Introduction
The liver has dual blood supplies: the portal vein
and the hepatic artery. When the portal vein is compromised, by
thrombosis for example, capacity of the liver regenerate is
limited. Liver dual arterial blood supply (LDABS) is a surgical
procedure that shunts arterial blood to the portal vein system for
enhancing liver blood supply to maintain liver regeneration. LDABS
is distinct from arterioportal fistula (1) as well as simple portal vein
arterialization, in which the portal vein is the only route to
supply blood to the liver because of injury, thrombosis or surgical
removal of the proper hepatic artery (2,3).
LDABS could extend the usefulness of portal vein
arterialization (4) and has been
used to manage portal vein thrombosis before and after orthotopic
liver transplantation (OLT) or auxiliary liver transplantation
(ALT) (5,6). LDABS could maintain hepatic function
and morphology in some patients for as long as 3 years (7).
The mechanisms by which LDABS maintains liver
regeneration are not fully understood, but clearly differ
substantially from liver regeneration that occurs after PH. Unlike
PH, LDABS involves loss of blood supply from the portal vein,
changes in blood components and hemodynamics of the portal vein
system, and changes in liver anatomy.
In the current study, we used whole-genome oligo
microarray analysis to examine the molecular changes in a rat model
of PH plus LDABS. Key genes identification was validated using a
MAPK signaling PCR array. The results of this study may help expand
our understanding of signaling pathways that underlie liver
regeneration and provide a firmer foundation for further developing
LDABS as a clinical tool.
Materials and methods
The study protocol was approved by the Laboratory
Animal Ethics Committee of Inner Mongolia Medical University.
Briefly, male Sprague-Dawley rats (Vital River Laboratory Animal
Technology; Beijing, China) randomly received PH alone or PH
followed by LDABS (n=20/group). Rats were sacrificed immediately at
0, 6, 48 and 168 h after the surgery (n=5 per time point). Remnant
liver was collected for whole genome oligo microarrays. Trunk blood
(2 ml) was collected from the infrahepatic inferior vena cava.
PH
The operation was performed by two surgeons using
clean but not sterile technique under a microscope (Leica M525 F20,
Germany). Rats were fasted for 24 h prior to operation. Anesthesia
was induced by inhalation of 3–4% isoflurane, and maintained by
inhalation of 1–2% isoflurane. After anesthesia induction, a median
incision was cut on the abdomen. The left and middle lobes of the
liver were resected using bloodless hepatectomy (Fig. 1), as previously reported (8).
LDABS
After the left and middle lobes of the liver were
resected, the infrahepatic caval vein was isolated. The right renal
artery was isolated and clamped. The right renal vein was isolated
and ligated with a 1–0 silk suture adjacent to the infrahepatic
caval vein. The right kidney was removed. The pyloric vein was
ligated with an 8–0 nylon suture and divided; the proximal and
distal ends of the portal vein were clamped, and the main portal
vein was isolated and dissected transversely at the midpoint. The
right renal artery was anastomosed with the proximal end of the
portal vein using a MicroRenathane catheter (length: 10 mm; inner
diameter: 0.5 mm). Then the clamps were removed successively from
the portal vein and right renal artery to allow portal vein
arterialization.
The distal end of the portal vein was anastomosed
with the right renal vein, as described (9). The clamps were removed successively
from the right renal vein and portal vein to allow portacaval shunt
(Fig. 1). After irrigation with
warm lactated Ringer's solution (5 ml), the abdomen was closed.
Rat body temperature was maintained at 36°C using a
heating blanket throughout the procedure. Rats received daily low
molecular weight heparin (50 IU/ml, s.c.). Antibiotics and
analgesics were not used.
Evaluation of liver regeneration
Liver regeneration was assessed by visual inspection
with naked eyes as well as microscopic examination of hepatic cells
and portal areas following hematoxylin-eosin staining. Alanine
transaminase (ALT) and albumin (ALB) levels in serum were measured
using an automated analyzer. Regeneration is quantitatively
expressed as liver regeneration rate (LRR), based on liver weight
(LW), and calculated as follows:
LRR=LW at autopsy-estimated residual LW
at time of surgeryResected LW×100%
Residual liver weight at the time of surgery was
estimated based on the assumption that the left and middle hepatic
lobes account for 70 percent of total liver weight (8).
