Introduction
Type 2 diabetes mellitus (T2DM) is a chronic
metabolic syndrome that has an increasing prevalence, especially in
China (1). T2DM is characterized
by high blood glucose levels, relative insufficiency of insulin
secretion from pancreatic beta cells and insulin resistance, worse
still, persistent increased blood glucose will result in vascular
complications including microvascular complications (2,3). The
life-threatening T2DM associated microvascular complications
include long-term damage, dysfunction and failure of the vital
organs such as retinopathy, nephropathy neuropathy and
cardiovascular diseases (4,5). Of
note, the metabolic changes of diabetes induce endothelial cells
(ECs) dysfunction, which is critical to the initiation and
progression of vascular complications (6). Accumulating evidence indicates that
hyperglycemia induced by T2DM could increase cell apoptosis, which
has emerged as one of the key mechanisms leading to ECs damage
(7). Thus, there is an urgent need
to identify the therapeutic strategies against ECs damage, which
could be useful for prevention and treatment of diabetic vascular
lesions.
DJ-1 was first identified as a novel oncogene
(8), and subsequent studies have
identified its role in the pathogenesis of neurodegenerative
disorders, such as Parkinson's disease (9) and Alzheimer's disease (10). Indeed, DJ-1 exerts ubiquitously in
variety of EC types, containing human umbilical vein endothelial
cells (HUVECs) (11) and corneal
ECs (12). Mutations in the gene
encoding DJ-1 can cause familial Parkinsonism and overexpression of
DJ-1 protects neurons against oxidative stress-induced cell death
(13,14). Besides, various studies have shown
that DJ-1 could decrease oxidative damage and increase antioxidant
gene levels, which contributing to its pro-survival activity
(15–17). The evidence described above
suggests DJ-1 possesses potential therapeutic activities for
oxidative stress associated ECs dysfunction. Although a recent
study showed that overexpression of DJ-1 protects endothelial
progenitor cells against angiotensin II-induced dysfunction by
reducing reactive oxygen species (ROS) production (18), it is still unclear whether DJ-1
could also play an antioxidant role in ECs injuries.
It has been well established that high glucose (HG)
could induce endothelial apoptosis, dysfunction and inflammation,
resulting in ECs injury (19–21).
Given above findings, we therefore studied the potential protective
effects of DJ-1 on HG-induced ECs damage and investigated the
relationship between its effect and the modulation of PI3K/Akt-eNOS
signaling pathway.
Materials and methods
Cell culture and treatment
HUVECs were purchased from AllCells Biotechnology
Co., Ltd. (Shanghai, China). The culture medium was DMEM with
either 5.5 mM (control group) or 25 mM (HG group) D-glucose,
containing 10% fetal bovine serum, penicillin (100 U/ml) and
streptomycin (100 mg/ml). Cells were incubated in a humidified
incubator with 5% CO2 at 37°C. Recombinant adenoviral
vectors, including green fluorescent protein expression (GFP)
vectors pAdEasy-1 pShuttle-bSYN and pGEM-3ZF (+) and carrying a
human DJ-1 (PARK7) gene were constructed utilizing the AdEasy
Vector system (22). pAdEasy-DJ-1
was linearized with PacI and transfected into HUVECs to generate
adenovirus that encoded DJ-1 (Ad-DJ-1). The viral titers of
adenoviral Ad-DJ-1 and Ad-GFP used for transfection were
1.0×109 and 2.5×109 pfu/ml.
EdU incorporation assay
Cell proliferation was determined with EdU
incorporation assay. In brief, cells were seeded into 96-well
plates at 1×104 cells/well and then 50 µM of EdU was
added to each well with the incubation for 4 h. HUVECs were fixed
with 4% formaldehyde and permeated with 0.5% Triton X-100 for 20
min. After washing with PBS, 100 µl of 1X Apollo reaction cocktail
was added for an additional 30 min. Then HUVECs were stained with
100 µl of Hoechst 33342 for 30 min and the EdU positive cells (red
cells) was counted under an inverted Nikon microscope (Nikon
Corporation, Tokyo, Japan) at magnification, ×200.
Determination of cellular
apoptosis
Cell apoptotic rates were measured by flow
cytometric analysis using Annexin V-FITC/propidium iodide (PI)
staining (Beyotime, Shanghai, China). In brief, HUVECs in groups
were trypsinized and rinsed with PBS. Subsequently, cells were
resuspended in Annexin V binding buffer and stained with 10 µl
Annexin V-FITC for 15 min under dark. Then, 5 µl of PI was also
added for an additional 5 min. Stained cells were analyzed by flow
cytometry (FACS Calibur; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Measurement of ROS production
For ROS detection, an Image-iT LIVE Green ROS
Detection kit (Invitrogen, Carlsbad, CA, USA) was used. HUVECs were
incubated with DMEM containing 10 µM 2,7-dichlorodihydrofluorescein
diacetate (H2DCF-DA) for 30 min and then washed with
PBS. The results were obtained using flow cytometry analysis.
Biochemical assay
HUVECs (1×105 cells/ml) were plated in
6-well plates for 18 h and treated with the method described above.
The appropriate volume of supernatant was collected to determine
the release of lactate dehydrogenase (LDH) and nitric oxide (NO),
the content of malondialdehyde (MDA), and the activities of
superoxide dismutase (SOD) (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) according to the manufacturer's
instructions.
