Introduction
Spinal cord injury (SCI) frequently occurs as a
result of traffic, falling, industrial or athletic accidents
(1,2). The subsequent symptoms resulting from
these injuries are associated with a high disability rate, high
cost and low mortality rate. There is a high incidence rate of SCI
in China. As a serious nervous system injury, the majority of SCI
cases result in paralysis, pain and burden to patients and their
families (3). Therefore,
therapeutic intervention to aid treatment of these symptoms is of
primary concern (4).
There are two primary mechanisms involved in the
development of SCI, including primary mechanical injury and
sequential injury. The latter was proposed in 1911 and has been
accepted and acts as a foundation to current research
investigations (3). The
self-destruction fracture degree of sequential organization
involves multiple factors and exceeds even that of primary injury
mechanism (5). Prevention of
sequential injury may reserve the residual functions of the
surviving nerve tissue (6). In
addition, it may correct microcirculation dysfunction, which is an
important part of secondary injury. The mechanisms currently known
to participate in sequential injury include immune inflammatory
reaction, vascular mechanism, lipid peroxidation and free radical
theory, theory of amino acid, calcium mediated mechanism and
electrolyte imbalance and inflammatory mechanisms (2). Of these, the immune inflammatory
reaction results from early microcirculation dysfunction and is
important in the development of sequential injury (7).
SCI, as a severe central nervous system injury,
lacks an effective therapeutic method and development of treatment
for patients is of primary concern (8). Considerable research has previously
been conducted regarding injury mechanism, treatment and other
aspects of SCI. The injury mechanism has been elucidated however,
no breakthrough progress regarding specific treatment has been
obtained and therefore SCI remains a worldwide issue.
The inflammatory reaction is the primary mediator of
the sequential injury in SCI (9),
exhibiting a key role in the pathophysiological mechanism of the
injury. SCI may trigger a series of molecular events, leading to
activation of inflammatory cells in myeloid tissue resulting from
circulatory system infiltration, release a large amount of
proinflammatory medium and neurotoxins, and generate oxygen free
radicals and nitroso compounds which may lead to cell injury
(10,11).
Chlorogenic acid is a phenylpropanoid substance
synthesized during aerobic respiration in plants. The molecular
formula is C16H18O9 and the
molecular weight is 345.30 g/mol (12). It is one of the primary active
components of numerous Chinese herbal medicines, including
lonicera japonica, eucommia ulmoides and oriental
wormwood (13). It is additionally
an important active ingredient of various fruits and vegetables.
Chlorogenic acid has a variety of effects including free radical
scavenging, antisepsis and anti-inflammation, anti-virus, reducing
blood glucose, anti-hyperlipidemia, and acts to protect the liver
and as a cholagogue (13).
Chlorogenic acid reduces tumor necrosis factor (TNF)-α, interleukin
(IL)-1β and IL-6 production by suppressing the toll like receptor
4-mediated nuclear factor (NF)-κB signaling pathway (14,15).
It has previously been demonstrated that chlorogenic acid
additionally exhibits anti-cancer and anti-AIDS effects and may
therefore be used as a foundation medicine to design and develop
anti-cancer and anti-AIDS drugs. Furthermore, due to its actions as
an anti-oxidant, chlorogenic acid may be used in the pharmaceutical
industry and its actions applied in daily chemical, food and
further fields (14). The present
study investigated the effects of administration of chlorogenic
acid on SCI and the potential underlying mechanism.
Materials and methods
Animals and experimental protocol
Female Wistar rats (n=50, weight, 250–300 g; age,
8–10 weeks) were purchased from the Center for Experimental Animals
of Central South University (Changsha, China), and then maintained
at 22–24°C, under a 12 h dark/light cycle, relative humidity
40–60%, with free access to standard laboratory diet and water
ad libitum. The use of Wistar rats was approved by the
Animal Care and Use Committee of Central South University.
Models and grouping
All rats were randomly assigned into five groups (10
rats/group): Sham; SCI; Chlorogenic acid (10 mg/kg); Chlorogenic
acid (30 mg/kg); Chlorogenic acid (100 mg/kg). The Wistar rats were
intraperitoneally injected with ketamine (80 mg/kg) and xylazine
(10 mg/kg) for anesthesia. The SCI model was established as
previously described (16).
Following this, the T8 and T9 vertebral peduncles of narcotized
rats were removed. The same laminectomy without compression was
carried out for control group. The following treatments were
administered to the rats after inducing the SCI model for 24 h:
Sham group, rats were gavaged with normal saline; SCI group, SCI
rats were gavaged with normal saline; Chlorogenic acid groups, SCI
rats were gavaged with 10, 30 or 100 mg/kg Chlorogenic acid once
every three days for 3 weeks.
