Introduction
Gliomas are considered as one of the most harmful
cancers, which frequently leads to the development of serious
problems and in the majority of cases patients succumb to the
disease (1). There are many
complications in diagnosing and treating gliomas. Gliomas can be
observed mostly in children and adults aged between 50 and 60 years
old (2). Malignant gliomas are
therefore a crucial cause of mortality in young people and the
improvement in survival rates could save the lives of many people.
The common treatment is composed of a cytoreductive operation
followed by radiotherapy, however the diagnosis still remains poor
with a survival period of nine months and ~5–10% of patients
surviving up to two years (3).
Gliomas are locally aggressive tumors that have poor
diagnosis even following the treatment with a combination of
operation, chemotherapy and radiotherapy. Previously, there has
been a small development in analyzing the molecular genetics of
these brain tumors, but still the origin of cell types are
uncertain and the molecular origin of tumor metastasis are not well
understood (4). Clear analyses
about the origination and development of disease may recognize new
targets for treating tumors. Flavonoids are a class of polyphenolic
compounds that are familiar constituents in the human diet.
Flavonoids occur worldwide in plants and contain various beneficial
characteristics. It was reported that people living in western
countries are consuming a significant amount of dietary flavonoids.
They are rich in antioxidant properties against cancer cells and
can be used as anticancer drugs in the form of apoptosis inducers
and cell proliferation inhibitors (5).
Galangin, also termed as 3,5,7-trihydroxyflavone, is
a member of the flavonoids and occurs in Alpinia officinarum
herbal plants that are used in Asian countries for therapeutic
treatment. These plants contain a rich source of honey and the
elements in this plant contains major constituents of propolis,
which is a natural balsam secreted by honey bees (6–8).
Galangin exhibited different pharmacological properties including
antioxidative, antimutagenic and radical scavenging (9–11).
Literature studies have indicated that galangin presents anticancer
effects against different cancer cells such as hepatocellular
carcinoma cells, colon cancer cells, ovarian cancer cells, human
mammary tumor cells, melanoma, prostate cancer cells and
promyelocytic leukemia cells (12–18).
Galangnin-induced apoptosis through the mitochondrial pathway and
G0/G1 cell cycle arrest upon the reduction of cyclins E, A9 and
D310. Galangin triggered autophagy and apoptosis at different
concentrations via elevation of p53 in HepG2 cell lines (19). TRAIL-induced apoptosis was observed
in prostate cancer cells upon treatment with galangin (18). Though galangin displayed cell
proliferation and apoptosis in various cancer cells, the knowledge
on exact effect and its associated molecular mechanism of galangin
participated in glioma cancer still remains doubtful. Hence, the
authors are interested to investigate whether galangin has any
potential effects against glioma cancer using the A172 glioma
cancer cell line.
Materials and methods
Materials
Galangin, MTT reagent and dimethyl sulfoxide (DMSO)
were acquired from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
All the antibodies used against extracellular signal-regulated
kinase (Erk)1/2, p-Erk1/2, protein kinase B (AKT), p-AKT and ADAM9,
secondary antibodies, β-actin and small interfering (si)ERK were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
ADAM9 protein, MEK inhibitor (U0126) and the ARY021 human protease
array kit were ordered from Abcam (Cambridge, UK).
Cell culture and methods
The A172 human glioma cancer cell line was purchased
from American Type Culture Collection (Manassas, VA, USA). These
cell lines were cultured in RPMI-1640 medium supplemented with 10%
FBS, penicillin and streptomycin (100 U/ml + 100 mg/ml) and then
maintained in an incubator with humidified atmosphere (5%
CO2) at 37°C. Galangin (10 mM) stock solution was
prepared using DMSO solvent, kept at −20°C for storage and then
diluted further to carry out the other experiments.
Cytotoxicity
MTT assay was performed to determine the cell
cytotoxicity. In this experiment, the human glioma cancer cell line
(A172) at a density of 4×103 cells/well were cultured in
24-well plates and then treated with different galangin
concentrations (5–25 µM) for different time periods, 24 and 72 h.
Later, the cell lines were incubated with MTT reagent (0.4 mg/ml)
for another ~5 h. Following discarding the medium, each well was
added with ~50 µl isopropanol and then absorbance was measured at
590 nm using a spectrophotometer.
