Introduction
Malignant glioma is a primary brain tumor that
frequently results in a high rate of morbidity and mortality
(1). Gliomas do not metastasize
but infiltrate locally, invading into adjacent tissues and exhibit
a poor prognosis (2). Currently,
glioma treatment involves a combination of radiotherapy,
chemotherapy and surgical resection (3). However, the results of treatment are
not satisfactory and the development of novel and efficient
therapeutic strategies is required. Evaluation of natural products
has led to the identification of a number of compounds that exhibit
inhibitory effects on the growth of glioma cells. These molecules
are currently in clinical trials for the treatment of glioma
(3–5).
Artemisinin is isolated from the herbaceous plant,
Artemisia annua in China. Artemisinin was used in China for
the treatment of malaria and has exhibited promising results
(6,7). Chemical modification of artemisinin
led to the development of certain analogs, including
dihydroartemisinin, which exhibited anti-malarial activity
comparable to or improved compared with artemisinin (6). In vitro studies have revealed
that derivatives of artemisinin possess potential as inhibitors of
proliferation in malignant tumors (8–11).
The anti-proliferative effect of artemisinin was demonstrated to be
greater in breast and ovarian cancer compared with lung cancer. The
advantage of using dihydroartemisinin as an antitumor agent is it
is non-toxic to normal cells (11,12).
Therefore, dihydroartemisinin can be used for the treatment of
glioma due to its anti-proliferative action. A glioma cell line
that is resistant to chemotherapy and commonly used to study
gliomas is C6 rat cells (13,14).
The present study aimed to investigate the effect of
dihydroartemisinin on the proliferation of C6 glioma cells.
Dihydroartemisinin treatment exhibited an anti-proliferative effect
on the C6 glioma cell growth.
Materials and methods
Cell line and culture
The C6 rat glioma cell line was purchased from the
Cell Bank of the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) and antibiotics, penicillin (100 U/ml)/streptomycin (100
µg/ml) (1:100; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in an
incubator with humidified atmosphere of 5% CO2 at
37°C.
Analysis of apoptosis
Apoptosis in the C6 rat glioma cell line was
measured using the Annexin V-FITC Apoptosis Detection kit (BD
Biosciences, San Jose, CA, USA). Following culture for 24 h the
cells were seeded into 6-well plates at a density of
3×106 cells/well and were treated with dimethyl
sulfoxide (DMSO) alone as a control or with 5, 10, 15, 20 or 30 µM
dihydroartemisinin. Following incubation for 48 h, the cells were
washed two times with ice-cold PBS. The cells were then resuspended
in 100 µl binding buffer and incubated with 3 µl Annexin
V-fluorescein isothiocyanate (FITC; BD Biosciences) and 10 µl
propidium iodide (PI; BD Biosciences) at room temperature for 15
min. The experiment was repeated three times.
Cell cycle analysis
C6 rat glioma cells were seeded at a density of
3×106 cells into each of the 10 cm culture dishes. The
dishes contained complete RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) and the cells were cultured for 24 h. The cells
were then treated DMSO as a control or with 10, 20 or 30 µM
dihydroartemisinin for 48 h. Following treatment, the cells were
harvested, fixed in 70% ethanol at −2°C for 24 h and washed with
PBS. The cells were then subjected to staining with a 5% PI
solution according to manufacturer's instructions. Analysis of the
cells was performed using a FACSCalibur flow cytometer with Cell
Quest software Pro (5.1 version; BD Biosciences, Franklin Lakes,
NJ, USA). The ModFit LT software package (version 2.0; Verity
Software House, Inc., Topsham, ME, USA) was used to analyze the
data.
Analysis of the cyclic
3′,5′-monophopshate (cAMP) level
C6 rat glioma cells at a density of 1×104
cells/well in 24-well plates, following treatment with 5, 10, 15,
20 and 30 µM dihydroartemisinin for 48 h, were washed with PBS. The
cells were treated with a radioimmunoprecipitation lysis buffer and
then subjected to centrifugation to collect the supernatant, which
was treated with mixture of 0.1 N HCl and Dulbecco's modified
Eagle's medium/F12 (Biological Industries Israel Beit-Haemek Ltd.,
Beit Haemek, Israel). The 30 µl supernatant samples were collected
and the concentration of cAMP was determined using an immunoassay.
