Introduction
Diabetic nephropathy (DN) is the leading cause of
end-stage renal disease (ESRD) in diabetes, with an incidence of
20–40% worldwide (1,2). DN is characterized by progressive
renal interstitial fibrosis. A previous study reported that high
glucose (HG) and lysophosphatidylcholine (LPC) levels were
associated with the development and progression of DN (3); these two factors have been
demonstrated to stimulate platelet-activating factor (PAF)
expression and extracellular matrix (ECM) secretion by the
mesangial cells (MCs) of the kidney (4).
Protein kinase C (PKC)βI is an isoenzyme in the PKC
family and is involved in a number of biological processes,
including cell proliferation, differentiation, apoptosis and
angiogenesis (5), in addition to
having a role in the pathogenesis of DN (6,7). PKC
is aberrantly activated in the diabetic kidney, which leads to an
increase in PKCβI activity and deposition of ECM proteins,
including fibronectin (Fn) and collagen (Col) type IV (8,9). In
addition, transforming growth factor (TGF)-β1 has an important role
in ECM accumulation during renal fibrosis (10), and it has been implicated in the
occurrence of DN (11–13). However, the underlying molecular
mechanism between PAF, PKC, TGF-β1 and the ECM in DN remains to be
elucidated. The present study investigated the association among
the aforementioned factors in a DN model consisting of human (H)MCs
exposed to high HG) and LPC treatments. Reverse
transcription-quantitative polymerase chain reaction and western
blotting was used to detect PKCβI and TGF-β1 expression, and then
an ELISA assay was used to detect the expression levels of the
ECM-associated molecules collagen IV and fibronectin in the
supernatant. To clarify the function of PKCβI, immunocytochemistry
was used to demonstrated the subcellular localization of PKCβI. The
results of the present study suggested that PAF stimulated ECM
deposition in HMCs via activation of the PKC-TGF-β1 axis in a DN
model.
Materials and methods
Cell culture
HMCs donated by the Zhongda Hospital affiliated with
Southeast University (Nanjing, China) were maintained in Dulbecco's
modified Eagle's medium containing 10% fetal calf serum
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in
an atmosphere containing 5% CO2 at 37°C.
The cells were divided into six groups: Control (5.5
mM D-glucose; Enzo Life Sciences, Inc., Farmingdale, NY, USA); PAF
(2×10−8 M PAF C-16; Cayman Chemical Company, Ann Arbor,
MI, USA); PAF + PKCβI inhibitor LY333531 (Enzo Life Sciences, Inc.;
2×10−8 M PAF and 2×10−7 M LY333531); HG + LPC
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; 30 mM D-glucose and
20 mg/l LPC); PAF + HG + LPC (2×10−8 PAF, 30 mM
D-glucose and 20 mg/l LPC); and PAF + HG + LPC + LY333531
(2×10−8 PAF, 30 mM D-glucose, 20 mg/l LPC and
2×10−7 M LY333531) (4).
ELISA analysis
The expression levels of Fn and Col IV in the cell
culture supernatants were detected using specific ELISA kits (cat
nos. CSB-EL005745HU and CSB-E04551h) according to the
manufacturer's protocol (JingMei Biotech, Shenzheng, China).
Samples were analyzed in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and was
reverse transcribed into cDNA using the Revert Aid First Strand
cDNA Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. RT-qPCR assay was
performed using a SYBR_Premix ExTaq II kit (Takara Biotechnology
Co., Ltd., Dalian, China) was performed using in the CFX96
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) to determine the relative expression levels of
target genes. The sequences of forward and reverse primers: PKCβI,
5′-GGGGGCGACCTCATGTAT-3′ and 5′-GCAATTTCTGCAGCGTAAAA-3′; and GAPDH,
5′-ACACCCACTCCTCCACCTTT-3′ and 5′-TTACTCCTTGGAGGCCATGT-3′. Primers
were designed using Premier Oligo version 5 and Primer version 6.22
(Premier Biosoft International, Palo Alto, CA, USA). The
thermocycling program used was as follows: 95°C for 30 sec,
followed by 40 cycles of 60°C for 30 sec and 72°C for 30 sec.
Relative changes in expression level were calculated using the
quantification cycle (2−ΔΔCq) method (14). Each sample was prepared in
triplicate and the results are expressed as the mean of three
independent experiments.
Western blotting
Cells were resuspended in lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) for 30
min and sonicated for 2 min at 20 W, followed by centrifugation at
12,000 × g for 10 min at 4°C. The supernatant was collected and 50
µg/lane protein (concentration determined using the bicinchoninic
assay kit (Thermo Fisher Scientific, Inc.) was separated using
SDS-PAGE on a 10% gel (Bio-Rad Laboratories, Inc.) and transferred
to a nitrocellulose membrane (Bio-Rad Laboratories, Inc.), which
was blocked in Tris-buffered saline/Tween-20 (TBST) with 5% non-fat
milk for 1 h at 37°C. The membrane was subsequently incubated with
primary antibodies against TGF-β1 (cat no. sc-146; 1:2,000), PKCβI
(cat no. sc- 209; 1:1,000) and GAPDH (cat no. sc-25778; 1:500)
(both from Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C. Following washing with TBST, the membranes were
incubated with a horseradish peroxidase-conjugated labeled goat
anti-rabbit secondary antibody (cat no. sc-2004; 1:500; Santa Cruz
Biotechnology, Inc.) for 1 h at 4°C, followed by additional three
washes with TBST. Protein bands were visualized by enhanced
chemiluminescence (GE Healthcare, Chicago, IL, USA). The Scion
Image system version 4.03 (National Institutes of Health, Bethesda,
MD, USA) was used to quantify band intensity and data are expressed
as the mean of three independent experiments.
