Introduction
Breast cancer is a major health concern for women,
and its incidence is rapidly increasing worldwide (1). The latest statistical data from the
National Cancer Center reported that there were ~268,600 new cases
of breast cancer and 69,500 breast cancer-associated mortalities in
females in China in 2015. In addition, breast cancer has the
highest incidence among gynecological carcinomas (2). Radiotherapy has an important role in
the comprehensive treatment of breast cancer, in addition to
surgery, chemotherapy and endocrine therapy. The majority of
patients diagnosed with early-stage breast cancer prefer
conservative surgery followed by radiotherapy rather than
mastectomy due to the low incidence of injury associated with the
former treatment, which has become the primary strategy to cure
breast cancer (3,4). However, the clinical outcome of
adjuvant radiotherapy may be limited due to intrinsic
radioresistance that directly influences the prognosis and survival
of patients. Therefore, it is necessary to investigate efficient
radiosensitization methods that improve the clinical outcome of
patients undergoing radiotherapy.
Recently, various studies have focused on targeted
therapy, including the use of phosphatidylinositol 3-kinase (PI3K),
mechanistic target of rapamycin (mTOR) and γ-secretase inhibitors
in breast cancer (5–7). mTOR belongs to the family of
serine/threonine protein kinases, and its two subtypes, mTORC1 and
mTORC2, differ structurally (8).
mTORC1 directly activates eukaryotic translation initiation factor
4E binding protein 1 (4EBP1) and ribosomal protein S6 kinase β1 to
facilitate translation, the cell cycle and angiogenesis, while
mTORC2 induces the phosphorylation of Akt at serine 473 to further
activate the PI3K/Akt/mTOR pathway in a positive feedback
manner.
mTOR, as one of the major downstream components of
the PI3K/Akt pathway, is functionally involved in protein
translation and is involved in various biological processes,
including tumor survival, proliferation and autophagy. mTOR is
reported to be excessively activated in several tumor types as a
result of hotspot mutations in
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
(PIK3CA) (9–11). Therefore, mTOR has become a
potential therapeutic target, particularly in breast cancer that
harbors hotspot mutations in PIK3CA. Several clinical trials have
demonstrated superior antitumor activity of different mTOR
inhibitors in patients with breast cancer, and everolimus has been
approved by the FDA for the treatment of advanced breast cancer
(12).
Torin2 is a novel, second-generation ATP-competitive
mTOR inhibitor that demonstrates dual criteria as it suppresses
mTORC1 and mTORC2, and also reduces the DNA damage repair response
(13). Several preclinical studies
have reported decreased proliferation and migration induced by
Torin2 in lung cancer, hepatocarcinoma, thyroid cancer and
epithelial ovarian cancer (14–16).
However, it is unclear whether Torin2 may enhance the
radiosensitivity of cancer cell lines, particularly in breast
cancer where PIK3CA mutations occur at a high frequency. Therefore,
the present study aimed to investigate the effect of Torin2 on the
radiosensitivity of breast cancer cells and to explore the
associated underlying mechanism of this synergy.
Materials and methods
Cell culture and inhibitor
MCF-7 and MDA-MB-231 human breast cancer cells were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's
medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Biological Industries
Israel Beit Haemek Ltd., Beit Haemek, Israel), 100 µg/ml penicillin
and 100 mg/ml streptomycin in a humidified incubator at 37°C with
5% CO2. Exponentially growing cells were trypsinized for
subculture or for further experiments. The selective mTOR
inhibitor, Torin2 (cat. no. SML1224), was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell Counting Kit-8 (CCK-8) assay
Exponentially growing MCF-7 and MDA-MB-231 cells
were seeded in 100 µl media consist of DMEM with 10% FBS at a
density of 5×103 cells/well in 96-well plates and were
incubated overnight at 37°C. The following day, cells were treated
with 0, 25, 50, 100, 200, 400 and 800 nM Torin2 for 48 h at 37°C;
wells containing cells and medium only were used as controls.
Colorimetric readings were taken at 450 nm following incubation
with CCK-8 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) for 2 h
at 37°C. All assays were performed independently at least three
times. The 20 and 50% inhibitory concentration (IC20 and
IC50) values for each cell line were calculated using
the median-effect equation derived by Chou (17).
Clonogenic assays
MCF-7 cells were seeded at the density of
1×105 cells/ml in 30-mm dishes and allowed to adhere in
the incubator overnight at 37°C. Cells were subsequently pretreated
with or without 20 nM Torin2 (close to IC20 value) for 1
h prior to irradiation at 0–10 Gy ≤1 min. Cells were incubated for
a further 24 h at 37°C and trypsinized to plate in 60-mm dishes,
with 3×103 cells per dish, for a period of 2 weeks. The
colonies that formed were stained with 0.1% crystal violet
(Beyotime Institute of Biotechnology, Haimen, China) at room
temperature for 2 h, and colonies with >50 cells were counted
using Quantity One software version 4.4.0 (Bio-Rad Laboratories,
Inc.). A linear-quadratic model was used to evaluate their
radiosensitivity (18).
Flow cytometry analysis
MCF-7 cells at the density of 1×105
cells/ml were seeded in 60-mm dishes allowed to adhere overnight at
37°C and were treated with or without Torin2 at a concentration of
20 nM for 1 h at 37°C prior to irradiation with X-rays at 2 or 4 Gy
≤30 sec. Cells were subsequently washed twice with PBS and
suspended with pre-cooled 70% alcohol for fixing overnight.
