Introduction
Allogeneic bone marrow transplantation (allo-BMT)
can be a lifesaving therapy for a variety of malignancies and
non-malignant diseases. But the efficacy of allo-BMT is hampered by
a major and lethal complication, graft-versus-host disease (GvHD)
(1). GvHD can be classified into
acute GvHD (aGvHD) and chronic GvHD (cGvHD), and aGvHD usually
occurs within 100 days of allo-BMT. As a rapidly progressive
syndrome, aGvHD is characterized by profound wasting,
immunosuppression and tissue damage in many organs including the
gut, liver and skin (2–4). aGvHD is a serious immune pathological
response, in which naïve donor T cells recognize the host
allogeneic antigen by antigen presenting cells and finally cause
recipient tissue injury (5,6).
Increasing evidence from experiments illustrated that T cell
activation was vital for the induction of aGvHD through
dysregulating of inflammatory cytokine cascades and agents that
suppressed allo-reactive T cells have shown promise in the therapy
of aGvHD (7).
It was reported that JAK-STAT signaling had a vital
role in regulating effector T cell differentiation, homeostasis and
integrating cytokine secretion (8). As the important member of the STAT
family, STAT3 is critical for T cell alloactivation in aGvHD
(9–11) and prolonged activation of STAT3,
which is a dominant event occurring in host T cells and GvHD
targeted organs (12). Murine
recipients of allo-BMT with T lymphocytes lacking STAT3 did not
exhibit the typical symptoms of GvDH, but displayed conspicuous
survival instead (13). Hill et
al (14) reported that
knockout of SOCS3, which is the negative regulator of JAK2/STAT3,
markedly increased the incidence of GvHD in the mice model. In
addition, several inhibitors or antibodies such as ruxolitinib,
CP-690550, TG101348 and PIAS3, block the JAK2/STAT3 signaling
pathway and alleviate the severity of GvHD (15–21).
Nifuroxazide has been initially identified as an
intestinal broad-spectrum antibiotic since the 1980s and
illustrated as a potent inhibitor of the JAK/STAT signaling
recently (22). Nifuroxazide has
been demonstrated to inhibit STAT3 phosphorylation by suppressing
the Jak family kinases Jak2 and Tyk2, and cause a decrease in
viability of myeloma cells but with no cytotoxic effect of normal
peripheral blood mononuclear cells (22), illustrating the security to a
certain extent in clinical practice. Although not available for
clinical use in certain countries currently, nifuroxazide is
available in many countries worldwide and may have broader efficacy
as well. In the present study, the authors hypothesized that
nifuroxazide therapy would attenuate the development of aGVHD using
an experimental murine model.
Materials and methods
Animals and drugs
The recipient mice were 8–12-week-old BALB/c male
mice (n=20 per group), and the donor mice were 8–12-week-old
C57BL/6 male mice. All mice were obtained from the Experimental
Animal Center of Zhengzhou University (Zhengzhou, China) and were
fed for about one week to adapt to the environment. Subsequently,
in the next week, the recipient mice were fed with sterile food and
acidified water. All experiments were performed according to the
Institutional Animal Care and Use Committee Guidelines and were
approved by the Ethics Committee of Xinxiang Medical University
(Xinxiang, China). The nifuroxazide used was obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) and dissolved in
dimethylsulfoxide.
Allogeneic BMT
Prior to transplantation, the recipient mice were
exposed to 7.5 Gy total body irradiation (60Co source).
Eight donor C57BL/6 mice were sacrificed, and the bone marrow cells
and the splenocytes were isolated and adjusted to
1×108cell/ml, respectively. Following 4 h, the mixture
of bone marrow cells (50 µl) and the splenocytes (50 µl) from donor
mice were injected through tail veins.
Treatment with nifuroxazide
The entire recipient mice were divided randomly into
two groups with 20 mice each group. The drug of nifuroxazide (2
mg/ml) was continuously injected intravenously into the recipient
mice (100 µl/mouse) at 4–11 days following transplantation, while
the control group received saline. The survival time, clinical
manifestations and body weight changes were monitored over time and
the clinical scores of aGVHD were made according to the work of
Cooke et al (23).
Western blotting
Liver tissues were collected from
nifuroxazide-treated and saline-treated mice of the allo-BMT model
respectively. Tissues were lysed in radioimmunoprecipitation assay
buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2
EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium
pyrophosphate, 1 mM b-glycerophosphate, 1 mM
Na3VO4, 1 mg/ml leupeptin]. Protein
concentration was determined using a bicinchoninic protein assay
kit (cat no. P0012S; Beyotime Institute of Biotechnology, Haimen,
China). A total of 50 µg sample per lane was separated by 12%
SDS-PAGE and then transferred to a polyvinylidene difluoride
membrane. Membranes were blocked in bovine serum albumin blocking
buffer (cat no. P0023B; Beyotime Institute of Biotechnology) for 1
h at room temperature. Subsequently, membranes were stained with
primary antibodies against β-actin (1:1,000), STAT3 (1:1,000) and
p-STAT3 (1:500) (cat nos. 3700, 9139 and 9145 respectively; Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight, and
subsequently incubated with an appropriate horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat nos. 129256,
127655; OriGene Technologies, Inc., Rockville, MD, USA) for 1 h at
room temperature. Finally, the immunocomplexes were visualized
using a BeyoECL Plus kit (Beyotime Institute of Biotechnology). The
gray analysis of bands were quantified using Quantity One software
(version 4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Histopathological analysis
Following treatment for ten days, the liver and
small intestine from mice in the GvHD control group and
nifuroxazide-treated group were fixed in 4% paraformaldehyde and
then the tissues were sectioned at 7 µm thicknesses, deparaffinized
using xylene, then dehydrated using a gradient of ethanol, and
finally stained with hematoxylin and eosin.