Immunocytochemistry
Cells (2×104/ml) were cultured on
coverslips in 24-well plates for 24 h, and subsequently fixed with
4% paraformaldehyde for 5 min at −20°C and blocked at room
temperature for 30 min in 0.2% Triton X-100 in PBS. The cells were
incubated with anti-PKCβI antibody (1:50) (cat no. 07-870; EMD
Millipore, Billerica, MA, USA) overnight at 4°C, followed by
fluorescein isothiocyanate-conjugated secondary antibody (1:400;
cat no. K532511-8; Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA) for 1 h in the dark at room temperature. Following three
washes in PBS, coverslips were placed on the slides and the cells
were visualized using confocal microscopy. Fluorescence intensity
(wavelength of 490 nm) was analyzed using Image J software (version
number: 1.48u; (National Institutes of Health).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Data were analyzed using SPSS software version 13.0
(SPSS, Inc., Chicago, IL, USA). Differences between groups were
assessed using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
PKCβI expression is upregulated in
HMCs in the presence of PAF, HG and LPC
PKCβI mRNA expression level was increased in the
PAF, HG + LPC, and PAF + HG + LPC groups compared with control
group (P<0.05). The expression was increased in the PAF + HG +
LPC group compared with cells treated with HG and LPC alone
(P<0.05), this increase in PKCβI expression was reversed by
treatment with the PKCβI inhibitor LY333531 (P<0.05; Table I; Fig.
1).
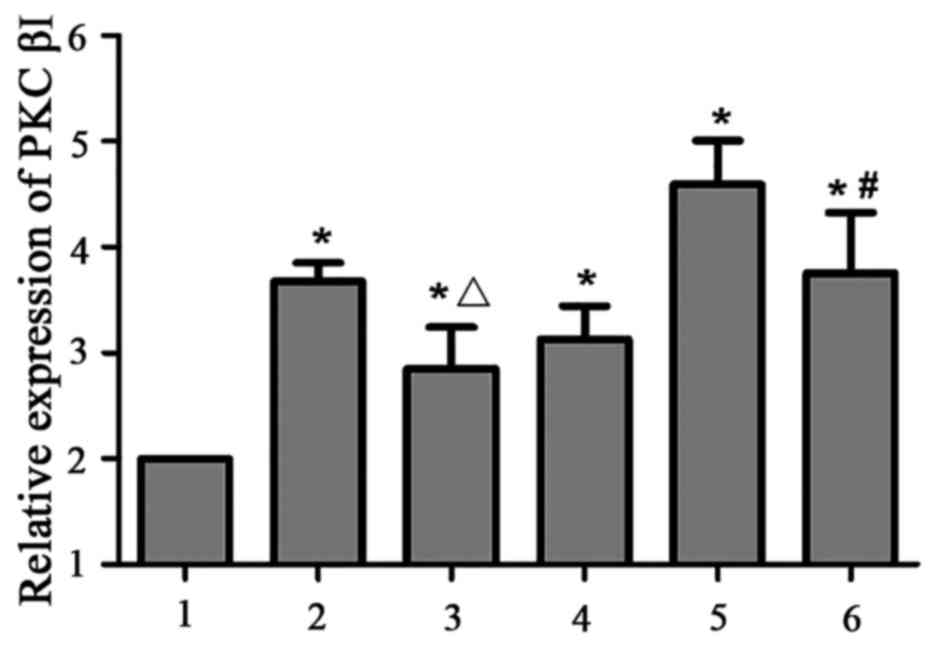 | Figure 1.PKCβI mRNA expression in human
mesangial cells in various treatment groups. Expression levels were
determined relative to GAPDH using the reverse
transcription-quantitative polymerase chain reaction. 1, control;
2, PAF; 3, PAF + LY333531; 4, HG + LPC; 5, PAF + HG + LPC; 6, PAF +
HG + LPC + LY333531. Data are presented as the mean ± standard
error of the mean of three independent experiments. *P<0.05 vs.
control group; ΔP<0.05 vs. PAF group;
#P<0.05 vs. PAF + HG + LPC group. PKCβI, protein
kinase CβI; PAF, platelet activating factor; LY333531, PKCβI
inhibitor; HG, high glucose; LPC, lysophosphatidylcholine. |
 | Table I.PKCβI mRNA expression in each
treatment group. |
Table I.
PKCβI mRNA expression in each
treatment group.
| Group | Expression |
|---|
| Control |
1.00±0.00 |
| PAF |
2.68±0.17a |
| PAF + LY333531 |
1.85±0.39a,b |
| HG + LPC |
2.12±0.31a |
| PAF + HG + LPC |
3.59±0.41a |
| PAF + HG + LPC +
LY333531 |
2.76±0.57a,c |
A similar trend was observed for PKCβI protein
expression, which was increased in the PAF, HG + LPC and PAF + HG +
LPC groups compared with control cells (P<0.05; Fig. 2). The observed upregulation in
PKCβI expression levels was reduced following treatment with
LY333531 (P<0.05).