The supernatant was treated with mouse monoclonal anti-cAMP primary
antibody (cat. no. 250532, 1:100; BI Biotech India Pvt., Ltd., New
Dehli, India) to analyze the expression level of cAMP as previously
described (15). The secondary
antibody used was NorthernLights™ 557 conjugated
anti-mouse immunoglobulin G (cat. no. NL007, 1:5,000; BI Biotech
India Pvt., Ltd.).
Analysis of bromodeoxyuridine (BrdU)
labeling
A total of 1×104 cells/well in 24-well
plates, following treatment with dihydroartemisinin for 48 h, were
washed and then distributed onto cover slips in 24-well plates. The
coverslips were treated with 0.5% alcoholic solution of
H2O2 followed by treatment with HCl. The
coverslips were washed with PBS and then treated with rat anti-BrdU
antibodies (cat. no. ab152095, 1:100; Abcam, Cambridge, UK) at room
temperature overnight. The coverslips were washed again with PBS
and then incubated with secondary antibodies (1:5,000) for 1 h at
room temperature.
Scanning electron microscopy
(SEM)
The cells were distributed onto the microslides at a
density of 2×106 cells/well in 24-well culture plates.
The plates were supplemented with RPMI-1640 medium. The cells were
treated with 5, 10, 15, 20 or 30 µM dihydroartemisinin or with DMSO
alone as a control for 48 h. The cells were fixed with
glutaraldehyde (2.5%) in a sodium cacodylate buffer [0.1 M (pH
7.4)] for 1 h at 4°C and then for 72 h. Then, cells were fixed with
osmium tetraoxide for 1 h at 4°C. Dehydration of the cells was
performed by treatment with gradient acetone and then with amyl
acetate. The slides were dried, coated with an alloy of gold and
palladium and examined under scanning electron microscopy
(magnification, ×750; Jeol-JSM-5200).
Statistical analysis
The results are presented as the mean ± standard
error from three independent experiments. The data was analyzed
using one-way analysis of variance with the Bonferroni post-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
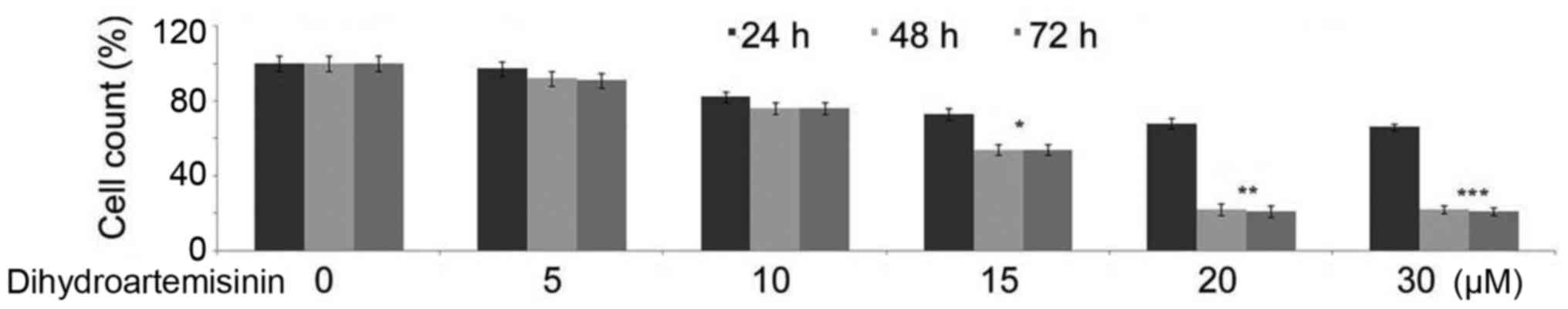
Inhibition of C6 glioma cell growth by
treatment with dihydroartemisinin
The untreated control cultures of C6 glioma cells
were demonstrated to be growing at an increased rate during the 48
h compared with the treated cells. However, incubation of C6 glioma
cells with 15, 20 or 30 µM dihydroartemisinin for 48 h led to a
significant reduction in the cell count (Fig. 1). The reduction in the cell count
in cultures treated with 20 µM concentration of dihydroartemisinin
was demonstrated to be −0.8-fold compared with the control cultures
at 48 h.