Flow cytometry analysis
Spleens were isolated and harvested at 2 weeks
following allo-BMT, passed through a 40 mm nylon cell strainer
filter, and then collected in PBS. RBCs were removed with Red Blood
Cell Lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China). Cells were washed and resuspended in PBS at
1×107 cells/ml. A total of 100 µl resuspended cells were
placed on ice and labeled with anti-CD3, anti-CD4, anti-CD8, and
anti-CD25 (cat nos. 05112-50-100, 06112-80-25, 10112-60-25,
07312-60-25; PeproTech, Inc., Rocky Hill, NJ, USA) labeled with
appropriate fluorochrome for 30 min in the dark. Then cells were
washed with iced PBS twice and resuspended in 1% paraformaldehyde.
The fluorescence intensity of cell was measured with flow cytometer
(Guava easyCyte; EMD Millipore, Billerica, MA, USA) with a minimum
of 10,000 cells collected.
ELISA
The peripheral blood was collected at day 14
following transplantation, and the levels of tumor necrosis
factor-α and interferon-γ in plasma were analyzed by ELISA kits
(cat nos. 0608150430 and 0608150421; RayBiotech, Norcross, GA, USA)
according to the manufacturer's instructions.
Statistical analysis
Student's t-tests or one-way analysis of variance
were performed to test whether mean values between different groups
were significantly different when appropriate. For survival
analysis, Kaplan-Meier method with log-rank test was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Treatment of nifuroxazide ameliorated
the symptoms of the recipient mice with aGvHD and improved their
living time
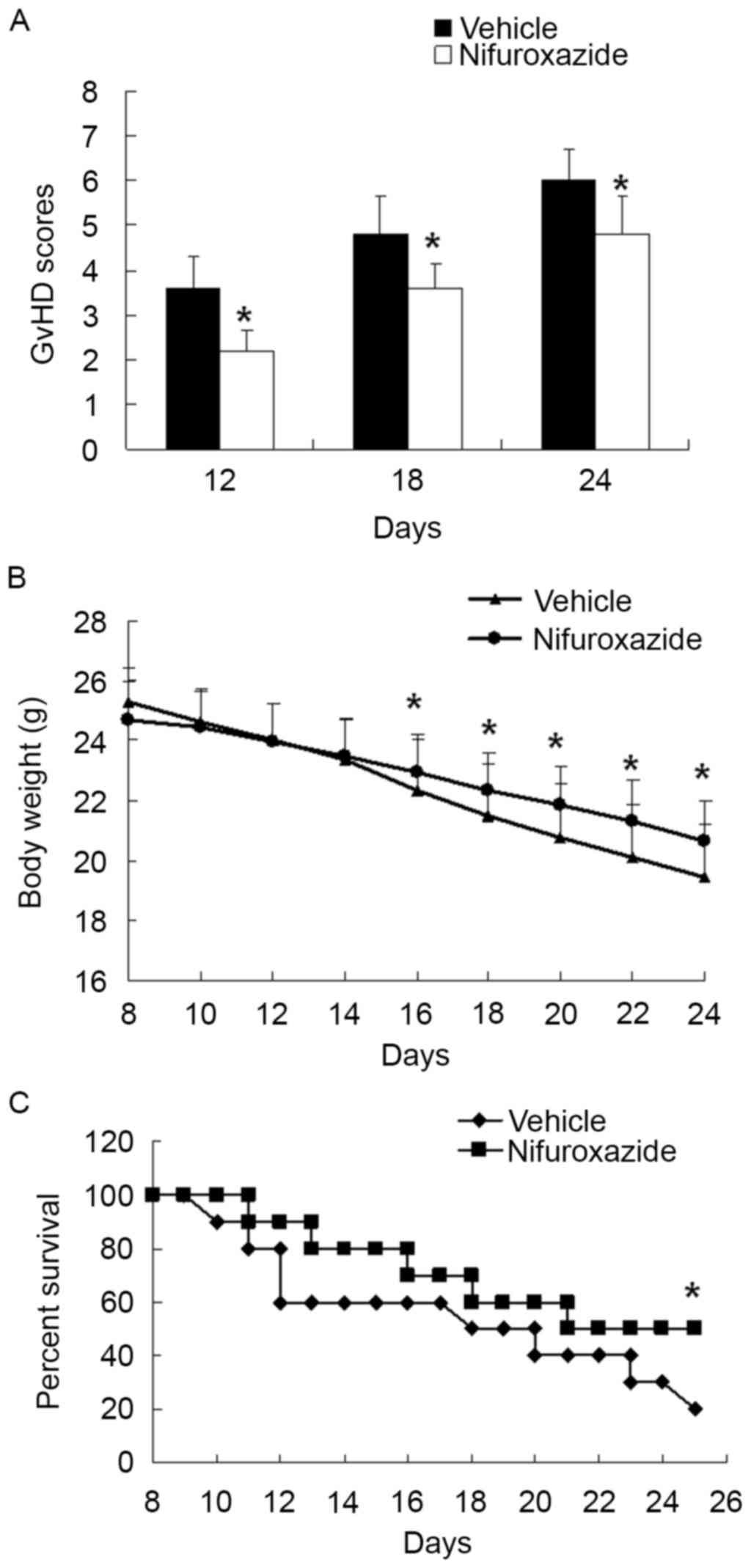
Although prophylactic treatment for aGVHD is
important, perhaps equally important is the therapy for established
GVHD, which is usual refractory to frontline therapy (24). The intravenous (i.v.) injection of
lethally irradiated BALB/c mice with 5×106 total bone
marrow cells and 5×106 splenocytes from C57BL/6 donors
leads to aGVHD, which is characterized by gradual weight loss,