Following digestion with 100 µg/ml RNaseA at 37°C for 30 min, 50
µg/ml propidium iodide (550825; BD Pharmingen; BD Biosciences, San
Diego, CA, USA) was added to the cells for 30 min at room
temperature and they were kept protected from light. The
fluorescence intensity of each cell was measured using a MoFlo XDP
flow cytometer with Kaluza Analysis Software version 1.3 (Beckman
Coulter, Inc., Brea, CA, USA).
Western blot analysis
MCF-7 cells were treated with 0, 200 and 400 nM
Torin2 for 2 h, or 200 nM Torin2 for 0, 2, 4 and 8 h at 37°C. In
another experiment, MCF-7 cells were treated with or without Torin2
at a concentration of 20 nM for 1 h at room temperature prior to
irradiation with X-rays at 0–10 Gy ≤1 min. Cells were lysed in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) on ice for 10 min 48 h after irradiation. Cell
lysates were centrifuged at 12,000 × g at 4°C for 15 min and the
supernatants were collected. Protein concentrations were measured
in the supernatant using a Pierce BCA Protein assay kit (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). A total of 50 µg
protein from each sample was separated by 10% SDS-PAGE and
transferred onto a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA), which was followed by blocking with 5% bovine
serum albumin (Bio-sharp, Hefei, China) at 37°C for 1 h, incubated
overnight at room temperature with the indicated primary antibodies
followed by incubation with corresponding secondary antibodies for
2 h at room temperature and detection using Pierce ECL Western
Blotting Substrate (Thermo Fisher Scientific, Inc.). β-tubulin was
used as the loading control. The signal intensity with background
correction was quantified using Quantity One software version 4.4.0
(Bio-Rad Laboratories, Inc.). The following antibodies were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA):
Phosphorylated (p)-Akt473 (cat. no. 9271, 1:1,000), Akt
(cat. no. 9272, 1:1,000), P-4EBP137/46 (cat. no. 2855,
1:1,000), 4EBP1 (cat. no. 9452, 1:1,000), γ-H2A histone family
member X (H2AX; cat. no. 9718, 1:1,000), p-ataxia
telangiectasia-mutated (ATM; cat. no. 5883, 1:1,000), p-ATR
serine/threonine kinase (ATR; cat. no. 2853, 1:1,000), β-tubulin
(cat. no. 2146, 1:5,000), horseradish peroxidase (HRP)-conjugated
goat anti-rabbit (cat. no. 7074, 1:5,000).
Statistical analysis
Data are presented as the mean ± standard deviation.
Each experiment was repeated at least three times. Significance was
determined using two-tailed unpaired Student's t-tests. All figures
and statistical analysis were performed with Origin version 8.0
(OriginLab, Northampton, MA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Torin2 inhibits cell proliferation by
downregulating the mTOR signaling pathway
CCK-8 assay results demonstrated that the viability
of MCF-7 and MDA-MB-231 cells was significantly decreased by Torin2
in a dose-dependent manner (Fig.
1A). However, Torin2 was more potent in MCF-7 cells, with
IC20 and IC50 values of 32.1 and 163.4 nM,
respectively, compared with MDA-MB-231 cells, which had
IC20 and IC50 values of 83.9 and 1012.8 nM,
respectively. In order to validate the drug-targeted effect,
western blot analysis was performed to evaluate the protein
expression of the major components of the Akt/mTOR pathway.
Consistent with the effects of Torin2 on cell growth, the basal
expression of p-Akt and P-4EBP1 were elevated in MCF-7 cells
compared with MDA-MB-231 cells (Fig.
1B). As demonstrated in Fig.
1C, p-Akt473 and P-4EBP137/46 expression
was abolished when MCF-7 cells were treated with 200 or 400 nM
Torin2 for 2 h. Furthermore, p-Akt473 and
P-4EBP137/46 expression was inhibited in MCF-7 cells by
treatment with 200 nM Torin2 for up to 8 h (Fig. 1D). As 4EBP1 and Akt are direct
substrates of the enzymes mTORC1 and mTORC2, respectively, these
results indicate that Torin2 may reduce the viability of MCF-7
cells by diminishing mTOR activity, therefore, MCF-7 cells were
selected for subsequent experiments.
