Introduction
At present, it has been identified that certain
diseases, which cause blindness, including glaucoma, diabetic and
hypertensive retinopathy, can cause ganglion cells damage or
progressive cell apoptosis (1,2) as
can optic nerve damage resulting from traumatic brain injury, which
accounts for 0.5–5% of traumatic brain injuries (3). However, since retinal ganglion cells
(RGCs) are terminally differentiated cells, unable to self-renew
(4) and thus leading to optic
nerve damage, there remains no clear effective treatment. To
address this, researchers have introduced stem cell transplantation
therapy, by which the patient was expected to recover visual acuity
(5,6).
Adipose derived stem cells (ADSCs), present in the
adipose tissue, are a particularly useful source of mesenchymal
stem cells (7). ADSCs can
differentiate into osteogenic, adipogenic, chondrogenic, myogenic
and a number of other cell lineages (8–12).
Zuk et al (13) first
isolated ADSCs from an adipose tissue cell suspension in 2001.
Kingham et al (14)
cultured ADSCs with glial cell growth factor 2, basic fibroblast
growth factor, platelet-derived growth factor and forskolin. ADSCs
were able to differentiate into Schwann-like cells. They all
expressed GFAP, S100 and p75 cell markers, used to characterize
glial cells. Previous studies have also demonstrated that induced
Schwann cells can express nerve growth factor, generate myelinated
fibers and promote axonal regeneration in the model of peripheral
nerve injury (15–17). Thus, ADSCs transplantation is a
potential means for the treatment of optic nerve crush; they can
differentiate into retinal ganglion cells to replace those
injured.
The present study, by making a rat optic nerve crush
injury model, identified that the number of RGCs decreased in the
optic nerve injury groups. However, the number of RGCs in the stem
cells transplantation group was higher compared with the PBS buffer
group. Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) results also demonstrated that growth-associated protein
(GAP)-43 mRNA expression levels were higher in the stem cell
transplantation group compared with the PBS buffer control group.
Apoptosis tests demonstrated that the stem cell transplantation
group was able to resist the apoptosis of retinal cells. The
present study provided meaningful insights for treatment following
optic nerve injury.
Materials and methods
Experimental animals and
maintenance
A total of 10 healthy male SD rats (age, 8–14 days;
weight, 15–20 g) and 75 adult male healthy SD rats (age, 2.5–3.0
months; weight, 250–350 g) in which the external and ocular fundus
examinations proved normal, were purchased from Suzhou Aiermaite
Technology Co., Ltd. (Suzhou, China). All animals were raised in a
specific-pathogen-free animal house with a room temperature of
22–24°C, 12-h light-dark cycle, and a relative humidity of 50–60%.
Rats had water and food ad libitum. All animal procedures
performed in this study were reviewed and approved by the Animal
Ethics Committee of the Affiliated Yantai Yuhuangding Hospital of
Qingdao University (Yantai, China).
Isolation and culture of rat
adipose-derived stem cells
The 8–14 day postnatal SD rats were sacrificed and
the subcutaneous fat isolated under sterile conditions. The
subcutaneous fat was repeatedly washed with PBS buffer which had
been mixed with 100 µg/ml streptomycin and 100 U/ml penicillin. The
tissue was chopped and digested with 0.1% type I collagenase
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and then
centrifuged (1,200 × g for 10 min at room temperature) to obtain
the primary cells. Primary cells were added to low glucose DMEM
medium containing 2 mmol/l of L-glutamine and supplemented with 10%
FBS (both from Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), and incubated at 37°C in an atmosphere of 5%
CO2. Non-adherent cells were removed through the
exchange of the cell medium during the incubation period. When the
cells spread across the surface of the culture bottle, cells were
passaged with 0.25% trypsin.
Identification of rat adipose-derived
stem cells
The rat adipose-derived stem cells were identified
using nestin as a marker and detected by immunocytochemistry. An
appropriate amount of well-grown cell suspension (cells from a 10
mm culture dish) was centrifuged at 1,200 × g for 5 min at room
temperature, the supernatant discarded and the cells resuspended in
PBS. The cells were placed on polylysine-treated glass slide with a
capillary pipette and incubated for 60 min at room temperature.