Whole-genome oligo microarray
analysis
Total RNA was harvested from the liver using TRIzol
(Invitrogen) and the RNeasy kit (Qiagen); this procedure included a
DNase digestion step. RNA was quantified using a Nanodrop ND-1000
apparatus (NanoDrop Technologies, Wilmington, DE, USA). RNA quality
was verified with denaturing gel electrophoresis. Samples were
amplified and labeled using the Agilent Quick Amp labeling kit and
hybridized using an Agilent 4×44K whole-genome oligo microarray in
Agilent SureHyb Hybridization Chambers. After hybridization and
washing, microarray slides were scanned using the Agilent DNA
microarray scanner (G2505B). Text files of results were extracted
using Agilent Feature Extraction Software (version 10.5.1.1) and
imported into Agilent GeneSpring GX software (version 10.0). Genes
differentially expressed between the 2 groups were defined as
>2.0 fold-change and P<0.05 between the 2 groups at each time
point. Identified genes were analyzed using the KEGG PATHWAY
Database (http://www.genome.jp/kegg/).
Two-sided Fisher's exact test was used to classify enriched
pathways. Enrichment was defined by (a/n)/(A/N),
where a represents the number of target genes; n, the
total number of genes in the particular pathway; A, the
total number of differentially expressed genes in all the pathways;
and N, the total number of genes in all the pathways.
MAPK signaling PCR array analysis
Regeneration-related genes differentially expressed
between the 2 groups were analyzed using a rat MAPK signaling PCR
Array (SuperArray Bioscience, Frederick, Maryland, USA). RNA was
extracted and converted to first-strand cDNA using the
RT2 First Strand Kit. The template was added to an
instrument-specific, ready-to-use RT2 SYBR Green qPCR
Master Mix. The resulting mixture was added to 96-well PCR array
plate (25 µl/well) pre-loaded with gene-specific primer sets (25
µl). PCR was performed and threshold cycle (Ct) values for all
genes on each PCR array were calculated using instrument-specific
software. Fold-changes in gene expression between the 2 groups were
calculated using the DDCt method. Gene interaction networks were
constructed using Pathway Studio (Ariadne Genomics, NX Amsterdam,
the Netherlands).
Statistical analysis
Continuous variables are expressed as mean ±
standard deviation (SD). Statistical analyses were carried out
using SPSS 18.0 (IBM, Chicago, IL, USA). Homogeneity of variance
was assessed using the F test and the collecting dates were
analyzed by repeated measurement analysis of variance. P<0.05
was defined as the threshold of significance.
Results
Histological examination
Both groups showed initial hepatic congestion and
subsidence over time; the remnant liver in the LDABS group was
brighter than that the control group (Fig. 1). Liver weight increased to a
similar extent in the 2 groups. Neither group showed evidence of
macroscopic necrotic findings, such as petechia and ecchymoses.
Liver tissue from the control group showed integral
hepatic lobules, hepatic cords radially arranged around a central
vein, distinct hepatic sinuses, and absence of inflammatory cell
infiltration in and around portal areas (Fig. 2). In contrast, liver tissue from
the LDABS group showed markedly dilated hepatic sinuses, peaking at
6 h postoperatively. Red blood cells (RBCs) were present to various
extents in selected sinuses, but without RBC deposits or
thrombosis. Interlobular veins were dilated. Normal bile canaliculi
were visible in portal areas. Pyknosis and necrosis were observed
in a small fraction of hepatocytes in the central zone. Hepatic
cords appeared normal. Tissue edema and vacuolar degeneration of
hepatocytes increased over time, peaking at 48 h postoperatively.
Hepatic sinus dilation and congestion decreased gradually over
time.
Liver function
ALT level increased to a peak at 6 h after the
surgery, and then gradually returned to the baseline in both groups
(Fig. 3). ALT was lower in the
LDABS group at 6 h (782.9±59.9 vs. 411.2±54.6, P<0.05), but not
other time points (P>0.05). ALB level decreased to a trough at 6
h, and then gradually returned to the baseline in both groups
(Fig. 4). ALB level was comparable
between the 2 groups at all time points (P>0.05).