Assessment of neuronal functional recovery.
Following administration of chlorogenic acid (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), the assessment of neuronal functional
recovery was executed using the Basso, Beattie and Bresnahan (BBB)
test. The BBB test indicates a locomotor rating scale of 0 (no
observable hind-limb movements) to 21 (normal locomotion) (17).
Wet/dry weight ratio
Following administration of chlorogenic acid, spinal
cord tissue was acquired and weighed immediately as wet weight, and
then dried weight to constant weight at 80°C for 72 h, and weighed
again. Wet/dry weight ratio was calculated by dividing the wet
weight by the dry weight.
Assessment of TNF-α, IL-1β and IL-6
levels and inducible nitric oxide synthase (iNOS) activity
Following administration of chlorogenic acid, spinal
cord tissue was acquired and homogenized in cool phosphate-buffered
saline. Then, the protein concentration was measured using a
bicinchoninic acid protein assay kit (BCA; Beyotime Institute of
Biotechnology, Nanjing, China). TNF-α (PT516), IL-1β (PI303) and
IL-6 (IL-6) levels and iNOS activity (S0025) were measured with
corresponding ELISA kits according to the manufacturer's protocol
(Beyotime institute of Biotechnology) and analyzed using an ELISA
reader.
Western blot analysis
Following administration of chlorogenic acid, spinal
cord tissue was acquired and homogenized in cool
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). Proteins were extracted using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) and the concentration was measured using a BCA
protein assay kit (Beyotime Institute of Biotechnology). Proteins
(50 µg) were separated by 10% SDS-PAGE and electrotransferred to
nitrocellulose membranes. The membranes were blocked with 5% nonfat
milk for 1 h at 37°C and incubated overnight with primary
antibodies against NF-κB (dilution, 1:1,000; cat. no. sc-7178), IκB
(dilution, 1:1,000; cat. no. sc-371), phosphorylated IκB (p-IκB;
dilution, 1:1,000; cat. no. sc-101713), phosphorylated p38 (p-p38;
dilution, 1:1,000; cat. no. sc-101759) and β-actin (dilution,
1:800; cat. no. sc-7210), all obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA), at 4°C. Membranes were then
incubated with the secondary goat anti-mouse or anti-rabbit IgG