depilation, lassitude, diarrhea and death. Thus, to determine
whether nifuroxazide treatment could modulate the development of
aGVHD, nifuroxazide was injected intraperitoneal (i.p.) to the mice
following allo-BMT as described previously. Compared with the
saline-treated group, the mice in the nifuroxazide-treated group
showed attenuation of weight loss and less severe clinical scores
of aGVHD (Fig. 1A and B).
Moreover, the mean survival time of mice in the nifuroxazide
treatment group extension was longer than the control group
(Fig. 1C). These results
demonstrated that aGVHD mice treated with the nifuroxazide
suppressed the development of aGVHD and significantly delayed
aGVHD-induced lethality.
The damage of GVHD target organs were
alleviated by nifuroxazide
A hallmark of aGVHD is tissue injury and
inflammation in target organs including the skin, liver and
intestinal tract. When observed 2 weeks following HSCT
(hematopoietic stem cell transplantation), saline-treated mice
exhibited marked inflammation with pustule formation and ulceration
in the skin, ballooning degeneration and severe hepatic congestion
in the liver, and blunting of villi, glandular organ rupture and an
inflammatory infiltrate in the lamina propria of the small
intestine. In contrast, mice receiving nifuroxazide had mostly
normal-appearing skin with minimal focal ulceration, mild edema and
hepatic congestion in the liver, and a less-pronounced injury in
the small intestine (Fig. 2).
Nifuroxazide controlled the
development of aGVHD through STAT3 modulation in a mouse model of
allo-BMT
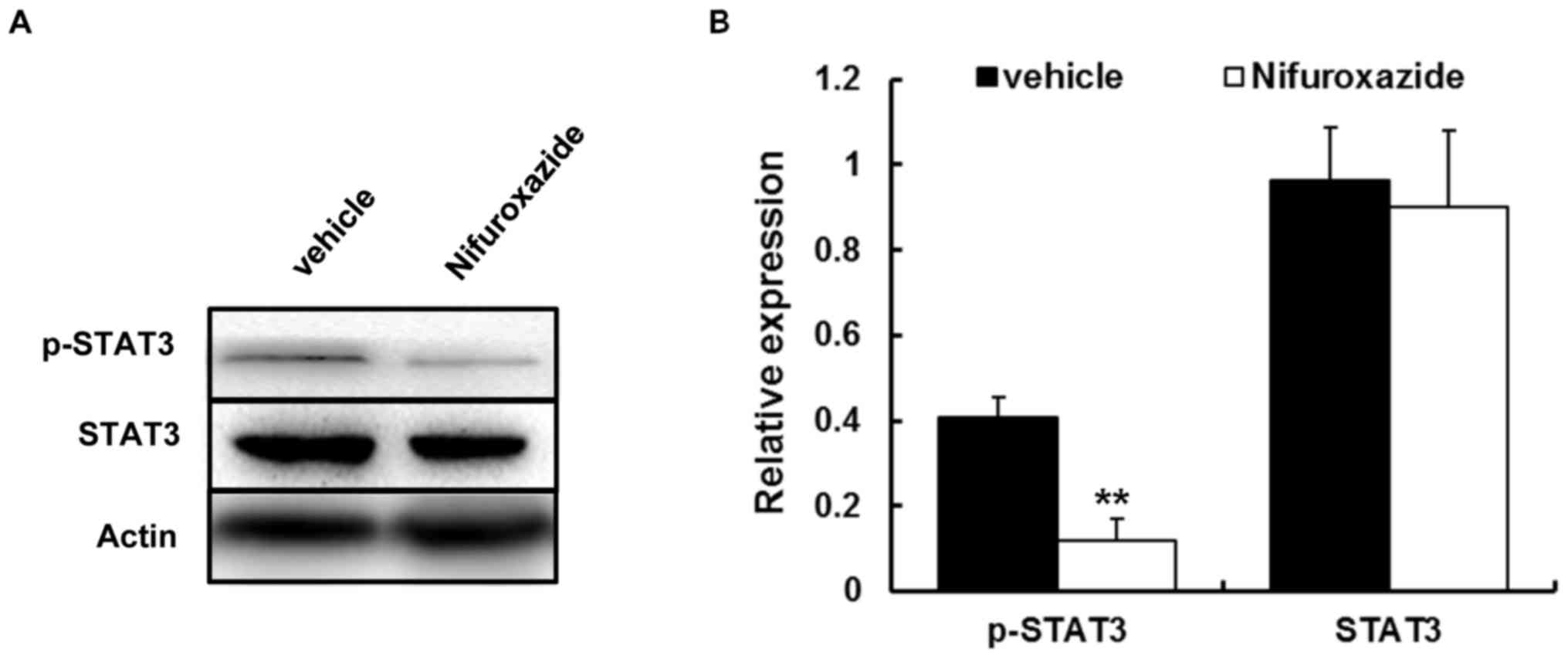
As nifuroxazide was proven as the inhibitor of
STAT3, in addition to the vital role in the pathogenesis of GVHD,
the authors next investigated whether this drug could affect STAT3
activity. Western blotting was carried out to quantify the STAT3
activity. The results showed that p-STAT3 activities in liver
isolated from each group were noticeably decreased by nifuroxazide
treatment (Fig. 3).
