Introduction
Cerebrovascular disease poses a substantial threat
to human health. Annually, ~1.5 million new patients are diagnosed
in China and the most common type is ischemic stroke, namely
cerebral ischemia (1). In recent
years, incidence trends have shifted towards younger individuals,
and the majority of patients experience nerve function defect
following the attack, which greatly affects the quality of life of
patients and is associated with economic and mental burdens to
society and the family and friends of patients (2). However, at present, no effective
treatment exists.
Cerebral ischemia reperfusion-induced injury (IRI)
is a highly complex pathological process that involves a series of
cellular and molecular events (3).
It is divided into the following three stages: Acute stage, which
primarily manifests as metabolic disorders and excitatory toxicity;
sub-acute stage, where the major pathological changes are
inflammation and apoptosis; and chronic stage, which primarily
consists of repair and regeneration (4). The sub-acute and chronic stages are
also collectively termed the ‘late stage’, as the boundary between
the two stages is difficult to determine (5).
A previous study demonstrated that inflammation has
an important role in cerebral IRI (6). The early accumulation of neutrophil
granulocytes at ischemic regions has been verified by
histopathology and biochemical methods (7). The most frequently used reliability
index to evaluate the degree of neutrophil granulocyte infiltration
in tissue is myeloperoxidase (8),
which is an enzyme that catalyzes peroxide reduction and is an
essential constituent of the oxygen-dependent sterilization system
of neutrophil granulocytes (9).
Pentoxifylline is a type of alkaloid that is formed
by introducing a hexanone group to theobromine extracted from cocoa
beans (10). It is a derivative of
methylxanthine and non-selective phosphodiesterase inhibitors
(11). Pentoxifylline has been
reported to increase the deformability of red blood cells, improve
the hemorheology of leukocytes, inhibit the adhesion and activation
of neutrophil granulocytes, expand capillaries, reduce blood
viscosity, increase the oxygen partial pressure of tissue and
eliminate free radicals (12,13).
Therefore, the present study was performed to investigate the
protective effects of pentoxifylline on cerebral IRI and the
potential underlying mechanisms.
Materials and methods
Animals and treatments
Male Sprague-Dawley rats weighing 260–300 g (n=36;
1.5 years old) were obtained from the Experimental Animal Centre of
Zhangqiu People's Hospital (Jinan, China) and were allowed free
access to laboratory chow and tap water in day-night quarters at
25°C with 50–60% humidity and a 12-h light/dark cycle. The
experiment was approved by the Committee on Animal Experiments of
Zhangqiu People's Hospital (14).
All rats were randomly divided into the following three
experimental groups (n=12 per group): Sham, sham-operated rats
pretreated intraperitoneally with normal saline for 3 days;
cerebral IRI model, cerebral IRI model rats pretreated
intraperitoneally with normal saline for 3 days; and pentoxifylline
treatment group, cerebral IRI model rats pretreated daily with 46.7
mg/kg pentoxifylline (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) intraperitoneally for 3 days. The chemical structure of
pentoxifylline
(3,7-dimethyl-1-(5-oxohexyl)-3,7-dihydro-1H-purine-2,6-dione) is
presented in Fig. 1.
Cerebral IRI model
Intraluminal filamentous occlusion of the middle
cerebral artery (MCA) was performed to induce the cerebral IRI
model for 1 h. Reperfusion of the MCA was initiated by removing the
MCA-occlusive filament after the 1 h of occlusion and then
reperfusion was performed for 1 h. The mean arterial blood
pressure, arterial blood gases and pH were monitored following
cannulation of the right femoral artery. A laser Doppler flowmeter
(Periflux System 5000; Perimed AB, Järfälla, Sweden) was used to
monitor regional cerebral blood flow. Sham-operated rats were
anesthetized (35 mg/kg pentobarbital sodium) and surgically opened
up without cerebral IRI induction. Brain samples were fixed using
4% paraformaldehyde for 24 h at room temperature and processed by
routine histological methods, embedded in paraffin blocks and
sectioned coronally into sequential 5-µm sections. Then, section
samples were stained using haematoxylin and eosin for 20 min at
room temperature. Subsequently, neurological deficit scores were
analyzed (0, normal; 1, moderate; 2, considerable; 3, severe)
following treatment with pentoxifylline for 3 days using a
fluorescence microscope (OLYMPUS BX51; Olympus Corporation, Tokyo,
Japan).