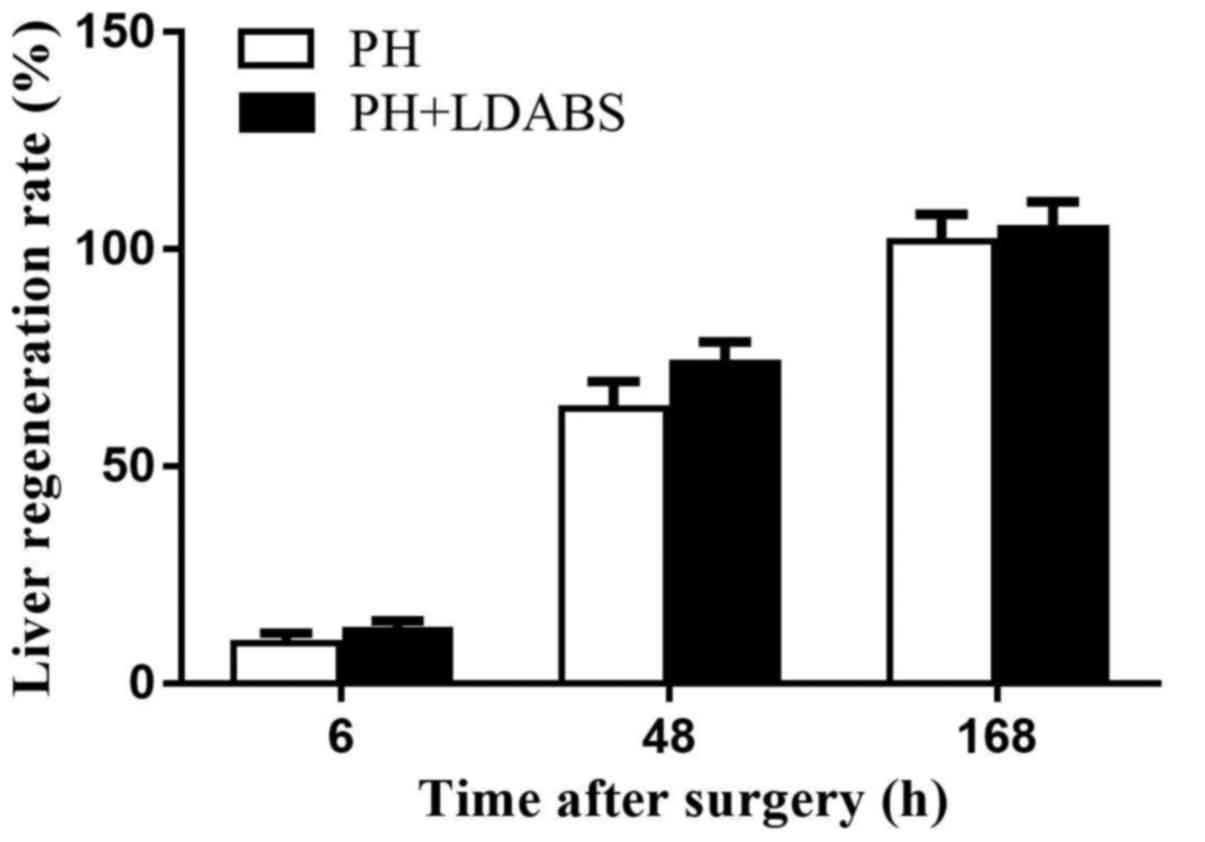
LRR
LRR gradually increased over time, reaching 100% by
168 h in both groups (Fig. 5), and
LRR did not differ between the 2 groups at all time points
(P>0.05).
Differential gene expression
In order to reveal molecular mechanisms of LDABS,
gene expression was compared between rats treated by PH in the
absence or presence of LDABS using whole-genome oligo microarray
analysis of hepatic tissue. After limiting the results to genes
showing >2-fold difference between the two conditions (with
P<0.05), pathway analysis was used to identify signaling
pathways altered in LDABS-mediated regeneration (Table I). Wayne chart analysis identified
up-regulated genes in arginine and proline metabolism signaling
pathway at all three time points, and down-regulated genes in the
following 11 signaling pathways (Fig.