antibodies (A0208 or A0216; dilution, 1:5,000; Beyotime Institute
of Biotechnology) for 1 h at 37°C and detected by an enhanced
chemiluminescent detection system (GE Healthcare Life Sciences,
Little Chalfont, UK) according to the manufacturer's protocol.
Statistical analysis
All data are expressed as the mean ± standard
deviation and analyzed using SPSS software, version 17.0 (SPSS
Inc., Chicago, IL, USA). Data were compared between groups using a
Student's t-test. One-way analysis of variance followed by
Bonferroni's post hoc test were utilized to determine the
significant difference among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Administration of chlorogenic acid
increases BBB scores in SCI rats
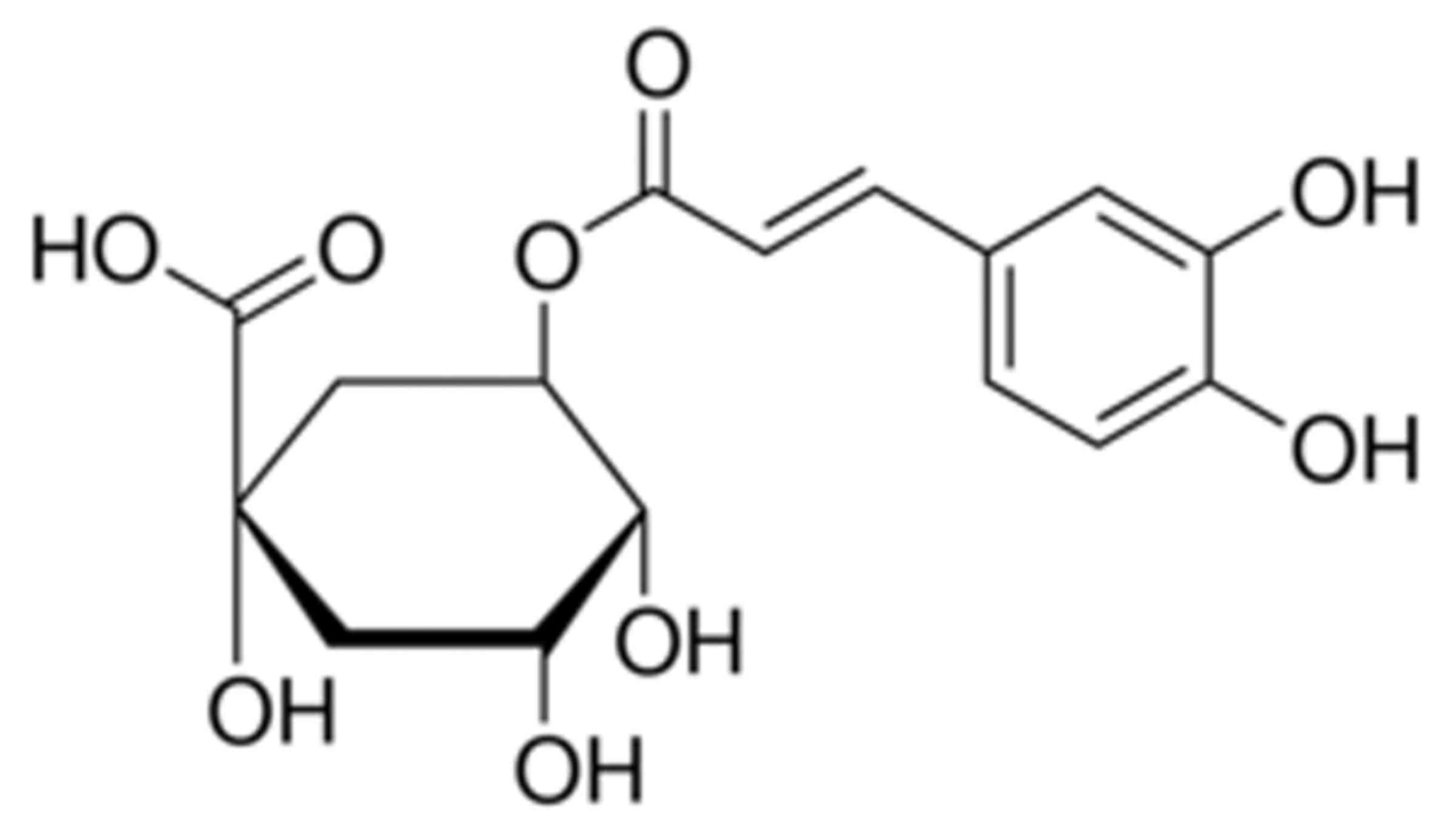
The chemical structure of chlorogenic acid is
presented in Fig. 1. The present
study investigated the effects of administration of chlorogenic
acid on neuronal function recovery, assessed using the BBB test.
When compared with sham-operated group, BBB scores were
significantly reduced in SCI rats (Fig. 2). However, treatment with
chlorogenic acid (30 and 100 mg/kg) significantly elevated the
SCI-induced inhibition of BBB scores in the rats (Fig. 2).
Administration of chlorogenic acid
decreases spinal cord wet/dry weight ratio in SCI rats
It was then investigated if chlorogenic acid
decreased spinal cord wet/dry weight ratio in the SCI rat.
Following a single day of treatment with chlorogenic acid, SCI rats
demonstrated an increased spinal cord wet/dry weight ratio compared
with the sham-operated group (Fig.
3). Treatment with chlorogenic acid significantly reduced the
SCI-induced increase in wet/dry weight ratio compared with the SCI
group rats (Fig. 3).
Administration of chlorogenic acid
suppresses inflammatory activity in SCI rat
In order to detect alteration in inflammatory
activity in rats with SCI following chlorogenic acid treatment,
assessment of TNF-α, IL-1β and IL-6 levels was performed. As
presented in Fig. 4, TNF-α, IL-1β
and IL-6 levels in SCI tissue were significantly increased compared
with the sham-operated group. Furthermore, TNF-α, IL-1β and IL-6
levels of SCI rats exposed to chlorogenic acid were decreased
compared with the SCI rats (Fig.
4).