Evaluation of cerebral infarct
volume
Rats were sacrificed by decapitation and cerebral
infarct tissues were rapidly acquired. Cerebral infarct tissues
were frozen at −80°C for storage. Subsequently, cerebral infarct
tissues were fixed with 4% paraformaldehyde in PBS for 24 h at room
temperature. Then, brains tissues were sliced into 5-µm uniform
coronal sections, which were stained with 2%
2,3,5-triphenyltetrazolium chloride solution for 1 h at 37°C.
Cerebral infarct volume was using a fluorescence microscope
(OLYMPUS BX51) and measured using OlyVIA software version 2.6 (both
Olympus Corporation).
Histology
Rats were sacrificed by decapitation and cerebral
infarct tissues were rapidly acquired. Cerebral infarct tissues
were fixed in 3% paraformaldehyde in PBS for 4 h on ice and then
transferred to 50% ethanol for 2 h. Tissues samples were cut into
histological sections (4-µm) and were stained with hematoxylin and
eosin (H&E) for 15 min at room temperature using light
microscope (magnification, ×20; Metallurgical Microscope; Shanghai
Optical Instrument Import & Export Co., Ltd., Shanghai, China)
to analyze the number of neurocytes.
Evaluation of tumor necrosis factor
(TNF)-α, interleukin (IL)-6, malondialdehyde (MDA) and superoxide
dismutase (SOD) activities
Rats were sacrificed by decapitation and blood
samples (0.5 ml) were collected from the femoral vein. Serum was
isolated from the blood following centrifugation at 1,000 × g for
20 min at 4°C. ELISA analyses were performed using ELISA kits for
TNF-α (cat. no. KRC3012; Biosource; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), IL-6 (cat. no. BMS625TEN; Biosource; Thermo
Fisher Scientific, Inc.), MDA (cat. no. A003-1; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) and SOD (cat. no.
A001-1-1; Nanjing Jiancheng Bioengineering Institute) to measure
their activities in serum samples, according to the manufacturer's
protocol.
Measurement of cyclooxygenase (COX)-2
and inducible nitric oxide synthase (iNOS) mRNA expression
Total RNA was prepared from cerebral infarct tissues
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. RNA samples (1 mg) were
used for cDNA synthesis, which was performed using the RevertAid
First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
temperature protocol was as follows: 37°C for 1 h and 42°C for 10
min. Quantitative (q)PCR was performed using SYBR Green PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) by the ABI
7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.) by
to measure the mRNA expression of COX-2 and iNOS (n=3). The
following primers were used: COX-2 sense,
5′-GTGGGATGACGAGCGACTGT-3′ and antisense
5′-TTTCAGGGAGAAGCGTTTGC-3′; iNOS sense, 5′-GCATCCCAAGTACGAGTGGT-3′
and antisense 5′-GAAGGCGTAGCTGAACAAGG-3′; and GAPDH sense,
5′-CCATCACTGCCACTCAGAAGA-3′ and antisense
5′-CATGAGGTCCACCACCCTGT-3′. The PCR conditions were 94°C for 5 min,
35 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 1 min,
and then 4°C for 10 min. miRNA expression were measured using the
2−∆∆Ct method (15).
Experiments were repeated three times.
Western blotting
Rats were sacrificed by decapitation and cerebral
infarct tissues were rapidly acquired. Cerebral infarct tissues
were frozen at −80°C. Subsequently, brain tissues were ground into
a powder with liquid nitrogen and lysed in radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) for 30 min at 4°C, which was followed by centrifugation at
12,000 × g for 5 min at 4°C. The supernatant was collected and the
protein concentration was determined using a BCA Protein assay kit
(Sangon Biotech Co., Ltd., Shanghai, China). Proteins (50 µg) were
separated on 12% SDS polyacrylamide gels (Sangon Biotech Co., Ltd.)
and electrophoretically transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA), which were then
blocked using 5% non-fat milk in TBST (TBS containing 0.1%
Tween-20) for 1 h at 37°C. Membranes were incubated overnight at
4°C with anti-COX-2 (cat. no. sc-7951; 1:2,000; Santa Cruz
Biotechnology), anti-iNOS (cat. no. sc-649; 1:2,000; Santa Cruz
Biotechnology), anti-cleaved caspase-3 (cat. no. 9662; 1:2,000;
Santa Cruz Biotechnology), anti-matrix metallopeptidase (MMP)-9
(cat. no. sc-10737; 1:2,000; Santa Cruz Biotechnology),
anti-phosphorylated (p)-p38 mitogen-activated protein kinase (cat.