Western blot analysis
Total protein extracts were obtained with RIPA lysis
buffer (Shanghai Biyuntian Bio-Technology Co., Ltd., Shanghai,
China) containing protease inhibitor cocktail (Sigma-Aldrich, St.
Louis, MO, USA). Protein lysates were then separated by 8–15%
SDS-PAGE, transferred to polyvinylidene fluoride membranes (PVDF;
MA, USA) and blocked with 1% bovine serum albumin (BSA). Then the
membranes were probed with specific primary antibodies against
PARK7/DJ-1 (1:10,000), Bcl-2 (1:500), Bax (1 µg/ml), caspase-3
(1:500; all from Abcam, Cambridge, MA, USA), and Akt (1:1,000),
p-Akt (1:2,000), eNOS (1:1,000), p-eNOS (1:1,000; all from Cell
Signaling Technology, Shanghai, China), and GAPDH (Sigma-Aldrich).
Subsequently, after washing with TBST for three times, the blots
were incubated in peroxidase conjugated immunoglobulin G
anti-rabbit secondary antibody (1:5,000; Sigma-Aldrich) for 2 h.
The immune complexes were visualized using an enhanced
chemiluminescence kit (Millipore, Billerica, MA, USA) and
quantified with the Quantity One v5.0 software (Bio-Rad
Laboratories, Inc.).
Statistical analysis
All statistical analyses were undertaken using the
SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). Data were
presented as mean ± standard deviation. Comparisons among groups
were carried out with a two-tailed Student t-test or one-way ANOVA.
The value of P<0.05 was considered to be statistically
significant.
Results
Overexpression of DJ-1 promotes
proliferation in HG-induced HUVECs
To determine whether DJ-1 affects the proliferation
of HG-induced HUVECs, the adenoviral vector Ad-DJ-1 or Ad-GFP was
used to overexpression of DJ-1 in HUVECs and the results were
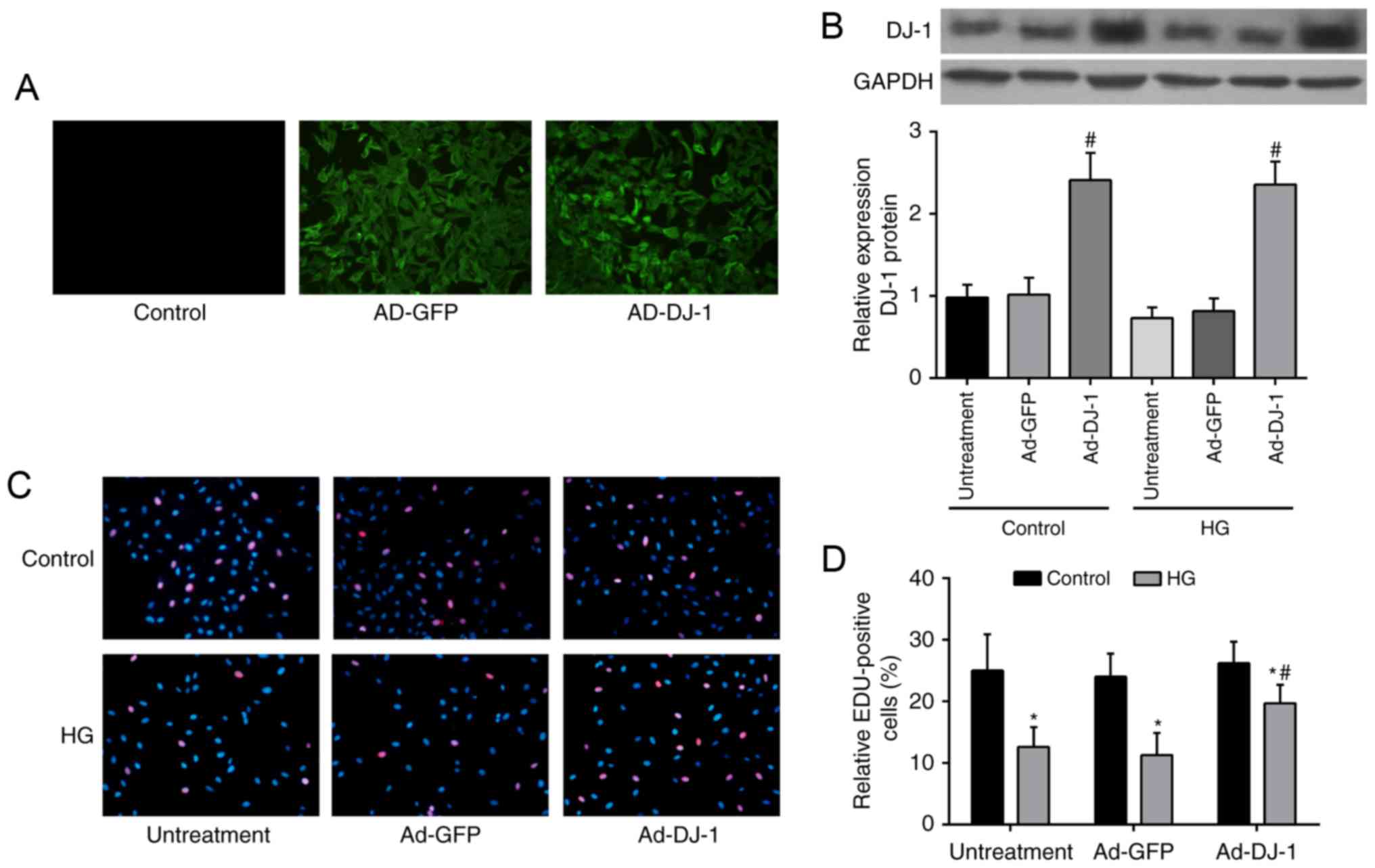
illustrated in Fig. 1A. The
western blot analysis showed that DJ-1 protein expression was
markedly increased in Ad-DJ-1 group, compared to that in Ad-GFP
group (P<0.05; Fig. 1B). HUVECs
exposed to HG showed significant decrease in proliferation ability
comparing with control group, whereas overexpression of DJ-1
reversed the inhibitory effect caused by HG (P<0.05; Fig. 1C and D).