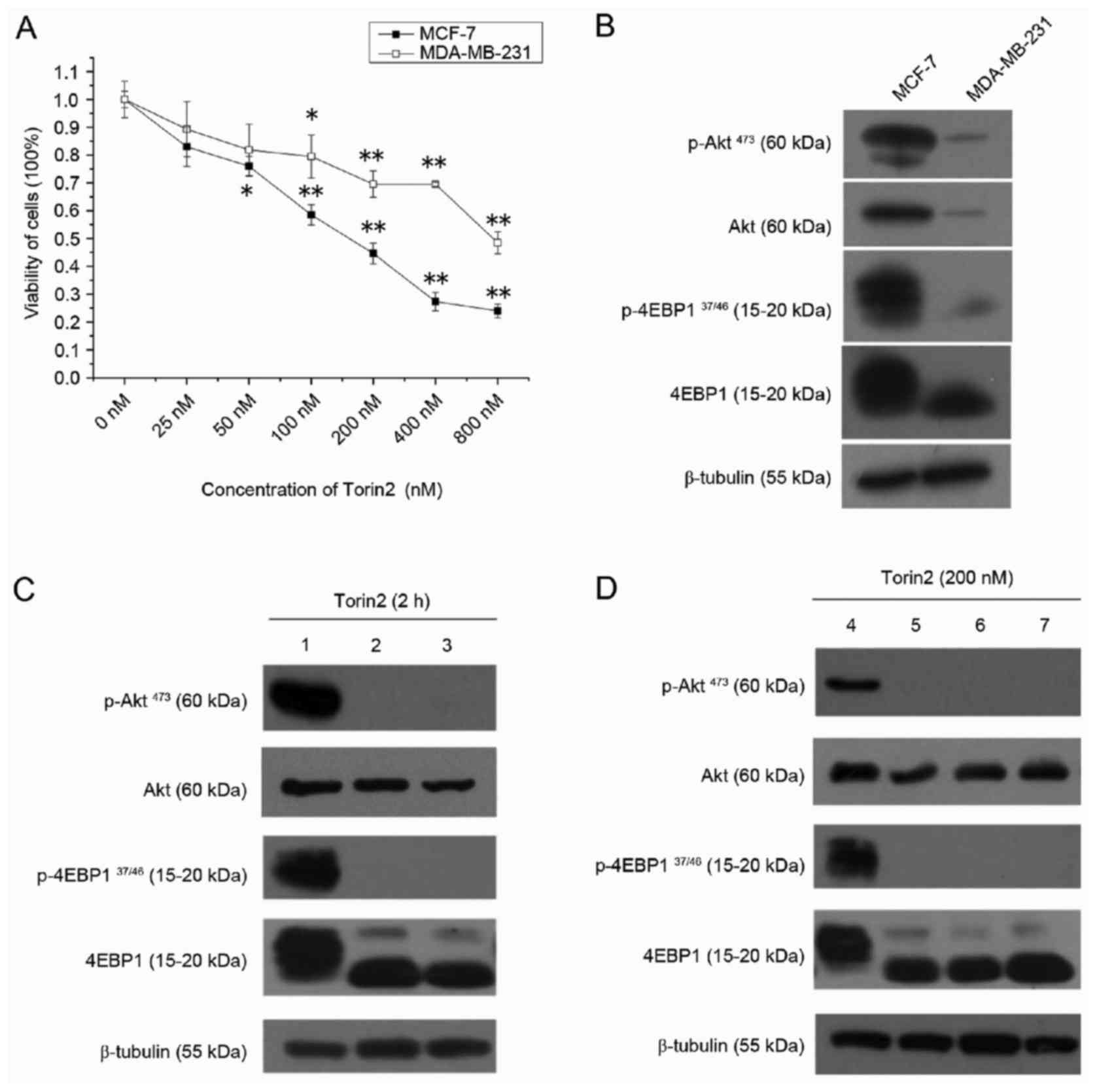 | Figure 1.Torin2 suppresses cell viability and
mTOR signaling pathway in breast cancer cells. (A) Viability assay
for MCF-7 and MDA-MB-231 cell lines treated with 0–800 nM Torin2
for 48 h. (B) Protein expression of major components of the mTOR
signaling pathway under routine culture conditions in MCF-7 and
MDA-MB-231 cell lines. (C) MCF-7 cells were exposed to 0, 200 and
400 nM Torin2 for 2 h and western blotting was performed. Lanes 1,
2 and 3 present results for cells treated with 0, 200 and 400 nM
Torin2, respectively. (D) MCF-7 cells were exposed to 200 nM Torin2
for 0, 2, 4 and 8 h and western blotting was performed. Lanes, 4,
5, 6 and 7 present results for cells treated with Torin2 for 0, 2,
4 and 8 h, respectively. β-tubulin was used as a loading control.
*P<0.05 and **P<0.01 vs. 0 nM group. mTOR, mechanistic target
of rapamycin; p, phosphorylated; 4EBP1, eukaryotic translation
initiation factor 4E binding protein 1. |
Torin2 enhances the radiosensitivity
of MCF-7 cells
Colony formation assay results demonstrated that
treatment with 20 nM Torin2 prior to irradiation sensitized cells
to radiation compared with cells treated with radiation alone
(Fig. 2A). The parameters of α and
β calculated using the linear-quadratic model were 0.264±0.029
Gy−1 and 0.029±0.005 Gy−2, respectively, for
cells treated with a combination of radiation and Torin2, and
0.057±0.016 Gy−1 and 0.031±0.002 Gy−2,
respectively, for those treated with radiation alone (Fig. 2B). These data indicate that
pharmacological inhibition of mTOR using Torin2 enhanced the
radiosensitivity of MCF-7 cells in vitro.
Torin2 increases the level of γ-H2AX
induced by radiation
To investigate the potential mechanism of enhanced
radiosensitivity induced by Torin2, the protein expression of
p-H2AX, which is a well-established indicator of DNA double-strand
breaks (DSBs) induced by radiation, was evaluated in MCF-7 cells
treated with or without 20 nM Torin2 followed by an increasing dose
of radiation. Induction of γ-H2AX expression by irradiation in
MCF-7 cells was dose dependent, and the expression was markedly
elevated at 6 and 8 Gy (Fig. 3A).
However, compared with the induced expression of γ-H2AX in MCF-7
cells treated with radiation alone, expression was further induced
in cells that were pretreated with Torin2 (fold change, 4.34±0.56
and 10.02±1.00 at 6 and 10 Gy, respectively; Fig. 3B). These results indicate that
Torin2 may enhance the lethal effect of radiation on MCF-7 cells by
increasing DNA damage.