no. 4511; 1:3,000; Cell Signaling Technology, Inc.) and anti- GAPDH
(cat. no. sc-25778; 1:500; Santa Cruz Biotechnology). Membranes
were incubated with goat anti-rabbit horseradish
peroxidase-conjugated IgG (cat. no. sc-2004; 1:5,000; Santa Cruz
Biotechnology) for 1 h at 37°C. Protein expression was observed
with the BeyoECL Plus chemiluminescence reagent (Beyotime Institute
of Biotechnology) and analyzed using Image_Lab_3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean + standard error of
the mean. Statistical analysis was performed by one-way analysis of
variance and Dunnett's post-hoc test, which was performed using
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Protective effects of pentoxifylline
on neurological deficit score
To determine the protective effects of
pentoxifylline on neurological deficit score, neurological deficit
scores were evaluated. As presented in Fig. 2, there was a significant increase
in the neurological deficit score of the cerebral IRI model group,
compared with the sham-operated rats (control) group. However,
pretreatment with pentoxifylline significantly reduced the
neurological deficit score of cerebral IRI rats (Fig. 2).
Protective effects of pentoxifylline
on neurocytes
To further determine the protective effects of
pentoxifylline on neurocytes, neurocytes in brain sections were
stained using H&E. The number of neurocytes in the sham group
was higher compared with the cerebral IRI model group (Fig. 3). However, pentoxifylline treatment
increased neurocytes compared with the cerebral IRI model group
(Fig. 3).
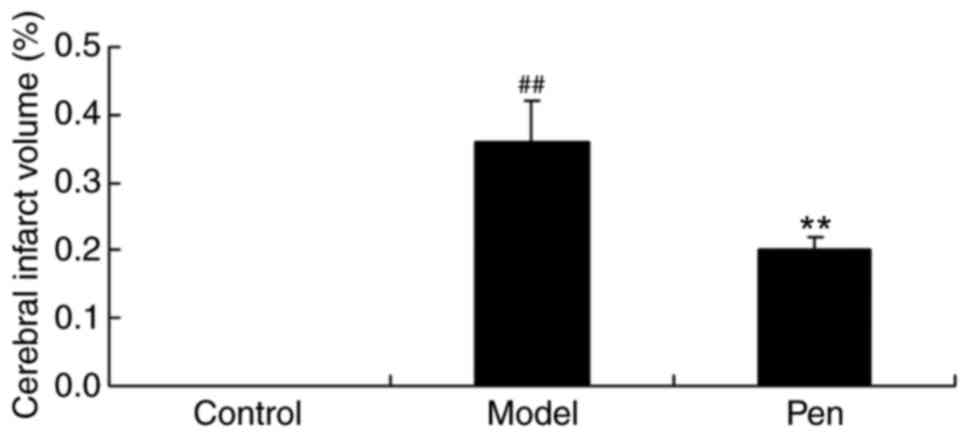
Protective effects of pentoxifylline
on cerebral infarct volume
The present study also determined the protective
effects of pentoxifylline on cerebral infarct volume. In the sham
group, cerebral infarct volume was lower compared with the cerebral
IRI model group (Fig. 4). However,
treatment with pentoxifylline significantly reduced the cerebral
infarct volume in rats with cerebral IRI (Fig. 4).
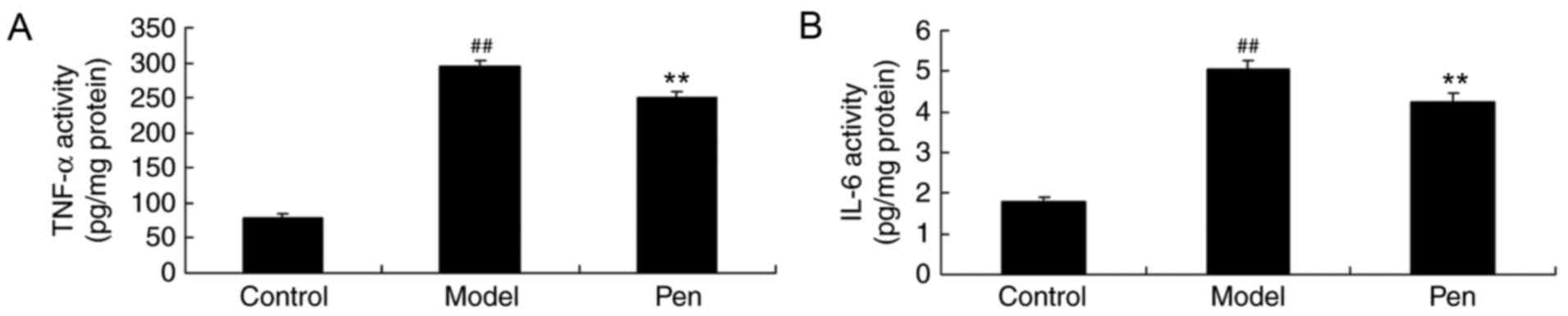
Effects of pentoxifylline on IL-6 and
TNF-α levels in cerebral IRI rats
The present study also determined the effects of
pentoxifylline on IL-6 and TNF-α levels in the serum of cerebral
IRI rats using ELISA kits. As demonstrated in Fig. 5, a significant increase in the
levels of TNF-α and IL-6 was observed in cerebral IRI rats compared
with the sham-operated group. Pretreatment with pentoxifylline
significantly suppressed the levels of IL-6 and TNF-α in the serum
of cerebral IRI rats (Fig. 5).