DJ-1 inhibits HG-induced apoptosis in
HUVECs
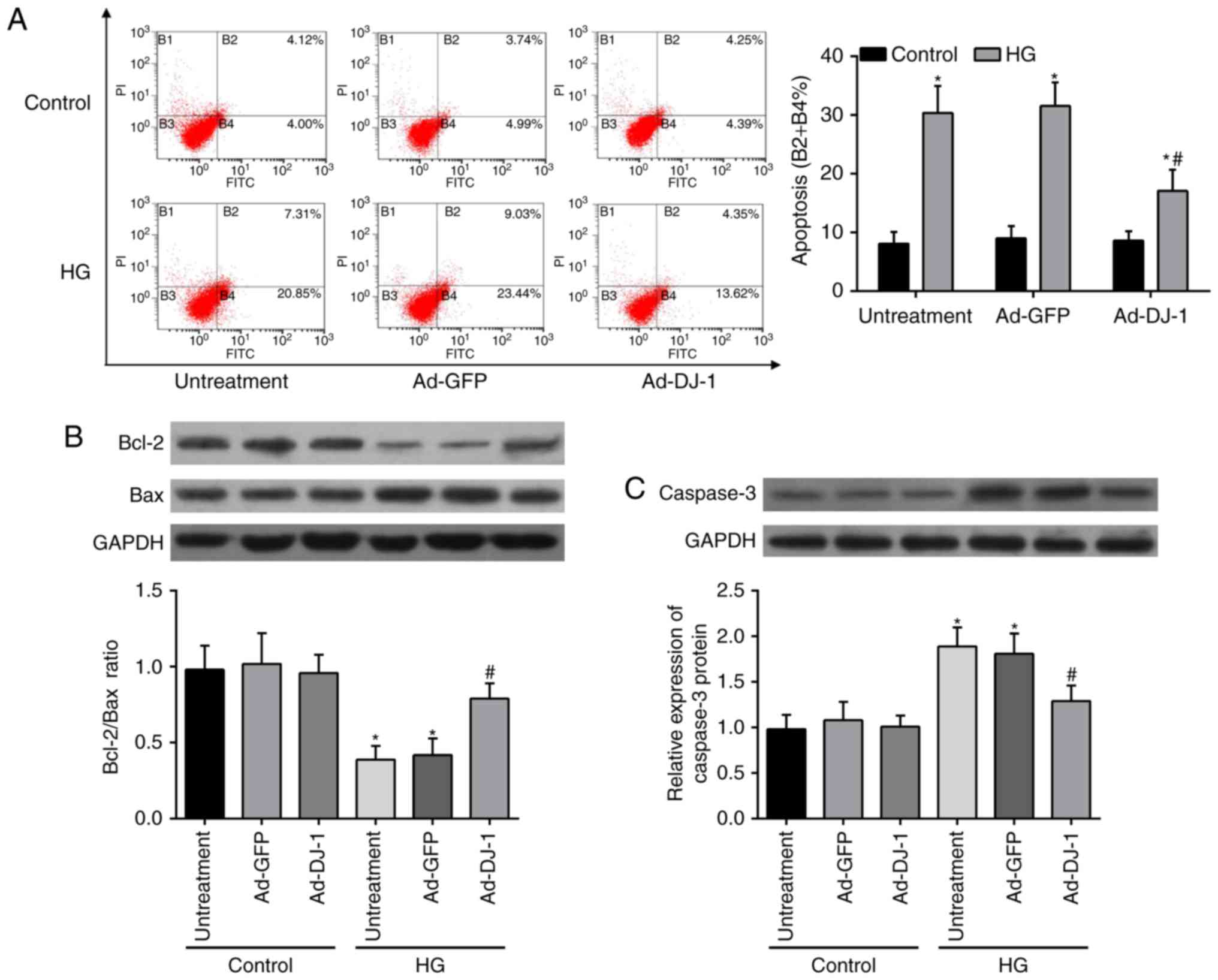
Flow cytometry analysis with Annexin V-FITC and PI
double staining was then used to determine the effect of DJ-1 on
HG-induced apoptosis. In comparison with the control group, the
results of flow cytometry displayed that HG caused an obvious
increase on the cell apoptosis rates of HUVECs. Unexpectedly, this
injury was restored by overexpression of DJ-1 (Fig. 2A). In addition, the expressions of
pro-apoptotic proteins, including Bax (Fig. 2B) and caspase-3 (Fig. 2C), were elevated, whereas
anti-apoptosis protein Bcl-2 (Fig.
2B) was decreased in HG-induced HUVECs. Overexpression of DJ-1
significantly reversed these effects.