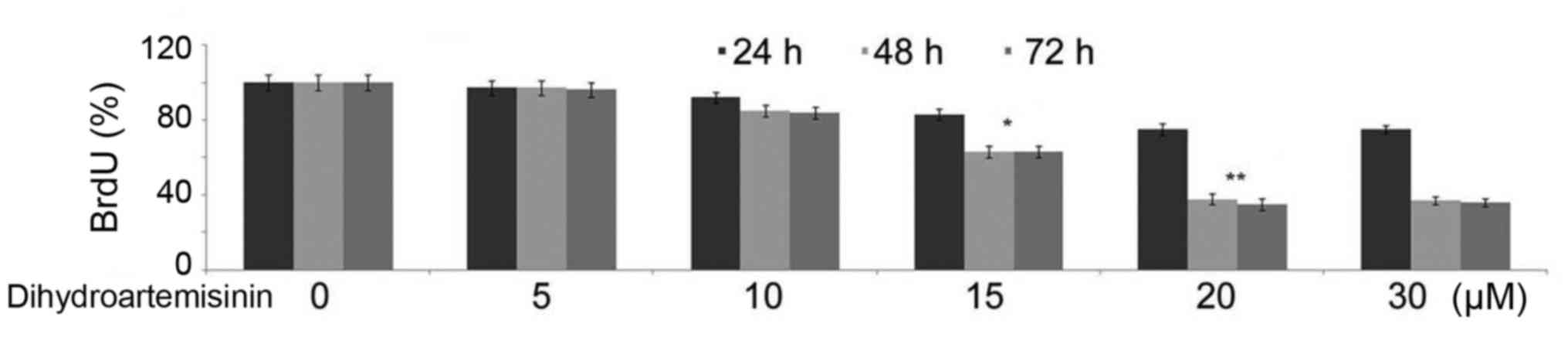
Suppression of BrdU-labeling index
(LI) in C6 glioma cells by treatment with dihydroartemisinin
Incubation of C6 glioma cells with 10, 15, 20 or 30
µM dihydroartemisinin for 48 h led to a reduction in the BrdU-LI
compared with the control cultures (Fig. 2). The BrdU-LI positive C6 glioma
cell population was reduced to 37% in the cultures treated with 20
µM dihydroartemisinin for 48 h compared with the control cultures.
Reduction in the BrdU-LI indicated a decrease in the uptake of BrdU
by C6 glioma cells.
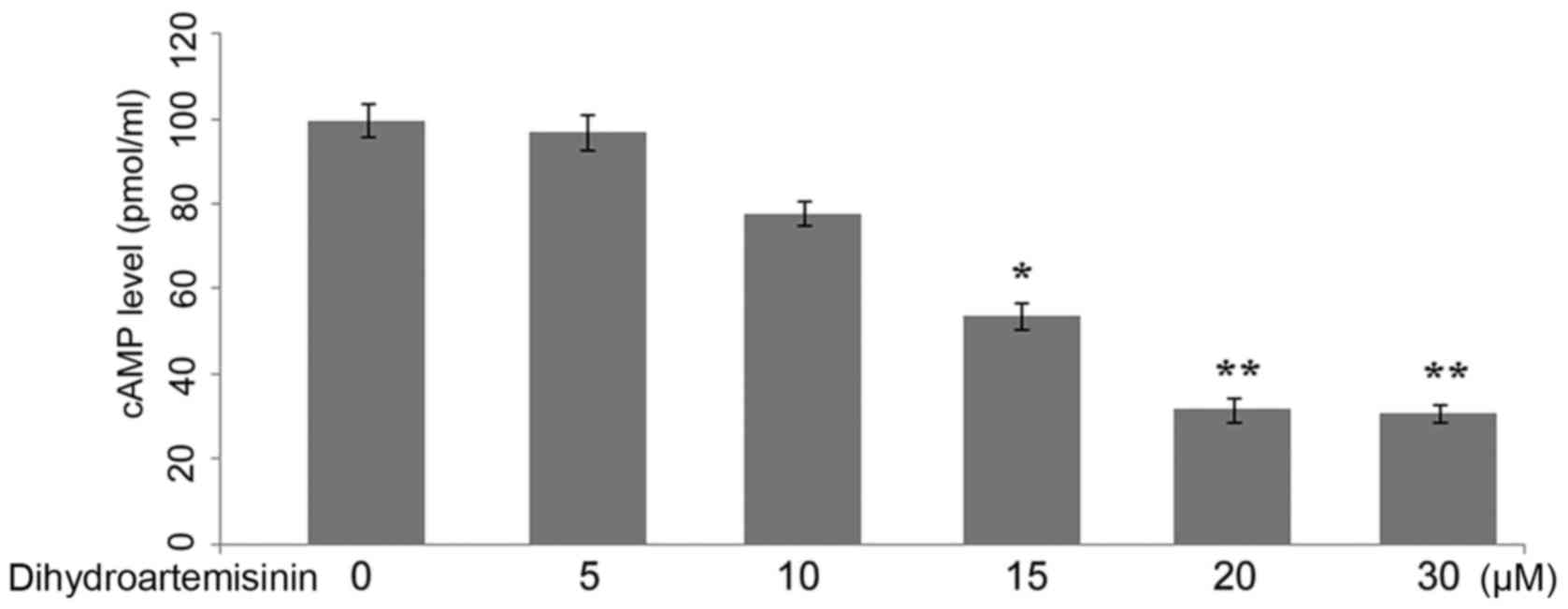
cAMP level reduction by treatment with
dihydroartemisinin in C6 glioma cells
In the control (treated with DMSO alone) C6 glioma
