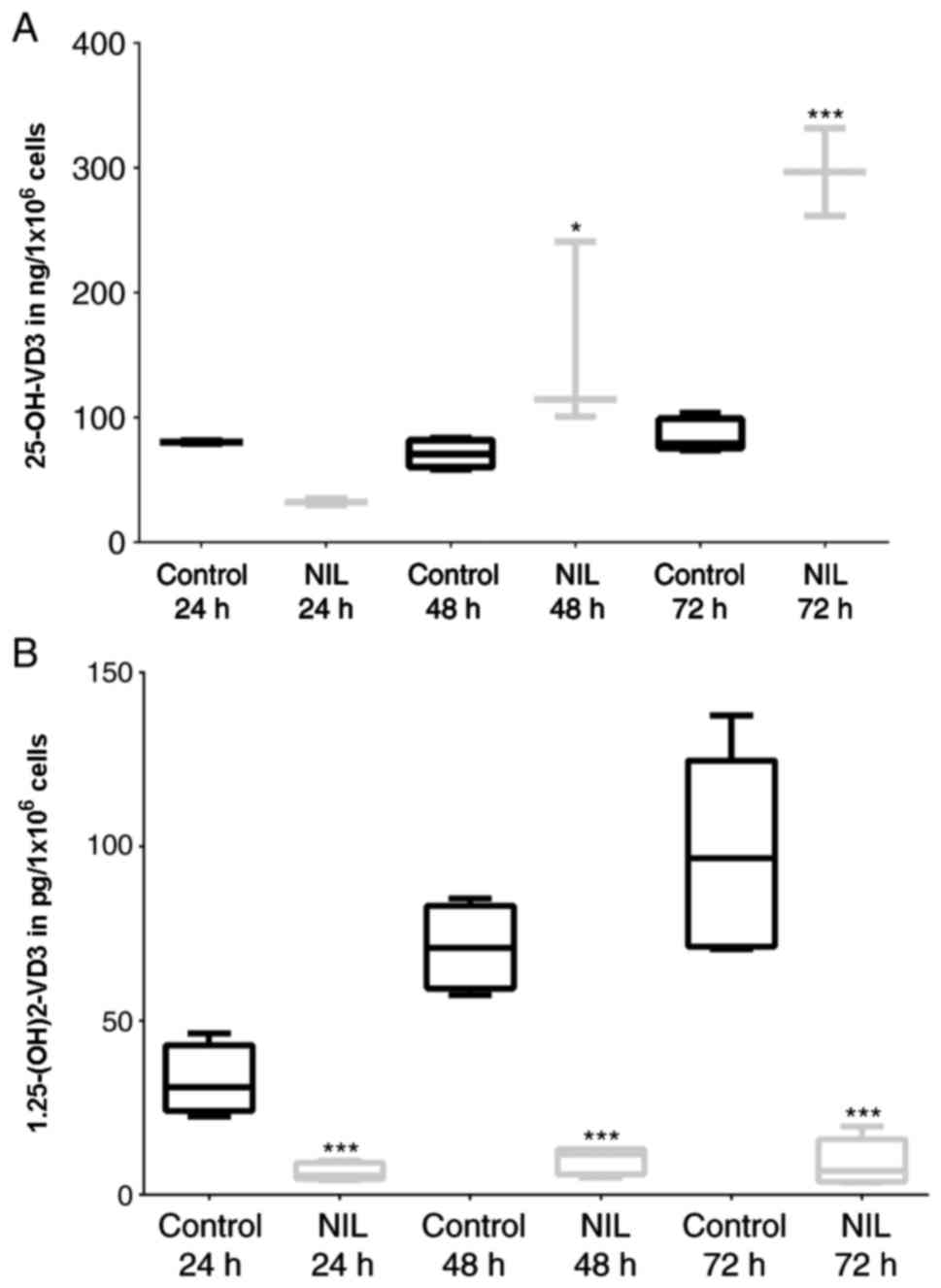
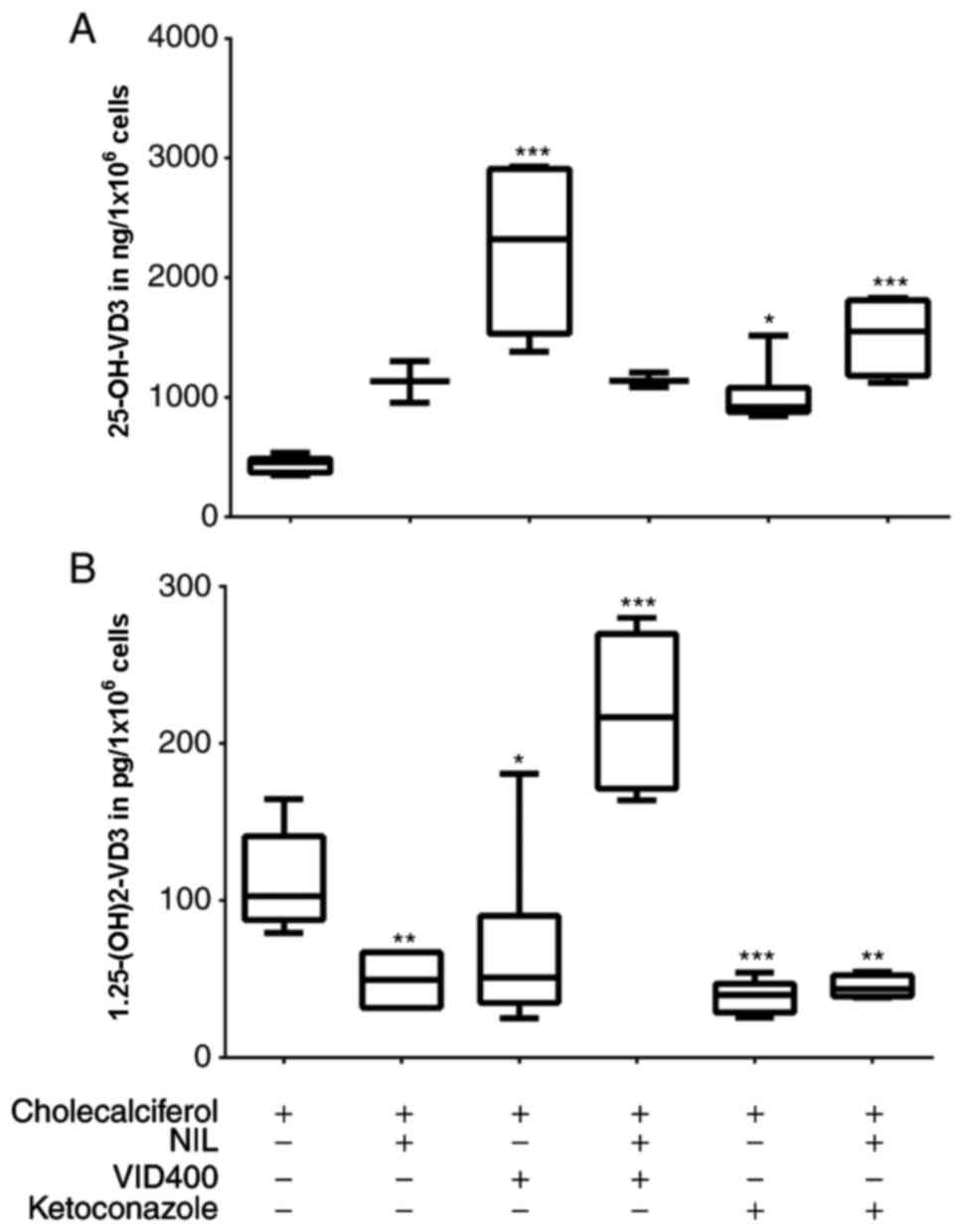
|
1
|
Jabbour E, El AS, Cortes J and Kantarjian
H: Nilotinib: A novel Bcr-Abl tyrosine kinase inhibitor for the
treatment of leukemias. Expert Opin Investig Drugs. 17:1127–1136.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tipping AJ, Mahon FX, Zafirides G, Lagarde
V, Goldman JM and Melo JV: Drug responses of imatinib
mesylate-resistant cells: Synergism of imatinib with other
chemotherapeutic drugs. Leukemia. 16:2349–2357. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Capdeville R, Silberman S and Dimitrijevic
S: Imatinib: The first 3 years. Eur J Cancer. 38 Suppl 5:S77–S82.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daley GQ, Van Etten RA and Baltimore D:
Induction of chronic myelogenous leukemia in mice by the
P210bcr/abl gene of the Philadelphia chromosome. Science.
247:824–830. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen MH, Williams G, Johnson JR, Duan J,
Gobburu J, Rahman A, Benson K, Leighton J, Kim SK, Wood R, et al:
Approval summary for imatinib mesylate capsules in the treatment of
chronic myelogenous leukemia. Clin Cancer Res. 8:935–942.