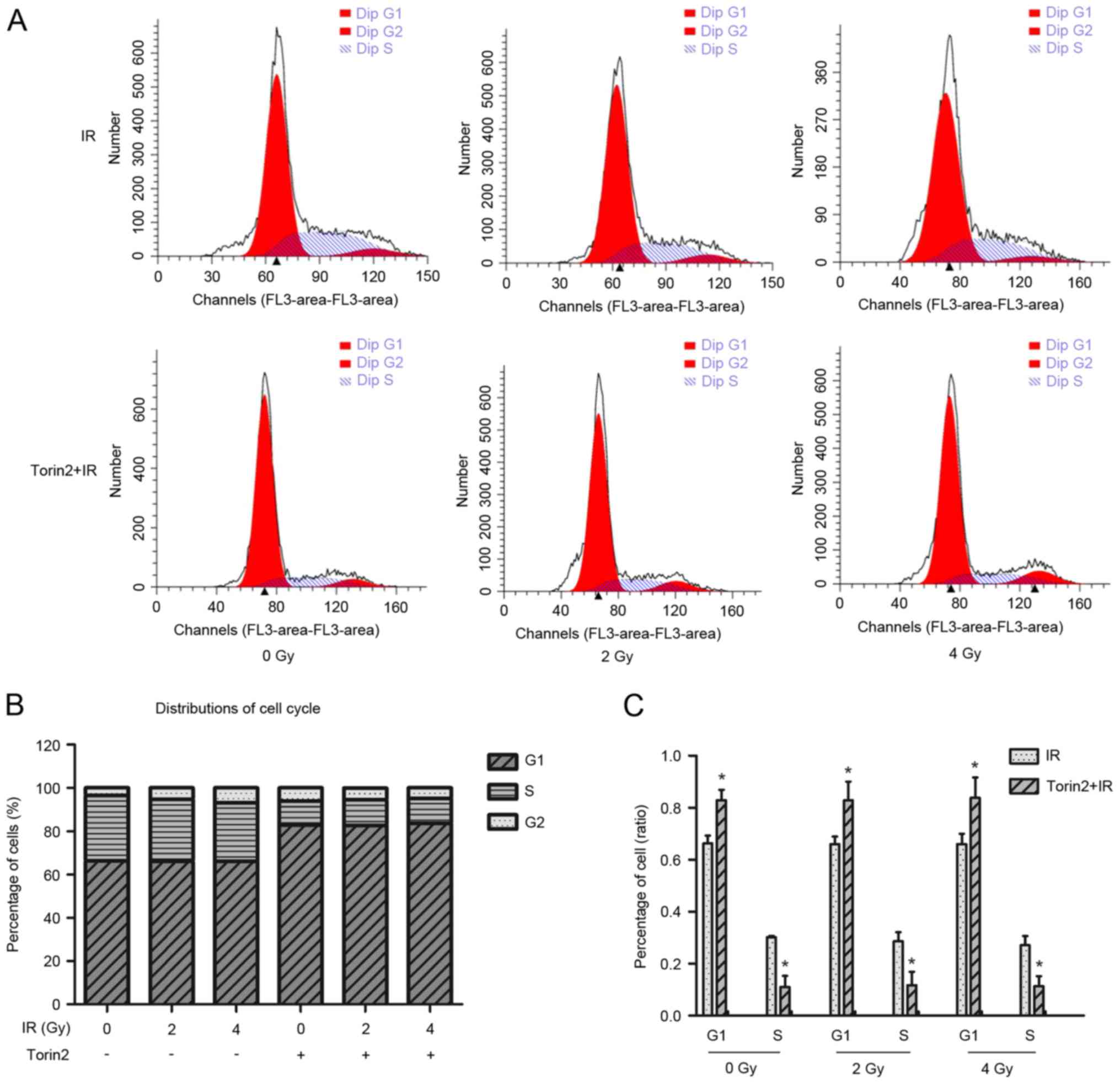
Torin2 induces cell cycle arrest in
the G1/S phase
Flow cytometry was performed to investigate the
effect of Torin2 on cell cycle arrest (Fig. 4A). The G1 phase cell population
increased by 16.57%, whereas the S phase population significantly
decreased by 19.17%, in MCF-7 cells treated with Torin2 alone
compared with control cells without Torin2 or irradiation treatment
(Fig. 4B). The percentage of cells
in G1 and S phases were significantly increased and decreased,
respectively, compared with cells treated with radiation alone at
different radiation doses (Fig.
4C). However, the combination of Torin2 and irradiation at 2
and 4 Gy did not enhance alterations in cell cycle distribution in
MCF-7 cells compared with cells treated with Torin2 alone.