cells, the level of cAMP was demonstrated to be increased compared
with the cells treated with dihydroartemisinin. Incubation of the
C6 glioma cells with dihydroartemisinin led to a
concentration-dependent reduction in the level of cAMP after 48 h
of treatment (Fig. 3). The level
of cAMP was reduced significantly in C6 glioma cells on incubation
with 20 µM dihydroartemisinin for 48 h (P<0.01).
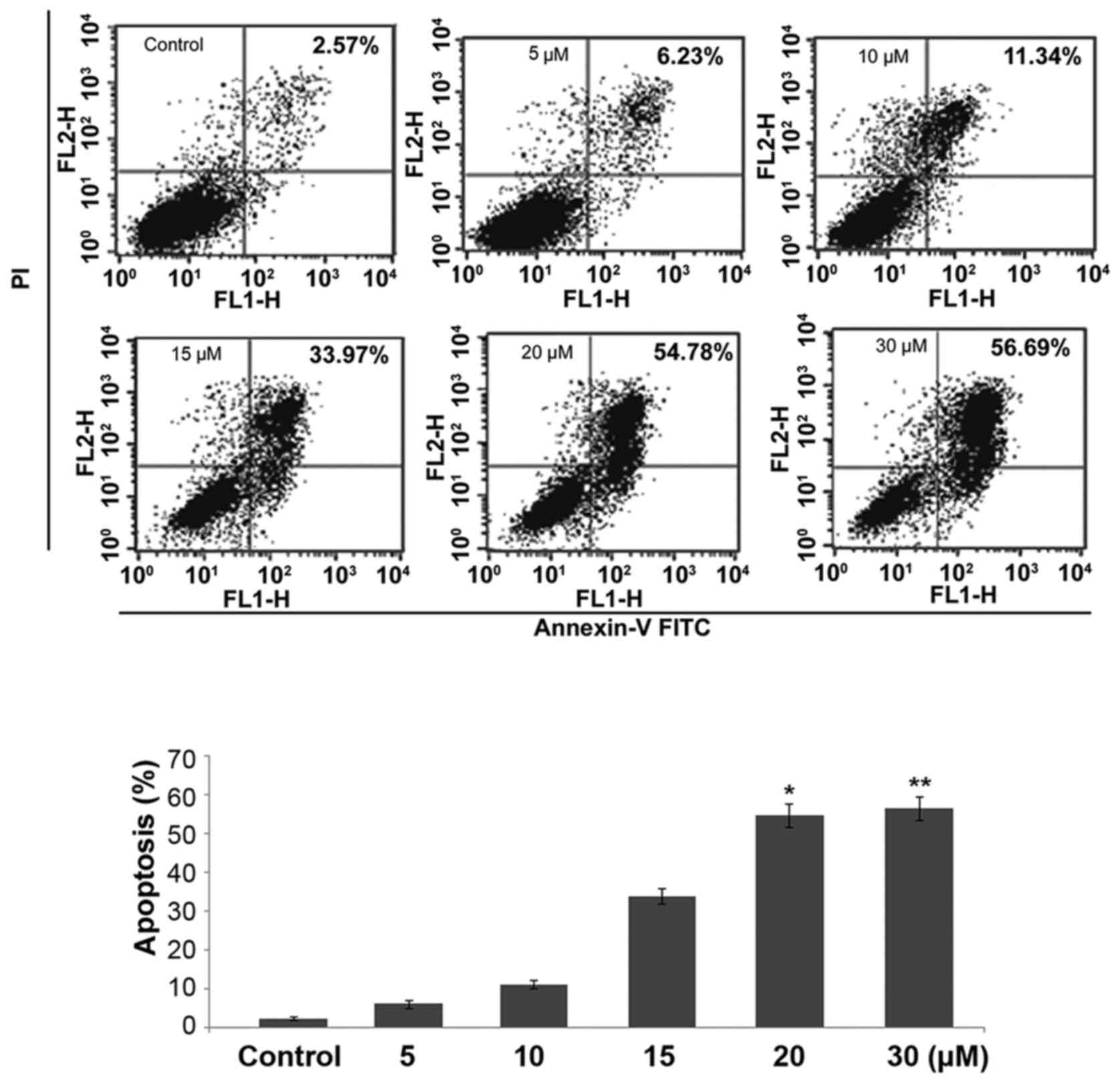
Apoptosis induction by treatment with
dihydroartemisinin in C6 glioma cells
Incubation of C6 glioma cell cultures with a range
of dihydroartemisinin concentrations for 48 h caused an increase in
the percentage of apoptotic cells compared with the control. The
percentage of apoptotic cells in the cultures incubated with 20 µM