2002.PubMed/NCBI
|
|
6
|
Pasternak G, Hochhaus A, Schultheis B and
Hehlmann R: Chronic myelogenous leukemia: Molecular and cellular
aspects. J Cancer Res Clin Oncol. 124:643–660. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Champagne MA, Capdeville R, Krailo M, Qu
W, Peng B, Rosamilia M, Therrien M, Zoellner U, Blaney SM and
Bernstein M: Children's Oncology Group phase 1 study: Imatinib
mesylate (STI571) for treatment of children with Philadelphia
chromosome-positive leukemia: Results from a children's oncology
group phase 1 study. Blood. 104:2655–2660. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Druker BJ, Tamura S, Buchdunger E, Ohno S,
Segal GM, Fanning S, Zimmermann J and Lydon NB: Effects of a
selective inhibitor of the Abl tyrosine kinase on the growth of
Bcr-Abl positive cells. Nat Med. 2:561–566. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Druker BJ, Talpaz M, Resta DJ, Peng B,
Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R,
Ohno-Jones S and Sawyers CL: Efficacy and safety of a specific
inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid
leukemia. N Engl J Med. 344:1031–1037. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grigg A and Hughes T: Role of allogeneic
stem cell transplantation for adult chronic myeloid leukemia in the
imatinib era. Biol Blood Marrow Transplant. 12:795–807. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Millot F, Guilhot J, Nelken B, Leblanc T,
De Bont ES, Békassy AN, Gadner H, Sufliarska S, Stary J,
Gschaidmeier H, et al: Imatinib mesylate is effective in children
with chronic myelogenous leukemia in late chronic and advanced
phase and in relapse after stem cell transplantation. Leukemia.
20:187–192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roy L, Guilhot J, Krahnke T,
Guerci-Bresler A, Druker BJ, Larson RA, O'Brien S, So C, Massimini
G and Guilhot F: Survival advantage from imatinib compared with the
combination interferon-alpha plus cytarabine in chronic-phase
chronic myelogenous leukemia: Historical comparison between two
phase 3 trials. Blood. 108:1478–1484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hobernicht SL, Schweiger B, Zeitler P,
Wang M and Hunger SP: Acquired growth hormone deficiency in a girl
with chronic myelogenous leukemia treated with tyrosine kinase
inhibitor therapy. Pediatr Blood Cancer. 56:671–673. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deguchi Y, Kimura S, Ashihara E, Niwa T,
Hodohara K, Fujiyama Y and Maekawa T: Comparison of imatinib,
dasatinib, nilotinib and INNO-406 in imatinib-resistant cell lines.
Leuk Res. 32:980–983. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim TD, le Coutre P, Schwarz M, Grille P,
Levitin M, Fateh-Moghadam S, Giles FJ, Dörken B, Haverkamp W and
Köhncke C: Clinical cardiac safety profile of nilotinib.
Haematologica. 97:883–889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kantarjian H, Giles F, Wunderle L, Bhalla
K, O'Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W,
et al: Nilotinib in imatinib-resistant CML and Philadelphia
chromosome-positive ALL. N Engl J Med. 354:2542–2551. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shima H, Tokuyama M, Tanizawa A, Tono C,
Hamamoto K, Muramatsu H, Watanabe A, Hotta N, Ito M, Kurosawa H, et
al: Distinct impact of imatinib on growth at prepubertal and
pubertal ages of children with chronic myeloid leukemia. J Pediatr.
159:676–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berman E, Nicolaides M, Maki RG, Fleisher
M, Chanel S, Scheu K, Wilson BA, Heller G and Sauter NP: Altered
bone and mineral metabolism in patients receiving imatinib
mesylate. N Engl J Med. 354:2006–2013. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fierro F, Illmer T, Jing D, Schleyer E,
Ehninger G, Boxberger S and Bornhäuser M: Inhibition of
platelet-derived growth factor receptorbeta by imatinib mesylate
suppresses proliferation and alters differentiation of human
mesenchymal stem cells in vitro. Cell Prolif. 40:355–366. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fitter S, Dewar AL, Kostakis P, To LB,
Hughes TP, Roberts MM, Lynch K, Vernon-Roberts B and Zannettino AC:
Long-term imatinib therapy promotes bone formation in CML patients.
Blood. 111:2538–2547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmid H, Jaeger BA, Lohse J and Suttorp
M: Longitudinal growth retardation in a prepuberal girl with
chronic myeloid leukemia on long-term treatment with imatinib.