Cell migration and invasion
assays
In the current assay, Matrigel was not used to coat
the cell culture chambers. The experiment was carried out in such a
way that the A172 cells at a density of 4×104 per well
were cultured in the upper compartment using serum free medium,
whereas the lower compartment was added with 10% FBS containing
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). It was then treated with different galangin
concentrations (5–25 µM) and incubated for 6 h. Following
incubation, a cotton swab was used to clean the cells located
inside the chambers and the migrated cells to the lower portion
were stained using Giemsa (0.1%). The migration was carried out via
poly-vinylpyrrolidine free polycarbonate membrane with a pore size
of 8 mm. A light microscope at the magnification ×200 was used for
counting and non-treated cells are treated as controls.
A cell invasion assay was performed similarly to
that of the cell migration assay as mentioned in previous method.
In this assay, initially the chamber membranes were coated using
Matrigel (50 µg/ml) followed by cell culturing at a density of
4×104 cells/well in the upper compartment. The cell
invasion via Matrigel and polycarbonate membranes to the lower
portion was recorded at 12 h incubation, the same way as mentioned
in cell migration experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In this experiment, TRIzol plus RNA extraction kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract
total RNAs and the synthesis of cDNAs (from 4×104 A 172
cells) was performed using cDNA synthetic kit/Superscript III
Platinum One Step RTqPCR kit (Thermo Fisher Scientific, Inc.) based
on previous methods presented in the reported literature (20). Briefly, TaqMan probe reaction
mixture (SYBR-Green I dye from Invitrogen; Thermo Fisher
Scientific, Inc.) in a standard cycling program were used along
with specific forward and reverse primers (flurogenic primers) of
ADAM9 (forward, 5′-GCTACGCACCTCCAAATTGT-3′ and reverse
5′-GGCCTCAAGTCATTGGAA-3′) and β-actin (forward
5′-AGTTTAGGACTTGACC-3′ and reverse,
5′-TTAAGCCCTGAAATCCT-3′-designed using Primer3 program) were run in
a Step One Plus RT PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) to amplify the mRNAs of ADAM9 and β-actin. The
relative fold change in mRNA expression levels were quantified
using the 2−ΔΔCq method (20).
Western blot analysis
To carry out western blotting, lysis buffer [50 mM
Tris-HCl (pH 7.5), 0.5% sodium deoxycholate, 150 mM NaCl, 0.1% SDS,
1 mM dithiothreitol, 5 mM EDTA, 50 mM sodium fluoride] was used to
extract the cells total protein and estimated using Bradford
method. Equal concentration of protein extracts (50 µg) were added
to each well for SDS-PAGE (10%) separation electrotransferred onto
polyvinylidene difluoride (PVDF) membranes. The membrane was
blocked with TBS containing Tween-20 and 5% skimmed milk for 2 h at
37°C and incubated with primary antibodies anti-rabbit monoclonal
ADAM9 (cat. no. 2099S; 1:1,000), Erk1/2 (cat. no. 4376S; 1:1,000),
p-Erk1/2 (cat. no. 4370S; 1:1,000), AKT (cat. no. 4685S; 1:1,000),
p-AKT (cat. no. 4060S; 1:1,200) antibodies and β-actin (cat. no.
4970S; 1:1,000) for 2 h at 37°C and again incubated with a
secondary antibody with conjugated to anti-rabbit immunoglobulin G
horseradish peroxidase (cat. no. 7074S; 1:10,000) for 2 h at 37°C.
All the antibodies (primary/secondary) are bought from Cell
Signaling Technology, Inc. The visualization of bounded proteins
was determined using the enhanced chemiluminescence detection
system (GE Healthcare Life Sciences, Chalfont, UK). The image/band
intensity was detected using a LAS 4000 gel documentation system
from GE Healthcare Life Sciences (Chalfont, UK) using Image J
software from the National Institutes of Health (version 2.8;
Bethesda, MD, USA).
Cell transfection
Initially, the human A172 cells were transfected
using siErk with the help of Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) and kept for next 2 days to undergo transfection.
Once the cells were transfected, galangin was added and harvested
to extract total RNA/protein. Later, the above discussed methods
(RT-qPCR and western blot section) were used to measure the
mRNA/protein levels.