Then the PBS was removed using a filter paper. Following a brief
wash in fresh PBS, the cells were immersed in −20°C acetone for 10
min fixation. Cells were ventilated dry for 10 min and examined
microscopically under an inverted microscope. Extraneous tissue was
removed and the cells washed with PBS for 2 min. The cells were
incubated with 0.5% Triton X-100S twice at room temperature, each
for 5 min, then the cells were incubated with 3% hydrogen peroxide
at room temperature for 15 min. Following a brief rinse in hydrogen
peroxide, the cells were washed with PBS for 2 min. The remaining
steps were according to the streptavidin biotin complex assay
method using a SABC kit (SA1022; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) according to the manufacturer's
instructions. Cells were incubated with an anti-nestin primary
antibody (1:500, bs-0008R-HRP; BIOSS, Beijing, China) at room
temperature for 45 min.
Grouping of experimental animals and
transplantation
The 75 adult SD rats were randomly divided into stem
cell transplantation therapy group (group A, n=30), phosphate
buffer control group (group P, n=30) and sham group (group S,
n=15). Groups A and P were used to establish the partial damage
model of optic nerve.
Animal model of optic nerve
injury
Following anesthesia with 10% chloral hydrate
intraperitoneal injection (3 ml/kg body weight), rats were placed
on the operating disk to open the paropia under a binocular
microscope (all animals were operated on the right eye). The
Tenon's capsule was opened, the lateral rectus muscle separated and
cut and dissection performed along the scleral surface to the optic
nerve. The optic nerve posterior 2–3 mm of the eyeball was held for
15 sec using a small aneurysm clip. The third generation of ASCs
cells were digested with trypsin and made into a 2×104
cell/µl suspension in 0.1 M PBS buffer solution. Group A was slowly
injected with 1.5 µl ASCs suspension from the cornea of eyeball to
the vitreous body using a micro glass tube. Group P was injected
with the same quantity of 0.1 M PBS.
Following cell transplantation, the eyeball was
repositioned and the Tenon's capsule and external canthus sutured.
The retinal blood supply was observed under direct ophthalmoscope.
Conventional antibiotic ointment was applied following the
operation.
On day 3, 7, 14, 21 and 28 following treatment, rats
were sacrificed (6 rats at each time point) and the eyes removed.
Group S serving as control was injected with the same amount of 0.1
M PBS and 3 rats were sacrificed at each time point. The
morphological changes of the retina were observed under light
microscope following hematoxylin and eosin (H&E) staining at
room temperature and the ganglion cells were imaged and counted by
microscopic image analysis (Olympus Viewer 3; Olympus Corporation,
Tokyo, Japan).
RT-qPCR and western blotting were used to detect the
changes of GAP-43 mRNA and the expression of apoptosis-related
proteins in the optic nerve at day 3, 7, 14, 21 and 28 following
injury.
Retinal morphology observation and
RGCs counting
The fresh eye was dehydrated in a graded series of
alcohol and treated with xylene. Following embedding in paraffin,
it was sectioned vertical to the retina at 5 µm. Following H&E
staining and neutral resin mounting, retinal morphology was
observed under the light microscope. The layer of the center of the
retina was set as a reference section and two sections anterior and
posterior to the center layer and the center section were selected
for analysis. Then, 5 randomly selected high magnification (×40)
views of each section were used for image analysis, using Olympus
Viewer 3, to count the number of RGCs.
The expression of GAP-43 mRNA in optic
nerve
The GAP-43 mRNA expression changes at day 3, 7, 14,
21 and 28 following optic nerve injury and in normal optic nerve
was detected by RT-qPCR. Total RNA was extracted using an RNeasy
kit (Shanghai Sangon Biotech Co., Ltd., Shanghai, China). Total RNA
concentration was determined by measuring the absorbance at 260 nm.
The RT reaction was carried out using a First-Strand cDNA Synthesis
kit (Toyobo Life Science, Osaka, Japan). The primers were purchased
from Shanghai Sangon Biotech, Co., Ltd. The primer sequences were
as follows: GAP-43 forward, 5-GCT TCC GTG GAC ACA TAA CAA GGA-3 and
reverse 5-CTT AAA GTT CAG GCA TGT TCT TGG T-3′; GAPDH forward,
5-GGC AAG TTC AAC GGC ACA GT-3 and reverse, 5-CGC CAG TAG ACT CCA
CGA CA-3′. The qPCR reaction was performed using SYBR Premix ExTaq
(Takara Biotechnology Co., Ltd., Dalian, China). The qPCR cycling
conditions were 94°C for 30 sec, 62°C for 15 sec and 72°C for 15
sec, for 35 cycles. Quantification was performed using the
2−ΔΔCq method (18).
Triplicate experiments were performed with triplicate samples.