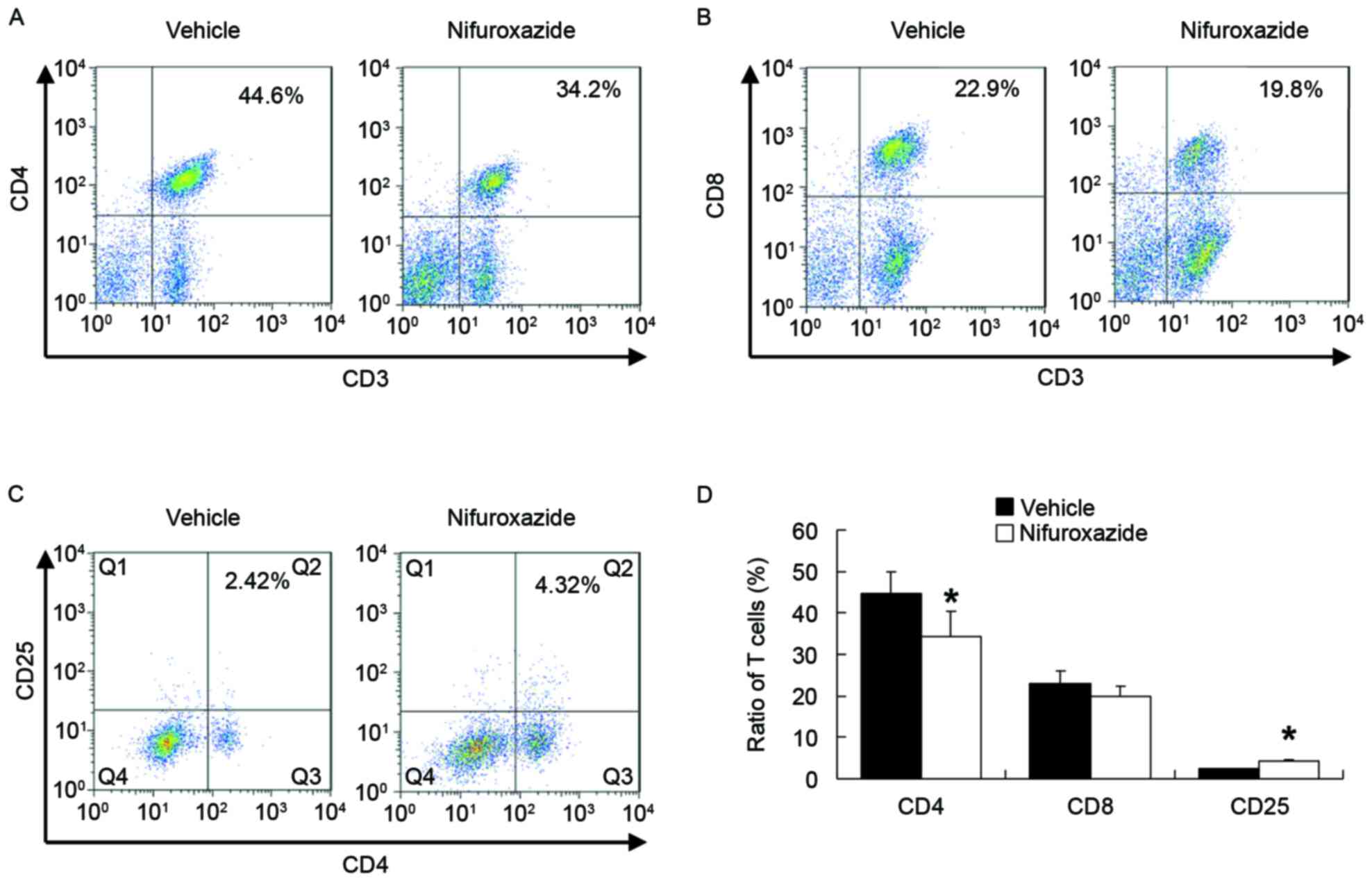
Nifuroxazide therapy inhibited effect T cells
activation and regulated CD4+CD25+
T cells expression. The inflammatory processes that observed
in aGVHD target organs are driven by the T lymphocytes activation
(25). Correspondingly, less
CD4+ but not CD8+ T lymphocytes were
identified in the spleen of mice treated with nifuroxazide than in
saline-treated controls (Fig. 4A and
B). As the CD4+CD25+ T cells serve a
negative role in GVHD progression (26), the authors next detected the
CD4+CD25+ T cells following nifuroxazide
treatment. The results demonstrated that when compared with the
saline treated group, number of CD4+CD25+ T
cells in nifuroxazide treated group was significantly increased
(Fig. 4C), indicating that the
therapeutic effect using nifuroxazide partly not only due to the
inhibition of the CD4+ T cells but also due to the
increase of the CD4+CD25+ T cells. The small
but statistically significant increase in
CD4+CD25+ T cells is consistent with the
possibility that the increase of CD4+CD25+ T
cells contributes to the efficacy of the drug.