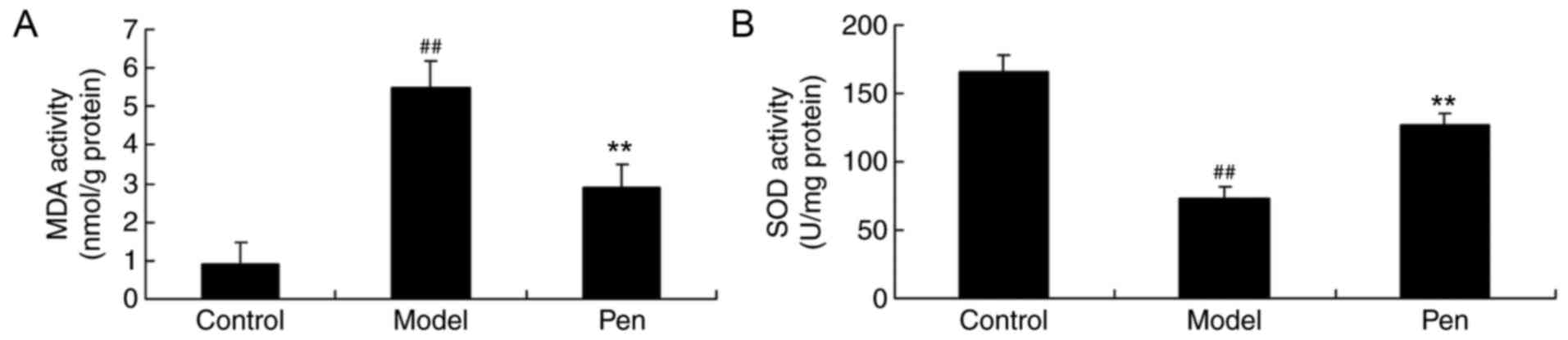
Effects of pentoxifylline on MDA and
SOD levels in cerebral IRI rats
Furthermore, the effects of pentoxifylline on MDA
and SOD levels in the serum of cerebral IRI rats were investigated
by ELISA. Compared with the sham-operated rats, there was a
significant increase in MDA and reduction in SOD levels in the
serum of cerebral IRI model rats (Fig.
6). However, the MDA and SOD levels were significantly reversed
by treatment with pentoxifylline in cerebral IRI rats (Fig. 6).
Effects of pentoxifylline on COX-2 and
iNOS mRNA and protein expression in cerebral IRI rats
Reverse transcription-qPCR (RT-qPCR) analysis and
western blotting was performed to determine the changes in COX-2
and iNOS mRNA and protein expression, respectively. The
sham-operated group exhibited a significant decrease in COX-2 and
iNOS mRNA and protein expression, compared with cerebral IRI model
rats (Fig. 7). However, treatment
with pentoxifylline significantly suppressed the COX-2 and iNOS
mRNA and protein expression in cerebral IRI rats (Fig. 7).
Effects of pentoxifylline on cleaved
caspase-3, MMP-9 and p38 protein expression in cerebral IRI
rats
In order to determine the effects of pentoxifylline
on cleaved caspase-3, MMP-9 and p-p38 MAPK protein expression in
cerebral RI rats, western blotting was performed. The protein
expression of cleaved caspase-3 and p-p38 MAPK was significantly
increased in cerebral IRI rats, while the expression of MMP-9 was
significantly reduced, compared with sham control rats (Fig. 8). However, the protein expression
of cleaved caspase-3, MMP-9 and p38 was significantly reversed with
pentoxifylline administration in cerebral IRI rats (Fig. 8).