concentration of dihydroartemisinin was demonstrated to be 54.78%
compared with 2.57% in the control cultures (Fig. 4). At 5, 10, 15, 20 or 30 µM
concentrations of dihydroartemisinin the population of apoptotic
cells was demonstrated to be 6.23, 11.34, 33.97, 54.78 and 56.69%,
respectively.
Treatment with dihydroartemisinin
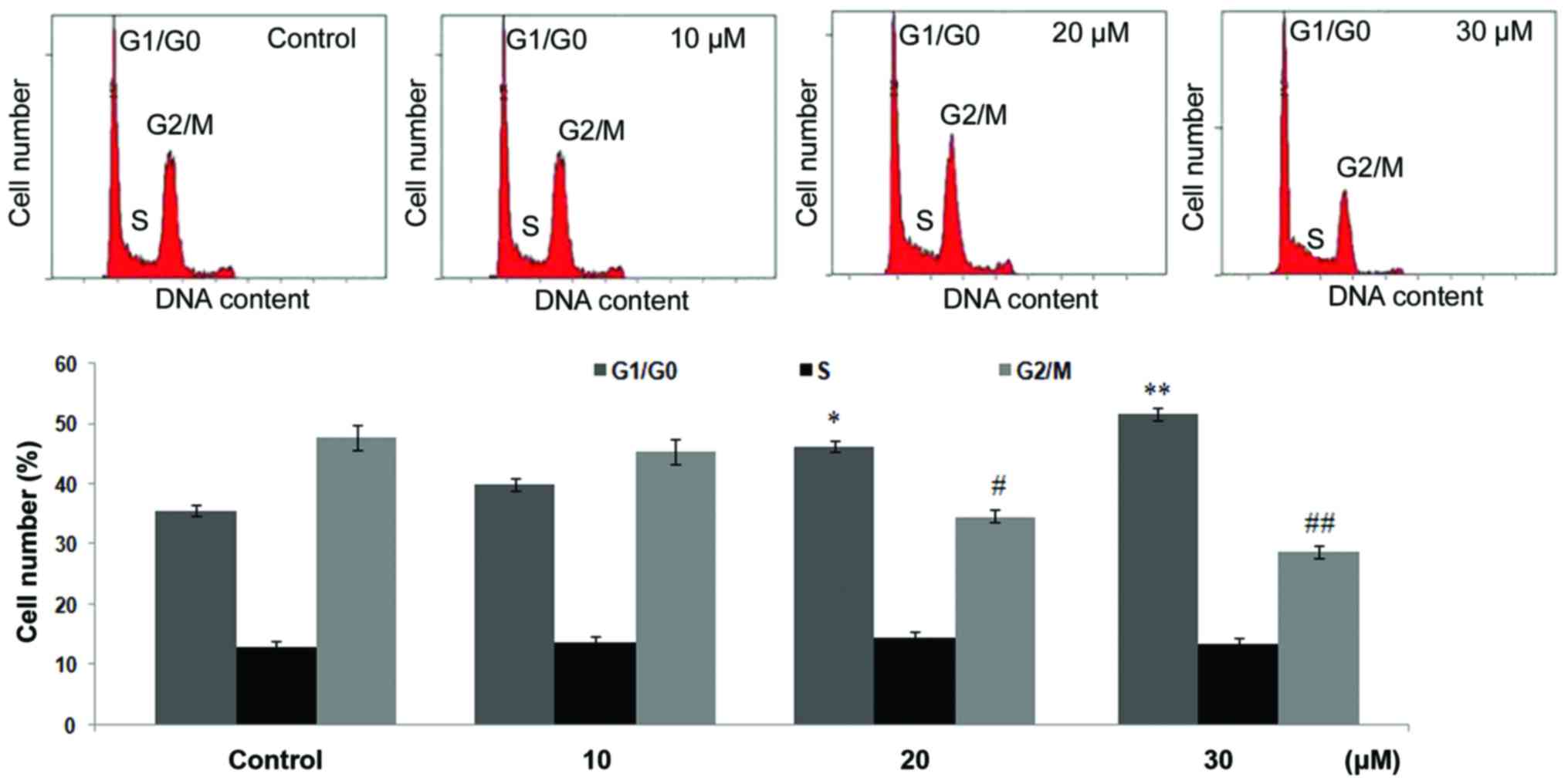
causes cell cycle arrest at G0/G1 phase
Incubation of the C6 glioma cells with
dihydroartemisinin for 48 h led to a significant reduction in the
percentage of cells in G2/M phase with an increase in cells in
G0/G1 phase (P<0.005; Fig. 5).
The population of cells in G2/M phase at 10, 20 and 30 µM
concentrations of dihydroartemisinin was demonstrated to be 45.43,
