physiol
Pharmacol. 87:756–763. 2009.PubMed/NCBI
|
|
5
|
Alkreathy H, Damanhouri ZA, Ahmed N,
Slevin M, Ali SS and Osman AM: Aged garlic extract protects against
doxorubicin-induced cardiotoxicity in rats. Food Chem Toxicol.
48:951–956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu MF, Tang PL, Qian ZM and Ashraf M:
Effects by doxorubicin on the myocardium are mediated by oxygen
free radicals. Life Sci. 68:889–901. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simůnek T, Stérba M, Popelová O, Adamcová
M, Hrdina R and Gersl V: Anthracycline-induced cardiotoxicity:
Overview of studies examining the roles of oxidative stress and
free cellular iron. Pharmacol Rep. 61:154–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singal PK, Li T, Kumar D, Danelisen I and
Iliskovic N: Adriamycin-induced heart failure: Mechanism and
modulation. Mol Cell Biochem. 207:77–86. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spallarossa P, Garibaldi S, Altieri P,
Fabbi P, Manca V, Nasti S, Rossettin P, Ghigliotti G, Ballestrero
A, Patrone F, et al: Carvedilol prevents doxorubicin-induced free
radical release and apoptosis in cardiomyocytes in vitro. J Mol
Cell Cardiol. 37:837–846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu W, Zou Y, Aikawa R, Harada K, Kudoh S,
Uozumi H, Hayashi D, Gu Y, Yamazaki T, Nagai R, et al: MAPK
superfamily plays an important role in daunomycin-induced apoptosis
of cardiac myocytes. Circulation. 100:2100–2107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang YJ, Zhou ZX, Wang GW, Buridi A and
Klein JB: Suppression by metallothionein of doxorubicin-induced
cardiomyocyte apoptosis through inhibition of p38 mitogen-activated
protein kinases. J Biol Chem. 275:13690–13698. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo RM, Xu WM, Lin JC, Mo LQ, Hua XX, Chen
PX, Wu K, Zheng DD and Feng JQ: Activation of the p38 MAPK/NF-κB
pathway contributes to doxorubicin-induced inflammation and
cytotoxicity in H9c2 cardiac cells. Mol Med Rep. 8:603–608. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Zhao Z, Liu J, Huang N, Long D, Wang
J, Li X and Liu Y: MEK/ERK and p38 MAPK regulate chondrogenesis of
rat bone marrow mesenchymal stem cells through delicate interaction
with TGF-beta 1/Smads pathway. Cell Prolif. 43:333–343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Force T and Bonventre JV: Growth factors
and mitogen-activated protein kinases. Hypertension. 31:152–161.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hussein RH and Khalifa FK: The protective
role of ellagitannins flavonoids pretreatment against
N-nitrosodiethylamine induced-hepatocellular carcinoma. Saudi J
Biol Sci. 21:589–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lohani M, Ahuja M, Buabeid MA, Dean S,
Dennis S, Suppiramaniam V, Kemppainen B and Dhanasekaran M:
Anti-oxidative and DNA protecting effects of flavonoids-rich
Scutellaria lateriflora. Nat Prod Commun. 8:1415–1418.
2013.PubMed/NCBI
|
|
17
|
Huxley RR and Neil HA: The relation
between dietary flavonol intake and coronary heart disease
mortality: A meta-analysis of prospective cohort studies. Eur J
Clin Nutr. 57:904–908. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bast A, Kaiserová H, den Hartog GJ, Haenen
GR and van der Vijgh WJ: Protectors against doxorubicin-induced
cardiotoxicity: Flavonoids. Cell Biol Toxicol. 23:39–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du Y, Guo H and Lou H: Grape seed
polyphenols protect cardiac cells from apoptosis via induction of
endogenous antioxidant enzymes. J Agric Food Chem. 55:1695–1701.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bagchi D, Sen CK, Ray SD, Das DK, Bagchi
M, Preuss HG and Vinson JA: Molecular mechanisms of
cardioprotection by a novel grape seed proanthocyanidin extract.