Statistical analysis
All the given experimental data were expressed as
mean ± standard error of the mean. All the experiments in this work
were performed independently in triplicate. Measurement of
statistical differences between the groups were recorded using
one-way analysis of variance followed by Dunnett's multiple
comparison post hoc test with the help of GraphPad Prism software
(version, 5; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Cell migration and invasion
assays
The investigation results of cell migration and
invasion assays on A172 cells is presented in Fig. 1. The results indicated that
galangin treatment reduced the A172 cell migration and invasion. In
order to study the galangin effect on cell viability, MTT assay
(Fig. 1B) was carried out on the
A172 glioma cancer cell line. Upon treatment of the A172 cell line
with galangin at different concentrations (5–25 µM), no significant
inhibition on A172 cell viability was observed. To elucidate the
galangin potentials on A172 cell migration and invasion, a specific
galangin concentration was chosen in such a way that it did not
show significant effects on A172 cell growth. The data indicated
that treatment of various concentrations of galangin (5–25 µM) for
~24 h on the A172 cell line markedly decreased the migration
potential of the A172 cell line in a concentration dependent
fashion when compared to that of non-treated cells. The percentage
of migration was from 16 to 84% in A172 cells, whereas the
percentage of cell invasion was from 14 to 81% (Fig. 1C and D). All these results
indicated that galangin strongly inhibited A172 cell migration and
invasion under non-toxic doses.
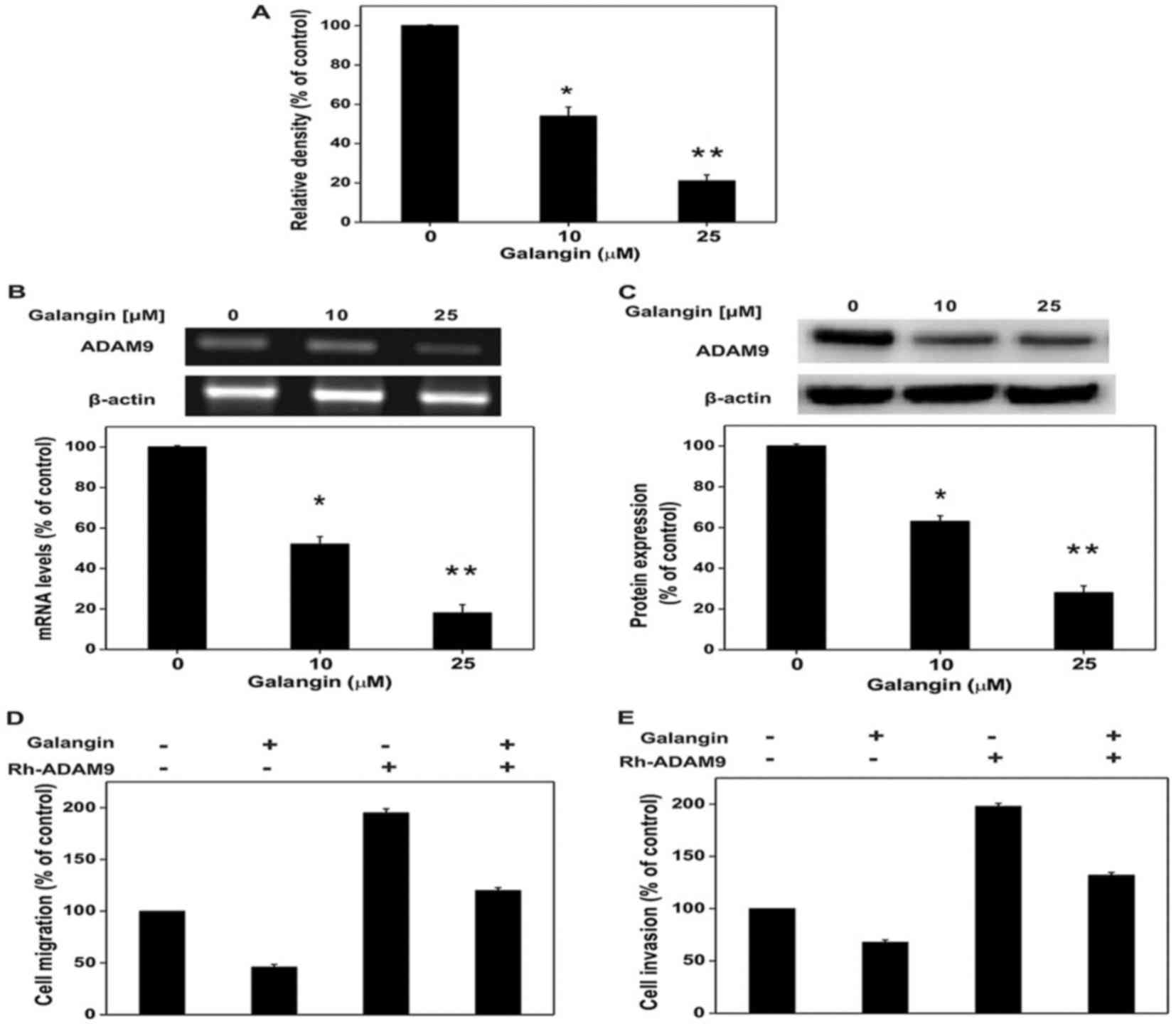
ADAM9 expression in A172 cells
The authors conducted an experiment using a human
protease inhibitor array kit in order to exhibit the molecular
mechanisms underlying the reducing effects of galangin on A172 cell
migration and invasion. This investigation helps to display the
invasion associated protein actions inside the cells. An earlier
report indicated that ADAM9 has a special role in cell migration
and cell invasion in glioma cancer cell lines (21). The study observed ADAM9 expression
following treatment with different doses of galangin and the
results clearly indicated that galangin reduced ADAM9 expression
(Fig. 2A). To further elucidate
the protease array results, western blot analysis and RT-qPCR was
conducted using galangin and demonstrated that it hindered mRNA and
ADAM9 protein expression in the A172 cell line (Fig. 2B and C). Results of these studies
revealed that galangin hinders the A172 cell migration and invasion
potential, which could be due to the decrease of ADAM9 protein