6): cytokine-cytokine receptor interaction, malaria, HTLV-I
infection, NF-kappa B signaling pathway, Chagas disease (American
trypanosomiasis), amoebiasis, rheumatoid arthritis, Toll-like
receptor signaling pathway, MAPK signaling pathway, toxoplasmosis,
and ECM-receptor interaction. In all these signaling pathways only
the MAPK signaling pathway, NF-kappa B signaling pathway, and
Toll-like receptor signaling pathway which regulated post PH liver
regeneration were involved in LDABS-mediated liver
regeneration.
 | Table I.Whole-genome oligo microarray analysis
of genes differentially expressed between rats with PH alone vs.
with PH plus LDABS. |
Table I.
Whole-genome oligo microarray analysis
of genes differentially expressed between rats with PH alone vs.
with PH plus LDABS.
| Time point, h | Number of upregulated
genes | Signaling pathways
enriched with upregulated genes | Number of
downregulated genes | Signaling pathways
enriched with downregulated genes |
|---|
| 6 | 2,267 | 39 | 1,566 | 38 |
| 48 | 1,653 | 37 | 928 | 36 |
| 168 | 1,235 | 18 | 668 | 42 |
MAPK signaling pathway involvement
using PCR array analysis
Since our study identified MAPK signaling pathway
were involved in LDABS-mediated liver regeneration, we validated
our results for 84 genes associated with MAPK signaling pathway
using MAPK signaling pathway PCR array analysis. The results
confirmed that several changes were expressed at different levels
(>2 fold-change) in the absence or presence of LDABS (Table II), The genes list is as
follows:
 | Table II.MAPK signaling pathway polymerase
chain reaction array analysis to confirm differential expression of
mitogen-activated protein kinase signaling-related genes in liver
dual arterial blood supply-mediated liver regeneration. |
Table II.
MAPK signaling pathway polymerase
chain reaction array analysis to confirm differential expression of
mitogen-activated protein kinase signaling-related genes in liver
dual arterial blood supply-mediated liver regeneration.
| Time point, h | Number of upregulated
genes | Number of
downregulated genes |
|---|
| 6 | 16 | 5 |
| 48 | 10 | 5 |
| 168 | 3 | 14 |
A. Time point 6 h
Number of upregulated genes (16): Ccnd1, Cdk4, Cdkn1c, Col1a1, Creb1,
Egr1, Map4k1, Mapk10, Mapk11, Mapk12, Mapk8ip1, Mef2c, Mknk1, Mos,
Nfatc4 and Rb1. Number of downregulated genes (5): Cdkn1a, Dlk1, Map2k3, Mapk14 and
Mapk7.
B. Time point 48 h
Number of upregulated genes (10): Dlk1, Egr1, Ets2, Fos, Hspb1, Jun,
Kras, Myc, Actb and Ldha. Number of downregulated genes (17): Ccne1, Cdkn1a, Col1a1, Egr1, Ets1,
Fos, Hspb1, Kras, Ksr1, Mapk10, Mapk13, Mapk8ip3, Mef2c, Mos, Myc,
Nfatc4 and Tp53.
C. Time point 168 h
Number of upregulated genes (3): Map2k6, Nfatc4, Rb1. Number of
downregulated genes (14): Ccnd2,
Cdk6, Cdkn2a, Cdkn2d, Dlk1, E2f1, Egr1, Fos, Jun, Kras, Ksr1,
Map4k1, Mapk10 and Mapk8ip3.
The up regulation of genes was significantly
increased at 6 h, while the down regulation of genes was
significantly increased at 48 and 168 h. These findings suggest
that MAPK signaling pathway is involved in post PH regeneration in
the absence or presence of LDABS, but that the pathway functions
differently (or to a different extent) in the presence of
LDABS.
Network analysis to identify key
genes
Our microarray results and data from the literature
were used to construct an interaction network of genes
differentially expressed in LDABS-mediated liver regeneration
(Figs. 7–9), Combined the list of genes in Table II, Rb1, Ccnd1, Cdk4,
Mapk10 and Creb1 genes in the initiation phase, Kras, Tp53,
Myc, Ccne1 and Hspb1 genes in the proliferation phase, Kras,
Rb1, Jun, Ccnd2 and Mapk10 genes in the termination phase were
identified as key genes in LDABS-mediated liver regeneration using
MAPK signaling PCR array analysis.