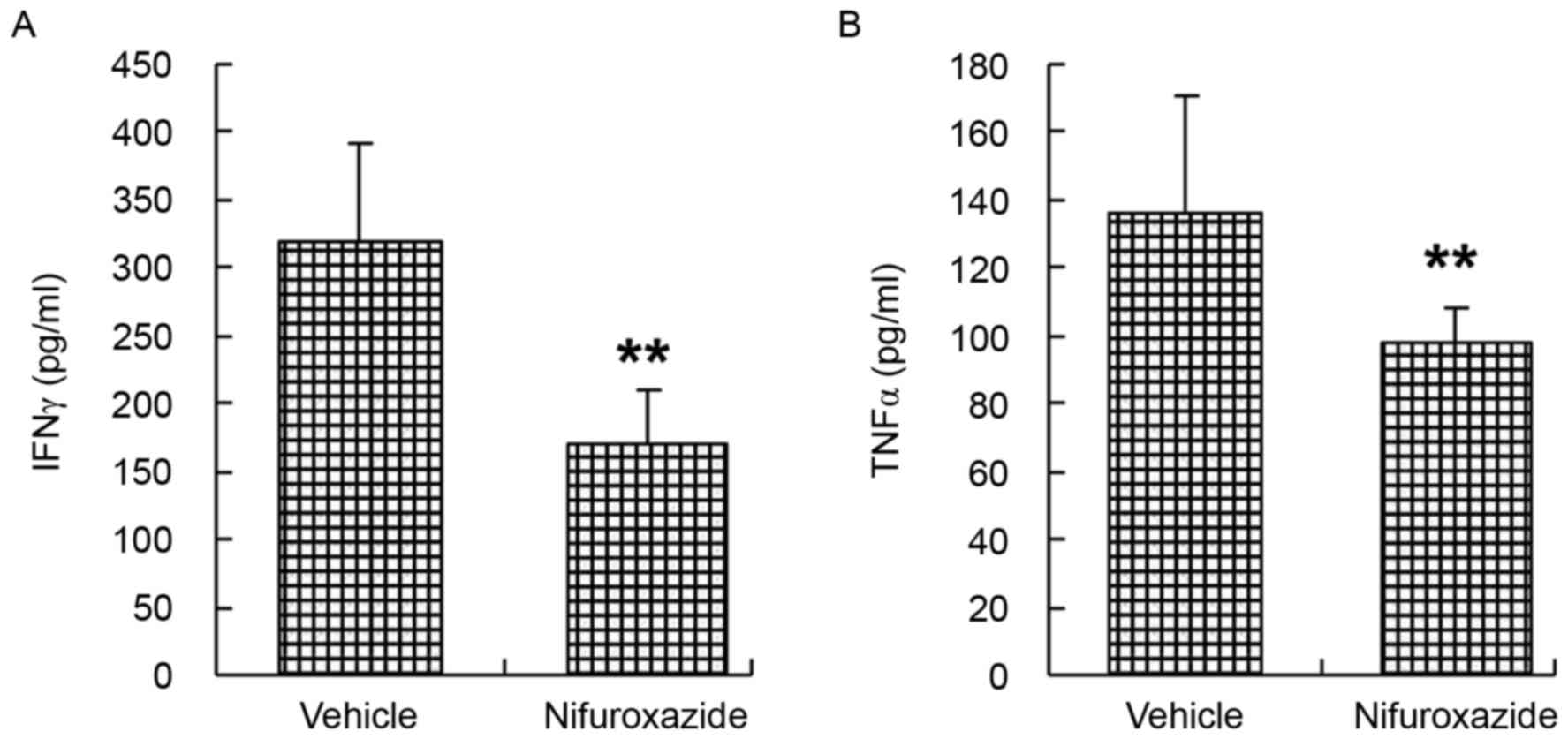
Treated with nifuroxazide suppressed
the production of inflammatory cytokine
The progression of aGvHD is accompanied by producing
and releasing proinflammatory cytokines (27,28).