Administration of chlorogenic acid
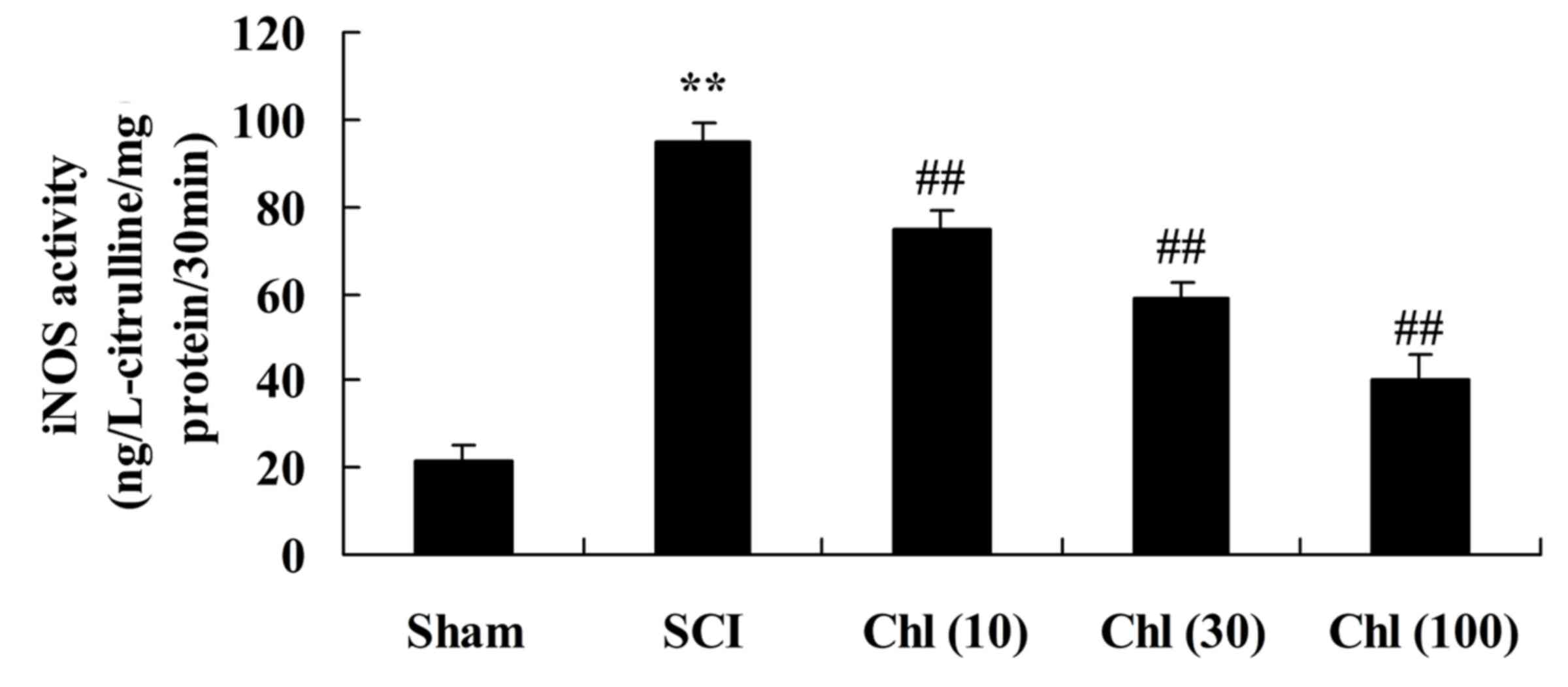
suppresses iNOS activity in SCI rat
To investigate iNOS activity in SCI rats following
chlorogenic acid treatment, ELISA kits were used. SCI induced
increased iNOS activity in the rats compared with the sham-operated
group (Fig. 5). Following
treatment with chlorogenic acid, iNOS activity was significantly
reduced compared with SCI rats (Fig.
5).
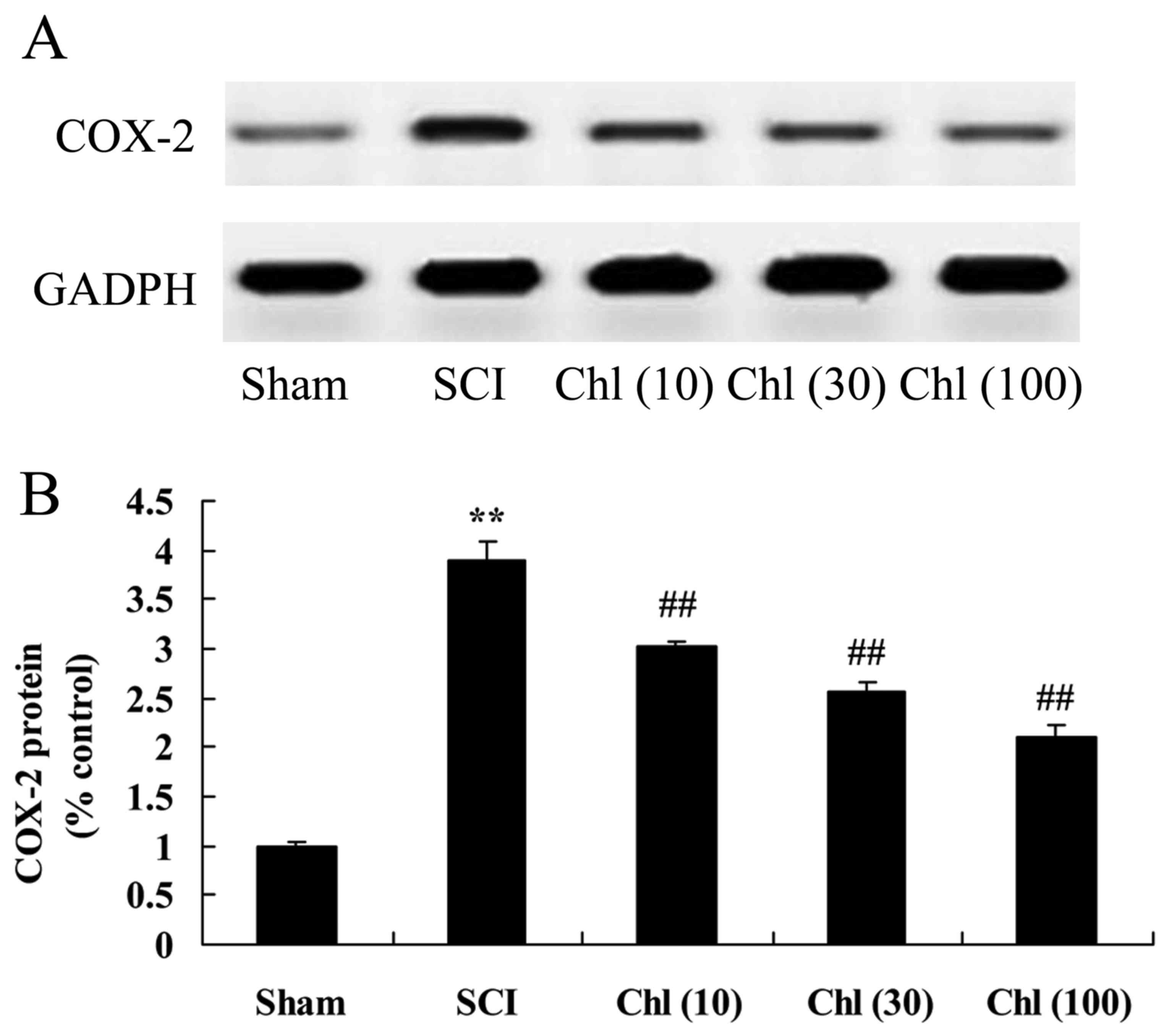
Administration of chlorogenic acid
suppresses COX-2 protein expression in SCI rat
The present study investigated if administration of
chlorogenic acid suppressed COX-2 protein expression in SCI rats.
As presented in Fig. 6, COX-2
protein expression levels in the SCI model group were increased
compared with control group. Treatment with chlorogenic acid
significantly suppressed COX-2 protein expression in SCI rats,
compared with the SCI rat model group (Fig. 6).
Administration of chlorogenic acid
suppresses toll like receptor (TLR) 4 protein expression in SCI
rat
TLR4 protein expression in the SCI rat treated with
chlorogenic acid was assessed using western blot analysis. TLR4
protein expression levels in the SCI rat were significantly
increased, compared with control group (Fig. 7). The increase in TLR4 protein
expression was then significantly suppressed by administration of
chlorogenic acid in SCI rats, compared with SCI rat model group