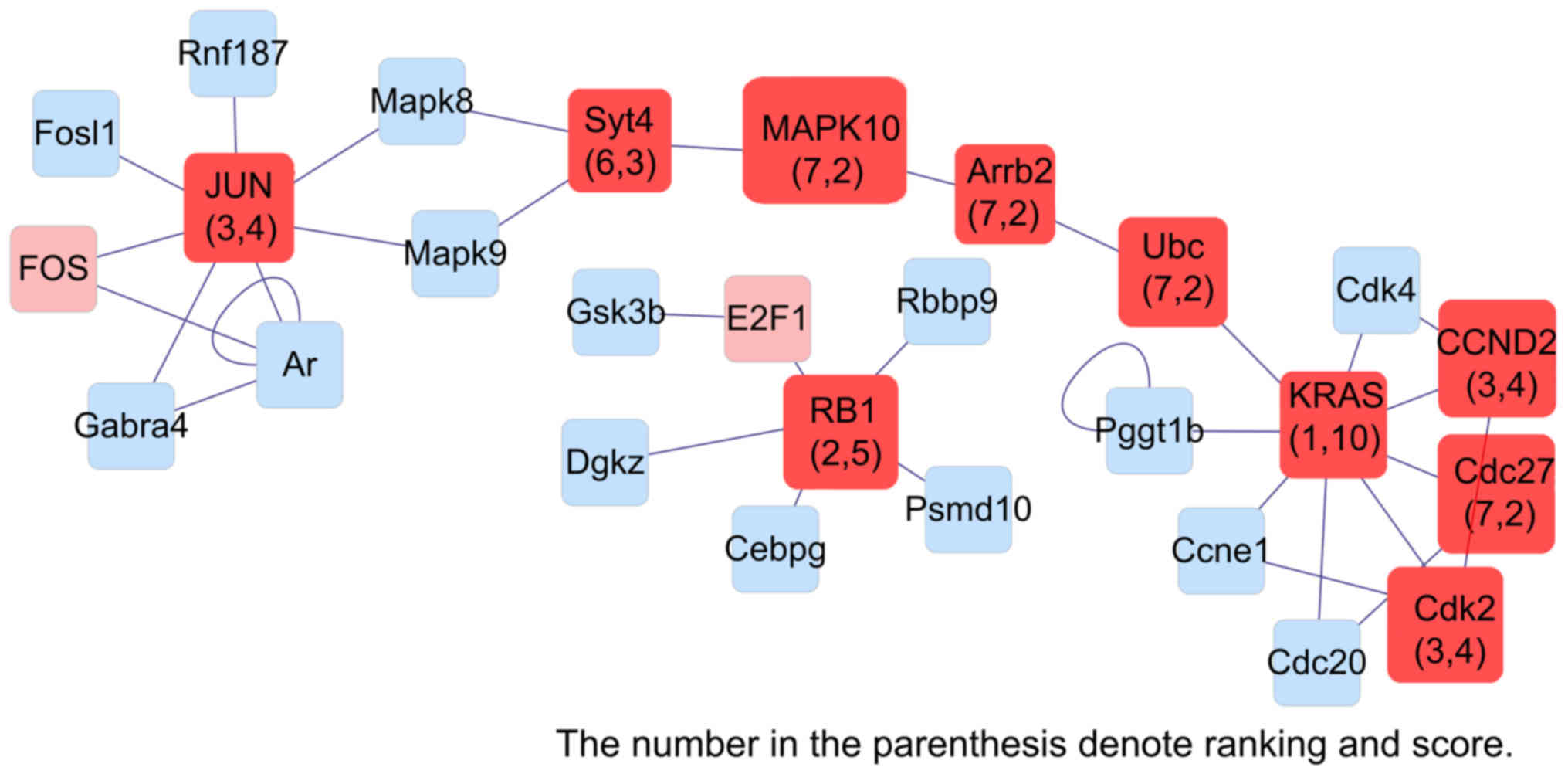 | Figure 9.Interaction network of genes
differentially expressed in liver dual arterial blood
supply-mediated liver regeneration and identification of key genes
involved at 168 h. Kras, Kirsten rat sarcoma viral oncogene
homolog; Rb1, Retinoblastoma 1; JUN, Jun proto-oncogene, AP-1
transcription factor subunit; Cdk2, Cyclin-dependent kinase 2;
CCND2, Cyclin D2; Syt4, synaptotagmin; MAPK10, Mitogen-activated
protein kinase 10; Arrb2, Arrestin Beta 2; Cdc27, cell-division
cycle 27. |
Discussion
Portal vein thrombosis (PVT) represents a major
barrier for liver transplantation because diffuse PVT is widely
considered a contraindication for liver transplantation. In 1995,
Erhard et al (5) described
what would become known as the LDABS, procedure that allowed a
patient with advanced liver cirrhosis to undergo OLT. All
components of the portal vein system in this patient showed diffuse
PVT, with the exception of a segment of inferior mesenteric vein,
and embolectomy proved unsuccessful. Blood flow to the transplanted
liver was restored by connecting the portal vein and hepatic artery
of the liver to the abdominal aorta of the recipient via the donor
iliac blood vessels. Subsequent portal hypertension was addressed
by portacaval shunt. Normal liver function with no fibrotic changes
(confirmed by hepatic biopsy) was maintained for 12-month follow
up. Nearly a decade later, a modified LDABS procedure involving was
carried out patient with advanced liver cirrhosis scheduled to
undergo OLT (10). This patient
showed diffuse PVT in the portal vein system. Blood flow to the
transplanted liver was restored by connecting the portal vein to
the hepatic artery and the hepatic artery of the donor liver to the
abdominal aorta. That patient survived for >20 months.
In a literature research, we identified 10 patients
with PVT who underwent LDABS since 1995 to reconstruct blood flow
in a transplanted liver (5,7,10–13).
All patients survived for long periods except two deaths caused by
liver necrosis or liver fibrosis, possibly due to portal vein
hyperperfusion. These results suggest that LDABS can enable OLT
upon diffuse PVT.
Evidence suggests that LDABS can also be effective
for managing PVT after OLT, helping to prevent or defer repeat
transplantation. In a case report, a patient received two OLTs, the
first transplantation due to biliary atresia and the second due to
transplanted liver lymphoma (6).