34.67 and 28.79%, respectively compared with 47.67% in the control.
Dihydroartemisinin at 10, 20 and 30 µM concentrations increased the
G0/G1 phase cell population to 39.98, 46.32 and 51.65%,
respectively in comparison with 35.64% in the control. In S phase
the cell population was demonstrated to be 12.98, 13.67 and 14.59%,
respectively at 10, 20 and 30 µM dihydroartemisinin compared with
13.45% in the control (Fig.
5).
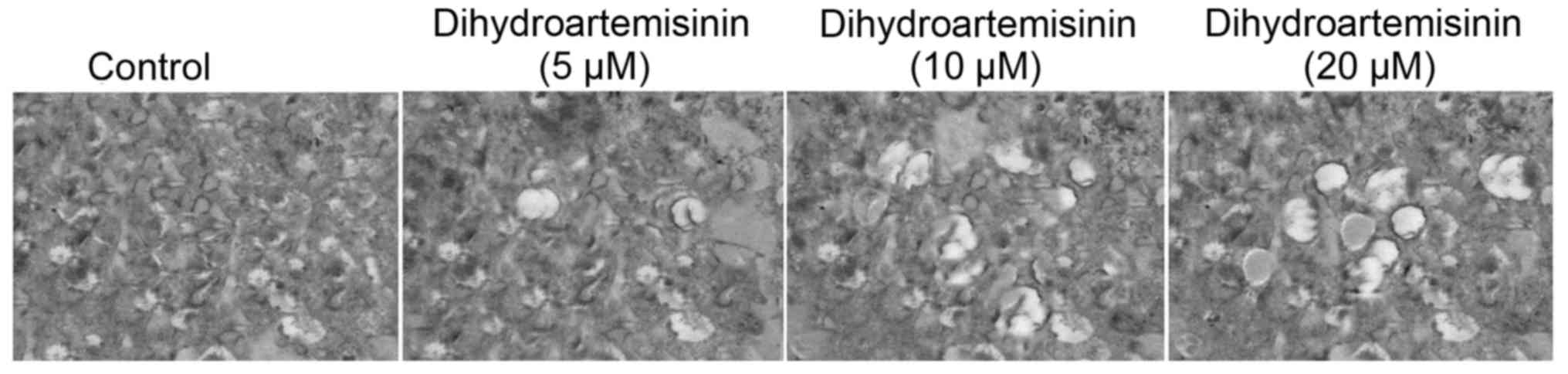
Assessment of cell morphology by
SEM
The control cells exhibited a spindle-shaped
morphology and were actively undergoing mitosis following 48 h of
culture. The cells treated with dihydroartemisinin were round with
small surface extensions (Fig.