Western blot analysis
Western blotting was performed to determine the
expression of apoptosis related protein B-cell lymphoma 2 (Bcl-2)
and Bcl-2-associated X protein (Bax). Rats at day 3, 7, 14, 21 and
28 following optic nerve injury and normal optic nerve were
selected for protein analysis. After the rats were sacrificed at
each time point, the right eye and optic nerve junction were
removed immediately and placed in 0.9% physiological saline. The
eyeball was opened along the pars coronal plane of the ciliary
body, and the anterior segment of the eye discarded. Following the
separation of the retina and the severing of the optic nerve, the
eye was dried with filter paper, put into a frozen storage tube and
placed in liquid nitrogen immediately. The eyes were kept in liquid
nitrogen for 24 h and then stored in −80°C for future study. The
expression levels of apoptosis related protein Bcl-2 and Bax
proteins in the optic nerve of rats were detected by western
blotting.
Radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Wuhan, China) was used to
perform protein extraction at 4°C for 30 min. The protein
concentration was detected using the bicinchoninic acid method and
the lysates (30 µg protein/lane) were separated by 12% SDS-PAGE.
The proteins were electrotransferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA) and expression levels
were detected using dilutions of the primary antibodies, as
follows: Rabbit anti-Bcl-2 (1:1,000; #2872), rabbit anti-Bax
(1:1,000; #2772) (both from Cell Signaling Technology, Inc.,
Danvers, MA, USA) and mouse anti-β-actin (1:5,000; ab6276; Abcam,
Cambridge, USA). The membranes were washed in 0.05% Tween-20/TBS
and then incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody (1:5,000; goat
anti-rabbit, ZB-2301; goat anti-mouse, ZDR5307; OriGene
Technologies, Inc., Beijing, China). Bound antibodies were
visualized using an enhanced chemiluminescence reagent (EMD
Millipore) and quantified by densitometry using ChemiDoc XRS+ image
analyzer (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Densitometric analyses of bands were adjusted with β-actin as a
loading control. Triplicate experiments with triplicate samples
were performed.
Statistical analysis
SPSS version 19.0 (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. Data were expressed as mean ±
standard deviation. The comparison between groups was performed by
one way analysis of variance, followed by Dunnett's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ASCs purification and
identification
Following digestion with collagenase, a uniform
milky cell suspension was obtained from adipose tissue. The
adherent growth was observed after 24 h, the cells were small,
round and of differing sizes. Then, 2–4 days later, the cells
became elongated. The majority of the cells were spindle shaped
with an oval nucleus. The proliferation peak in colony growth was
reached at day 3. After 7–10 days culture, colonies overlapped each
other and up to 70–80% fusion was observed. The adherent growth and
proliferation time was clearly shortened in subcultured cells. The
cells were spindle shaped, with few protrusions and almost the same
size. They showed fibroblast-like growth and were arranged in
bundles or spirals. The cells were able to maintain a strong
capability for proliferation and uniform fibroblast-like morphology
following several subcultures. The results of the nestin
immunocytochemistry test were positive (data not shown).
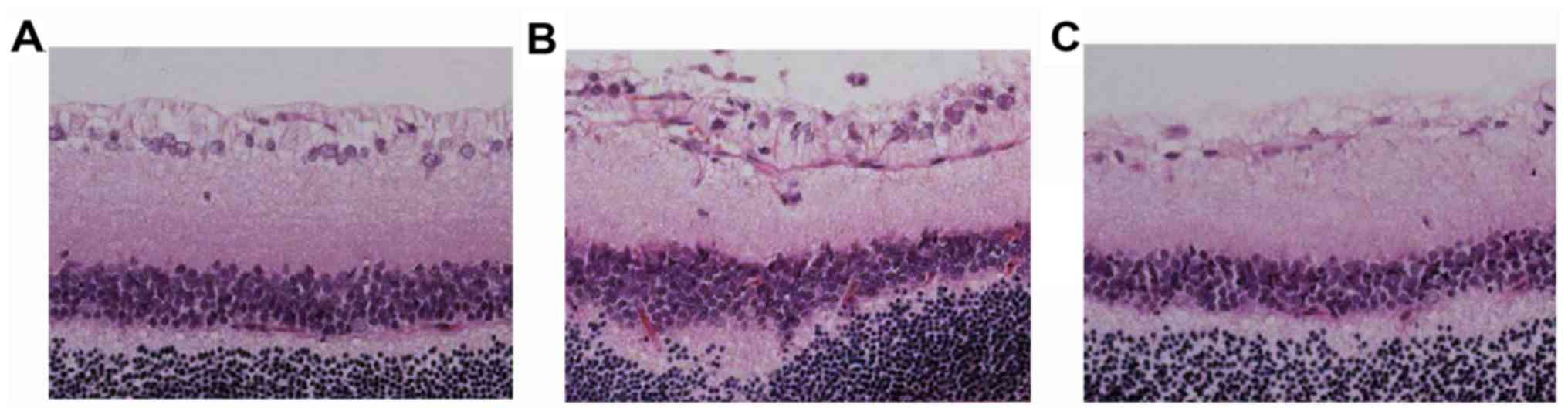
Morphological changes in the
retina
Clear boundaries between the layers of the retina
were observed in the normal control group. Retina divided from its
inner to outer layers by a ganglion cell layer, inner nuclear layer
and outer nuclear layer. The layers are in a compact parallel
arrangement. RGCs are a monolayer of tightly packed round or
oval-shaped cells (Fig. 1).