DJ-1 possesses antioxidative property
in HG-induced HUVECs
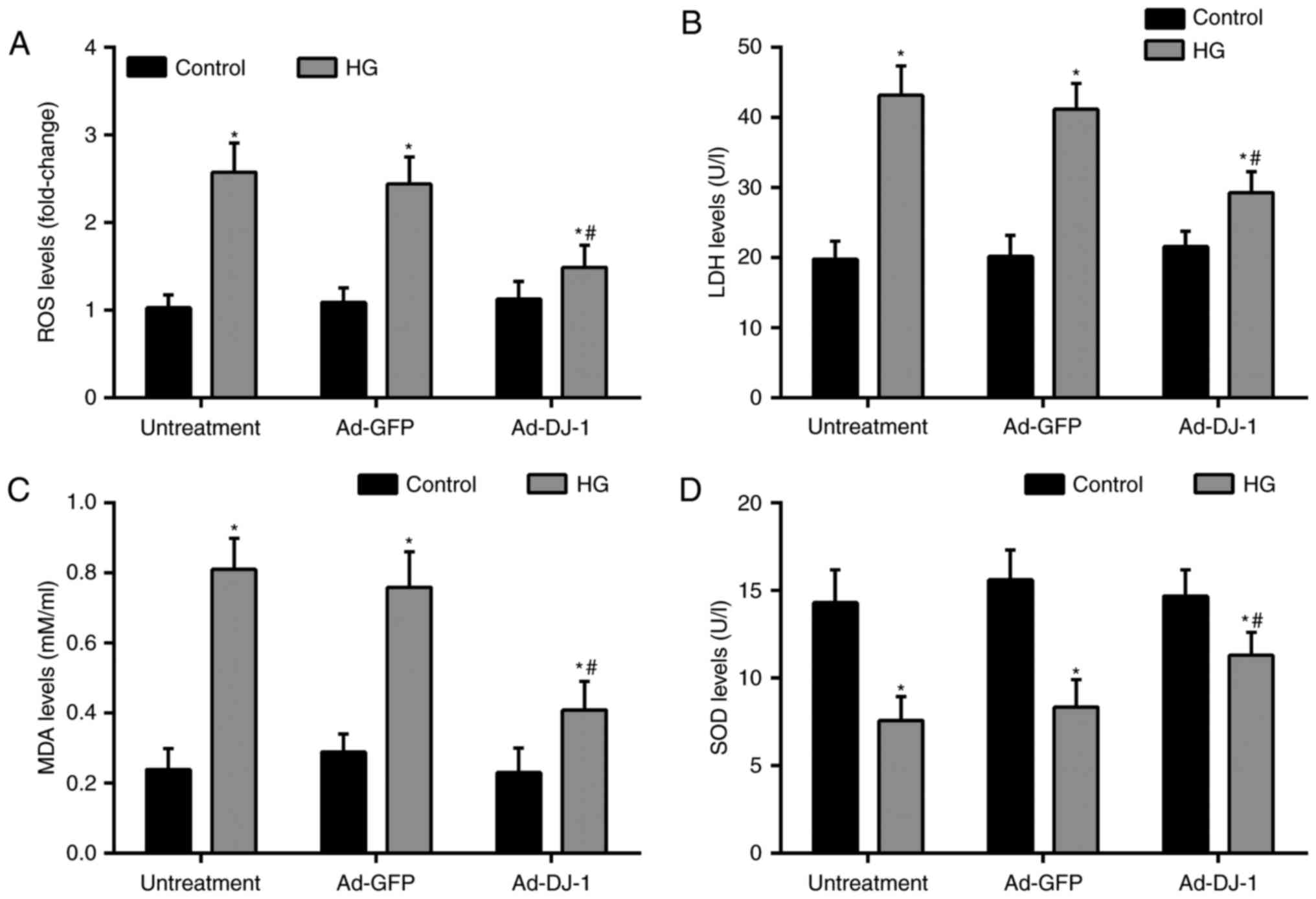
Oxidative stress plays a critical role in ECs
apoptosis. Thereby, we investigated the levels of markers of
oxidative stress, including ROS, LDH, MDA, and SOD in HUVECs.
Treatment of cells with HG dramatically caused ROS generation
compared with the control group. While, overexpression of DJ-1
suppressed ROS production in HUVECs exposed to HG (Fig. 3A). Besides, the subsequent tests
showed that compared with the control group, HG significantly
increased the levels of LDH, MDA, and reduced the level of SOD in
the supernatant. Similarly, overexpression of DJ-1 effectively
reduced above elevated oxidative stress markers (Fig. 3B-D).