(Fig. 7).
Administration of chlorogenic acid
suppresses myeloid differentiation primary response (MyD)88 protein
expression in SCI rats
The present study additionally analyzed whether
chlorogenic acid suppressed MyD88 protein expression in SCI rats.
There was a significant increase in MyD88 protein expression in SCI
rats, compared with control group (Fig. 8). Chlorogenic acid then
significantly suppressed MyD88 protein expression in SCI rats,
compared with SCI rat model group (Fig. 8).
Administration of chlorogenic acid
suppresses nuclear factor (NF)-κB expression in SCI rat
The effect of chlorogenic acid on NF-κB protein
expression in SCI rats was analyzed using western blot analysis. As
presented in Fig. 9, NF-κB protein
expression was increased compared with sham-operated group.
Pretreatment with chlorogenic acid significantly reduced NF-κB
protein expression in the SCI rat (Fig. 9).
Administration of chlorogenic acid has
differing effects on IκB and p-IκB expression in SCI rat
The present study investigated the in vivo
effects of chlorogenic acid on IκB and p-IκB expression, and it was
demonstrated that IκB protein expression levels in the SCI rat were
decreased compared with the sham group, whereas p-IκB expression
levels were increased. Administration of chlorogenic acid
significantly increased activated IκB protein expression and
suppressed p-IκB expression in SCI rats (Fig. 10).