Recipient mice were irradiated and transplanted with total bone
marrow cells and splenocytes cells and then were injected with
either nifuroxazide or saline as before. Serum samples from each
group were analyzed on day 14 following transplantation for
detecting levels of IFN-γ and TNF-α. The authors reported that two
weeks after allo-BMT, serum levels of IFN-γ and TNF-α were
aggrandized above baseline in saline-treated mice, while the
circulating levels of these cytokines were reduced by nifuroxazide
treatment (Fig. 5).
Discussion
Though the pathophysiology of aGvHD is complicated,
accumulating evidence suggests that suppression the activity of
alloreactive T cells can potentially limit the morbidity associated
with the disorder. However, many agents carry with obvious side
effects that restrict their usage, such as delayed immune
reconstitution and increased incidence of infection (24). The induction of aGvHD is a process
of donor T cells response to host alloantigens and causes T-cell
mediated tissue damage. It is worth noting that STAT3 binds to its
ligands and triggers the allogeneic T cells activation.
Nifuroxazide, which is used as an antimicrobial drug, has been
demonstrated the potent of STAT3 inhibitor, and exhibits antitumor
effect both in hematological malignancy and solid tumors. There are
numerous STAT3 inhibitors. Among them, the authors chose
nifuroxazide because of the safeness currently available (22,29,30).
The intended effect of nifuroxazide was observed, i.e. blocking
STAT3 by inhibiting its phosphorylation, however, it was unclear
whether the nifuroxazide could prevent GvHD.
As previously reported, lethal TBI (total body
irradiation) can destroy the immune system and result in an
immunodeficiency state of mice (31). The experiments in the current study
still indicated that the mice with TBI and without any particular
treatment exhibited an obvious body weight loss within two days,
and would die from irradiation related damage or infection very
soon. Nevertheless, the mice engrafted the donor T cells exhibited
the characteristic of severe GvHD, including less activity, arched
back and ruffled fur, which led to their intestine, liver skin
injury and even death.
The doses of nifuroxazide used in the experiment are
derived from the articles and pre-experiments. Injections occurred
daily when administration was delayed until 7 days following
allogeneic BMT. The results demonstrated that post-treatment of
nifuroxazide reduced the incidence and severity of mice with aGvHD,
whereas a previous article reported that post-treatment using JAK2
inhibition ameliorated GvHD syndromes (32). Consequently, the histopathology
observation demonstrated a decrease of damage in the target
organs.
The authors then investigated whether the
immunosuppressive effects of nifuroxazide on GvHD, which blocked
STAT3 activation, stemmed from the decrease of alloreactive
T-cells. It has been suggested that, following BMT, alloreative T
cells enter peripheral lymphoid organs rapidly within hours, while
alloreactive T lymphocytes migrate to target organs slowly within a
few days (25,33). Flow cytometry indicated that STAT3
blockage with nifuroxazide significantly reduced CD4+ T
lymphocytes activation in spleens at day 14.
Furthermore, the dysregulation of inflammatory
cytokine cascades in aGvHD initiation is known to serve a critical
role in the pathogenesis of aGvHD. In particular, IFN-γ (34–36)
and TNF-α (37) have been
established as vital effector factors of tissue damage originating
from BMT-conditioning regimen. A marked decrease was identified in
the concentrations of IFN-γ and TNF-α in plasma following
nifuroxazide treatment. Though not detected, nuclear factor-κB, a
proinflammatory transcription factor, having a crosstalk both with
STAT3 and TLR9 signaling pathway, may also be inhibited in the
treatment. The immunological effects of nifuroxazide need to be
clearly illustrated through further researches.
So far, the drugs, as the therapeutic approaches of
GvHD, have usually resulted in severe immunosuppression occurring
at the expense of graft-versus-leukemia, while through inhibiting
STAT3 signaling pathway, nifuroxazide has proven the antitumor
effects in hematological oncology (22) and solid tumors (38,39),
suggesting that apart from attenuating the symptom of GvHD,
treatment with nifuroxazide has the potential to be a potent
antitumor agent. Most importantly, there were no observed severe
side effects from the experiment. Further studies ought to be
carried out to detect the effect of prophylactic application of
nifuroxazide on GvHD (including aGvHD or cGvHD).
In conclusion, the current study illustrated that
nifuroxazide therapy may attenuate GvHD and improve the survival
rate, and the existence of a potential effect in terms of
regulating T cell differentiation, with a significant decrease in
the secretion of IFN-γ and TNF-α cytokines. These effects were
mediated by the blockade of the STAT3 pathways. On this basis,
these observations illustrated that the nifuroxazide may be
efficacious for post-transplant of GvHD, providing a potent
clinical choice as a prophylactic or as a second-line therapy for
aGvHD.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81300442 and
81301947) and grants from the Scientific Research Fund of Xinxiang
Medical University (grant nos. 2013QN112 and 2014QN115) and the
doctor launch fund of Xinxiang Medical University (grant nos.
505016, 505017).
References
|
1
|
Gratwohl A, Baldomero H and Passweg J:
Hematopoietic stem cell transplantation activity in Europe. Curr
Opin Hematol. 20:485–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blazar BR, Murphy WJ and Abedi M: Advances
in graft-versus-host disease biology and therapy. Nat Rev Immunol.