DJ-1 activates PI3K/Akt-eNOS pathway
in HG induced HUVECs
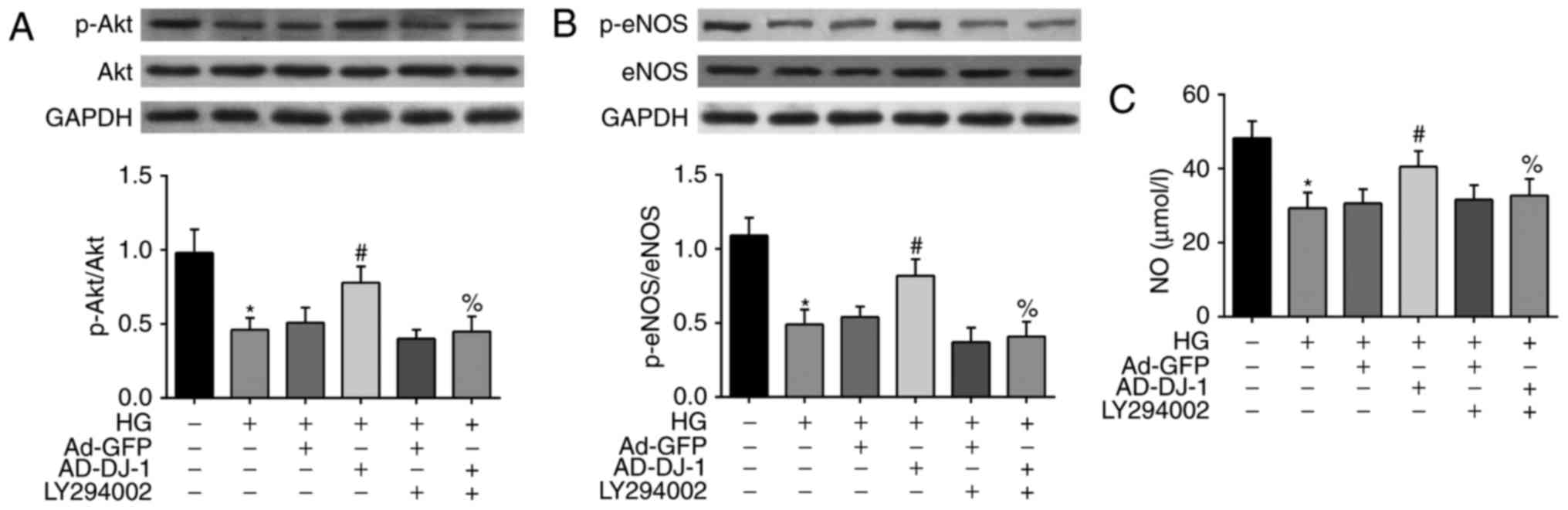
To reveal the mechanism underlying the above
protective effects of DJ-1 on HG-induced HUVECs, we investigated
whether PI3K/Akt-eNOS signaling pathway was involved. As shown in
Fig. 4A, the treatment of cells
with HG inhibited the phosphorylation of Akt. Interestingly,
overexpression of DJ-1 significantly increased p-Akt level.
However, LY294002, the PI3K inhibitor of Akt pathway, markedly
suppressed the effect of DJ-1 on Akt phosphorylation level. As eNOS
is an important downstream target of Akt, we then examined the
alteration of eNOS and p-eNOS protein in HG-induced HUVECs. As a
result, HG also inhibited the phosphorylation of eNOS in HUVECs,
and this effect was reversed by overexpression of DJ-1. Similarly,
the effects of DJ-1 on p-eNOS protein expression was blocked by
LY294002 (Fig. 4B). Besides, we
found that HG treatment significantly decreased the NO production
in the culture medium, while overexpression of DJ-1 reversed this
tendency (Fig. 4C).
Discussion
In the present studies, we found that DJ-1
significantly promoted HUVECs proliferation and protected it from
HG-induced cell apoptosis through suppressing the oxidative stress.
Moreover, we demonstrated that the protective effects of DJ-1 on
ECs function rely heavily on the PI3K/Akt-eNOS signaling pathway.
These findings provided new information about the role of DJ-1 in
protecting ECs from HG mediated injury, representing a novel
therapeutic strategy in the treatment of ECs damaged associated
diabetic vascular lesions.
A variety of reports demonstrated that
diabetes-associated hyperglycemia could induce apoptosis in
pancreatic islet ECs (23),
pancreatic beta-cells (24), and
HUVECs (25), via an intrinsic
apoptotic pathway. Similarly, we used (25) mM D-glucose to simulate
hyperglycemia in this work and found cultured HUVECs showing
reduction in cell proliferation ability and possessed of high cell
apoptotic rates after treatment with HG. Of note, emerging evidence
indicates a link between HG-induced apoptosis of ECs and ROS
production (26). And as expected.
We also showed HG triggered oxidative stress in HUVECs, which was
detected via ROS production, levels of LDH, MDA, and SOD in cell
supernatant.
The growing body of evidences demonstrated that DJ-1
is involved in various regulatory functions, including
transcriptional regulation and anti-oxidative stress regulation
(27). DJ-1 is a homodimeric
protein, belonging to the Thi/Pfp1 superfamily and is abundant in
most living things from humans to bacteria (28). With the increase in blood glucose
levels, the levels of DJ-1 increase in pancreatic β-cells, to
inhibit oxidative stress induced ROS (29). However, decreased expression of DJ1
has been detected in the islets of elderly T2DM patients in a
gender dependent manner (29).
These showed conflicting results concerning DJ-1 expression in this
metabolic disease. Here, we focused on the function of DJ-1 on
HG-induced cell dysfunction rather than the expression levels of
DJ-1 in ECs. It has been shown that DJ-1 protects the morphology
and function of the mitochondria and protects against cell injury
(30), and in the present study,
we revealed that DJ-1 reversed HG-induced HUVECs apoptosis. In
accordance with our findings, Wang and Gao found that DJ-1
silencing in HeLa cells increased cell apoptotic rates while DJ-1
overexpression significantly inhibited cell apoptosis (31). In addition, DJ-1 transgene protects
cortical neurons from H2O2-induced apoptosis
and re-expression of DJ-1 into the cortical neurons from
DJ-1-knockout mice could reduce H2O2-induced
cell death via Akt1 signaling pathway (32). The Bcl-2 family proteins,
consisting pro-apoptotic (Bax) and anti-apoptotic (Bcl-2)
molecules, are known to participate in the regulation of the
apoptotic pathway (33). DJ-1
increased the reduction of Bcl-2/Bax ratio and reduced the
upregulation of caspase-3, caused by HG in the current study.