Administration of chlorogenic acid suppresses p-p38
mitogen activated protein kinase (MAPK) expression in SCI rat. The
present study finally investigated the effects of chlorogenic acid
on p-p38 MAPK expression in SCI rat. As presented in Fig. 11, p-p38 MAPK protein expression
was enhanced by SCI compared with sham-operated group. However,
administration of chlorogenic acid significantly suppressed p-p38
MAPK protein expression in SCI rats (Fig. 11).
Discussion
Due to the complicated pathophysiological basis of
SCI, identification of an effective therapeutic method has become
one of the most difficult challenges in the medical field (18). Numerous tests and experiments have
been conducted on neurofunctional deficit following SCI, which have
significantly enriched understanding of the SCI pathological
mechanism (19). However, there is
almost no current research focused at the clinical application
stage. At present, SCI treatment research primarily focuses on two
aspects: To limit or reduce the sequential injury to protect nerve
function, and to take measures to promote nerve regeneration
(20). The data from the present
study revealed that administration of chlorogenic acid increased
BBB scores and decreased spinal cord wet/dry weight ratio in SCI
rats.
Previously, with an increase in research regarding
nitric oxide, the effect of secondary spinal cord injury has become
of primary concern and research interest (21). Under the physiological status,
nitric oxide maintains and organizes the blood circulation
(21). Under abnormal conditions,
nitric oxide may participate in the inflammatory reaction and cell
apoptosis, and neuronal apoptosis has been demonstrated to be
important in the secondary injury of the spinal cord (22,23).
It has previously been demonstrated that COX-2 exerts various
pathological roles in the body, including a role in the
inflammatory reaction, sensation of pain, cellular damage and
cancer (24). However, another
study suggested that COX-2 additionally affects the normal
physiological functioning of the human body (24). In the central nervous system, COX-2
is expressed in glutamic-acid nerve cells in seahorses and in the
human cortex, and is important in synaptic activity, long-term
synaptic plasticity and nerve and blood vessel interactions during
functional congestive periods. The present study demonstrated that
chlorogenic acid significantly inhibited iNOS activity and COX-2
protein expression in SCI rats.
The presence of inflammatory cytokines may further
induce the degree of sequential SCI, due to the fact that following
injury, inflammatory cells, particularly neutrophile granulocytes,
gather at the injured area (25).
Neutrophils release elastase and reactive oxygen free radicals
which destroy the integrity of the vascular endothelium, increase
degree of tissue edema and necrosis and deteriorate neural
functions. They additionally result in neuronal and oligodendrocyte
apoptosis via caspase-3; stimulate astrocyte proliferation, lead to
local glial scar formation, restrain the axon regeneration,
upregulate expression of associated inflammatory genes and
subsequently induce the inflammatory response following the SCI
(26,27). Therefore, the presence of
inflammatory cytokines is one of the important events at the early
stages following SCI. In the present study, chlorogenic acid
significantly reduced TNF-α, IL-1β and IL-6 levels of SCI rats
through the TLR4/MyD88/NF-κB signaling pathway. Furthermore, Shi
et al (28) indicates that
chlorogenic acid reduces liver inflammation through the inhibition
of TLR4/MyD88/NF-κB signaling pathway. Ruifeng et al
(15) suggested that chlorogenic
acid reduces TNF-α, IL-1β and IL-6 production by suppressing
TLR4-mediated NF-κB signaling pathway.
The NF-κB family is the primary regulatory factor of
inflammatory gene expression. It regulates the expression of
numerous cytokines and regulates the inflammatory reaction in
central nervous system injury (29). In central nervous system trauma,
excitatory damage, ischemic damage and neurodegenerative diseases,
abnormal activated NF-κB may induce neuronal apoptosis. Abnormal
activation of NF-κB, co-localization staining of activated NF-κB
and its target gene product iNOS have been revealed in nerve cells
of SCI. A large number of genes with expressions levels regulated
by NF-κB were detected in SCI, including the pro inflammatory
cytokines, TNF-α, IL-1β, IL-6, iNOS and matrix metalloproteinases
(MMPs) (30). It was demonstrated
that chlorogenic acid significantly inhibited NF-κB protein
expression in SCI rats. Chen and Wu (13) indicated that chlorogenic acid
suppresses IL-1β-induced inflammation via iNOS and COX-2 in human
chondrocytes.