12:443–458. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Socié G and Blazar BR: Acute
graft-versus-host disease: From the bench to the bedside. Blood.
114:4327–4336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wysocki CA, Panoskaltsis-Mortari A, Blazar
BR and Serody JS: Leukocyte migration and graft-versus-host
disease. Blood. 105:4191–4199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson BE, Zheng H, Taylor PA,
Matte-Martone C, McNiff JM, Jain D, Demetris AJ,
Panoskaltsis-Mortari A, Ager A, Blazar BR, et al: Memory T cells in
GVHD and GVL. Biol Blood Marrow Transplant. 14(1 Suppl 1): S19–S20.
2008.
|
|
6
|
Shlomchik WD, Couzens MS, Tang CB, McNiff
J, Robert ME, Liu J, Shlomchik MJ and Emerson SG: Prevention of
graft versus host disease by inactivation of host
antigen-presenting cells. Science. 285:412–415. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Pira G, Di Cecca S, Montanari M,
Moretta L and Manca F: Specific removal of alloreactive T-cells to
prevent GvHD in hemopoietic stem cell transplantation: Rationale,
strategies and perspectives. Blood Rev. 30:297–307. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spoerl S, Mathew NR, Bscheider M,
Schmitt-Graeff A, Chen S, Mueller T, Verbeek M, Fischer J, Otten V,
Schmickl M, et al: Activity of therapeutic JAK 1/2 blockade in
graft-versus-host disease. Blood. 123:3832–3842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu SX, Alpdogan O, Lin J, Balderas R,
Campos-Gonzalez R, Wang X, Gao GJ, Suh D, King C, Chow M, et al:
STAT-3 and ERK 1/2 phosphorylation are critical for T-cell
alloactivation and graft-versus-host disease. Blood. 112:5254–5258.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujino M and Li XK: Role of STAT3 in
regulatory T lymphocyte plasticity during acute
graft-vs.-host-disease. JAKSTAT. 2:e245292013.PubMed/NCBI
|
|
11
|
Radojcic V, Pletneva MA, Yen HR, Ivcevic
S, Panoskaltsis-Mortari A, Gilliam AC, Drake CG, Blazar BR and
Luznik L: STAT3 signaling in CD4+ T cells is critical
for the pathogenesis of chronic sclerodermatous graft-versus-host
disease in a murine model. J Immunol. 184:764–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma HH, Ziegler J, Li C, Sepulveda A,
Bedeir A, Grandis J, Lentzsch S and Mapara MY: Sequential
activation of inflammatory signaling pathways during
graft-versus-host disease (GVHD): Early role for STAT1 and STAT3.
Cell Immunol. 268:37–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laurence A, Amarnath S, Mariotti J, Kim
YC, Foley J, Eckhaus M, O'Shea JJ and Fowler DH: STAT3
transcription factor promotes instability of nTreg cells and limits
generation of iTreg cells during acute murine graft-versus-host
disease. Immunity. 37:209–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hill GR, Kuns RD, Raffelt NC, Don AL,
Olver SD, Markey KA, Wilson YA, Tocker J, Alexander WS, Clouston
AD, et al: SOCS3 regulates graft-versus-host disease. Blood.
116:287–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Das R, Komorowski R, Beres A,
Hessner MJ, Mihara M and Drobyski WR: Blockade of interleukin-6
signaling augments regulatory T-cell reconstitution and attenuates
the severity of graft-versus-host disease. Blood. 114:891–900.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SH, Moon SJ, Park MJ, Kim EK, Moon YM
and Cho ML: PIAS3 suppresses acute graft-versus-host disease by
modulating effector T and B cell subsets through inhibition of
STAT3 activation. Immunol Lett. 160:79–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carniti C, Gimondi S, Vendramin A,
Recordati C, Confalonieri D, Bermema A, Corradini P and Mariotti J:
Pharmacologic inhibition of JAK1/JAK2 signaling reduces
experimental murine acute GVHD while preserving GVT effects. Clin
Cancer Res. 21:3740–3749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaekel N, Behre G, Behning A, Wickenhauser
C, Lange T, Niederwieser D and Al-Ali HK: Allogeneic hematopoietic
cell transplantation for myelofibrosis in patients pretreated with
the JAK1 and JAK2 inhibitor ruxolitinib. Bone Marrow Transplant.