6).
Discussion
Efficient treatment of glioblastoma multiforme is a
challenge for clinicians worldwide. Evaluation of natural products
has led to the identification of a number of compounds, which
exhibit an inhibitory effect on the growth of glioma cells
(16–18). In the present study, the effect of
dihydroartemisinin on C6 glioma cell proliferation was studied. The
present study demonstrated that dihydroartemisinin treatment
reduced the proliferation of C6 glioma cells, reduced the
proportion of cells in S phase, reduced the level of cAMP and
induced apoptosis.
The results of the present study demonstrated that
dihydroartemisinin treatment inhibits the growth of C6 glioma cells
with maximum inhibition 48 h following treatment. Analysis of the
effect of dihydroartemisinin on the cell cycle revealed cells
arrested in G0/G1 phase. Treatment of C6 glioma cells with
dihydroartemisinin increased the percentage of cells in G0/G1 phase
with a simultaneous decrease in cells in G2/M phase. Earlier
studies have demonstrated that platelet-derived growth factor
receptors (PDGFRs) cause an increase in the levels of cAMP on
activation (19,20). A higher rate of proliferation of C6
glioma cells has been demonstrated to be associated with PDGFs.
cAMP is produced mainly in cellular mitochondria from ATP (21,22).
In glioma cells the presence of native β-adrenoreceptors (ADRs) and
functional PDGFRs causes proliferation by increasing the level of
cAMP (19,20). The α and β-ADRs, which are coupled
to G-proteins, lead to the accumulation of cAMP in the cells. In
the present study, a reduction in the level of cAMP was
demonstrated by dihydroartemisinin 48 h following treatment.
Therefore, a reduction in the level of cAMP in C6 glioma cells
following treatment with dihydroartemisinin may be the involved in
the inhibition of cell growth. Inhibition of cell proliferation
indicated that the effect of dihydroartemisinin on the induction of
cell apoptosis should be examined further. The results from Annexin
V-FITC/PI staining demonstrated an increase in the percentage of
apoptotic cells following 48 h of incubation with
dihydroartemisinin.
Alterations in the permeability of the mitochondrial
membrane causes the release of cytochrome c and the activation of
caspases, resulting in apoptosis (23–25).
In the present study results from SEM demonstrated rounding of
cells with the formation of small surface projections on treatment
with dihydroartemisinin for 48 h. In conclusion, treatment of the
glioma cells with dihydroartemisinin exhibits antitumor effects by
induction of apoptosis. Therefore, it may be valuable to evaluate
dihydroartemisinin in animal models for the treatment of
glioma.
References
|
1
|
Curran WJ Jr, Scott CB, Horton J, Nelson
JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO,
Krisch RE, et al: Recursive partitioning analysis of prognostic
factors in three Radiation Therapy Oncology Group malignant glioma
trials. J Natl Cancer Inst. 85:704–710. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: Genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang SM, Lamborn KR, Malec M, Larson D,
Wara W, Sneed P, Rabbitt J, Page M, Nicholas MK and Prados MD:
Phase II study of temozolomide and thalidomide with radiation
therapy for newly diagnosed glioblastoma multiforme. Int J Radiat
Oncol Biol Phys. 60:353–357. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fine HA, Wen PY, Maher EA, Viscosi E,
Batchelor T, Lakhani N, Figg WD, Purow BW and Borkowf CB: Phase II
trial of thalidomide and carmustine for patients with recurrent
highgrade gliomas. J Clin Oncol. 21:2299–2304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marx GM, Pavlakis N, McCowatt S, Boyle FM,
Levi JA, Bell DR, Cook R, Biggs M, Little N and Wheeler HR: Phase
II study of thalidomide in the treatment of recurrent glioblastoma
multiforme. J Neurooncol. 54:31–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meshnick SR: Artemisinin: Mechanisms of
action, resistance and toxicity. Int J Parasitol. 32:1655–1660.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Neill PM: Medicinal chemistry: A worthy
adversary for malaria. Nature. 430:838–839. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI
|
|
9
|
Huang XJ, Ma ZQ, Zhang WP, Lu YB and Wei
EQ: Dihydroartemisinin exerts cytotoxic effects and inhibits
hypoxia inducible factor-1alpha activation in C6 glioma cells. J
Pharm Pharmacol. 59:849–856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nam W, Tak J, Ryu JK, Jung M, Yook JI, Kim
HJ and Cha IH: Effects of artemisinin and its derivatives on growth
inhibition and apoptosis of oral cancer cells. Head Neck.