During the activation process of NF-κB, which is
regulated by IKKβ, IKKβ phosphorylates I-κBα protein, leading to
ubiquitination and degradation of I-κBα protein (31). Following SCI, the protein
expression of phosphorylated I-κBα in myeloid tissue increases
significantly. BMS-345541 intervention may reduce >50% of the
protein expression of phosphorylated I-κBα (32). These results further demonstrate
that the IKKβ kinase activity in the tissue following SCI
significantly increases, and that this increase may be inhibited by
BMS-345541 intervention (13). In
the present study, chlorogenic acid significantly suppressed p-IκB
and p38 MAPK protein expression and activated IκB protein
expression in SCI rats. Chen and Wu (13) indicate that chlorogenic acid
suppresses IL-1β-induced rabbit chondrocytes through IκB. These
results suggest that NF-κB and p38 signaling pathways are involved
in the effects of chlorogenic acid on SCI.
The p38 MAPK signaling pathway is one of the classic
three branches of the MAPK signal pathway, which widely
participates in the inflammatory reaction, radioactive injury and
stress reactions. It has previously been demonstrated that the p38
MAPK signal pathway promotes MMP-9 expression following SCI and
enters the blood-spinal cord barrier. The results of the present
study demonstrated that chlorogenic acid significantly suppressed
p38 MAPK protein expression in SCI rats.
In conclusion, the findings demonstrated that
administration of chlorogenic acid alleviated spinal cord injury
via anti-inflammatory activities in vivo. The therapeutic
effect of chlorogenic acid is associated with suppression of
TLR4/MyD88/NF-κB/IκB, and down-regulation of p38 MAPK expression.
Therefore, chlorogenic acid may act as a potential therapeutic
candidate for the treatment of SCI through its anti-inflammatory
properties mediated by the TLR4/MyD88/NF-κB and p38 MAPK signaling
pathways.
References
|
1
|
Casha S, Zygun D, McGowan MD, Bains I,
Yong VW and Hurlbert RJ: Results of a phase II placebo-controlled
randomized trial of minocycline in acute spinal cord injury. Brain.
135:1224–1236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ren Y and Young W: Managing inflammation
after spinal cord injury through manipulation of macrophage
function. Neural Plast. 2013:9450342013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thrasher TA, Ward JS and Fisher S:
Strength and endurance adaptations to functional electrical
stimulation leg cycle ergometry in spinal cord injury. Neuro
Rehabilitation. 33:133–138. 2013.PubMed/NCBI
|
|
4
|
van Leeuwen CM, van der Woude LH and Post
MW: Validity of the mental health subscale of the SF-36 in persons
with spinal cord injury. Spinal Cord. 50:707–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zangrillo A, Buratti L, Carozzo A,
Casiraghi G, Landoni G, Lembo R, Pasin L, Marone EM, Melissano G
and Chiesa R: Intrathecal lactate as a predictor of early-but not
late-onset spinal cord injury in thoracoabdominal aneurysmectomy. J
Cardiothorac Vasc Anesth. 28:473–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldshmit Y, Frisca F, Kaslin J, Pinto AR,
Tang JK, Pébay A, Pinkas-Kramarski R and Currie PD: Decreased
anti-regenerative effects after spinal cord injury in spry4−/−
mice. Neuroscience. 287:104–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Margraf S, Lögters T, Reipen J, Altrichter
J, Scholz M and Windolf J: Neutrophil-derived circulating free DNA
(cf-DNA/NETs): A potential prognostic marker for posttraumatic
development of inflammatory second hit and sepsis. Shock.
30:352–358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meves JM and Zheng B: Extrinsic inhibitors
in axon sprouting and functional recovery after spinal cord injury.
Neural Regen Res. 9:460–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mortazavi MM, Verma K, Harmon OA,
Griessenauer CJ, Adeeb N, Theodore N and Tubbs RS: The microanatomy
of spinal cord injury: A review. Clin Anat. 28:27–36. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kigerl KA, de Rivero Vaccari JP, Dietrich
WD, Popovich PG and Keane RW: Pattern recognition receptors and
central nervous system repair. Exp Neurol. 258:5–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adibhatla RM and Hatcher JF: Phospholipase
A(2), reactive oxygen species, and lipid peroxidation in CNS
pathologies. BMB Rep. 41:560–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng S, Cao J, Feng Q, Peng J and Hu Y:
Roles of chlorogenic Acid on regulating glucose and lipids
metabolism: A review. Evid Based Complement Alternat Med.
2013:8014572013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen WP and Wu LD: Chlorogenic acid
suppresses interleukin-1β-induced inflammatory mediators in human
chondrocytes. Int J Clin Exp Pathol. 7:8797–8801. 2014.PubMed/NCBI
|
|
14
|
Del Rio D, Stalmach A, Calani L and
Crozier A: Bioavailability of coffee chlorogenic acids and green
tea flavan-3-ols. Nutrients. 2:820–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruifeng G, Yunhe F, Zhengkai W, Ershun Z,
Yimeng L, Minjun Y, Xiaojing S, Zhengtao Y and Naisheng Z:
Chlorogenic acid attenuates lipopolysaccharide-induced mice
mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur
J Pharmacol. 729:54–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ravikumar R, Fugaccia I, Scheff SW, Geddes
JW, Srinivasan C and Toborek M: Nicotine attenuates morphological
deficits in a contusion model of spinal cord injury. J Neurotrauma.