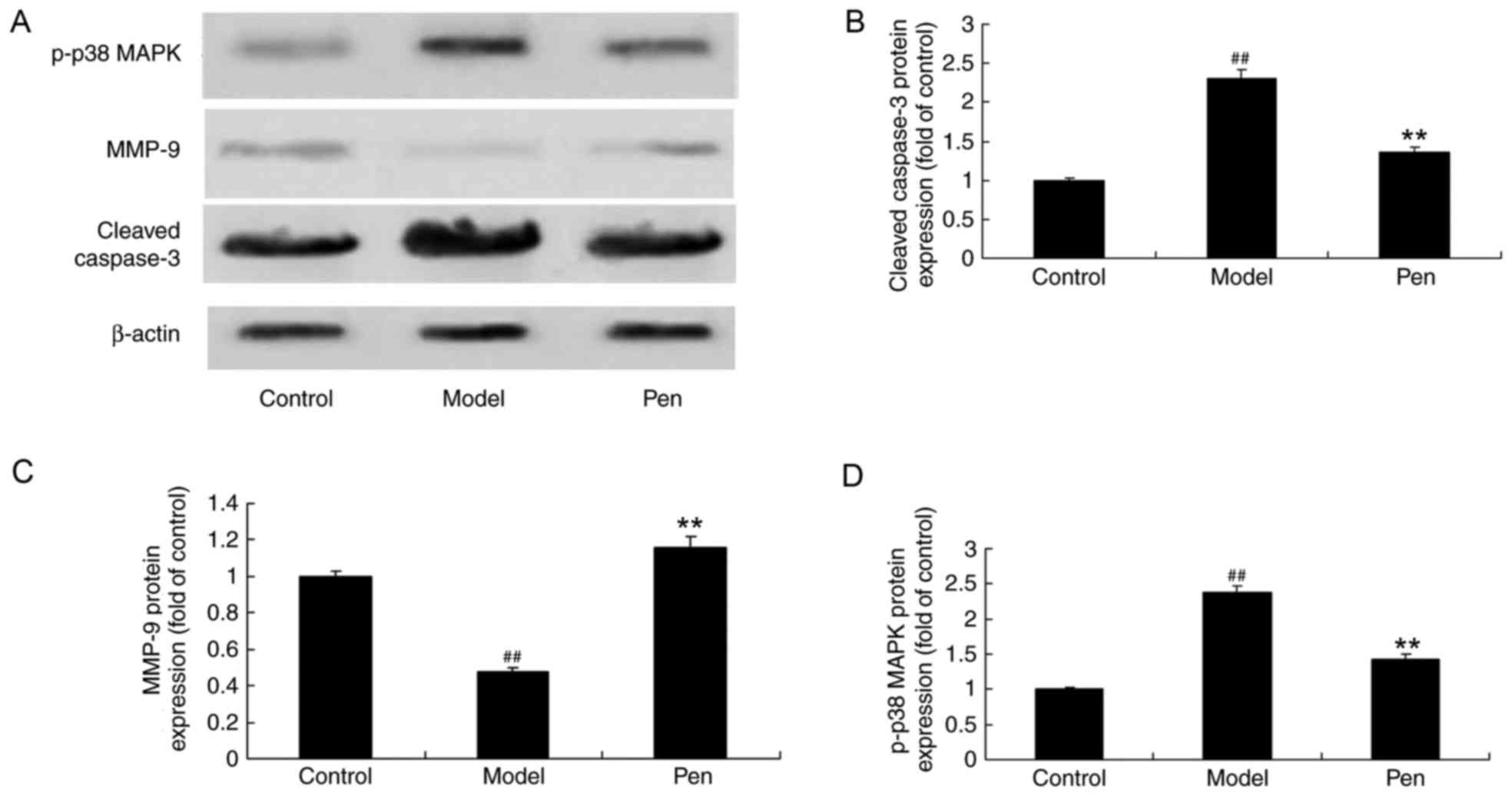 | Figure 8.Pentoxifylline treatment affects
cleaved caspase-3, MMP-9 and p-p38 MAPK protein expression in
cerebral IRI rats. (A) Representative western blot demonstrating
the effects of pentoxifylline on cleaved caspase-3, MMP-9 and p-p38
MAPK protein expression. Densitometric and statistical analysis of
(B) cleaved caspase-3, (C) MMP-9 and (D) p-p38 MAPK protein
expression in cerebral IRI rats. ##P<0.01 vs. control
group; **P<0.01 vs. model group. MMP, matrix metallopeptidase;
p-, phosphorylated-; MAPK, mitogen-activated protein kinase; IRI,
ischemia reperfusion injury; control, sham-operated group; model,
cerebral ischemia reperfusion injury model group; pen,
pentoxifylline-treated group. |
Discussion
Ischemic cerebral vascular disease is a common
neurological disease, and its high disability and death rates
negatively affect families and society (16). At present, the principle of
treatment is to restore the blood supply to the ischemic area,
however, the reperfusion injury that follows this may exacerbate
the brain dysfunction and cause further damage to the tissue, which
is termed IRI injury (17). IRI
often occurs in the recanalization of cerebral vascular
embolization, heart failure correction, shock correction, IRI
microcirculation recanalization and cardiopulmonary-cerebral
resuscitation (18). With the
increased understanding of the pathophysiological mechanism of
cerebral IRI, the inhibition of reperfusion injury has become an
important part of the treatment of ischemic cerebrovascular disease
(19), and the effectiveness and
safety of these treatments is the focus in the clinic. In the
present study, pretreatment with pentoxifylline significantly
reduced the neurological deficit score and cerebral infarct volume
in rats with cerebral IRI. Taken together, these results indicate
that pentoxifylline may be a potential candidate drug for cerebral
IRI.