29:335–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh NP and Lai H: Selective toxicity of
dihydroartemisinin and holotransferrin toward human breast cancer
cells. Life Sci. 70:49–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen T, Li M, Zhang R and Wang H:
Dihydroartemisinin induces apoptosis and sensitizes human ovarian
cancer cells to carboplatin therapy. J Cell Mol Med. 13:1358–1370.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Reilly T, Wartmann M, Maira SM,
Hattenberger M, Vaxelaire J, Muller M, Ferretti S, Buchdunger E,
Altmann KH and McSheehy PM: Patupilone (epothilone B, EPO906) and
imatinib (STI571, Glivec) in combination display enhanced
antitumour activity in vivo against experimental rat C6 glioma.
Cancer Chemother Pharmacol. 55:307–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lokker NA, Sullivan CM, Hollenbach SJ,
Israel MA and Giese NA: Platelet-derived growth factor (PDGF)
autocrine signaling regulates survival and mitogenic pathways in
glioblastoma cells: Evidence that the novel PDGF-C and PDGF-D
ligands may play a role in the development of brain tumors. Cancer
Res. 62:3729–3735. 2002.PubMed/NCBI
|
|
15
|
Kaygisiz Z, Erkasap N, Yazihan N, Sayar K,
Ataoglu H, Uyar R and Ikizler M: Erythropoietin changes
contractility, cAMP, and nitrite levels of isolated rat hearts. J
Physiol Sci. 56:247–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buchdunger E, Cioffi CL, Law N, Stover D,
Ohno-Jones S, Druker BJ and Lydon NB: Abl protein-tyrosine kinase
inhibitor STI571 inhibits in vitro signal transduction mediated by
c-kit and platelet-derived growth factor receptors. J Pharmacol Exp
Ther. 295:139–145. 2000.PubMed/NCBI
|
|
17
|
Yu C, Krystal G, Dent P and Grant S:
Flavopiridol potentiates STI571-induced mitochondrial damage and
apoptosis in BCR-ABL-positive human leukemia cells. Clin Cancer
Res. 8:2976–2984. 2002.PubMed/NCBI
|
|
18
|
Uziel O, Fenig E, Nordenberg J, Beery E,
Reshef H, Sandbank J, Birenbaum M, Bakhanashvili M, Yerushalmi R,
Luria D and Lahav M: Imatinib mesylate (Gleevec) downregulates
telomerase activity and inhibits proliferation in
telomerase-expressing cell lines. Br J Cancer. 92:1881–1891. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sokołowska P and Nowak JZ: Constitutive
activity of beta-adrenergic receptors in C6 glioma cells. Pharmacol
Rep. 57:659–663. 2005.PubMed/NCBI
|
|
20
|
Grobben B, De Deyn PP and Slegers H: Rat
C6 glioma as experimental model system for the study of
glioblastoma growth and invasion. Cell Tissue Res. 310:257–270.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jaiswal BS and Conti M: Calcium regulation
of the soluble adenylyl cyclase expressed in mammalian spermatozoa.
Proc Natl Acad Sci USA. 100:pp. 10676–10681. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baillie GS and Houslay MD: Arrestin times
for compartmentalised cAMP signalling and phosphodiesterase-4
enzymes. Curr Opin Cell Biol. 17:129–134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antonsson B and Martinou JC: The Bcl-2
protein family. Exp Cell Res. 256:50–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salvesen GS and Dixit VM: Caspases:
Intracellular signaling by proteolysis. Cell. 91:443–446. 1997.
View Article : Google Scholar : PubMed/NCBI
|