49:179–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park HB, Oh K, Garmaa N, Seo MW, Byoun OJ,
Lee HY and Lee DS: CP-690550, a Janus kinase inhibitor, suppresses
CD4+ T-cell-mediated acute graft-versus-host disease by
inhibiting the interferon-γ pathway. Transplantation. 90:825–835.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Betts BC, Abdel-Wahab O, Curran SA, St
Angelo ET, Koppikar P, Heller G, Levine RL and Young JW: Janus
kinase-2 inhibition induces durable tolerance to alloantigen by
human dendritic cell-stimulated T cells yet preserves immunity to
recall antigen. Blood. 118:5330–5339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tawara I, Koyama M, Liu C, Toubai T,
Thomas D, Evers R, Chockley P, Nieves E, Sun Y, Lowler KP, et al:
Interleukin-6 modulates graft-versus-host responses after
experimental allogeneic bone marrow transplantation. Clin Cancer
Res. 17:77–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nelson EA, Walker SR, Kepich A, Gashin LB,
Hideshima T, Ikeda H, Chauhan D, Anderson KC and Frank DA:
Nifuroxazide inhibits survival of multiple myeloma cells by
directly inhibiting STAT3. Blood. 112:5095–5102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cooke KR, Kobzik L, Martin TR, et al: An
experimental model of idiopathic pneumonia syndrome after bone
marrow transplantation: I. The roles of minor H antigens and
endotoxin. Blood. 88:3230–3239. 1996.PubMed/NCBI
|
|
24
|
Kim SS: Treatment options in
steroid-refractory acute graft-versus-host disease following
hematopoietic stem cell transplantation. Ann Pharmacother.
41:1436–1444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beilhack A, Schulz S, Baker J, Beilhack
GF, Wieland CB, Herman EI, Baker EM, Cao YA, Contag CH and Negrin
RS: In vivo analyses of early events in acute graft-versus-host
disease reveal sequential infiltration of T-cell subsets. Blood.
106:1113–1122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dijke IE, Hoeppli RE, Ellis T, Pearcey J,
Huang Q, McMurchy AN, Boer K, Peeters AM, Aubert G, Larsen I, et
al: Discarded human thymus is a novel source of stable and
long-lived therapeutic regulatory T Cells. Am J Transplant.
16:58–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujii N, Hiraki A, Aoe K, Murakami T,
Ikeda K, Masuda K, Matsuo K, Shinagawa K, Ishimaru F, Sugi K, et
al: Serum cytokine concentrations and acute graft-versus-host
disease after allogeneic peripheral blood stem cell
transplantation: Concurrent measurement of ten cytokines and their
respective ratios using cytometric bead array. Int J Mol Med.
17:881–885. 2006.PubMed/NCBI
|
|
28
|
Ju XP, Xu B, Xiao ZP, Li JY, Chen L, Lu SQ
and Huang ZX: Cytokine expression during acute graft-versus-host
disease after allogeneic peripheral stem cell transplantation. Bone
Marrow Transplant. 35:1179–1186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cipolla BG, Havouis R and Moulinoux JP:
Polyamine contents in current foods: A basis for polyamine reduced
diet and a study of its long term observance and tolerance in
prostate carcinoma patients. Amino Acids. 33:203–212. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cipolla BG, Havouis R and Moulinoux JP:
Polyamine reduced diet (PRD) nutrition therapy in hormone
refractory prostate cancer patients. Biomed Pharmacother.
64:363–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qiao S, Ren H, Shi Y and Liu W: Allogeneic
compact bone-derived mesenchymal stem cell transplantation
increases survival of mice exposed to lethal total body
irradiation: A potential immunological mechanism. Chin Med J
(Engl). 127:475–482. 2014.PubMed/NCBI
|
|
32
|
Joly F, Breton M, Wolf C, Ninio E and
Colard O: Heterogeneity of arachidonate and paf-acether precursor
pools in mast cells. Biochim Biophys Acta. 1125:305–312. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Panoskaltsis-Mortari A, Price A, Hermanson
JR, Taras E, Lees C, Serody JS and Blazar BR: In vivo imaging of
graft-versus-host-disease in mice. Blood. 103:3590–3598. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burman AC, Banovic T, Kuns RD, Clouston
AD, Stanley AC, Morris ES, Rowe V, Bofinger H, Skoczylas R, Raffelt
N, et al: IFNgamma differentially controls the development of
idiopathic pneumonia syndrome and GVHD of the gastrointestinal
tract. Blood. 110:1064–1072. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Delisle JS, Gaboury L, Bélanger MP, Tassé
E, Yagita H and Perreault C: Graft-versus-host disease causes
failure of donor hematopoiesis and lymphopoiesis in
interferon-gamma receptor-deficient hosts. Blood. 112:2111–2119.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yi T, Chen Y, Wang L, Du G, Huang D, Zhao
D, Johnston H, Young J, Todorov I, Umetsu DT, et al: Reciprocal
differentiation and tissue-specific pathogenesis of Th1, Th2, and
Th17 cells in graft-versus-host disease. Blood. 114:3101–3112.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Couriel D, Saliba R, Hicks K, Ippoliti C,
de Lima M, Hosing C, Khouri I, Andersson B, Gajewski J, Donato M,
et al: Tumor necrosis factor-alpha blockade for the treatment of
acute GVHD. Blood. 104:649–654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu Y, Ye T, Yu X, Lei Q, Yang F, Xia Y,
Song X, Liu L, Deng H, Gao T, et al: Nifuroxazide exerts potent
anti-tumor and anti-metastasis activity in melanoma. Sci Rep.
6:202532016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang F, Hu M, Lei Q, Xia Y, Zhu Y, Song X,
Li Y, Jie H, Liu C, Xiong Y, et al: Nifuroxazide induces apoptosis
and impairs pulmonary metastasis in breast cancer model. Cell Death
Dis. 6:e17012015. View Article : Google Scholar : PubMed/NCBI
|