expression. Before investigating the pharmacological properties of
galangin on reduced ADAM9 expression, the authors checked whether
galangin could hinder RhADAM9-induced A172 cell migration and
invasion. Results presented in Fig. 2D
and E clearly indicated that galangin hindered both
RhADAM9-induced A172 cell migration and invasion in the A172 glioma
cell line. These investigations revealed that the anti-metastatic
ability of galangin results from repressing the ADAM9 expression in
the A172 cell line.
Erk1/2 phosphorylation inducement in
A172 cells
A previous report clearly indicated that Erk1/2
activation is associated functions of cell migration and invasion
in human glioma cells (22).
Hence, the authors tested galangin against the A172 glioma cell
line for Erk1/2 activation in addition to the AKT signaling
pathway. The results obtained from ERK1/2 activation and the AKT
signaling pathway demonstrated that galangin effectively induced
Erk1/2 phosphorylation whereas it failed to report a significant
effect on AKT phosphorylation (Fig.
3). Furthermore, no notable quantity of proteins (t-Erk1/2 and
t-AKT) was obtained. All these data clearly evidenced the role of
activation of Erk1/2 in the galangin inhibited the A172 cell
migration and invasion mechanism.
Reduction of ADAM9 expression via
Erk1/2 activation
In order to investigate the mechanistic pathway for
galangin induced cell invasion inhibition, the authors first
pre-treated the A172 glioma cell line with 15 µM MEK inhibitor,
U0126, for ~3 h and then further added galangin for next 12 h
before finally subjecting to western blot analysis, RT-qPCR and
cell migration and invasion studies. Data obtained from these
studies indicated that U0126 decreased galangin and triggered
expression of Erk1/2, protecting galangin from protein hindrance
and expressions of ADAM9 mRNA in A172 cells, as presented in
Fig. 4A. Results from cell
migration and cell invasion assays suggested that the pretreatment
of MEK inhibitor completely blocked galangin-induced inhibition of
A172 cell migration and invasions (Fig. 4B and C). Based on these results,
ERK1/2 activation was identified to serve a specific function in
galangin-mediated inhibition to cell migration and invasion.
Moreover, A172 cells transiently transfected with siERK displayed
effective reduction in total expression of ERK1/2 and terminated
galangin-induced inhibition to ADAM9 expression (Fig. 5A). In addition, it was identified
that siERK expression in A172 cells also blocked galangin-induced
inhibition to cell migration and invasion (Fig. 5B and C). All the obtained results
clearly indicated that galangin hinders A172 glioma cell invasion
via activation of ERK1/2.