On day 3 after the second transplantation, diffuse PVT was detected
in the portal vein system. After LDABS that connected the donor
portal vein with the recipient abdominal aorta via donor iliac
blood vessels, liver function recovered over despite of eventual
death due to multiple organ failure.
Several cases have also been reported in which LDABS
enabled ALT to be performed in patients who were otherwise
ineligible because of acute hepatic failure, congenital metabolic
liver disease or benign end stage liver disease. Of the 7 cases of
LDABS-based ALT that we found in the literature (5,6,14–16),
two showed completely regenerated host liver and survived the
removal of the transplanted liver; one underwent OLT because of
liver failure 10 days after ALT; and four died of multiple organ
failure, cytomegaloviral pneumonia or sepsis, none of which was
associated with the surgical technique. These results suggest that
LDABS-based ALT can substantially benefit carefully selected
patients because the transplanted liver can substitute for the
failed host liver and effectively provide hepatic function.
The fact that previous studies of LDABS in patients
are limited to individual case studies justifies the use of
preclinical animal models to explore the procedure in more detail.
A previous study from this laboratory showed similar extent of
liver regeneration in rats with PH plus LDABS vs. PH alone
(17). The present study further
verified these previous findings.
The molecular mechanisms of LDABS-mediated liver
regeneration are unclear. Lack of understanding in this aspect
poses an obstacle to further development and clinical
implementation of the technique. Liver regeneration requires strict
spatiotemporal coordination of numerous hepatic genes activated by
cytokines and growth factors, which are in turn regulated by
various signaling pathways (18–20),
including pathways dependent on MAPK, JAK/STAT, NF-kappa B, Notch,
Hedgehog, Toll-like receptor, CXCR, Wnt, and RHO. The up- or
down-regulation of genes in these signaling pathways may promote or
suppress hepatocellular proliferation or apoptosis. Previous work
from our group suggested that the mechanisms behind LDABS-mediated
liver regeneration differ from regeneration after PH alone.
Specifically, fluorescent quantitative RT-PCR in our previous study
revealed differential expression of TNF-α, HGF and TGF-β1 in the
liver of rats with PH alone vs. with PH plus LDABS (21).