Haematologica. 94:1177–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hijiya N, Schultz KR, Metzler M, Millot F
and Suttorp M: Pediatric chronic myeloid leukemia is a unique
disease that requires a different approach. Blood. 127:392–399.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kimoto T, Inoue M and Kawa K: Growth
deceleration in a girl treated with imatinib. Int J Hematol.
89:251–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariani S, Giona F, Basciani S, Brama M
and Gnessi L: Low bone density and decreased inhibin-B/FSH ratio in
a boy treated with imatinib during puberty. Lancet. 372:111–112.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jaeger BA, Tauer JT, Ulmer A, Kuhlisch E,
Roth HJ and Suttorp M: Changes in bone metabolic parameters in
children with chronic myeloid leukemia on imatinib treatment. Med
Sci Monit. 18:CR721–CR728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lehmann B, Sauter W, Knuschke P, Dressler
S and Meurer M: Demonstration of UVB-induced synthesis of 1
alpha,25-dihydroxyvitamin D3 (calcitriol) in human skin by
microdialysis. Arch Dermatol Res. 295:24–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lehmann B and Meurer M: Vitamin D
metabolism. Dermatol Ther. 23:2–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holick MF: Resurrection of vitamin D
deficiency and rickets. J Clin Invest. 116:2062–2072. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DeLuca HF: Overview of general physiologic
features and functions of vitamin D. Am J Clin Nutr. 80 6
Suppl:1689S–1696S. 2004.PubMed/NCBI
|
|
31
|
Schuster I, Egger H, Herzig G, Reddy GS,
Schmid JA, Schüssler M and Vorisek G: Selective inhibitors of
vitamin D metabolism-new concepts and perspectives. Anticancer Res.
26:2653–2568. 2006.PubMed/NCBI
|
|
32
|
Bogh MK, Schmedes AV, Philipsen PA,
Thieden E and Wulf HC: Interdependence between body surface area
and ultraviolet B dose in vitamin D production: A randomized
controlled trial. Br J Dermatol. 164:163–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kremer R, Campbell PP, Reinhardt T and
Gilsanz V: Vitamin D status and its relationship to body fat, final
height, and peak bone mass in young women. J Clin Endocrinol Metab.
94:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Davis CD and Dwyer JT: The ‘sunshine
vitamin’: Benefits beyond bone? J Natl Cancer Inst. 99:1563–1565.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mathieu C and Badenhoop K: Vitamin D and
type 1 diabetes mellitus: State of the art. Trends Endocrinol
Metab. 16:261–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pettifor JM: Rickets and vitamin D
deficiency in children and adolescents. Endocrinol Metab Clin North
Am. 34(537–553): vii2005.
|
|
37
|
Mehlig LM, Garve C, Tauer JT, Suttorp M
and Bauer A: Inhibitory effects of imatinib on vitamin
D3 synthesis in human keratinocytes. Mol Med Rep.
11:3143–3147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Helou M, Ning Y, Yang S, Irvine P,
Bachmann LM, Godder K and Massey G: Vitamin D deficiency in
children with cancer. J Pediatr Hematol Oncol. 36:212–217. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lips P: Vitamin D status and nutrition in
Europe and Asia. J Steroid Biochem Mol Biol. 103:620–625. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tibullo D, Giallongo C, La Cava P,
Berretta S, Stagno F, Chiarenza A, Conticello C, Palumbo GA and Di
Raimondo F: Effects of imatinib mesylate in osteoblastogenesis. Exp
Hematol. 37:461–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
O'Sullivan S, Naot D, Callon K, Porteous
F, Horne A, Wattie D, Watson M, Cornish J, Browett P and Grey A:
Imatinib promotes osteoblast differentiation by inhibiting PDGFR
signaling and inhibits osteoclastogenesis by both direct and
stromal cell-dependent mechanisms. J Bone Miner Res. 22:1679–1689.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dewar AL, Zannettino AC, Hughes TP and
Lyons AB: Inhibition of c-fms by imatinib: Expanding the spectrum
of treatment. Cell Cycle. 4:851–853. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dewar AL, Cambareri AC, Zannettino AC,
Miller BL, Doherty KV, Hughes TP and Lyons AB: Macrophage
colony-stimulating factor receptor c-fms is a novel target of
imatinib. Blood. 105:3127–3132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dewar AL, Domaschenz RM, Doherty KV,
Hughes TP and Lyons AB: Imatinib inhibits the in vitro development
of the monocyte/macrophage lineage from normal human bone marrow
progenitors. Leukemia. 17:1713–1721. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Owen S, Hatfield A and Letvak L: Imatinib
and altered bone and mineral metabolism. N Engl J Med. 355:627–629.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
O'Sullivan S, Horne A, Wattie D, Porteous
F, Callon K, Gamble G, Ebeling P, Browett P and Grey A: Decreased
bone turnover despite persistent secondary hyperparathyroidism
during prolonged treatment with imatinib. J Clin Endocrinol Metab.