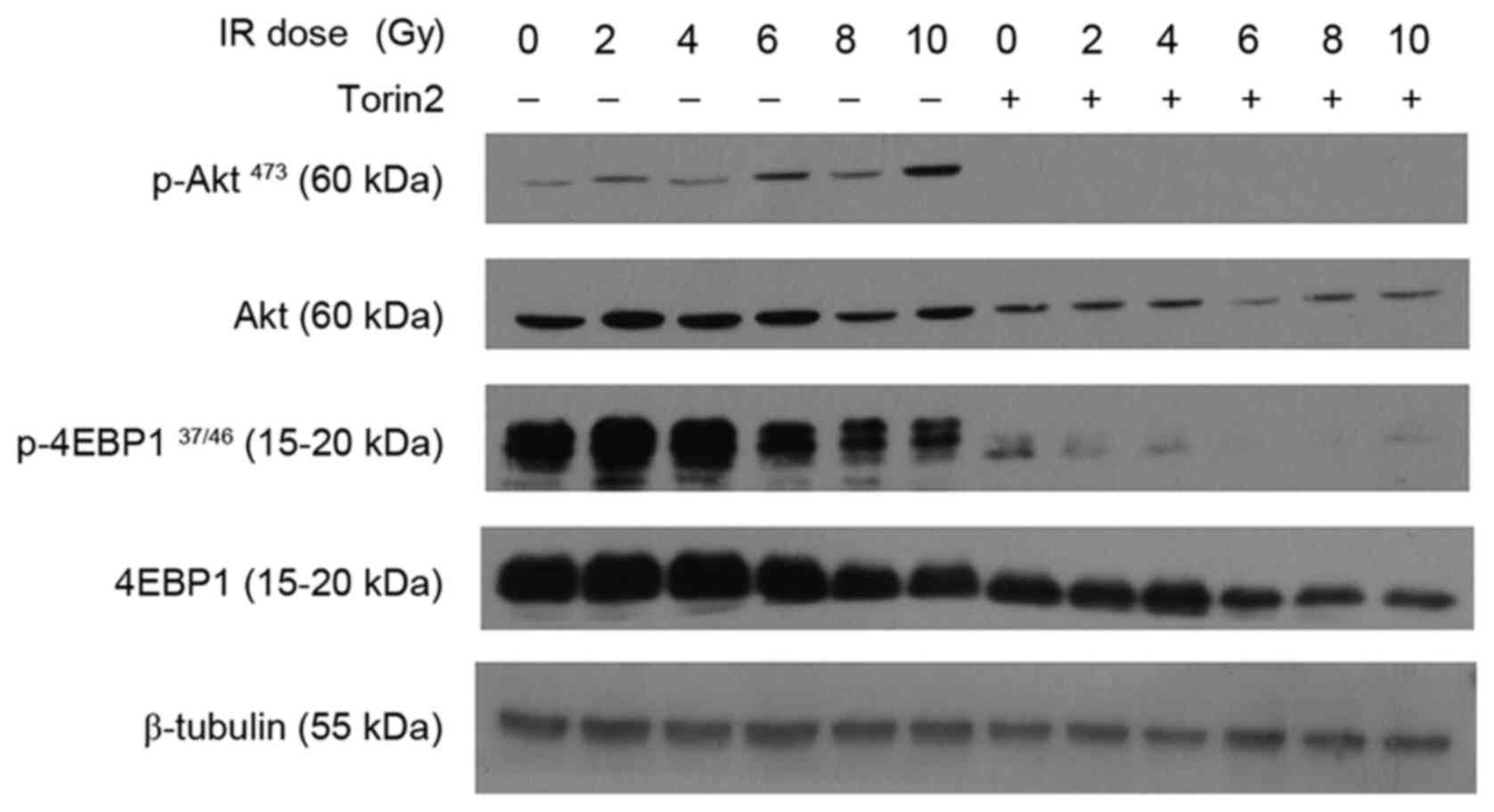
Torin2 downregulates the expression of
the mTOR signaling pathway
A previous study reported that hyperactivity of the
Akt/mTOR axis may confer radioresistance to glioblastoma cell lines
(19). In the present study,
radiation alone induced marginally elevated p-Akt expression in
MCF-7 cells at low doses, and markedly increased p-Akt expression
at higher doses (Fig. 5). P-4EBP1
was induced at low radiation doses and decreased when the dose was
increased between 6 and 10 Gy. Sustained inhibition of p-Akt and
almost complete downregulation of P-4EBP1 was observed in MCF-7
cells pretreated with Torin2. These results, combined with those
for γ-H2AX expression, indicate that Torin2 may enhance the
radiosensitivity of MCF-7 cells through inhibition of Akt/mTOR
signaling and subsequent impairment of DNA repair machinery.
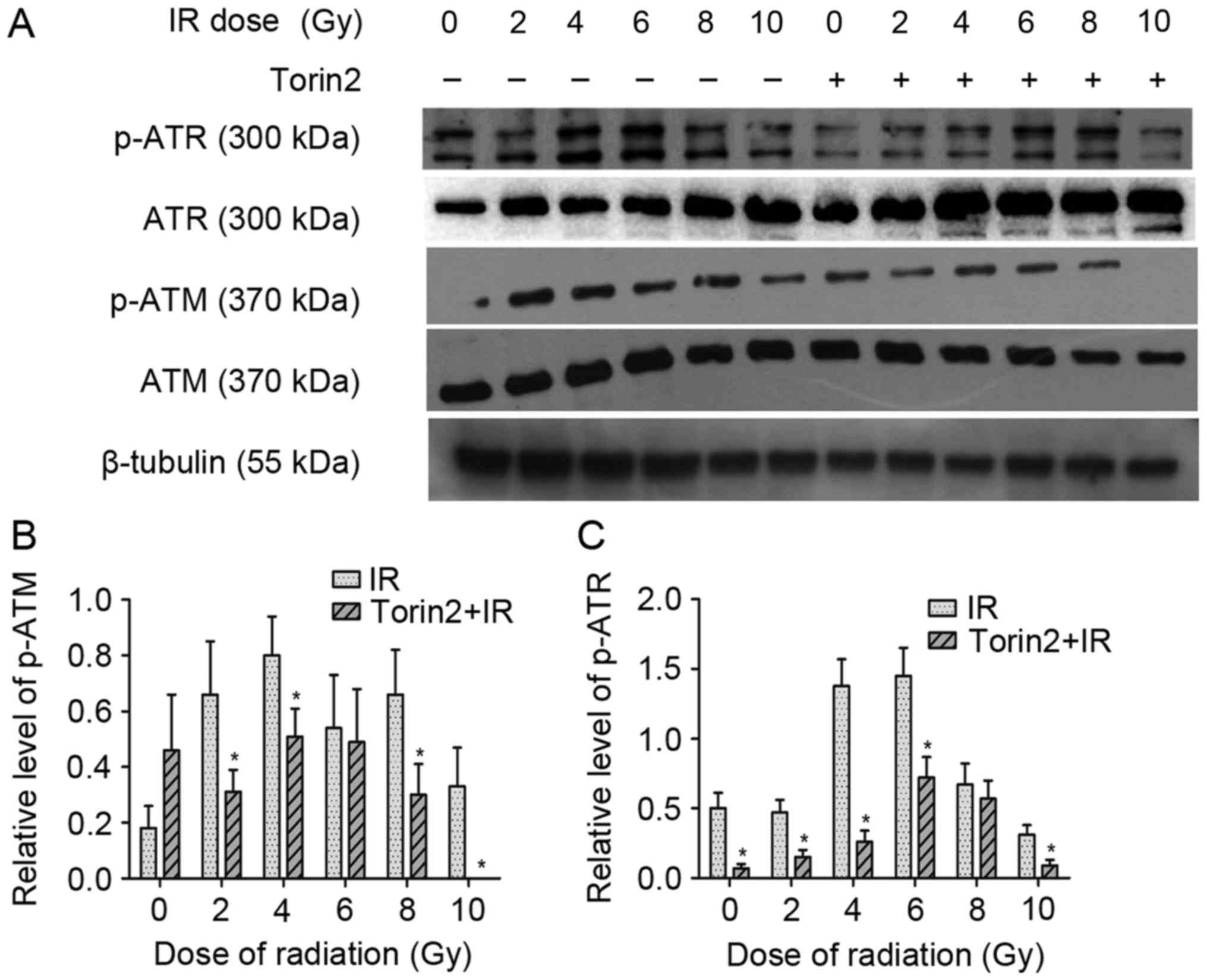
Torin2 sensitizes breast cancer cells
to radiation by attenuating DNA repair mechanisms
To confirm whether the attenuation of DNA repair
mechanisms induced by Torin2 contributes to radiosensitization of
MCF-7 cells, the present study analyzed the protein expression of
p-ATM and p-ATR (Fig. 6). The
results demonstrated that irradiation alone markedly enhanced the
expression of p-ATR. Although MCF-7 cells treated with radiation
alone and those pretreated with Torin2 exhibited a similar
expression of ATR and ATM, the expression of p-ATM and p-ATR in
Torin2-treated MCF-7 cells was significantly diminished compared
with the expression observed in MCF-7 cells treated with radiation
alone at the majority of radiation doses. These results
demonstrated that the effect of Torin2 on the radiosensitization of
MCF-7 cells may be partially attributed to impaired DSB repair due
to inactivation of the Akt/mTOR signaling pathway.
Discussion
Excessive activation of the PI3K/Akt/mTOR pathway
was reported in 30–50% cases of breast cancer, which may be
attributed to mutations in the oncogene PIK3CA (20–22).
Liu et al (23) reported
that the mTOR inhibitor INK128 radiosensitized breast cancer cell
lines. Consistent with the results of their study, the results of