In groups A and P, the inner and outer nuclear
layers were thinned and disordered during the 5 time-points; the
number of RGCs gradually decreased and the arrangement gradually
became disordered, the cells swelling, increasing multi-core
shrinkage and the occurrence of abnormal nuclei. The degree of
injury was relatively low in group A.
Compared with the normal control group S, groups P
and A had no significant changes at day 3 following optic nerve
injury. However, the evident changes were observed in the retinal
structure for group P at day 7 following optic nerve injury. The
retinal layers were disordered, the inner and outer nucleus layer
became thin, the number of cells was reduced, the nuclei in RGC
layer were dispersed and the size of the nuclei partly reduced,
which deepened the staining and demonstrated chromatin condensation
in the nuclei. The situation deteriorated further at day 14. The
inner and outer nucleus layers in group P were clearly thinner, the
cell number in RGC layer was decreased and more condensed nuclei
were observed. Otherwise, large and shallow stained ganglion cells
were reduced and chromatin significantly concentrated in group
A.
It was also noted that the inner and outer nucleus
layer in group P experienced further thinning, a further reduction
in the cell number and increased disorder in cell arrangement at
day 21 and 28 following injury. The RGC layer was clearly sparse
and the cell number was reduced. The damage in group A was
relatively low.
Number of RGCs
The number of RGCs in groups P and A was reduced
compared with the normal control group at day 3 following optic
nerve injury. The number of cells in the two groups began to reduce
significantly after day 7 (P<0.05). At day 14, the number of
injured RGCs cells accounted for ~70–81% of the total reduced cell
number. After day 14, the cell number continued to decrease;
however, the rate of decrease slowed.
Compared with group A, the number of RGCs were lower
in group P at all the detected time points except at the day 3
where no significant difference was observed between group A and
group P. The RGC number in group P was significantly lower compared
with group A at day 7 and 14 (P<0.01). By day 21 and 28, the
difference between the two groups was reduced. The changes at each
time point are presented in Table
I.
 | Table I.Changes of retinal ganglion cells at
various time points after optic nerve injury. |
Table I.
Changes of retinal ganglion cells at
various time points after optic nerve injury.
| Duration of injury,
days | Group S | Group P | Group A |
|---|
| 3 |
246.1±15.6 |
232.6±12.6 |
235.5±11.9 |
| 7 |
247.7±13.2 |
193.9±9.5a |
214.9±12.8a,c |
| 14 |
241.9±11.7 |
140.3±10.7a |
161.6±9.3a,c |
| 21 |
249.4±12.3 |
135.4±8.2a |
149.2±7.7a,c |
| 28 |
246.5±10.9 |
119.2±9.1a |
131.3±8.6a,b |
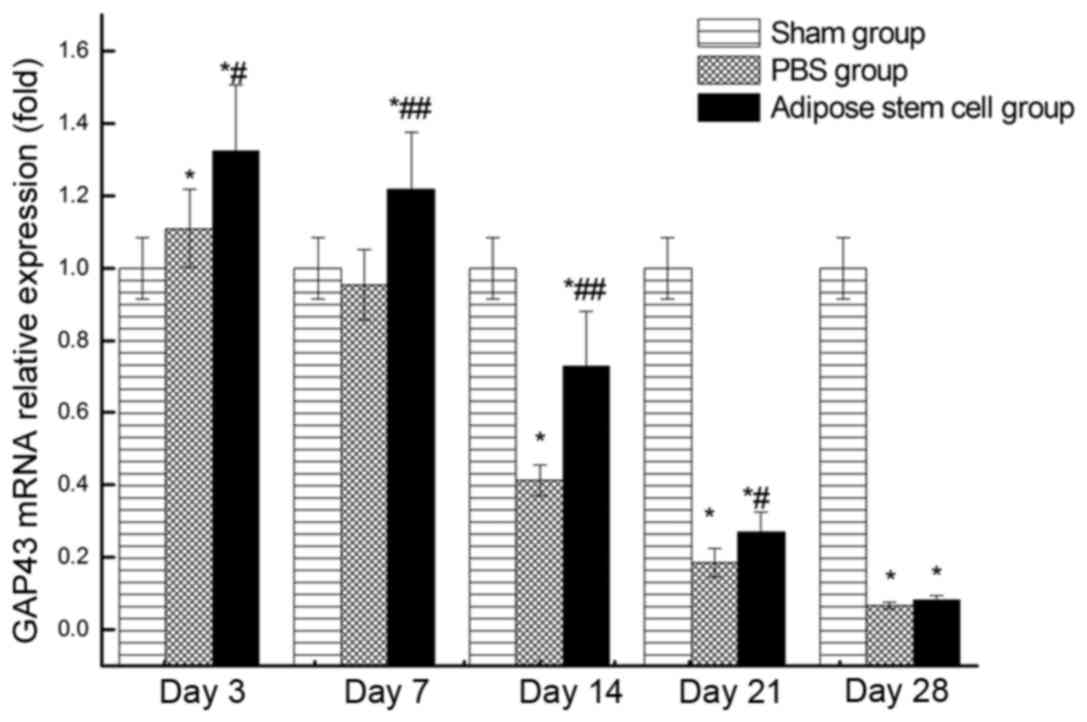