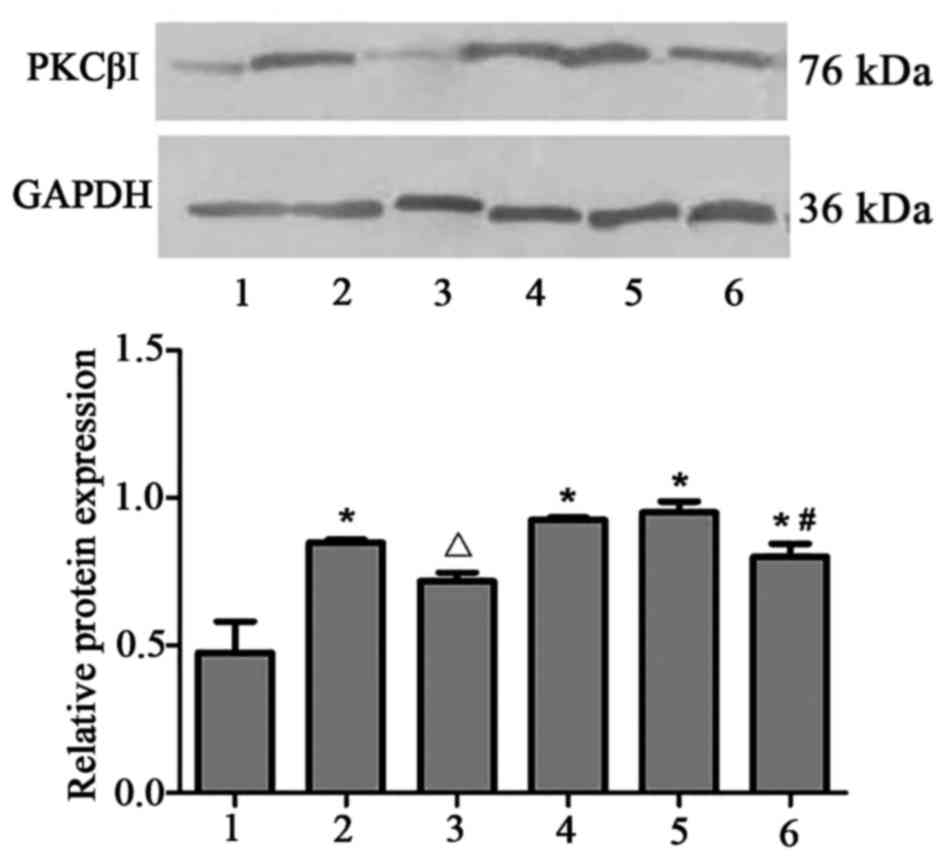 | Figure 2.PKCβI protein expression in human
mesangial cells under various treatment conditions. The protein
expression level was determined using western blotting, with GAPDH
used as a loading control. 1, control; 2, PAF; 3, PAF + LY333531;
4, HG + LPC; 5, PAF + HG + LPC; 6, PAF + HG + LPC + LY333531. Data
are presented as the mean ± standard error of the mean of three
independent experiments. *P<0.05 vs. control group;
ΔP<0.05 vs. PAF group; #P<0.05 vs. PAF
+ HG + LPC group. PKCβI, protein kinase CβI; PAF, platelet
activating factor; LY333531, PKCβI inhibitor; HG, high glucose;
LPC, lysophosphatidylcholine. |
TGF-β1 expression is upregulated in
HMCs in the presence of PAF, HG and LPC
TGF-β1 mRNA (Table
II; Fig. 3) and protein
(Fig. 4) expression levels were
upregulated in HMCs treated with PAF, HG and LPC, compared with the
control (P<0.05). The increased expression was not observed in
the presence of LY333531.
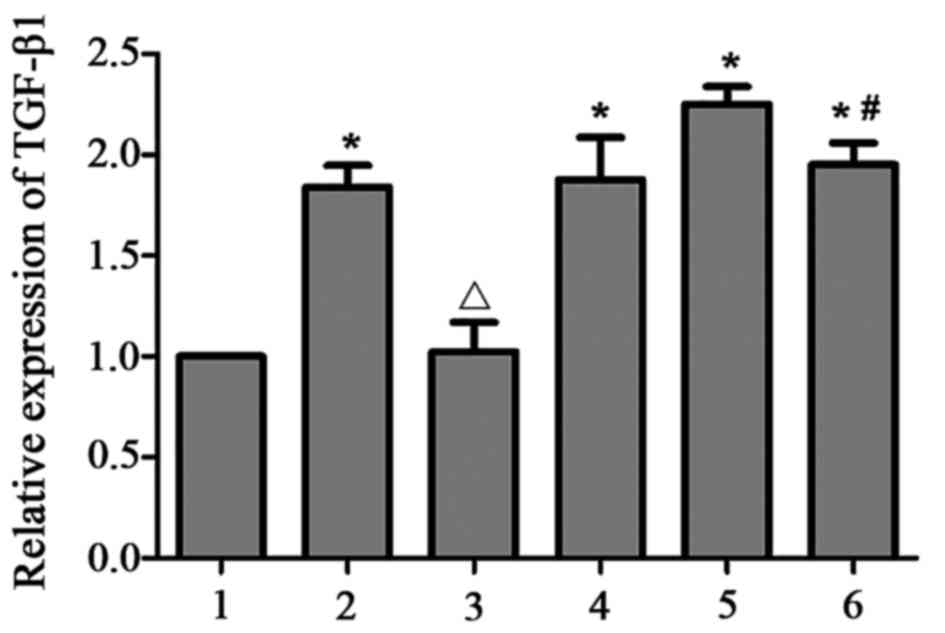 | Figure 3.TGF-β1 mRNA expression in human
mesangial cells under various treatment conditions. Expression
levels were determined relative to GAPDH using the reverse
transcription-quantitative polymerase chain reaction. 1, control;
2, PAF; 3, PAF + LY333531; 4, HG + LPC; 5, PAF + HG + LPC; 6, PAF +
HG + LPC + LY333531. Data are presented as mean ± standard error of
the mean of three independent experiments. *P<0.05 vs. control
group; ΔP<0.05 vs. PAF group; #P<0.05
vs. PAF + HG + LPC group. TGF-β1, transforming growth factor-β1;
PAF, platelet activating factor; LY333531, PKCβI inhibitor; HG,
high glucose; LPC, lysophosphatidylcholine. |
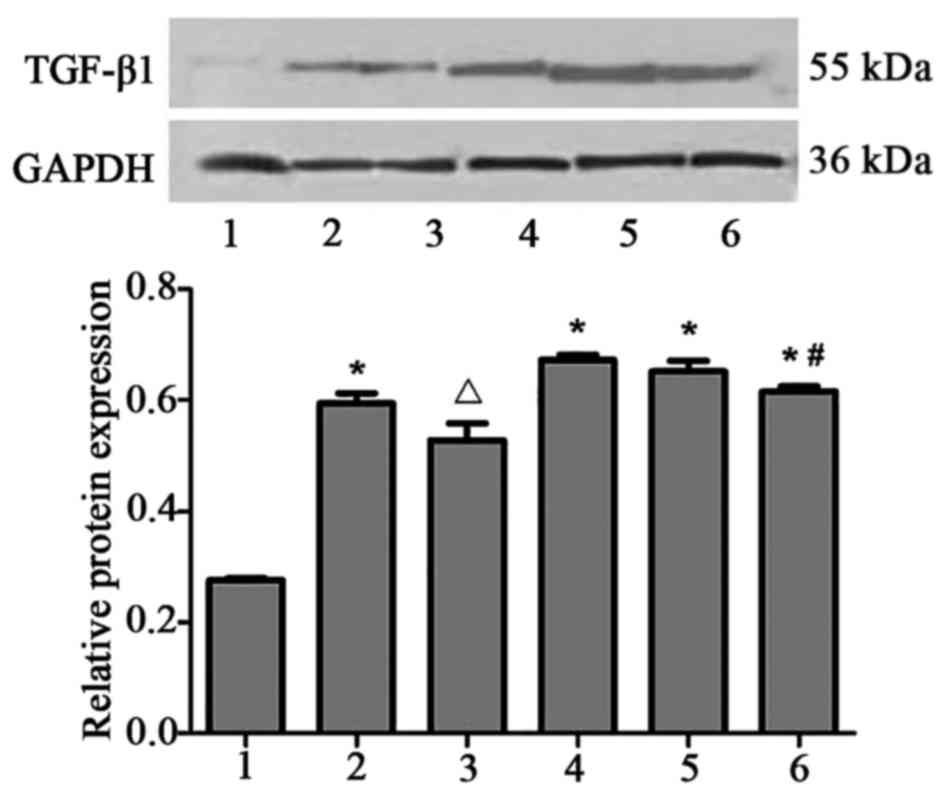 | Figure 4.TGF-β1 protein expression in human
mesangial cells under various treatment conditions. Protein
expression level was determined using western blotting, with GAPDH
used as a loading control. 1, control; 2, PAF; 3, PAF + LY333531;
4, HG + LPC; 5, PAF + HG + LPC; 6, PAF + HG + LPC + LY333531. Data
are presented as mean ± standard error of the mean of three
independent experiments. *P<0.05 vs. control group;
ΔP<0.05 vs. PAF group; #P<0.05 vs. PAF
+ HG + LPC group. TGF-β1, transforming growth factor-β1; PAF,
platelet activating factor; LY333531, PKCβI inhibitor; HG, high
glucose; LPC, lysophosphatidylcholine. |
 | Table II.TGF-β1 mRNA expression in each