94:1131–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
El Hajj Dib I, Gallet M, Mentaverri R,
Sévenet N, Brazier M and Kamel S: Imatinib mesylate (Gleevec)
enhances mature osteoclast apoptosis and suppresses osteoclast bone
resorbing activity. Eur J Pharmacol. 551:27–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grey A, O'Sullivan S, Reid IR and Browett
P: Imatinib mesylate, increased bone formation, and secondary
hyperparathyroidism. N Engl J Med. 355:2494–2495. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jönsson S, Olsson B, Ohlsson C, Lorentzon
M, Mellström D and Wadenvik H: Increased cortical bone
mineralization in imatinib treated patients with chronic
myelogenous leukemia. Haematologica. 93:1101–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vandyke K, Fitter S, Dewar AL, Hughes TP
and Zannettino AC: Dysregulation of bone remodeling by imatinib
mesylate. Blood. 115:766–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Genc DB, Ozkan MA and Buyukgebiz A:
Vitamin D in childhood cancer: A promising anticancer agent?
Pediatr Endocrinol Rev. 10:485–493. 2013.PubMed/NCBI
|
|
52
|
Cheng JB, Levine MA, Bell NH, Mangelsdorf
DJ and Russell DW: Genetic evidence that the human CYP2R1 enzyme is
a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA.
101:7711–7715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lehmann B, Rudolph T, Pietzsch J and
Meurer M: Conversion of vitamin D3 to 1alpha,25-dihydroxyvitamin D3
in human skin equivalents. Exp Dermatol. 9:97–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Peng B, Lloyd P and Schran H: Clinical
pharmacokinetics of imatinib. Clin Pharmacokinet. 44:879–894. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gschwind HP, Pfaar U, Waldmeier F,
Zollinger M, Sayer C, Zbinden P, Hayes M, Pokorny R, Seiberling M,
Ben-Am M, et al: Metabolism and disposition of imatinib mesylate in
healthy volunteers. Drug Metab Dispos. 33:1503–1512. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rochat B: Role of cytochrome P450 activity
in the fate of anticancer agents and in drug resistance: Focus on
tamoxifen, paclitaxel and imatinib metabolism. Clin Pharmacokinet.
44:349–366. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xie Z, Munson SJ, Huang N, Portale AA,
Miller WL and Bikle DD: The mechanism of 1,25-dihydroxyvitamin D(3)
autoregulation in keratinocytes. J Biol Chem. 277:36987–36990.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schuster I, Egger H, Reddy GS and Vorisek
G: Combination of vitamin D metabolites with selective inhibitors
of vitamin D metabolism. Recent Results Cancer Res. 164:169–188.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schuster I, Egger H, Nussbaumer P and
Kroemer RT: Inhibitors of vitamin D hydroxylases:
Structure-activity relationships. J Cell Biochem. 88:372–380. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yee SW, Campbell MJ and Simons C:
Inhibition of Vitamin D3 metabolism enhances VDR signalling in
androgen-independent prostate cancer cells. J Steroid Biochem Mol
Biol. 98:228–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yin OQ, Gallagher N, Tanaka C, Fisher D,
Sethuraman V, Zhou W, Lin TH, Heuman D and Schran H: Effects of
hepatic impairment on the pharmacokinetics of nilotinib: An
open-label, single-dose, parallel-group study. Clin Ther.
31:2459–2469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gupta RP, Hollis BW, Patel SB, Patrick KS
and Bell NH: CYP3A4 is a human microsomal vitamin D 25-hydroxylase.
J Bone Miner Res. 19:680–688. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nguyen M, Boutignon H, Mallet E, Linglart
A, Guillozo H, Jehan F and Garabedian M: Infantile hypercalcemia
and hypercalciuria: New insights into a vitamin D-dependent
mechanism and response to ketoconazole treatment. J Pediatr.
157:296–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Segersten U, Björklund P, Hellman P,
Akerström G and Westin G: Potentiating effects of nonactive/active
vitamin D analogues and ketoconazole in parathyroid cells. Clin
Endocrinol (Oxf). 66:399–404. 2007. View Article : Google Scholar : PubMed/NCBI
|