22:240–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chio CC, Lin JW, Chang MW, Wang CC, Kuo
JR, Yang CZ and Chang CP: Therapeutic evaluation of etanercept in a
model of traumatic brain injury. J Neurochem. 115:921–929. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gwak YS, Kang J, Unabia GC and Hulsebosch
CE: Spatial and temporal activation of spinal glial cells: Role of
gliopathy in central neuropathic pain following spinal cord injury
in rats. Exp Neurol. 234:362–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu PG, Feng H, Yuan SJ, Zhang RW, Li M, Hu
R, Liu ZS and Yin J: Effect of preconditioning with hyperbaric
oxygen on neural cell apoptosis after spinal cord injury in rats. J
Neurosurg Sci. 57:253–258. 2013.PubMed/NCBI
|
|
20
|
Fan T, Wang CC, Wang FM, Cheng F, Qiao H,
Liu SL, Guo W and Xiang FY: Experimental study of the protection of
ischemic preconditioning to spinal cord ischemia. Surg Neurol.
52:299–305. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo J, Zhu Y, Yang Y, Wang X, Chen B,
Zhang W, Xie B, Zhu Z, Yue Y and Cheng J: Electroacupuncture at
Zusanli (ST36) ameliorates colonic neuronal nitric oxide synthase
upregulation in rats with neurogenic bowel dysfunction following
spinal cord injury. Spinal Cord. 54:1139–1144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao W and Li J: Targeted siRNA delivery
reduces nitric oxide mediated cell death after spinal cord injury.
J Nanobiotechnology. 15:382017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brown R, Celermajer D, Macefield V and
Sander M: The effect of nitric oxide inhibition in spinal cord
injured humans with and without preserved sympathetic control of
the vasculature. Front Neurosci. 10:952016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yue K, Wang X, Wu Y, Zhou X, He Q and Duan
Y: microRNA-7 regulates cell growth, migration and invasion via
direct targeting of PAK1 in thyroid cancer. Mol Med Rep.
14:2127–2134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang WG, Xiu RJ, Xu ZW, Yin YX, Feng Y,
Cao XC and Wang PS: Protective effects of Vitamin C against spinal
cord injury-induced renal damage through suppression of NF-κB and
proinflammatory cytokines. Neurol Sci. 36:521–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nelissen S, Vangansewinkel T, Geurts N,
Geboes L, Lemmens E, Vidal PM, Lemmens S, Willems L, Boato F,
Dooley D, et al: Mast cells protect from post-traumatic spinal cord
damage in mice by degrading inflammation-associated cytokines via
mouse mast cell protease 4. Neurobiol Dis. 62:260–272. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Genovese T, Esposito E, Mazzon E, Di Paola
R, Caminiti R, Bramanti P, Cappelani A and Cuzzocrea S: Absence of
endogenous interleukin-10 enhances secondary inflammatory process
after spinal cord compression injury in mice. J Neurochem.
108:1360–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi H, Dong L, Jiang J, Zhao J, Zhao G,
Dang X, Lu X and Jia M: Chlorogenic acid reduces liver inflammation
and fibrosis through inhibition of toll-like receptor 4 signaling
pathway. Toxicology. 303:107–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silverman N and Maniatis T: NF-kappaB
signaling pathways in mammalian and insect innate immunity. Genes
Dev. 15:2321–2342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park SY, Jin ML, Kim YH, Kim Y and Lee SJ:
Anti-inflammatory effects of aromatic-turmerone through blocking of
NF-κB, JNK, and p38 MAPK signaling pathways in amyloid
beta-stimulated microglia. Int Immunopharmacol. 14:13–20. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun SC, Harhaj EW, Xiao G and Good L:
Activation of I-kappaB kinase by the HTLV type 1 Tax protein:
Mechanistic insights into the adaptor function of IKKgamma. AIDS
Res Hum Retroviruses. 16:1591–1596. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meunier A, Latrémolière A, Dominguez E,
Mauborgne A, Philippe S, Hamon M, Mallet J, Benoliel JJ and Pohl M:
Lentiviral-mediated targeted NF-κB blockade in dorsal spinal cord
glia attenuates sciatic nerve injury-induced neuropathic pain in
the rat. Mol Ther. 15:687–697. 2007. View Article : Google Scholar
|