the present study demonstrated that the novel mTOR inhibitor Torin2
inhibited the proliferation and enhanced the radiosensitivity of
MCF-7 cells. Torin2 also markedly inhibited the phosphorylation of
4EBP1 and Akt, which are direct substrates of mTORC1 and mTORC2,
respectively, indicating that the activities of mTORC1 and mTORC2
may be inhibited by Torin2. Unlike other traditional mTOR
inhibitors that inhibit only mTORC1, Torin2 inhibits both isoforms.
To the best of our knowledge, the present study is the first to
report that the novel mTOR inhibitor Torin2 inhibited the
proliferation and enhanced the radiosensitivity of breast cancer
cells. Furthermore, growth inhibition assay results also
demonstrated that MDA-MB-231 cells are more resistant Torin2 than
MCF-7 cells. MCF-7 cells belong to the luminal subtype of breast
cancer and are primarily characterized by a high frequency of
PIK3CA mutations, while MDA-MB-231 cells belong to the basal-like
subtype and exhibit the epithelial-mesenchymal transition phenotype
(24). Therefore, PI3K/Akt/mTOR is
a growth-dependent pathway in MCF-7 cells but not in MDA-MB-231
cells.
Research on genomics and proteomics has revealed
that the mechanisms of radioresistance in cancer are complex, and
involve the tumor microenvironment and genomic and epigenetic
alterations (25). Aberrant
activation of oncogenic signaling pathways such as the
PI3K/Akt/mTOR pathway has an important role in radioresistance. The
mechanisms of radioresistance due to activation of the
PI3K/Akt/mTOR pathway include intrinsic radioresistance, tumor cell
proliferation and hypoxia (26).
Activated mTOR induced by irradiation has been observed in several
cancer types, including lung and prostate cancer (8,27).
In the present study, upregulated mTOR activity, reflected by
phosphorylation status, was also observed in MCF-7 cells treated
with radiation alone. However, mTOR activity was suppressed in
MCF-7 cells treated with Torin2 prior to irradiation. Therefore,
the ability of Torin2 to enhance the radiosensitivity of MCF-7
cells may be partially attributed to reduced mTOR activity, which
reduces the response to DNA damage repair.