treatment group. |
Table II.
TGF-β1 mRNA expression in each
treatment group.
| Group | Expression |
|---|
| Control |
1.00±0.00 |
| PAF |
1.84±0.11a |
| PAF + LY333531 |
1.02±0.15b |
| HG + LPC |
1.88±0.21a |
| PAF + HG + LPC |
2.25±0.09a |
| PAF + HG + LPC +
LY333531 |
1.95±0.11a,c |
ECM production is induced in HMCs in
the presence of PAF, HG and LPC
The expression levels of two ECM proteins, Fn and
Col IV, in the supernatant of cultured HMCs were significantly
upregulated following treatment with PAF, HG and LPC, compared with
the control group (P<0.05; Fig.
5), with increased levels observed in cells treated with all
three factors compared with HG and LPC group (P<0.05). This
effect was reduced following treatment with LY333531 (Table III).
 | Figure 5.Fn and Col IV levels in human
mesangial cell culture supernatants, as detected by ELISA analysis.
1, control; 2, PAF; 3, PAF + LY333531; 4, HG + LPC; 5, PAF + HG +
LPC; 6, PAF + HG + LPC + LY333531. Data are presented as mean ±
standard error of the mean of three independent experiments.
*P<0.05 vs. control group; ΔP<0.05 vs. PAF group;
#P<0.05 vs. PAF + HG + LPC group. Fn, fibronectin;
Col IV, collagen type IV; PAF, platelet activating factor;
LY333531, PKCβI inhibitor; HG, high glucose; LPC,
lysophosphatidylcholine. |
 | Table III.Expression of the extracellular
matrix components Fn and Col IV in the different treatment
groups. |
Table III.
Expression of the extracellular
matrix components Fn and Col IV in the different treatment
groups.
| Group | Fn, mg/l | Col IV, µg/l |
|---|
| Control |
3.90±0.43 |
4.54±0.74 |
| PAF |
7.05±0.05a |
13.71±0.88a |
| PAF + LY333531 |
3.81±0.13b |
5.31±0.81b |
| HG + LPC |
7.89±0.34a,c |
16.32±1.55a,c |
| PAF + HG + LPC |
9.11±0.10a |
22.89±0.34a |
| PAF + HG + LPC +
LY333531 |
5.23±0.24a,c |
11.40±0.72a,c |
PKCβI protein is translocated from the
cytoplasm to the nucleus of HMCs following treatment with PAF, HG
and LPC
In the control group, PKCβI was diffusely
distributed throughout the cytoplasm, with no membrane or nuclear
localization. Treatment with PAF, HG and LPC increased PKCβI
protein levels, and induced the translocation of the protein from
the cytoplasm to the nucleus(P<0.05). Treatment with LY333531
did not alter in the subcellular localization of PKCβI protein
(Table IV; Figs. 6 and 7).