Mutat Res 523–524. 1–97. 2003.
|
|
21
|
Kong X, Qiu D, Ye X, Bao J, Sui Z, Fan J
and Xiang W: Physicochemical and crystalline properties of
heat-moisture-treated rice starch: Combined effects of moisture and
duration of heating. J Sci Food Agric. 95:2874–2879. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmad SF, Attia SM, Bakheet SA, Zoheir KM,
Ansari MA, Korashy HM, Abdel-Hamied HE, Ashour AE and Abd-Allah AR:
Naringin attenuates the development of carrageenan-induced acute
lung inflammation through inhibition of NF-κb, STAT3 and
pro-inflammatory mediators and enhancement of IκBα and
anti-inflammatory cytokines. Inflammation. 38:846–857. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choe SC, Kim HS, Jeong TS, Bok SH and Park
YB: Naringin has an antiatherogenic effect with the inhibition of
intercellular adhesion molecule-1 in hypercholesterolemic rabbits.
J Cardiovasc Pharmacol. 38:947–955. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ali MM and El Kader MA: The influence of
naringin on the oxidative state of rats with streptozotocin-induced
acute hyperglycaemia. Z Naturforsch C. 59:726–733. 2004.PubMed/NCBI
|
|
25
|
Wang XY, Yang CT, Zheng DD, Mo LQ, Lan AP,
Yang ZL, Hu F, Chen PX, Liao XX and Feng JQ: Hydrogen sulfide
protects H9c2 cells against doxorubicin-induced cardiotoxicity
through inhibition of endoplasmic reticulum stress. Mol Cell
Biochem. 363:419–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozyürek M, Akpinar D, Bener M, Türkkan B,
Güçlü K and Apak R: Novel oxime based flavanone, naringin-oxime:
Synthesis, characterization and screening for antioxidant activity.
Chem Biol Interact. 212:40–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Mao W, Ding B and Liang CS:
ERKs/p53 signal transduction pathway is involved in
doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am
J Physiol Heart Circ Physiol. 295:H1956–H1965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ozcelik B, Kartal M and Orhan I:
Cytotoxicity, antiviral and antimicrobial activities of alkaloids,
flavonoids, and phenolic acids. Pharm Biol. 49:396–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan TW, Chou YE, Yang WH, Hsu CJ, Fong YC
and Tang CH: Naringin suppress chondrosarcoma migration through
inhibition vascular adhesion molecule-1 expression by modulating
miR-126. Int Immunopharmacol. 22:107–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rajadurai M and Prince PS: Preventive
effect of naringin on cardiac mitochondrial enzymes during
isoproterenol-induced myocardial infarction in rats: A transmission
electron microscopic study. J Biochem Mol Toxicol. 21:354–361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rajadurai M and Prince PS: Naringin
ameliorates mitochondrial lipid peroxides, antioxidants and lipids
in isoproterenol-induced myocardial infarction in Wistar rats.
Phytother Res. 23:358–362. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Wang C, Peng J, Liang J, Jin Y, Liu
Q, Meng Q, Liu K and Sun H: Naringin inhibits TNF-a induced
oxidative stress and inflammatory response in HUVECs via Nox4/NF-κB
and PI3K/Akt pathways. Curr Pharm Biotechnol. 15:1173–1182. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kanno S, Tomizawa A, Hiura T, Osanai Y,
Shouji A, Ujibe M, Ohtake T, Kimura K and Ishikawa M: Inhibitory
effects of naringenin on tumor growth in human cancer cell lines
and sarcoma S-180-implanted mice. Biol Pharm Bull. 28:527–530.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takemura G and Fujiwara H:
Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms
to management. Prog Cardiovasc Dis. 49:330–352. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maejima Y, Adachi S, Morikawa K, Ito H and
Isobe M: Nitric oxide inhibits myocardial apoptosis by preventing
caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol.
38:163–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Christiansen S and Autschbach R:
Doxorubicin in experimental and clinical heart failure. Eur J
Cardiothorac Surg. 30:611–616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen M, Fan ZC, Liu XJ, Deng JL, Yang Q
and Huang DJ: Cell transplantation with a catheter-based approach:
An efficient method for the treatment of heart failure with
multiple lesions. Cell Prolif. 39:471–477. 2006. View Article : Google Scholar : PubMed/NCBI
|