Furthermore, it was recognized that loss of DJ-1
increases ROS production (34). In
corneal ECs, downregulation of DJ-1 increases caspase-3 activation
and phospho-p53 under ultraviolet A oxidative stress and the
decline in DJ-1 levels results to increased oxidative damage
(12). Excessive DJ-1 expression
also inhibited oxidative stress-induced HepG2 cell death (35) and pancreatic β-cell death (36). Thereby, the effects of DJ-1 against
oxidative stress induced intracellular ROS production, were then
explored in HUVECs. We showed that DJ-1 alleviates the HUVECs
damage induced by HG by suppressing oxidative stress through
detecting ROS, LDH, MDA and SOD levels. In accordance with our
results, Shen et al demonstrated that overexpression of DJ-1
exerts protective effects against HG-induced tubular epithelial
cells injury, as evidenced by increased SOD activity, the decreased
release of LDH and the decreased MDA content (37).
Nevertheless, how DJ-1 regulates ROS is still not
completely clear. DJ-1 is oxidized on its cysteine residues which
are also critical for the ability of DJ-1 to manage ROS (38). Studies showed that DJ-1 exerts its
antioxidant ability through interaction with nuclear factor
erythroid 2-related factor2 (Nrf2) (12), paraoxonase-2 (39), receptor of activated C kinase 1, or
activation of signaling pathway such as PI3K/Akt/mTOR (40), NF-κB and MAPK pathway (36). Of note, the PI3K/Akt signaling
pathway is widely present in cells playing a regulatory role for
eNOS, and is also involved in cell proliferation and apoptosis
(41). As a vascular endothelial
protective factor, eNOS exerts its role by adjusting the
biosynthesis of NO. So far, there remains a lack of investigation
on the effects of DJ-1 on PI3K/Akt-eNOS signaling pathway in HG
induced HUVECs. Once activated, the phosphorylation of Akt can
directly phosphorylate eNOS and induce the subsequent production of
NO (42). We found that,
accompanied with the inhibition of oxidative stress induced HUVECs
apoptosis, DJ-1 also attenuated the decrease in the phosphorylation
of Akt and eNOS when exposed to HG, as well as increased NO levels.
It is known that impaired ECs function arises from decreased
production and/or bioactivity of NO induced by eNOS phosphorylation
(43,44). In addition, deficiencies in
generation of eNOS-derived NO have been proposed as mechanisms
responsible for ECs dysfunction in diabetes (45). Our subsequent chemical stressors
analysis demonstrated that PI3K specific inhibitors, LY294002,
significantly abolished the activation of this pathway induced by
overexpression of DJ-1, suggesting that DJ-1 might exert its
antiapoptotic effect by activating the PI3K/Akt-eNOS pathways.
Collectively, our preliminary study showed that DJ-1
could antagonize endothelial dysfunction by attenuating oxidative
stress via activation of the PI3K/Akt-eNOS signaling pathway. As
increasing evidence have validated the important role of oxidative
stress in the pathological process of diabetic vascular
complications (46–48), the results of the present study
highlights DJ-1 as a potential therapeutic target. Accordingly,
further studies should focus on the function of DJ-1 in the
hyperglycemia related ECs dysfunction in the future.
Acknowledgements
Financial support for this study was provided by
Xianning Central Hospital, the First Affiliated Hospital of Hubei
University of Science and Technology (no. 2016XYA004).
Glossary
Abbreviations
Abbreviations:
|
T2DM
|
type 2 diabetes mellitus
|
|
ECs
|
endothelial cells
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
HG
|
high glucose
|
|
ROS
|
reactive oxygen species
|
|
NO
|
nitric oxide
|
References
|
1
|
Wang L, Gao P, Zhang M, Huang Z, Zhang D,
Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al: Prevalence and ethnic
pattern of diabetes and prediabetes in China in 2013. JAMA:.