COX is a type of rate-limiting enzyme that catalyzes
the synthesis of prostaglandin and thromboxane from arachidonic
acid (20). There are two isomers
of COX; COX-1 exists in the majority of tissues as a structural
type and catalyzes the production of prostaglandins required to
maintain the normal physiological function (21,22),
and COX-2, which is abnormally expressed following cerebral
ischemia, is a key enzyme in the production of free radicals and
inflammatory mediators, and is involved in the development of
ischemic brain injury and closely associated with the prognosis of
cerebral ischemia (21,23). Oxidative damage and the mechanism
of death in ischemic neurons has received increased attention in
research. The results of the current study demonstrated that
pretreatment with pentoxifylline significantly suppressed IL-6 and
TNF-α levels, inhibited MDA and increased SOD levels, and reduced
COX-2 mRNA and protein expression in cerebral IRI rats. Marques
et al (24) reported that
pentoxifylline may attenuate the inflammatory process and apoptosis
via cleaved caspase-3 and COX-2 in rats with intestinal IRI. In
addition, Mayyas et al (25) observed that pentoxifylline
suppressed myocardial oxidative status following intake of a
western diet.
MMP-9 is a type of zinc-dependent proteolytic enzyme
(26). In the neuroinflammation
reaction, it is released by the stimulation of cytokines and
immediate-early factors, reacts to various proinflammatory stimuli
and is involved in the inflammatory reaction and pathophysiological
process of various nervous system diseases (27). MMP-9 has an important role in the
physiological and pathological processes of the central nervous
system (28). A previous study
indicated that MMP-9 also has an important role in the development
of ischemic cerebrovascular disease (29). It has been demonstrated that MMP-9
is expressed in the hippocampus, cortex and striatum of normal
brain tissue, although the expression level is very low (30). Following cerebral IRI injury, the
expression of MMP-9 in the ischemic brain tissue is reported to be
markedly enhanced (30). Notably,
the present study demonstrated that treatment with pentoxifylline
significantly reduced the iNOS mRNA and protein expression, and
induced MMP-9 protein expression in cerebral IRI rats. Garcia et
al (31) reported that
pentoxifylline decreased glycemia levels through suppression of NOS
and COX-2 expression in the pancreas of diabetic rats. In addition,
de Campos et al (32)
demonstrated that pentoxifylline attenuated pulmonary inflammation
via MMP-9 in experimental acute pancreatitis.
A previous study confirmed that the p38 MAPK
signaling pathway is closely associated with nerve cell damage
(33). During cerebral IRI, p38
MAPK is activated, and the inhibition of p38 MAPK may protect nerve
cells from IRI (34). Activation
of the p38 MAPK pathway is reported to regulate nitric oxide levels
by influencing the expression of iNOS (35). Nitric oxide is an important factor
that affects the expression of MMP-9, which directly degrades
collagen IV and subsequently leads to increases in blood brain
barrier permeability and the exosmosis of IgG from the blood vessel
(35). The results of the current
study indicate that treatment with pentoxifylline significantly
attenuated caspase-3 and p-p38 protein expression in cerebral IRI
rats. Costantini et al (36) reported that pentoxifylline
suppressed leukoreduced stored blood-induced neutrophil activation
via p38 MAPK and extracellular signal-regulated kinase
phosphorylation.
In conclusion, the results of the present study
demonstrated that pentoxifylline inhibits the neurological deficit
score and cerebral infarct volume in cerebral IRI rats, which may
occur via anti-inflammation, antioxidation and antiapoptotic
mechanisms as pentoxifylline suppressed COX-2 expression, increased
MMP-9 expression and downregulated p38 pathways in cerebral IRI
rats in vivo. Further research is required to evaluate the
potential beneficial effect of pentoxifylline on cerebral IRI in
the clinic.