Discussion
Regardless of developing novel therapies for
different cancer types, the diagnosis for many cancers remains
poor. Previously, researchers focused primarily on tumor research
in order to reveal the underlying mechanisms on cancer metastasis
and hence, various anti-cancer compounds have been identified.
Flavonoids are a class of polyphenolic compounds that can display
numerous positive effects on cancer treatments. Galangin is one of
the flavonoids that can exhibit pleiotropic anti-cancer properties
and serve an important role in maintaining different molecular
targets such as TNF-α, NF-κB, p38, ERK, JNK, PPARγ, COX-2, ICAM-1
SMADs and interleukins (23–30).
It is known that metastasis in tumors requires a sequence of signal
transductions and, if drugs block these pathways, the cancer spread
may be limited. Earlier reports have demonstrated that galangin
induces apoptosis in different cancer cells in using different
molecular mechanisms (21–23). Hepatocellular carcinoma cell
proliferation is also hindered using galangin by inducing
endoplasmic reticulum stress, AMPK activation and mitochondrial
dysfunctions through Bax elevation and Bcl-2 reduction (31–33).
Hence, a series of experiments were conducted using galangin to
evaluate its anti-cancer potentials against the A172 glioma cell
line.
Tumor metastasis is a complicated process in which
tumor cells exhibit different characteristics such as elevated
motility and invasive ability to finish the process. The important
stage in this process is stromal extracellular matrix degradation
(34). This process resulted in
the cytokine and growth factor activation and produces signals to
assist the tumor survival (35).
Various proteases including ADAMTSs, ADAMs and MMPs are involved in
these kinds of processes (36,37)
and hence, the authors aimed to target the ADAM9 gene in their
studies. Among different cancer types, ADAM9 is considered as an
oncogene (38–40). Some of the experimental results
indicated that ADMA9 serves an important function in tumor
metastasis. Data displayed in this work are the first illustrated
results presenting galangin hindrance ability against human glioma
tumor cell invasion mediated via ADAM9 expression. It is well known
that the transduction molecules such as MAPKs and AKT are
associated in maintaining various cellular processes such as cell
proliferation, differentiation and apoptosis. However, some
biological reports are controversial, depending on particular
properties of different cancer cell types (41,42).
Many studies demonstrated that Erk1/2, NF-κB, FAK, PI3K/AKT Jnk1/2,
p38 and MAPK also participate in glioma cell migration and invasion
(43–46). In one report, treatment of
sulforaphane hindered the cell migration and invasion against human
glioma cells (U87MG and U373MG) mediated through the activation of
Erk1/2. Data obtained from the present work also match with above
result and hence, the authors revealed the participation of Erk1/2
activation along with hindrance of ADAM9 expression in
galangin-inhibited cell invasion. From these experiments, the
authors identified no direct proof to show that Erk1/2 can maintain
or control ADAM9 expression. Hence, they decided to carry out new
experiments to completely explore the correlation between Erk1/2
and ADAM9 expression in near future.
In conclusion, to the best of the authors'
knowledge, the present study is the first to reveal that galangin
possess the effective tendency to act as a potential drug to treat
human glioma cancer mediated through Erk1/2 activation and ADAM9
expression pathways. In the near future, further experiments will
be conducted to examine the complete connection between Erk1/2 and
ADAM9 expression.
Acknowledgements
The present study was supported by the Tongji
Medical College (grant no. TMC-28487/15), Huazhong University of
Science and Technology (grant no. HUST-16).
References
|
1
|
Takahashi H and Teramoto A: Trial of
targeting therapy against malignant glioma using monoclonal
antibody. J Nippon Med Sch. 71:2–3. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Souhami R and Tobias J: Cancer and its
management. 2nd edition. Oxford: Blackwell Sciences; 1995
|
|
3
|
Bleehen NM and Stenning SP: A medical
research council trial of two radiotherapy doses in the treatment
of grades 3 and 4 astrocytoma. Br J Cancer. 64:769–774. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitange GJ, Templeton KL and Jenkins RB:
Recent advances in the molecular genetics of primary gliomas. Curr
Opin Oncol. 15:197–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramos S: Cancer chemoprevention and
chemotherapy: Dietary polyphenols and signalling pathways. Mol Nutr
Food Res. 52:507–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li BH and Tian WX: Presence of fatty acid
synthase inhibitors in the rhizome of Alpinia officinarum
hance. J Enzym Inhib Med Ch. 18:349–356. 2003. View Article : Google Scholar
|
|
7
|
Volpi N: Separation of flavonoids and
phenolic acids from propolis by capillary zone electrophoresis.