317:2515–2523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ashcroft FM and Rorsman P: Diabetes
mellitus and the β cell: The last ten years. Cell. 148:1160–1171.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oelze M, Schuhmacher S and Daiber A:
Organic nitrates and nitrate resistance in diabetes: The role of
vascular dysfunction and oxidative stress with emphasis on
antioxidant properties of pentaerithrityl tetranitrate. Exp
Diabetes Res. 2010:2131762010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beckman JA, Creager MA and Libby P:
Diabetes and atherosclerosis: Epidemiology, pathophysiology and
management. JAMA. 287:2570–2581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Advani A and Gilbert RE: The endothelium
in diabetic nephropathy. Semin Nephrol. 32:199–207. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhar A, Dhar I, Desai KM and Wu L:
Methylglyoxal scavengers attenuate endothelial dysfunction induced
by methylglyoxal and high concentrations of glucose. Br J
Pharmacol. 161:1843–1856. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagakubo D, Taira T, Kitaura H, Ikeda M,
Tamai K, Iguchi-Ariga SM and Ariga H: DJ-1, a novel oncogene which
transforms mouse NIH3T3 cells in cooperation with ras. Biochem
Biophys Res Commun. 231:509–513. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi M, Bradner J, Hancock AM, Chung KA,
Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Kim HM,
et al: Cerebrospinal fluid biomarkers for Parkinson disease
diagnosis and progression. Ann Neurol. 69:570–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitamura Y, Inden M, Kimoto Y, Takata K,
Yanagisawa D, Hijioka M, Ashihara E, Tooyama I, Shimohama S and
Ariga H: Effects of a DJ-1-binding compound on spatial learning and
memory impairment in a mouse model of Alzheimer's disease. J
Alzheimers Dis. 55:67–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kinumi T, Kimata J, Taira T, Ariga H and
Niki E: Cysteine-106 of DJ-1 is the most sensitive cysteine residue
to hydrogen peroxide-mediated oxidation in vivo in human umbilical
vein endothelial cells. Biochem Biophys Res Commun. 317:722–728.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu C, Chen Y, Kochevar IE and Jurkunas
UV: Decreased DJ-1 leads to impaired Nrf2-regulated antioxidant
defense and increased UV-A-induced apoptosis in corneal endothelial
cells. Invest Ophthalmol Vis Sci. 55:5551–5560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lev N, Ickowicz D, Melamed E and Offen D:
Oxidative insults induce DJ-1 upregulation and redistribution:
Implications for neuroprotection. Neurotoxicology. 29:397–405.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inden M, Taira T, Kitamura Y, Yanagida T,
Tsuchiya D, Takata K, Yanagisawa D, Nishimura K, Taniguchi T, Kiso
Y, et al: PARK7 DJ-1 protects against degeneration of nigral
dopaminergic neurons in Parkinson's disease rat model. Neurobiol
Dis. 24:144–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dongworth RK, Mukherjee UA, Hall AR, Astin
R, Ong SB, Yao Z, Dyson A, Szabadkai G, Davidson SM, Yellon DM and
Hausenloy DJ: DJ-1 protects against cell death following acute
cardiac ischemia-reperfusion injury. Cell Death Dis. 5:e10822014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim DK, Kim HS, Kim AR, Kim JH, Kim B, Noh
G, Kim HS, Beaven MA, Kim YM and Choi WS: DJ-1 regulates mast cell
activation and IgE-mediated allergic responses. J Allergy Clin
Immunol. 131:1653–1662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang S, Song S, Hong YK, Choi G, Suh YS,
Han SY, Lee M, Park SH, Lee JH, Lee S, et al: Drosophila DJ-1
decreases neural sensitivity to stress by negatively regulating
Daxx-like protein through dFOXO. PLoS Genet. 9:e10034122013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han T, Liu M and Yang S: DJ-1 alleviates
angiotensin II-induced endothelial progenitor cell damage by
activating the PPARgamma/HO-1 pathway. J Cell Biochem. Jun
10–2017.(Epub ahead of print).
|
|
19
|
Cai S, Khoo J and Channon KM: Augmented
BH4 by gene transfer restores nitric oxide synthase function in
hyperglycemic human endothelial cells. Cardiovasc Res. 65:823–831.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan W, Han D, Sun Z, Ma S, Gao L, Chen J,
Li X, Li X, Fan M, Li C, et al: Endothelial deletion of mTORC1
protects against hindlimb ischemia in diabetic mice via activation
of autophagy, attenuation of oxidative stress and alleviation of
inflammation. Free Radic Biol Med. 108:725–740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang ST, Wang F, Shao M, Wang Y and Zhu
HQ: MicroRNA-126 suppresses inflammation in endothelial cells under
hyperglycemic condition by targeting HMGB1. Vascul Pharmacol.
88:48–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong L, Liu FQ, Wang J, Wang XP, Hou XG,
Sun Y, Qin WD, Wei SJ, Zhang Y, Chen L and Zhang MX: Hyperglycemia
induces apoptosis of pancreatic islet endothelial cells via
reactive nitrogen species-mediated Jun N-terminal kinase
activation. Biochim Biophys Acta. 1813:1211–1219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Saxena G, Mungrue IN, Lusis AJ and
Shalev A: Thioredoxin-interacting protein: A critical link between
glucose toxicity and beta-cell apoptosis. Diabetes. 57:938–944.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong ZY and Tang Y: Upregulation of
periostin prevents high glucose-induced mitochondrial apoptosis in
human umbilical vein endothelial cells via activation of nrf2/ho-1
signaling. Cell Physiol Biochem. 39:71–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao H, Ma T, Fan B, Yang L, Han C, Luo J
and Kong L: Protective effect of trans-α-viniferin against high
glucose-induced oxidative stress in human umbilical vein
endothelial cells through the SIRT1 pathway. Free Radic Res.
50:68–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Won KJ, Jung SH, Jung SH, Lee KP, Lee HM,
Lee DY, Park ES, Kim J and Kim B: DJ-1/park7 modulates
vasorelaxation and blood pressure via epigenetic modification of
endothelial nitric oxide synthase. Cardiovasc Res. 101:473–481.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bonifati V, Rizzu P, van Baren MJ, Schaap
O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P,
Joosse M, et al: Mutations in the DJ-1 gene associated with
autosomal recessive early-onset parkinsonism. Science. 299:256–259.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jain D, Jain R, Eberhard D, Eglinger J,
Bugliani M, Piemonti L, Marchetti P and Lammert E: Age- and
diet-dependent requirement of DJ-1 for glucose homeostasis in mice
with implications for human type 2 diabetes. J Mol Cell Biol.