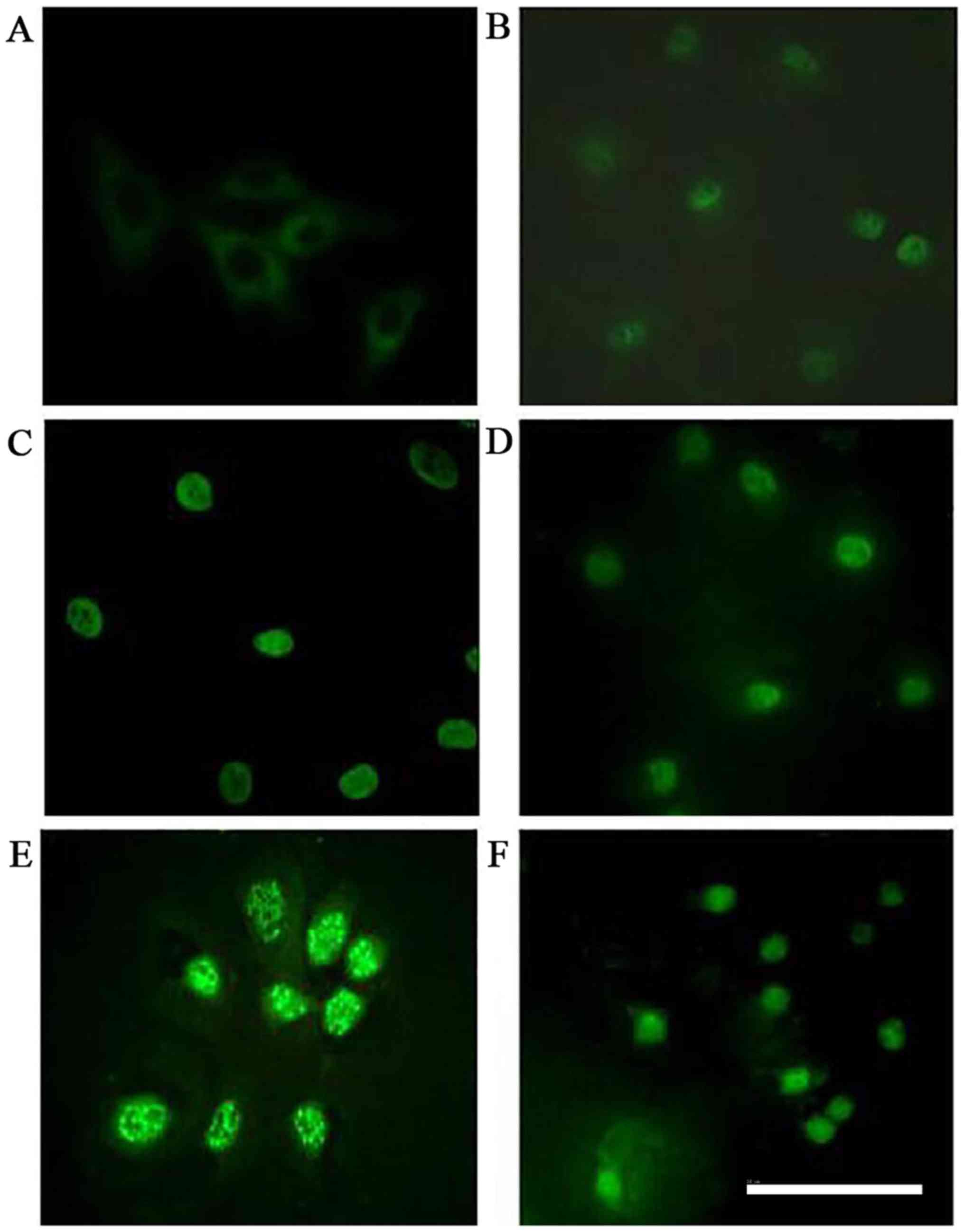 | Figure 6.Immunocytochemical analysis of PKCβI
localization in human mesangial cells under various treatment
conditions. PKCβI was detected by immunocytochemistry and
visualized by confocal microscopy in the (A) control, (B) PAF, (C)
PAF + LY333531, (D) HG + LPC, (E) PAF + HG + LPC, and (F) PAF + HG
+ LPC + LY333531 groups. Scale bar, 30µm. PKCβI, protein kinase
CβI; PAF, platelet activating factor; LY333531, PKCβI inhibitor;
HG, high glucose; LPC, lysophosphatidylcholine. |
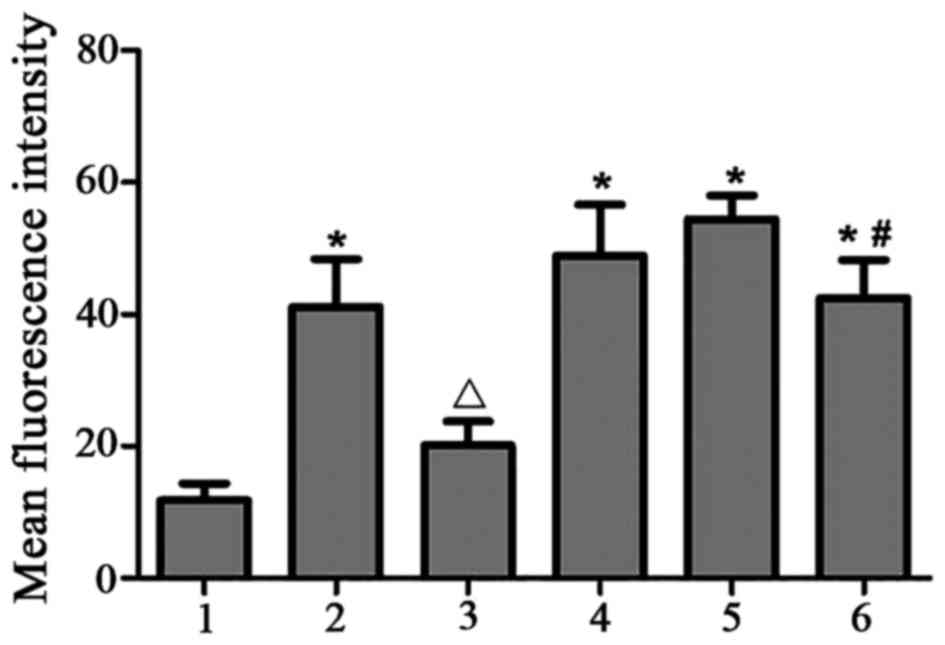 | Figure 7.Subcellular localization of PKCβI
protein in human mesangial cells under various treatment
conditions, based on mean fluorescence intensity. 1, control; 2,
PAF; 3, PAF + LY333531; 4, HG + LPC; 5, PAF + HG + LPC; 6, PAF + HG
+ LPC + LY333531. Data are presented as the mean ± standard error
of the mean of three independent experiments. *P<0.05 vs.