Electrophoresis. 25:1872–1878. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sulaiman GM, Al Sammarrae KW, Ad'hiah AH,
Zucchetti M, Frapolli R, Bello E, Erba E, D'Incalci M and Bagnati
R: Chemical characterization of Iraqi propolis samples and
assessing their antioxidant potentials. Food Chem Toxicol.
49:2415–2421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heo MY, Sohn SJ and Au WW:
Anti-genotoxicity of galangin as a cancer chemopreventive agent
candidate. Mutat Res. 488:135–150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cushnie TP and Lamb AJ: Assessment of the
antibacterial activity of galangin against 4-quinolone resistant
strains of Staphylococcus aureus. Phytomedicine. 13:187–191.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gwak J, Oh J, Cho M, Bae SK, Song IS, Liu
KH, Jeong Y, Kim DE, Chung YH and Oh S: Galangin suppresses the
proliferation of β-catenin response transcription positive cancer
cells by promoting adenomatous polyposis coli/Axin/glycogen
synthase kinase-3β-independent β-catenin degradation. Mol
Pharmacol. 79:1014–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ha TK, Kim ME, Yoon JH, Bae SJ, Yeom J and
Lee JS: Galangin induces human colon cancer cell death via the
mitochondrial dysfunction and caspase-dependent pathway. Exp Biol
Med (Maywood). 238:1047–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murray TJ, Yang X and Sherr DH: Growth of
a human mammary tumor cell line is blocked by galangin, a naturally
occurring bioflavonoid, and is accompanied by down-regulation of
cyclins D3, E, and A. Breast Cancer Res. 8:R172006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang HT, Luo H, Wu J, Lan LB, Fan DH, Zhu
KD, Chen XY, Wen M and Liu HM: Galangin induces apoptosis of
hepatocellular carcinoma cells via the mitochondrial pathway. World
J Gastroenterol. 16:3377–3384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang W, Lan Y, Huang Q and Hua Z:
Galangin induces B16F10 melanoma cell apoptosis via mitochondrial
pathway and sustained activation of p38 MAPK. Cytotechnology.
65:447–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang H, Chen AY, Rojanasakul Y, Ye X,
Rankin GO and Chen YC: Dietary compounds galangin and myricetin
suppress ovarian cancer cell angiogenesis. J Funct Foods.
15:464–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bestwick CS and Milne L: Influence of
galangin on HL-60 cell proliferation and survival. Cancer Lett.
243:80–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szliszka E, Czuba ZP, Bronikowska J,
Mertas A, Paradysz A and Krol W: Ethanolic extract of propolis
augments TRAIL-induced apoptotic death in prostate cancer cells.
Evid Based Complement Alternat Med. 2011:5351722011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wen M, Wu J, Luo H and Zhang H: Galangin
induces autophagy through upregulation of p53 in HepG2 cells.
Pharmacology. 89:247–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang G, Li Z, Tian N, Han L, Fu Y, Guo Z
and Tian Y: miR-148b-3p inhibits malignant biological behaviors of
human glioma cells induced by high HOTAIR expression. Oncol Lett.
12:879–86. 2016.PubMed/NCBI
|
|
21
|
Kim YH, Shin EK, Kim DH, Lee HH, Park JH
and Kim JK: Antiangiogenic effect of licochalcone A. Biochem
Pharmacol. 80:1152–1159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Green JA, Elkington PT, Pennington CJ,
Roncaroli F, Dholakia S, Moores RC, Bullen A, Porter JC, Agranoff
D, Edwards DR and Friedland JS: Mycobacterium tuberculosis
upregulates microglial matrix metalloproteinase-1 and −3 expression
and secretion via NF kappaB- and activator protein-1-dependent
monocyte networks. J Immunol. 184:6492–6503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huh JE, Jung IT, Choi J, Baek YH, Lee JD,
Park DS and Choi DY: The natural flavonoid galangin inhibits
osteoclastic bone destruction and osteoclastogenesis by suppressing
NF-κB in collagen-induced arthritis and bone marrow-derived
macrophages. Eur J Pharmacol. 698:57–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung YC, Kim ME, Yoon JH, Park PR, Youn
HY, Lee HW and Lee JS: Anti-inflammatory effects of galangin on
lipopolysaccharide-activated macrophages via ERK and NF-κB pathway
regulation. Immunopharmacol Immunotoxicol. 36:426–432. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Wu J, Lin B, Li X, Zhang H, Ding
H, Chen X, Lan L and Luo H: Galangin suppresses HepG2 cell
proliferation by activating the TGF-β receptor/Smad pathway.