4:221–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin TK, Liou CW, Chen SD, Chuang YC, Tiao
MM, Wang PW, Chen JB and Chuang JH: Mitochondrial dysfunction and
biogenesis in the pathogenesis of Parkinson's disease. Chang Gung
Med J. 32:589–599. 2009.PubMed/NCBI
|
|
31
|
Wang H and Gao W: DJ-1 expression in
cervical carcinoma and its effects on cell viability and apoptosis.
Med Sci Monit. 22:2943–2949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma J, Wu R, Zhang Q, Wu JB, Lou J, Zheng
Z, Ding JQ and Yuan Z: DJ-1 interacts with RACK1 and protects
neurons from oxidative-stress-induced apoptosis. Biochem J.
462:489–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhola PD and Letai A: Mitochondria-judges
and executioners of cell death sentences. Mol Cell. 61:695–704.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giaime E, Yamaguchi H, Gautier CA, Kitada
T and Shen J: Loss of DJ-1 does not affect mitochondrial
respiration but increases ROS production and mitochondrial
permeability transition pore opening. Plos One. 7:e405012012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jo HS, Yeo EJ, Shin MJ, Choi YJ, Yeo HJ,
Cho SB, Park JH, Lee CH, Eum WS and Choi SY: Tat-DJ-1 enhances cell
survival by inhibition of oxidative stress, NF-κB and MAPK
activation in HepG2 cells. Biotechnol Lett. 39:511–521. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jo HS, Cha HJ, Kim SJ, Yeo HJ, Cho SB,
Park JH, Lee CH, Yeo EJ, Choi YJ, Eum WS and Choi SY: Tat-DJ-1
inhibits oxidative stress-mediated RINm5F cell death through
suppression of NF-κB and MAPK activation. Med Chem Res.
25:2589–2598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen ZY, Sun Q, Xia ZY, Meng QT, Lei SQ,
Zhao B, Tang LH, Xue R and Chen R: Overexpression of DJ-1 reduces
oxidative stress and attenuates hypoxia/reoxygenation injury in
NRK-52E cells exposed to high glucose. Int J Mol Med. 38:729–736.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Canet-Avilés RM, Wilson MA, Miller DW,
Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko
GA and Cookson MR: The Parkinson's disease protein DJ-1 is
neuroprotective due to cysteine-sulfinic acid-driven mitochondrial
localization. Proc Natl Acad Sci USA. 101:9103–9108. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parsanejad M, Bourquard N, Qu D, Zhang Y,
Huang E, Rousseaux MW, Aleyasin H, Irrcher I, Callaghan S, Vaillant
DC, et al: DJ-1 interacts with and regulates paraoxonase-2, an
enzyme critical for neuronal survival in response to oxidative
stress. Plos One. 9:e1066012014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang B, Qin H, Wang Y, Chen W, Luo J, Zhu
X, Wen W and Lei W: Effect of DJ-1 overexpression on the
proliferation, apoptosis, invasion and migration of laryngeal
squamous cell carcinoma SNU-46 cells through PI3K/AKT/mTOR. Oncol
Rep. 32:1108–1116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie L, Wu Y, Fan Z, Liu Y and Zeng J:
Astragalus polysaccharide protects human cardiac microvascular
endothelial cells from hypoxia/reoxygenation injury: The role of
PI3K/AKT, Bax/Bcl-2 and caspase-3. Mol Med Rep. 14:904–910. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan J, Tie G, Park B, Yan Y, Nowicki PT
and Messina LM: Recovery from hind limb ischemia is less effective
in type 2 than in type 1 diabetic mice: roles of endothelial nitric
oxide synthase and endothelial progenitor cells. J Vasc Surg.
50:1412–1422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han F, Guo Y, Xu L, Hou N, Han F and Sun
X: Induction of haemeoxygenase-1 directly improves endothelial
function in isolated aortas from obese rats through the
Ampk-Pi3k/Akt-Enos pathway. Cell Physiol Biochem. 36:1480–1490.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang XM, Song SS, Xiao H, Gao P, Li XJ and
Si LY: Fibroblast growth factor 21 protects against high glucose
induced cellular damage and dysfunction of endothelial nitric-oxide
synthase in endothelial cells. Cell Physiol Biochem. 34:658–671.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xing Y, Lai J, Liu X, Zhang N, Ming J, Liu
H and Zhang X: Netrin-1 restores cell injury and impaired
angiogenesis in vascular endothelial cells upon high glucose by
PI3K/AKT-eNOS. J Mol Endocrinol. 58:167–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ceriello A, Testa R and Genovese S:
Clinical implications of oxidative stress and potential role of
natural antioxidants in diabetic vascular complications. Nutr Metab
Cardiovasc Dis. 26:285–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bansal S, Chawla D, Siddarth M, Banerjee
BD, Madhu SV and Tripathi AK: A study on serum advanced glycation
end products and its association with oxidative stress and
paraoxonase activity in type 2 diabetic patients with vascular
complications. Clin Biochem. 46:109–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pinna C, Morazzoni P and Sala A:
Proanthocyanidins from Vitis vinifera inhibit oxidative
stress-induced vascular impairment in pulmonary arteries from
diabetic rats. Phytomedicine. 25:39–44. 2017. View Article : Google Scholar : PubMed/NCBI
|