control group; ΔP<0.05 vs. PAF group;
#P<0.05 vs. PAF + HG + LPC group. PKCβI, protein
kinase CβI; PAF, platelet activating factor; LY333531, PKCβI
inhibitor; HG, high glucose; LPC, lysophosphatidylcholine. |
 | Table IV.Mean fluorescence intensity of PKCβI
in human mesangial cells under various treatment conditions. |
Table IV.
Mean fluorescence intensity of PKCβI
in human mesangial cells under various treatment conditions.
| Group | Mean fluorescence
intensity |
|---|
| Control |
11.80±2.57 |
| PAF |
41.14±7.21a |
| PAF + LY333531 |
20.19±3.60b |
| HG + LPC |
48.92±7.70a |
| PAF + HG + LPC |
54.45±3.57a |
| PAF + HG + LPC +
LY333531 |
42.50±5.70a,c |
Discussion
Diabetes mellitus is an important public health
concern, especially in developed countries (15), with DN being the primary cause of
ESRD worldwide (16–19). DN is caused by nerve damage
resulting from ECM deposition, mesangial expansion and basement
membrane thickening (20). The
accumulation of Fn and Col IV underlies chronic kidney diseases,
including progressive renal interstitial fibrosis (21). Metabolic disorders, such as
hyperlipidemia and hyperglycemia, are associated with the
occurrence and development of DN, with increased glucose and fat
levels having an adverse effect on glomerular capillary endothelial
cells and MCs, in addition to podocytes in the kidney (22), via stimulation of ECM secretion
(23) mediated by TGF-β/mothers
against decapentaplegic homolog 3 signaling. A HG/high fat diet may
upregulate Fn and Col IV expression, which may alter the structure
and function of renal tubules and lead to renal tubulointerstitial
fibrosis (24). PAF is a lipid
polymer, involved in the metabolism of arachidonic acid, that has a
role in DN by stimulating Fn secretion (25). The present study determined that Fn
and Col IV secretion were stimulated by PAF, HG and LPC, consistent
with previous studies (8,26,27).
The findings of the present study supported the hypothesis that HG
and LPC may be risk factors for renal fibrosis and DN.
PKC is a serine/threonine kinase expressed in
various mammalian tissues, which regulates a number of signaling
pathways (28,29). The present study revealed that PKC
was diffusely distributed throughout the cytoplasm in untreated
HMCs and translocated to the nucleus in the presence of PAF, HG and
LPC. DN may be delayed or prevented by inhibiting PKC (30,31);
enlargement of kidney volume and renal fibrosis were rescued by
PKCβI-knockout in a mouse model of DN (8). LY333531 is a Food and Drug
Administration-approved inhibitor of PKC-Β (32), which has been demonstrated to
promote myocardial angiogenesis in diabetes (33) and improve albuminuria and other
pathological features in DN rats via inhibition of PKC expression
(34). Treatment with LY333531 was
demonstrated to reduce mesangial matrix expansion and decrease the
urinary protein excretion rate in diabetic mice (35). In the present study, LY333531
treatment prevented the nuclear localization of PKCβI protein in
the presence of PAF, HG and LPC, which corresponded to the decrease
in Fn and Col IV secretion. The findings of the present study
suggested that PKCβI may have an important role in ECM deposition
by HMCs in DN.
TGF-β1 is a TGF-β superfamily member which regulates
a variety of cellular processes, including proliferation,
differentiation and apoptosis (36,37).
TGF-β1 has an important role in kidney hypertrophy (26), glomerular and renal tubular
basement membrane thickening, and renal tubulointerstitial fibrosis
(38,39), and previous studies have suggested
that it may modulate ECM secretion in DN. For example, plasmacytoma
variant translocation 1 was demonstrated to increase plasminogen
TGF-β1 in addition to Fn expression in MCs (40), whereas TGF-β1 inhibited the
expression of microRNA (miR)-26a to modulate DN progression in
diabetic mice (41). ECM
accumulation was increased via upregulation of miR-1207-5p in the
presence of glucose and TGF-β1, which was implicated in DN
pathogenesis (42). Additionally,
Fn and Col IV levels were suppressed by the knockdown of TGF-β1
(43). The present study revealed
that TGF-β1 mRNA and protein expression levels were upregulated in
HMCs, following treatment with PAF, HG and LPC compared with the
control group, which was accompanied by increased Fn and Col IV
secretion; these effects were abolished by treatment with
LY333531.
In conclusion, the findings of the present study
suggested that ECM deposition by MCs may be induced by HG and LPC
treatment and activation of PKCβI–TGF-β1 signaling via PAF.
Increased ECM deposition increases the risk of glomerular fibrosis
and DN in individuals with disorders of glucose and lipid
metabolism. The present findings reveal novel strategies for
managing DN by targeting the PKC-TGF-β1 signaling pathway in
MCs.