To further investigate the potential mechanisms of
radiosensitization by Torin2, flow cytometry analysis was performed
to evaluate the cell cycle progression in MCF-7 cells with or
without Torin2 pretreatment. The results demonstrated an increase
in the G1 phase cell population, which was accompanied by a reduced
proportion of cells in the S phase. Consistent with these results,
previous studies have reported that Akt inhibitors downregulated
the expression of cyclin D1, which led to G1/S arrest in various
cancer cells. It is well established that, compared with cells in
the S phase, those in the G1 phase are more sensitive to
irradiation (28–30). Therefore, radiosensitization may be
partially attributed to altered cell cycle distribution induced by
Torin2.
Cell membrane deconstruction and DNA damage induced
by irradiation, which enhance apoptosis, have important roles in
the anticancer effect of irradiation. Irradiation leads to DNA
single-strand breaks (SSBs) and DSBs, and the accumulation of DSBs
exerts lethal effects. ATR primarily participates in SSB repair,
whereas the ATM gene and DNA-dependent protein kinase C are
involved in the repair of DSBs through homologous recombination and
non-homologous end joining, respectively (31–33).
In the present study, increased γ-H2AX expression combined with
reduced p-ATR and p-ATM in MCF-7 cells treated with Torin2 and
irradiation was observed. These results indicate that
Torin2-enhanced radiosensitivity of MCF-7 cells may be partially
mediated by the suppression of ATR and ATM activity, and subsequent
diminished DNA repair capacity.
In conclusion, the present study demonstrated that
Torin2 inhibited the growth of MCF-7 cells and enhanced their
radiosensitivity. The enhanced radiosensitivity of MCF-7 cells by
Torin2 may be attributed to three factors: mTOR activity was
blocked by Torin2, which may lead to decreased radioresistance;
Torin2 induced G1/S arrest and inhibited cell proliferation, and
subsequently improved the radiosensitivity; and Torin2 enhanced the
radiosensitivity of MCF-7 cells by reducing the capacity for DNA
damage repair via inhibition of ATM and ATR activity. However, a
single breast cancer cell line was employed to obtain these results
in the present study, no animal model was used to validate the
results in vivo and association analysis with certain
genomic alterations was not performed. Therefore, further detailed
investigation of the radiosensitization mechanism of Torin2 is
required. However, the results of the present study may provide a
theoretical foundation to rationally utilize a combination of
irradiation and Torin2 therapy for breast cancer in clinical
practice.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81572959)
and the Chinese Society of Clinic Oncology (grant no.
Y-MX2015-041). The authors thank Medjaden Bioscience, Ltd. (Hong
Kong, SAR, P.R. China) for providing an English language
service.
References
|
1
|
Langlands FE, Horgan K, Dodwell DD and
Smith L: Breast cancer subtypes: Response to radiotherapy and
potential radiosensitisation. Br J Radiol. 86:201206012013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S4, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisher CM and Rabinovitch R: Frontiers in
radiotherapy for early-stage invasive breast cancer. J Clin Oncol.
32:2894–2901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HC, Kim SH, Suh YJ, Chung MJ, Kang DG,
Choi HJ and Lee JH: A prospective cohort study on postoperative
radiotherapy with TomoDirect using simultaneous integrated boost
technique in early breast cancer. Radiat Oncol. 9:2442014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Cosimo S, Bianchi GV, Bregni G and de
Braud F: Prognosis of women with early breast cancer and PIK3CA
mutations. Breast. 24:283–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guerrero-Zotano A, Mayer IA and Arteaga
CL: PI3K/AKT/mTOR: Role in breast cancer progression, drug
resistance and treatment. Cancer Metastasis Rev. 35:515–524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mamaeva V, Niemi R, Beck M, Özliseli E,
Desai D, Landor S, Gronroos T, Kronqvist P, Pettersen IK, McCormack
E, et al: Inhibiting notch activity in breast cancer stem cells by
glucose functionalized nanoparticles carrying gamma-secretase
Inhibitors. Mol Ther. 24:926–936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang L, Graham PH, Ni J, Hao J, Bucci J,
Cozzi PJ and Li Y: Targeting PI3K/Akt/mTOR signaling pathway in the
treatment of prostate cancer radioresistance. Crit Rev Oncol
Hematol. 96:507–517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
García-Carracedo D, Villaronga MÁ,
Álvarez-Teijeiro S, Hermida-Prado F, Santamaría I, Allonca E,
Suárez-Fernández L, Gonzalez MV, Balbín M, Astudillo A, et al:
Impact of PI3K/AKT/mTOR pathway activation on the prognosis of
patients with head and neck squamous cell carcinomas. Oncotarget.
7:29780–29793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loi S, Michiels S, Baselga J, Bartlett JM,
Singhal SK, Sabine VS, Sims AH, Sahmoud T, Dixon JM, Piccart MJ and
Sotiriou C: PIK3CA genotype and a PIK3CA mutation-related gene
signature and response to everolimus and letrozole in estrogen
receptor positive breast cancer. PLoS One. 8:e532922013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Hu H, Pan Y, Wang R, Li Y, Shen L,
Yu Y, Li H, Cai D, Sun Y and Chen H: PIK3CA mutations frequently
coexist with EGFR/KRAS mutations in non-small cell lung cancer and
suggest poor prognosis in EGFR/KRAS wildtype subgroup. PLoS One.