RT-qPCR
Relative expression level of GAP43 mRNA at each time
point following injury was demonstrated in Fig. 2. The highest expression in groups A
and P occurred at day 3. Although the expression of GAP43 mRNA in
groups A and P was slightly higher than the normal group at day 3,
the expression of these two groups decreased sharply later. The
expression of GAP43 mRNA in group P was lower than the normal
control group at day 14. Taken together, the expression of GAP43
mRNA in group A was significantly higher (P>0.05) than group P
at all the detected time points except day 28.
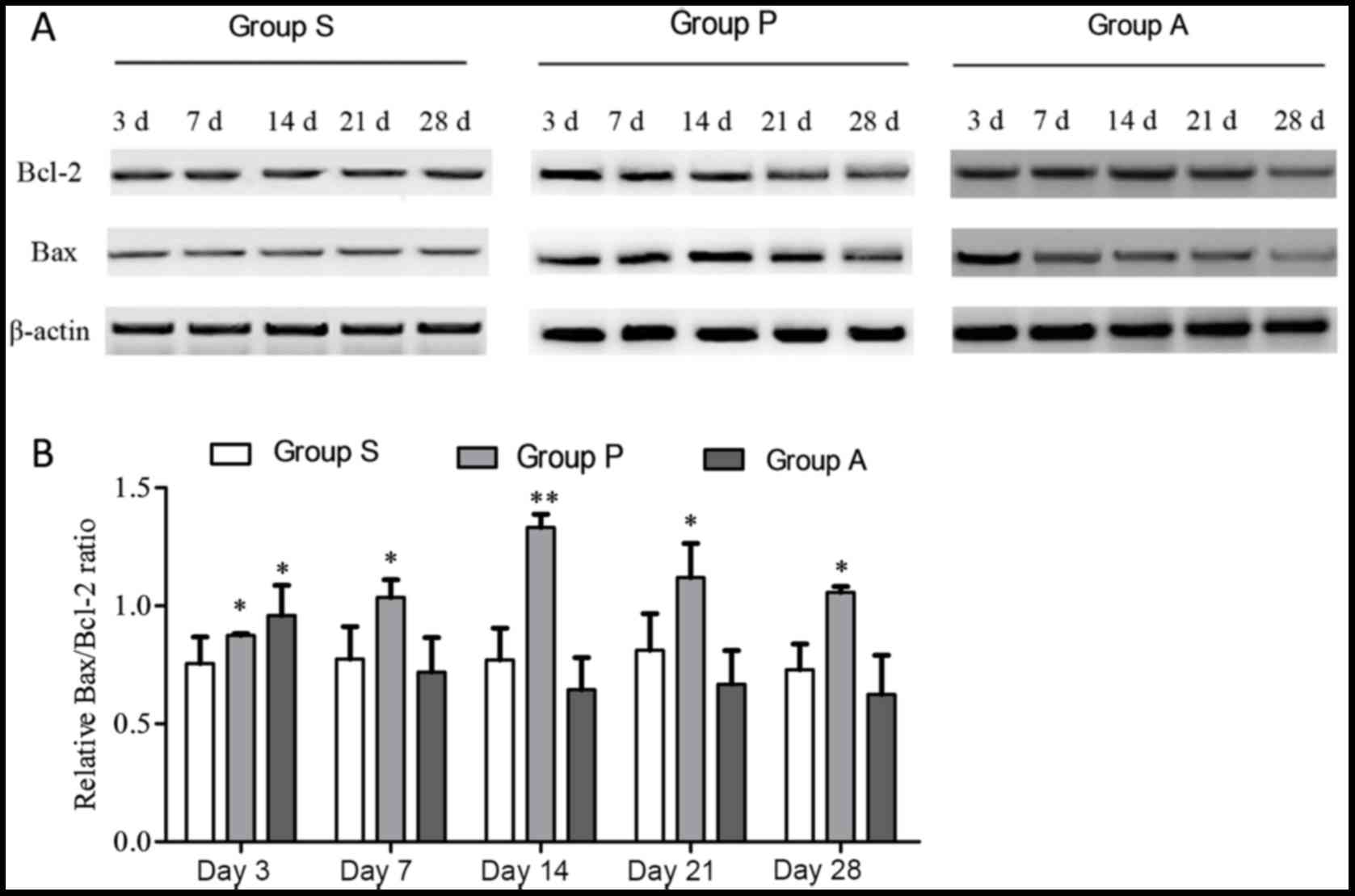
Detection of apoptosis-associated
proteins
When cells enter apoptosis, the expression level of
the anti-apoptosis protein Bcl-2 decreases and the expression level
of the other apoptosis-associated protein Bax, increases.
Therefore, western blotting was employed to detect the expression
of the two proteins in various time points following optic nerve
injury (Fig. 3). Analysis of the
Bax/Bcl2 ratio revealed that there were no statistical differences
across different time points in group S. At day 3, groups P and A
exhibited a higher Bax/Bcl2 ratio compared with group S
(P<0.05). The Bax/Bcl2 ratios in group P were higher than group
S at all time points, revealing an increasing trend from day 3 to
day 14 followed by a decrease to day 28 (Fig. 3B). However, although at day 3 the