References
|
1
|
Martins VL, Caley MP, Moore K, Szentpetery
Z, Marsh ST, Murrell DF, Kim MH, Avari M, McGrath JA, Cerio R, et
al: Suppression of TGFβ and angiogenesis by type VII collagen in
cutaneous SCC. J Natl Cancer Inst. 108:pii: djv293. 2015.PubMed/NCBI
|
|
2
|
Zhao Y, Wang D, Xu T, Liu P, Cao Y, Wang
Y, Yang X, Xu X, Wang X and Niu H: Bladder cancer cells re-educate
TAMs through lactate shuttling in the microfluidic cancer
microenvironment. Oncotarget. 6:39196–39210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodriguez DA, de Lima RF, Campos MS, Costa
JR, Biancardi MF, Marques MR, Taboga SR and Santos FCA:
Intrauterine exposure to bisphenol A promotes different effects in
both neonatal and adult prostate of male and female gerbils
(Meriones unguiculatus). Environ Toxicol. 31:1740–1750.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khoo NK, Cantu-Medellin N, St Croix C and
Kelley EE: In vivo immuno-spin trapping: Imaging the footprints of
oxidative stress. Curr Protoc Cytom. 74(12): 42.1–11. 2015.
|
|
5
|
Yue K, Trujillo-de Santiago G, Alvarez MM,
Tamayol A, Annabi N and Khademhosseini A: Synthesis, properties,
and biomedical applications of gelatin methacryloyl (GelMA)
hydrogels. Biomaterials. 73:254–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan Y, Martin LM, Bosco DB, Bundy JL,
Nowakowski RS, Sang QX and Li Y: Differential effects of acellular
embryonic matrices on pluripotent stem cell expansion and neural
differentiation. Biomaterials. 73:231–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koyano-Nakagawa N, Shi X, Rasmussen TL,
Das S, Walter CA and Garry DJ: Feedback mechanisms regulate Ets
variant 2 (Etv2) gene expression and hematoendothelial lineages. J
Biol Chem. 290:28107–28119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Warsinske HC, Ashley SL, Linderman JJ,
Moore BB and Kirschner DE: Identifying mechanisms of homeostatic
signaling in fibroblast differentiation. Bull Math Biol.
77:1556–1582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyamoto DT, Zheng Y, Wittner BS, Lee RJ,
Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, et
al: RNA-Seq of single prostate CTCs implicates noncanonical Wnt
signaling in antiandrogen resistance. Science. 349:1351–1356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gill SE, Rohan M and Mehta S: Role of
pulmonary microvascular endothelial cell apoptosis in murine
sepsis-induced lung injury in vivo. Respir Res. 16:1092015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang YR, Kim DH, Seo YK, Park D, Jang HJ,
Choi SY, Lee YH, Lee GH, Nakajima K, Taniguchi N, et al: Elevated
O-GlcNAcylation promotes colonic inflammation and tumorigenesis by
modulating NF-κB signaling. Oncotarget. 6:12529–12542. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thurber AE, Omenetto FG and Kaplan DL: In
vivo bioresponses to silk proteins. Biomaterials. 71:145–157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji H, Tang H, Lin H, Mao J, Gao L, Liu J
and Wu T: Rho/Rock cross-talks with transforming growth
factor-β/Smad pathway participates in lung fibroblast-myofibroblast
differentiation. Biomed Rep. 2:787–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng H, Zuo X, Zhang J, Liu X, Liu L, Xu
Q, Wu Z and Ji A: Α-lipoic acid protects against cerebral
ischemia/reperfusion-induced injury in rats. Mol Med Rep.
11:3659–3665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saiardi A, Guillermier C, Loss O, Poczatek
JC and Lechene C: Quantitative imaging of inositol distribution in
yeast using multi-isotope imaging mass spectrometry (MIMS). Surf
Interface Anal. 46 Suppl 1:S169–S172. 2014. View Article : Google Scholar
|
|
17
|
Hosseini Y, Agah M and Verbridge SS:
Endothelial cell sensing, restructuring, and invasion in collagen
hydrogel structures. Integr Biol (Camb). 7:1432–1441. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siddiqui A, Bhaumik D, Chinta SJ, Rane A,
Rajagopalan S, Lieu CA, Lithgow GJ and Andersen JK: Mitochondrial
quality control via the PGC1α-TFEB signaling pathway is compromised
by parkin Q311X mutation but independently restored by rapamycin. J
Neurosci. 35:12833–12844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhattacharya P, Pandey AK, Paul S, Patnaik
R and Yavagal DR: Aquaporin-4 inhibition mediates piroxicam-induced
neuroprotection against focal cerebral ischemia/reperfusion injury
in rodents. PLoS One. 8:e734812013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bassuk JA, Wu D, Lozano H, Arias J,
Kurlansky P, Lamas GA and Adams JA: Non-selective cyclooxygenase
inhibition before periodic acceleration (pGz) cardiopulmonary
resuscitation (CPR) in a porcine model of ventricular fibrillation.