9:e882912014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moulder SL, Rivera E, Ensor J,
Gonzalez-Angulo AM, Cristofanilli M, Murray JL, Booser D, Giordano
SH, Brewster A, Moore J, et al: Phase I trial of escalating doses
of weekly everolimus (RAD001) in combination with docetaxel for the
treatment of metastatic breast cancer (MBC). J Clin Oncol.
118:2378–2384. 2012.
|
|
13
|
Liu Q, Xu C, Kirubakaran S, Zhang X, Hur
W, Liu Y, Kwiatkowski NP, Wang J, Westover KD, Gao P, et al:
Characterization of Torin2, an ATP-competitive inhibitor of mTOR,
ATM, and ATR. Cancer Res. 73:2574–2586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hussain AR, Al-Romaizan M, Ahmed M,
Thangavel S, Al-Dayel F, Beg S, Uddin S, Siraj AK and Al-Kuraya KS:
Dual targeting of mTOR activity with Torin2 potentiates anticancer
effects of cisplatin in epithelial ovarian cancer. Mol Med.
21:466–478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sadowski SM, Boufraqech M, Zhang L, Mehta
A, Kapur P, Zhang Y, Li Z, Shen M and Kebebew E: Torin2 targets
dysregulated pathways in anaplastic thyroid cancer and inhibits
tumor growth and metastasis. Oncotarget. 6:18038–18049. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Wang X, Su Z, Fei H, Liu X and Pan
Q: The novel mTOR inhibitor Torin-2 induces autophagy and
downregulates the expression of UHRF1 to suppress hepatocarcinoma
cell growth. Oncol Rep. 34:1708–1716. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mukherjee B, Tomimatsu N, Amancherla K,
Camacho CV, Pichamoorthy N and Burma S: The Dual PI3K/mTOR
inhibitor NVP-BEZ235 is a potent inhibitor of ATM- and
DNA-PKCs-mediated DNA damage responses. Neoplasia. 14:34–43. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cejalvo JM, Perez-Fidalgo JA, Ribas G,
Burgués O, Mongort C, Alonso E, Ibarrola-Villava M, Bermejo B,
Martínez MT, Cervantes A and Lluch A: Clinical implications of
routine genomic mutation sequencing in PIK3CA/AKT1 and
KRAS/NRAS/BRAF in metastatic breast cancer. Breast Cancer Res
Treat. 160:69–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Basho RK, Gagliato DM, Ueno NT, Wathoo C,
Chen H, Shariati M, Wei C, Alvarez RH, Moulder SL, Sahin AA, et al:
Clinical outcomes based on multigene profiling in metastatic breast
cancer patients. Oncotarget. 7:76362–76373. 2016.PubMed/NCBI
|
|
22
|
Tabesh Azizi G, Izadi P, Fereidooni F,
Razavi Emami AN and Bazzaz Tavakkoly J: The high frequency of
PIK3CA mutations in Iranian breast cancer patients. Cancer Invest.
35:36–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu ZG, Tang J, Chen Z and Zhang H, Wang
H, Yang J and Zhang H: The novel mTORC1/2 dual inhibitor INK128
enhances radiosensitivity of breast cancer cell line MCF-7. Int J
Oncol. 49:1039–1045. 2016.PubMed/NCBI
|
|
24
|
Kao J, Salari K, Bocanegra M, Choi YL,
Girard L, Gandhi J, Kwei KA, Hernandez-Boussard T, Wang P, Gazdar
AF, et al: Molecular profiling of breast cancer cell lines defines
relevant tumor models and provides a resource for cancer gene
discovery. PLoS One. 4:e61462009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yahyanejad S, Theys J and Vooijs M:
Targeting Notch to overcome radiation resistance. Oncotarget.
7:7610–7628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim BM and Hong Y, Lee S, Liu P, Lim JH,
Lee YH, Lee TH, Chang KT and Hong Y: Therapeutic implications for
overcoming radiation resistance in cancer therapy. Int J Mol Sci.
16:26880–26913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heavey S, O'Byrne KJ and Gately K:
Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC.
Cancer Treat Rev. 40:445–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimura T: Acquired radioresistance of
cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J Radiat
Res. 52:539–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimura T, Kakuda S, Ochiai Y, Nakagawa H,
Kuwahara Y, Takai Y, Kobayashi J, Komatsu K and Fukumoto M:
Acquired radioresistance of human tumor cells by
DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene.
29:4826–4837. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimura T: Targeting the AKT/cyclin D1
pathway to overcome intrinsic and acquired radioresistance of
tumors of effective radiotherapy. Int J Radiat Biol. 93:381–385.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Javvadi P, Makino H, Das AK, Lin YF, Chen
DJ, Chen BP and Nirodi CS: Threonine 2609 phosphorylation of the
DNA-dependent protein kinase is a critical prerequisite for
epidermal growth factor receptor-mediated radiation resistance. Mol
Cancer Res. 10:1359–1368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bunimovich YL, Nair-Gill E, Riedinger M,
McCracken MN, Cheng D, McLaughlin J, Radu CG and Witte ON:
Deoxycytidine kinase augments ATM-Mediated DNA repair and
contributes to radiation resistance. PLoS One. 9:e1041252014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guy JB, Rancoule C, Méry B, Espenel S,
Wozny AS, Simonet S, Vallard A, Alphonse G, Ardail D,
Rodriguez-Lafrasse C and Magné N: Radiosensitivity and/or
radioresistance of head and neck cancers: Biological angle. Bull
Cancer. 103:41–47. 2016. View Article : Google Scholar : PubMed/NCBI
|