Toxicology. 326:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung CH, Jang SJ, Ahn J, Gwon SY, Jeon TI,
Kim TW and Ha TY: Alpinia officinarum inhibits adipocyte
differentiation and high-fat diet-induced obesity in mice through
regulation of adipogenesis and lipogenesis. J Med Food. 15:959–967.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lotito SB and Frei B: Dietary flavonoids
attenuate tumor necrosis factor alpha-induced adhesion molecule
expression in human aortic endothelial cells. Structure-function
relationships and activity after first pass metabolism. J Biol
Chem. 281:37102–371010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HH, Bae Y and Kim SH: Galangin
attenuates mast cell-mediated allergic inflammation. Food Chem
Toxicol. 57:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi JK and Kim SH: Inhibitory effect of
galangin on atopic dermatitis-like skin lesions. Food Chem Toxicol.
68:135–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O'Leary KA, de Pascual-Teresa S, Needs PW,
Bao YP, O'Brien NM and Williamson G: Effect of flavonoids and
vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat Res.
551:245–254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang HT, Luo H, Wu J, Lan LB, Fan DH, Zhu
KD, Chen XY, Wen M and Liu HM: Galangin induces apoptosis of
hepatocellular carcinoma cells via the mitochondrial pathway. World
J Gastroenterol. 16:3377–3384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su L, Chen X, Wu J, Lin B, Zhang H, Lan L
and Luo H: Galangin inhibits proliferation of hepatocellular
carcinoma cells by inducing endoplasmic reticulum stress. Food Chem
Toxicol. 62:810–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang H, Li N, Wu J, Su L, Chen X, Lin B
and Luo H: Galangin inhibits proliferation of HepG2 cells by
activating AMPK via increasing the AMP/TAN ratio in a
LKB1-independent manner. Eur J Pharmacol. 718:235–244. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pandey M, Mathew A and Nair MK: Global
perspective of tobacco habits and lung cancer: A lesson for third
world countries. Eur J Cancer Prev. 8:271–279. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sarkar FH and Li YW: Targeting multiple
signal pathways by chemopreventive agents for cancer prevention and
therapy. Acta Pharmacol Sin. 28:1305–1315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weng CJ and Yen GC: Chemopreventive
effects of dietary phytochemicals against cancer invasion and
metastasis: Phenolic acids, monophenol, polyphenol, and their
derivatives. Cancer Treat Rev. 38:76–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chetty C, Rao JS and Lakka SS: Matrix
metalloproteinase pharmacogenomics in non-small cell lung
carcinoma. Pharmacogenomics. 12:535–546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
López-Otín C, Palavalli LH and Samuels Y:
Protective roles of matrix metalloproteinases: From mouse models to
human cancer. Cell Cycle. 8:3657–3662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li M, Xiao T, Zhang Y, Feng L, Lin D, Liu
Y, Mao Y, Guo S, Han N, Di X, et al: Prognostic significance of
matrix metalloproteinase-1 levels in peripheral plasma and tumour
tissues of lung cancer patients. Lung Cancer. 69:341–347. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yoon SO, Shin S, Lee HJ, Chun HK and Chung
AS: Isoginkgetin inhibits tumor cell invasion by regulating
phosphatidylinositol 3-kinase/Akt-dependent matrix
metalloproteinase-9 expression. Mol Cancer Ther. 5:2666–2675. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Veit C, Genze F, Menke A, Hoeffert S,
Gress TM, Gierschik P and Giehl K: Activation of
phosphatidylinositol 3-kinase and extracellular signal-regulated
kinase is required for glial cell line-derived neurotrophic
factor-induced migration and invasion of pancreatic carcinoma
cells. Cancer Res. 64:5291–5300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Günther W, Skaftnesmo KO, Arnold H and
Terzis AJ: Molecular approaches to brain tumour invasion. Acta
Neurochir (Wien). 145:1029–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|