The rat model of 70% PH was used by many
investigators to study liver regeneration. After 70% liver
resection in 7–10 days, the residual liver tissue was completely
recovered. Liver regeneration consists of three stages: the
initiation phase (2–6 h after PH), the proliferation phase (12–72 h
after PH) and the termination phase (120–168 h after PH). So we
chose three time points (6, 48, 168 h) to represent the different
stages of liver regeneration.
The present study combined whole-genome oligo
microarrays with MAPK signaling PCR arrays. The results implicated
differentially expressed genes were enriched in 12 signaling
pathways using pathway analysis, but only the MAPK signaling
pathway, NF-kappa B signaling pathway, and Toll-like receptor
signaling pathway which regulated post PH liver regeneration were
involved in LDABS-mediated liver regeneration, we speculate that
these three pathways contribute to liver regeneration on different
time points or act on different downstream targets.
MAPK (mitogen activated protein kinase) belongs to a
class of intracellular serine/threonine protein kinase. Downstream
genes was activated by the activation of the MAPK signaling pathway
through continuous enzymatic reaction (MAPK kinase (MAP4Ks), MAPK
kinase (MAP3Ks), MAPK kinase (MAP2Ks), MAPKs (ERKs, JNK and P38))
to promote cell proliferation, differentiation and apoptosis
(22). Results showed the up
regulation of genes was significantly increased at 6 and 48 h,
while the down regulation of genes was significantly increased at
168 h among 84 genes of MAPK signaling pathway gene chip. It is
illustrated that there is dual regulation of MAPK signaling pathway
on liver regeneration at different stages.
Network analysis identified the Rb1,
Ccnd1, Cdk4, Mapk10 and Creb1 genes in the initiation phase,
Kras, Tp53, Myc, Ccne1 and Hspb1 genes in the proliferation
phase, Kras, Rb1, Jun, Ccnd2 and Mapk10 genes in the
termination phase were identified as key genes in LDABS-mediated
liver regeneration using MAPK signaling PCR array analysis.
However, the experiments of this study were based only to gene
expression, not the protein expression, the roles of these genes
should be further investigated, for example, using western blotting
and knockout mice.
In conclusion, MAPK signaling pathway play an
important role in regulation of LDABS-mediated liver regeneration.
Rb1, Ccnd1, Cdk4, Mapk10 and Creb1 genes in the
initiation phase, Kras, Tp53, Myc, Ccne1 and Hspb1 genes in
the proliferation phase, Kras, Rb1, Jun, Ccnd2 and Mapk10
genes in the termination phase were identified as key genes in
LDABS-mediated liver regeneration using MAPK signaling PCR array
analysis, the up- or down-regulation of these genes may promote or
suppress liver regeneration in the LDABS.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81260073,81560113); Natural Science
Foundation of Inner Mongolia Autonomous Region, China (2014MS0850);
Major Program of the Affiliated Hospital of Inner Mongolia Medical
University (NYFYZD2014006); Scientific Research Program in Colleges
and Universities of Inner Mongolia Autonomous Region, China
(NJZY113). Program for Young Talents of Science and Technology in
Universities of Inner Mongolia Autonomous Region (NJYT-17-A15).
The whole-genome oligo microarray and MAPK signaling
PCR array were carried out by Shanghai Kangchen Bio-tec, ltd.