Bax/Bcl2 ratio in group A was higher than group S, data analysis
showed that from day 7 to day 28, the Bax/Bcl-2 ratio in group A
decreased and had no statistical difference when compared with
Group S, indicating that the transplantation of adipose-derived
stem cells may resist the apoptosis of retinal cells.
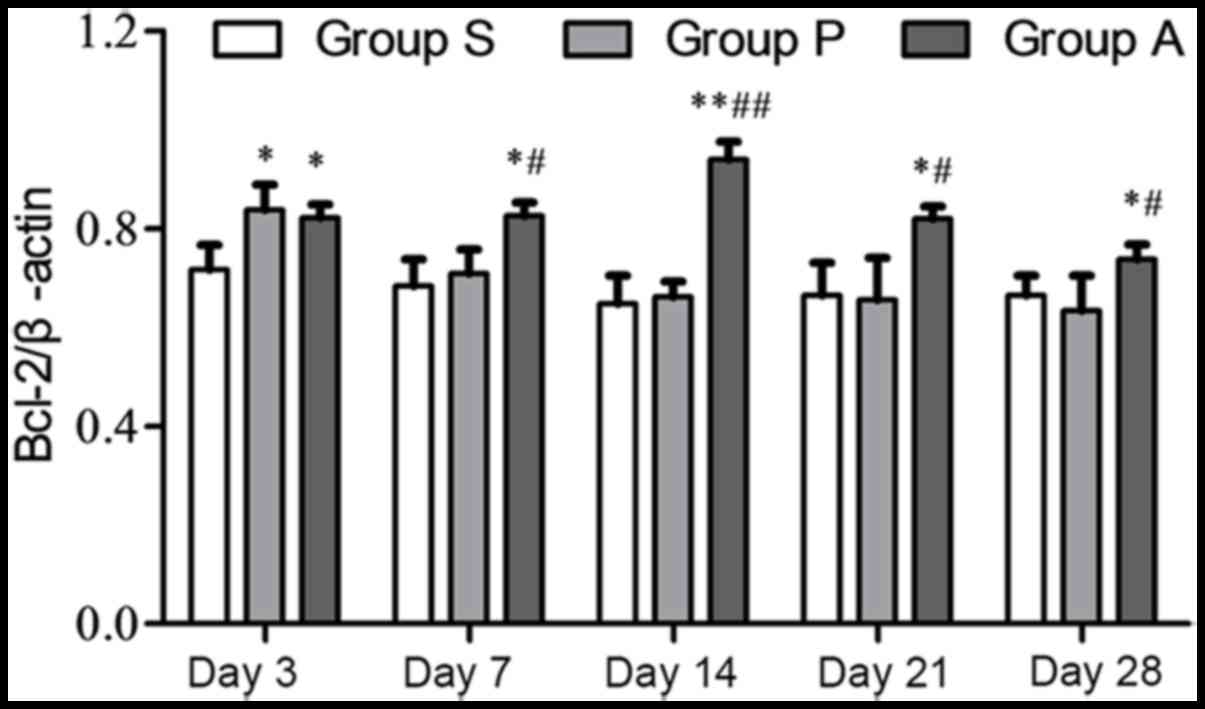
The Bax and Bcl-2 expression levels were also
analyzed independently from one another. The highest protein
expression of Bcl-2 in group P was at day 3 following injury. The
expression of Bcl-2 in group P was reduced from day 7 to 28
following injury. The expression of Bcl-2 in group A was increased
from day 3 to 14 following injury; however, it decreased at day 21
and 28 (Fig. 4). The expression of
Bcl-2 was significantly higher in group A compared with group P
from day 7 onwards following injury. The expression of the
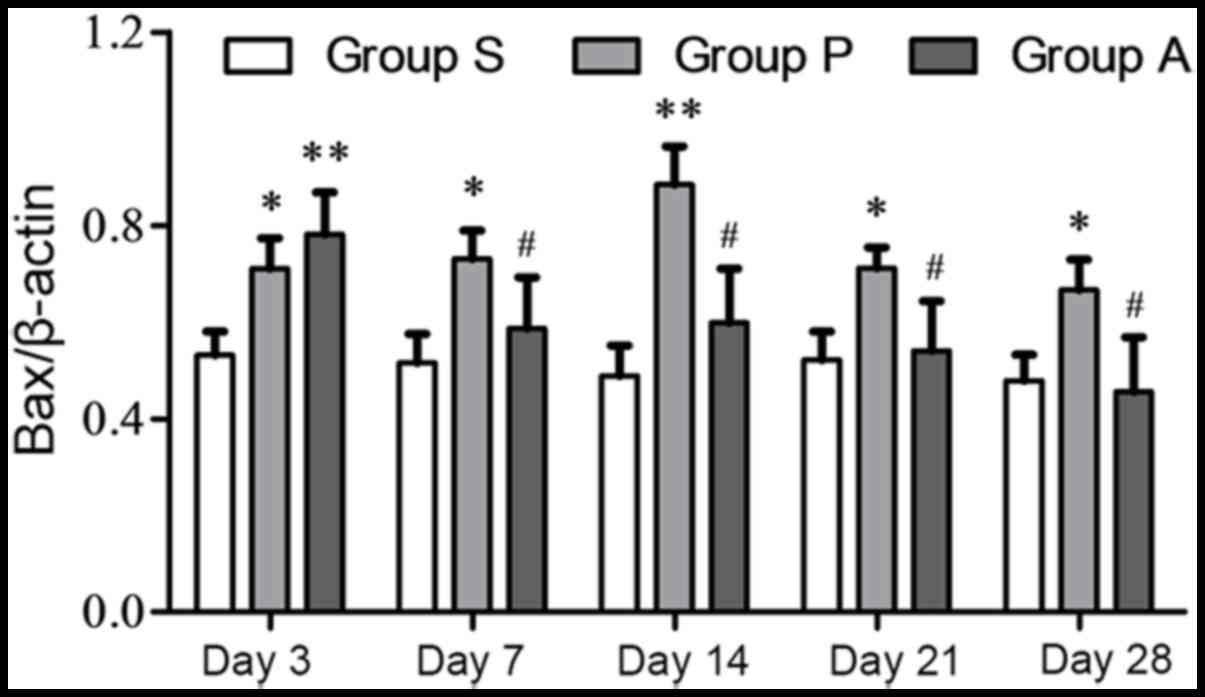
apoptosis protein Bax in group P was increased from day 3 to 14
following injury and it was decreased at day 21 and 28; however, in
group A Bax steadily decreased over the duration of the experiment
(Fig. 5). Furthermore, from day 7
to day 28, Bax expression in group A was significantly lower than
group P (P<0.05).
Discussion
ADSCs are multipotent stem cells with capacity to
differentiate and the faculty to secrete a variety of bioactive
molecules with trophic, paracrine, anti-inflammatory and
immunomodulatory functions (5).