Acknowledgements
The authors of the present study would like to thank
the Research Center of Guilin Medical University, and the
laboratory staff for their assistance. The present study was
supported by the National Natural Science Foundation of China
(grant nos. 81260134 and 81560148).
References
|
1
|
Schernthaner G, Mogensen CE and
Schernthaner GH: The effects of GLP-1 analogues, DPP-4 inhibitors
and SGLT2 inhibitors on the renal system. Diab Vasc Dis Res.
11:306–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang MH, Feng L, Zhu MM, Gu JF, Jiang J,
Cheng XD, Ding SM, Wu C and Jia XB: The anti-inflammation effect of
Moutan Cortex on advanced glycation end products-induced rat
mesangial cells dysfunction and High-glucose-fat diet and
streptozotocin-induced diabetic nephropathy rats. J Ethnopharmacol.
151:591–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie S, Lu K, Zhang Y, Song X, Tan M and
Wang C: Effects of Jiangya Xiaoke prescription on TGF-beta1 in
diabetic nephropathy rats with hypertension and its mechanisms. Int
J Clin Exp Med. 8:5129–5136. 2015.PubMed/NCBI
|
|
4
|
Zhou SX, Lei MX and Zhao JJ: The study of
the effects of platelet activating factor (PAF) on the relation
between the endothelial cell and mesangial cells exposed to high
glucose and high lysophosphatidylcholine. Chin J Diabetes.
18:591–593. 2010.(In Chinese).
|
|
5
|
Al-Khodor S and Abu KY: Triggering Ras
signalling by intracellular Francisella tularensis through
recruitment of PKCalpha and betaI to the SOS2/GrB2 complex is
essential for bacterial proliferation in the cytosol. Cell
Microbiol. 12:1604–1621. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noh H and King GL: The role of protein
kinase C activation in diabetic nephropathy. Kidney Int Suppl.
S49–S53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bryant DM, Roignot J, Datta A, Overeem AW,
Kim M, Yu W, Peng X, Eastburn DJ, Ewal AJ, Werb Z and Mostov KE: A
molecular switch for the orientation of epithelial cell
polarization. Dev Cell. 31:171–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meier M, Park JK, Overheu D, Kirsch T,
Lindschau C, Gueler F, Leitges M, Menne J and Haller H: Deletion of
protein kinase C-beta isoform in vivo reduces renal hypertrophy but
not albuminuria in the streptozotocin-induced diabetic mouse model.
Diabetes. 56:346–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Idris I and Donnelly R: Protein kinase C
beta inhibition: A novel therapeutic strategy for diabetic
microangiopathy. Diab Vasc Dis Res. 3:172–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muñoz-Felix JM, Oujo B and Lopez-Novoa JM:
The role of endoglin in kidney fibrosis. Expert Rev Mol Med.
16:e182014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang T, Chen SS, Chen R, Yu DM and Yu P:
Reduced beta 2 glycoprotein I improves diabetic nephropathy via
inhibiting TGF-β1-p38 MAPK pathway. Int J Clin Exp Pathol.
8:2321–2333. 2015.PubMed/NCBI
|
|
12
|
Hathaway CK, Gasim AM, Grant R, Chang AS,
Kim HS, Madden VJ, Bagnell CR Jr, Jennette JC, Smithies O and
Kakoki M: Low TGFβ1 expression prevents and high expression
exacerbates diabetic nephropathy in mice. Proc Natl Acad Sci USA.
112:pp. 5815–5820. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao P, Li L, Ji L, Wei Y, Li H, Shang G,
Zhao Z, Chen Q, Jiang T and Zhang N: Nrf2 ameliorates diabetic
nephropathy progression by transcriptional repression of TGFβ1
through interactions with c-Jun and SP1. Biochim Biophys Acta.
1839:1110–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duan JG, Chen XY, Wang L, Lau A, Wong A,
Thomas GN, Tomlinson B, Liu R, Chan JC, Leung TW, et al: Sex
differences in epidemiology and risk factors of acute coronary
syndrome in Chinese patients with type 2 diabetes: A long-term
prospective cohort study. PLoS One. 10:e1220312015. View Article : Google Scholar
|
|
16
|
Bakris GL, Pitt B, Weir MR, Freeman MW,
Mayo MR, Garza D, Stasiv Y, Zawadzki R, Berman L and Bushinsky DA:
AMETHYST-DN Investigators: Effect of patiromer on serum potassium
level in patients with hyperkalemia and diabetic kidney disease:
The AMETHYST-DN randomized clinical trial. JAMA. 314:151–161. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Panduru NM, Saraheimo M, Forsblom C, Thorn
LM, Gordin D, Wadén J, Tolonen N, Bierhaus A, Humpert PM and Groop
PH; FinnDiane Study Group, : Urinary adiponectin is an independent
predictor of progression to end-stage renal disease in patients
with type 1 diabetes and diabetic nephropathy. Diabetes Care.
38:883–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Nicola L, Provenzano M, Chiodini P,
Borrelli S, Garofalo C, Pacilio M, Liberti ME, Sagliocca A, Conte G
and Minutolo R: Independent role of underlying kidney disease on
renal prognosis of patients with chronic kidney disease under
nephrology care. PLoS One. 10:e1270712015. View Article : Google Scholar
|
|
19
|
Liu X, Yang G, Fan Q and Wang L: Proteomic
profile in glomeruli of type-2 diabetic KKAy mice using
2-dimensional differential gel electrophoresis. Med Sci Monit.
20:2705–2713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abe H: Recent progress in understanding
the molecular pathogenesis of diabetic nephropathy. Rinsho Byori.
59:179–186. 2011.(In Japanese). PubMed/NCBI
|
|
21
|
Rossert J, Terraz-Durasnel C and Brideau
G: Growth factors, cytokines, and renal fibrosis duringthe course
of diabetic nephropathy. Diabetes Metab. 26 Suppl 4:S16–S24.