References
|
1
|
Iwaki T, Miyatani H, Yoshida Y, Matsuura K
and Suminaga Y: Gastric variceal bleeding caused by an intrahepatic
arterioportal fistula that formed after liver biopsy: A case report
and review of the literature. Clin J Gastroenterol. 5:101–107.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu J, Wu H, Prasoon P and Zeng Y: Portal
vein arterialization in hilar cholangiocarcinoma: One case report
and literature review. Eur J Gastroenterol Hepatol. 24:229–232.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Melandro F, Lai Q, Levi Sandri GB,
Guglielmo N, Di Laudo M, Morabito V, Pretagostini R, Berloco PB and
Rossi M: A case of portal vein arterialization after a liver
transplant. Exp Clin Transplant. 11:287–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsivian M, Neri F, Prezzi D, Puviani L,
Pacile V, Bertelli R, Cavallari G, Mattioli B, Bianchi E, Piras GL,
et al: Portal vein arterialization in hepatobiliary surgery and
liver transplantation. Transplant Proc. 39:1877–1878. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Erhard J, Lange R, Giebler R, Rauen U, de
Groot H and Eigler FW: Arterialization of the portal vein in
orthotopic and auxiliary liver transplantation. A report of three
cases. Transplantation. 60:877–879. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Charco R, Margarit C, López-Talavera JC,
Hidalgo E, Castells L, Allende H, Segarra A, Moreíras M and Bilbao
I: Outcome and hepatic hemodynamics in liver transplant patients
with portal vein arterialization. Am J Transplant. 1:146–151. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Settmacher U, Stange B, Schaser KD, Puhl
G, Glanemann M, Steinmüller T, Heise M and Neuhaus P: Primary
permanent arterialization of the portal vein in liver
transplantation. Transpl Int. 16:430–433. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martins PN, Theruvath TP and Neuhaus P:
Rodent models of partial hepatectomies. Liver Int. 28:3–11. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiao JL, Wang ZY, Zhang JJ and Meng XK:
Assisting suspension triangulated continuous suture technique for
microvascular anastomosis in rat portocaval shunt. Microsurgery.
35:166–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nivatvongs S, Sirijindakul B and Nontasoot
B: Portal vein arterialization for liver transplantation with
extensive portomesenteric vein thrombosis: A case report.
Transplant Proc. 36:2267–2268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aspinall RJ, Seery JP, Taylor-Robinson SD
and Habib N: Comments on ‘arterialization of the portal vein in
orthotopic and auxiliary liver transplantation’. Transplantation.
62:1375–1376. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stange B, Glanemann M, Nussler NC,
Bechstein WO, Neuhaus P and Settmacher U: Indication, technique,
and outcome of portal vein arterialization in orthotopic liver
transplantation. Transplant Proc. 33:1414–1415. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ott R, Böhner C, Müller S, Aigner T,
Bussenius-Kammerer M, Yedibela S, Kissler H, Hohenberger W, Reck T
and Müller V: Outcome of patients with pre-existing portal vein
thrombosis undergoing arterialization of the portal vein during
liver transplantation. Transpl Int. 16:15–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Erhard J, Lange R, Rauen U, Scherer R,
Friedrich J, Pietsch M, de Groot H and Eigler FW: Auxiliary liver
transplantation with arterialization of the portal vein for acute
hepatic failure. Transpl Int. 11:266–271. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Margarit C, Bilbao I, Charco R, Lázaro JL,
Hidalgo E, Allende E and Murio E: Auxiliary heterotopic liver
transplantation with portal vein arterialization for fulminant
hepatic failure. Liver Transpl. 6:805–809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lange R, Rauen U, Janssen H, Erhard J and
de Groot H: Temporary heterotopic auxiliary liver transplantation
with arterialization of the portal vein as treatment of acute liver
failure. Transpl Int. 20:473–474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang JJ, Niu JX, Dong CX and Meng XK:
Portal vein arterialization used in partial hepatectomy maintains
liver regeneration. Sci Res Essays. 6:6325–6330. 2011.
|
|
18
|
Riehle KJ, Dan YY, Campbell JS and Fausto
N: New concepts in liver regeneration. J Gastroenterol Hepatol. 26
Suppl 1:S203–S212. 2011. View Article : Google Scholar
|
|
19
|
Nowatari T, Fukunaga K and Ohkohchi N:
Regulation of signal transduction and role of platelets in liver
regeneration. Int J Hepatol. 2012:5424792012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taub R: Liver regeneration: From myth to
mechanism. Nat Rev Mol Cell Biol. 5:836–847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niu JX, Dong CX, Zhang JJ and Meng XK:
TNF-α, HGF and TGF-β1 are involved in the liver regeneration
following partial hepatectomy using portal vein arterializations. J
Med Biochem. 31:135–139. 2012. View Article : Google Scholar
|
|
22
|
Cuschieri J and Maier RV:
Mitogen-activated protein kinase (MAPK). Crit Care Med. 33 12
Suppl:S417–S419. 2005. View Article : Google Scholar : PubMed/NCBI
|