Resuscitation. 77:250–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang T, Zhai L, Zhang H, Zhao L and Guo Y:
Picroside II Inhibits the MEK-ERK1/2-COX2 signal pathway to prevent
cerebral ischemic injury in rats. J Mol Neurosci. 57:335–351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brzozowski T, Konturek PC, Konturek SJ,
Pajdo R, Kwiecien S, Pawlik M, Drozdowicz D, Sliwowski Z and Pawlik
WW: Ischemic preconditioning of remote organs attenuates gastric
ischemia-reperfusion injury through involvement of prostaglandins
and sensory nerves. Eur J Pharmacol. 499:201–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salinas G, Rangasetty UC, Uretsky BF and
Birnbaum Y: The cycloxygenase 2 (COX-2) story: It's time to
explain, not inflame. J Cardiovasc Pharmacol Ther. 12:98–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marques GM, Rasslan R, Belon AR, Carvalho
JG, Neto Felice R, Rasslan S, Utiyama EM and Montero EF:
Pentoxifylline associated to hypertonic saline solution attenuates
inflammatory process and apoptosis after intestinal
ischemia/reperfusion in rats. Acta Cir Bras. 29:735–741. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mayyas F, Alzoubi KH and Al-Taleb Z: An
evaluation of the effect of pentoxifylline on blood pressure and
myocardial oxidative status following intake of western diet. Clin
Exp Hypertens. 37:666–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xin L, Hou Q, Xiong QI and Ding X:
Association between matrix metalloproteinase-2 and matrix
metalloproteinase-9 polymorphisms and endometriosis: A systematic
review and meta-analysis. Biomed Rep. 3:559–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun J, Zhu Y, Zhang L and Ma Y: Effects of
xuelian injection on cerebral TNF-α, IL-1β and MMP-9 in rats
experienced focal cerebral ischemia/reperfusion. Int J Clin Exp
Med. 7:2632–2638. 2014.PubMed/NCBI
|
|
28
|
Zheng M, Wei J, Tang Y, Yang C, Wei Y, Yin
X and Liu Q: ApoE-deficient promotes blood-brain barrier disruption
in experimental autoimmune encephalomyelitis via alteration of
MMP-9. J Mol Neurosci. 54:282–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim SJ and Lee SR: Protective effect of
melatonin against transient global cerebral ischemia-induced
neuronal cell damage via inhibition of matrix metalloproteinase-9.
Life Sci. 94:8–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xin H, Liang W, Mang J, Lin L, Guo N,
Zhang F and Xu Z: Relationship of gelatinases-tight junction
proteins and blood-brain barrier permeability in the early stage of
cerebral ischemia and reperfusion. Neural Regen Res. 7:2405–2412.
2012.PubMed/NCBI
|
|
31
|
Garcia FA, Pinto SF, Cavalcante AF,
Lucetti LT, Menezes SM, Felipe CF, Alves AP, Brito GA, Cerqueira GS
and Viana GS: Pentoxifylline decreases glycemia levels and
TNF-alpha, iNOS and COX-2 expressions in diabetic rat pancreas.
Springerplus. 3:2832014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Campos T, Deree J, Martins JO, Loomis
WH, Shenvi E, Putnam JG and Coimbra R: Pentoxifylline attenuates
pulmonary inflammation and neutrophil activation in experimental
acute pancreatitis. Pancreas. 37:42–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu XW, Ji EF, He P, Xing RX, Tian BX and
Li XD: Protective effects of the p38 MAPK inhibitor SB203580 on
NMDA-induced injury in primary cerebral cortical neurons. Mol Med
Rep. 10:1942–1948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu MH, Chio CC, Tsai KJ, Chang CP, Lin NK,
Huang CC and Lin MT: Obesity exacerbates rat cerebral ischemic
injury through enhancing ischemic adiponectin-containing neuronal
apoptosis. Mol Neurobiol. 53:3702–3713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Madhyastha R, Madhyastha H, Nakajima Y,
Omura S and Maruyama M: MicroRNA signature in diabetic wound
healing: Promotive role of miR-21 in fibroblast migration. Int
Wound J. 9:355–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Costantini TW, Deree J, Martins JO, Loomis
WH, Bansal V and Coimbra R: Pentoxifylline attenuates leukoreduced
stored blood-induced neutrophil activation through inhibition of
mitogen-activated protein kinases. Immunopharmacol Immunotoxicol.
32:74–81. 2010. View Article : Google Scholar : PubMed/NCBI
|