2000.
|
|
22
|
Zhou L: Research progress in impact of
high glucose and hyperlipidemia on glomerular cells. New Med.
286–289. 2014.(In Chinese).
|
|
23
|
Li L, Yin Q, Tang X, Bai L, Zhang J, Gou
S, Zhu H, Cheng J, Fu P and Liu F: C3a receptor antagonist
ameliorates inflammatory and fibrotic signals in type 2 diabetic
nephropathy by suppressing the activation of TGF-β/smad3 and IKBα
pathway. PLoS One. 9:e1136392014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li HG, Cai YJ and Zou JH: The effects of
high-gucrose and high-fat diet on tubulointerstitial fibrosis in
New Zanland white rabbits. Chinese J Zoology. 145–150. 2010.(In
Chinese).
|
|
25
|
Yoshikawa M, Matsumoto K, Iida M, Akasawa
A, Moriyama H and Saito H: Effect of extracellular matrix proteins
on platelet-activating factor-induced eosinophil chemotaxis. Int
Arch Allergy Immunol. 128 Suppl 1:S3–S11. 2002. View Article : Google Scholar
|
|
26
|
Yao LJ, Wang JQ, Zhao H, Liu JS and Deng
AG: Effect of telmisartan on expression of protein kinase C-alpha
in kidneys of diabetic mice. Acta Pharmacol Sin. 28:829–838. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wogensen L, Krag S, Chai Q and Ledet T:
The use of transgenic animals in the study of diabetic kidney
disease. Horm Metab Res. 37 Suppl 1:S17–S25. 2005. View Article : Google Scholar
|
|
28
|
Mishra S and Vinayak M: Ellagic acid
checks lymphoma promotion via regulation of PKC signaling pathway.
Mol Biol Rep. 40:1417–1428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
do Carmo A, Balça-Silva J, Matias D and
Lopes MC: PKC signaling in glioblastoma. Cancer Biol Ther.
14:287–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Manabe E, Handa O, Naito Y, Mizushima K,
Akagiri S, Adachi S, Takagi T, Kokura S, Maoka T and Yoshikawa T:
Astaxanthin protects mesangial cells from hyperglycemia-induced
oxidative signaling. J Cell Biochem. 103:1925–1937. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ochi S, Harigai M, Mizoguchi F, Iwai H,
Hagiyama H, Oka T and Miyasaka N: Leflunomide-related acute
interstitial pneumonia in two patients with rheumatoid arthritis:
Autopsy findings with a mosaic pattern of acute and organizing
diffuse alveolar damage. Mod Rheumatol. 16:316–320. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schwartz SG, Flynn HW Jr and Aiello LP:
Ruboxistaurin mesilate hydrate for diabetic retinopathy. Drugs
Today (Barc). 45:269–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang F, Huang D, Zhu W, Li S, Yan M, Wei M
and Li J: Selective inhibition of PKCbeta2 preserves cardiac
function after myocardial infarction and is associated with
improved angiogenesis of ischemic myocardium in diabetic rats. Int
J Mol Med. 32:1037–1046. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kelly DJ, Zhang Y, Hepper C, Gow RM,
Jaworski K, Kemp BE, Wilkinson-Berka JL and Gilbert RE: Protein
kinase C beta inhibition attenuates the progression of experimental
diabetic nephropathy in the presence of continued hypertension.
Diabetes. 52:512–518. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koya D, Haneda M, Nakagawa H, Isshiki K,
Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, et al:
Amelioration of accelerated diabetic mesangial expansion by
treatment with a PKC beta inhibitor in diabetic db/db mice, a
rodent model for type 2 diabetes. FASEB J. 14:439–447.
2000.PubMed/NCBI
|
|
36
|
Hinz B: The extracellular matrix and
transforming growth factor-β1: Tale of a strained relationship.
Matrix Biol. 47:54–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudła B: Transforming growth factor β1 (TGFβ1) in physiology
and pathology. Endokrynol Pol. 64:384–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang W, Xu C, Kahng KW, Noble NA, Border
WA and Huang Y: Aldosterone and TGF-beta1 synergistically increase
PAI-1 and decrease matrix degradation in rat renal mesangial and
fibroblast cells. Am J Physiol Renal Physiol. 294:F1287–F1295.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sam R, Wanna L, Gudehithlu KP, Garber SL,
Dunea G, Arruda JA and Singh AK: Glomerular epithelial cells
transform to myofibroblasts: Early but not late removal of
TGF-beta1 reverses transformation. Transl Res. 148:142–148. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alvarez ML and DiStefano JK: Functional
characterization of the plasmacytoma variant translocation 1 gene
(PVT1) in diabetic nephropathy. PLoS One. 6:e186712011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koga K, Yokoi H, Mori K, Kasahara M,
Kuwabara T, Imamaki H, Ishii A, Mori KP, Kato Y, Ohno S, et al:
MicroRNA-26a inhibits TGF-β-induced extracellular matrix protein
expression in podocytes by targeting CTGF and is downregulated in
diabetic nephropathy. Diabetologia. 58:2169–2180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alvarez ML, Khosroheidari M, Eddy E and
Kiefer J: Role of microRNA 1207-5P and its host gene, the long
non-coding RNA Pvt1, as mediators of extracellular matrix
accumulation in the kidney: Implications for diabetic nephropathy.
PLoS One. 8:e774682013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hwang M, Kim HJ, Noh HJ, Chang YC, Chae
YM, Kim KH, Jeon JP, Lee TS, Oh HK, Lee YS and Park KK: TGF-beta1
siRNA suppresses the tubulointerstitial fibrosis in the kidney of
ureteral obstruction. Exp Mol Pathol. 81:48–54. 2006. View Article : Google Scholar : PubMed/NCBI
|





