They are easy to harvest from adipose tissue (19). The low immunogenicity and
immunoregulatory potential for ADSCs allow their allogeneic use,
which makes them an alternative and promising treatment for severe
refractory autoimmune diseases including ophthalmological disorders
(11,20). In a previous study,
Arnalich-Montiel et al (21) injected a cell suspension of human
ASCs into a corneal stroma defect in a rabbit model. It was
determined that human ASCs survived in the rabbit stroma for at
least 12 weeks with a restoration of the stromal structure,
indicating that ASCs possess the potential to differentiate into
keratocytes (21). ADSCs obtained
from the present study have demonstrated consistency in their
isolation, high proliferation capacity, plastic adherence and
behavior in vitro, exhibiting the same immunohistochemistry
staining properties as those described for this species.
A previous study suggested that when transplanted
into the vitreous body of adult rats, dental pulp stem cells can
significantly promote RGC survival and axon regeneration (22). The findings of the current study
are consistent with those results. The present study demonstrated
the positive effects of transplanted adipose-derived stem cells on
RGC survival. The enhanced RGC survival may be attributed to
inhibition of apoptotic processed caused by optic nerve crush. The
higher level of expression of Bcl-2 in ADSCs transplantation group
protects RGCs from apoptosis. Axon regeneration was evaluated by
quantifying the expression level of GAP43 at the lesion site. The
increase in GAP43 expression suggested active axon regeneration in
the lesion site after ADSCs were transplanted.
In conclusion, the findings of the present study
indicated that ADSCs implantation is a safe, effective and
relatively simple therapy for optic nerve crush in rats. The
present study provided novel evidence that ADSCs are
neuroprotective for RGCs and supported the therapeutic potential of
ADSCs in the recovery of neural function. However, additional
studies are necessary to identify the negative factors that may be
released, including ADSCs injected as a suspension giving rise to
certain risks of migration into endogenous tissue and uncontrolled
proliferation.
References
|
1
|
Johnson TV and Martin KR: Cell
transplantation approaches to retinal ganglion cell neuroprotection
in glaucoma. Curr Opin Pharmacol. 13:78–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyer-Rüsenberg B, Pavlidis M, Stupp T and
Thanos S: Pathological changes in human retinal ganglion cells
associated with diabetic and hypertensive retinopathy. Graefes Arch
Clin Exp Ophthalmol. 245:1009–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guy WM, Soparkar CN, Alford EL, Patrinely
JR, Sami MS and Parke RB: Traumatic optic neuropathy and second
optic nerve injuries. JAMA Ophthalmol. 132:567–571. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berry M, Ahmed Z, Lorber B, Douglas M and
Logan A: Regeneration of axons in the visual system. Restor Neurol
Neurosci. 26:147–174. 2008.PubMed/NCBI
|
|
5
|
Levkovitch-Verbin H, Sadan O, Vander S,
Rosner M, Barhum Y, Melamed E, Offen D and Melamed S: Intravitreal
injections of neurotrophic factors secreting mesenchymal stem cells
are neuroprotective in rat eyes following optic nerve transection.
Invest Ophthalmol Vis Sci. 51:6394–6400. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson TV, Bull ND, Hunt DP, Marina N,
Tomarev SI and Martin KR: Neuroprotective effects of intravitreal
mesenchymal stem cell transplantation in experimental glaucoma.
Invest Ophthalmol Vis Sci. 51:2051–2059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mead B, Berry M, Logan A, Scott RA,
Leadbeater W and Scheven BA: Stem cell treatment of degenerative
eye disease. Stem Cell Res. 14:243–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim S, Cho H, Lee E, Won Y, Kim C, Ahn W,
Lee E and Son Y: Osteogenic stimulation of human adipose-derived
stem cells by pre-treatment with fibroblast growth factor 2. Cell
Tissue Res. 364:137–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gwak SJ, Bhang SH, Yang HS, Kim SS, Lee
DH, Lee SH and Kim BS: In vitro cardiomyogenic differentiation of
adipose-derived stromal cells using transforming growth
factor-beta1. Cell Biochem Funct. 27:148–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohammadi-Sangcheshmeh A, Shafiee A,
Seyedjafari E, Dinarvand P, Toghdory A, Bagherizadeh I, Schellander
K, Cinar MU and Soleimani M: Isolation, characterization, and
mesodermic differentiation of stem cells from adipose tissue of
camel. In Vitro Cell Dev Biol Anim. 49:147–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murphy MB, Moncivais K and Caplan AI:
Mesenchymal stem cells: Environmentally responsive therapeutics for
regenerative medicine. Exp Mol Med. 45:e542013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang JW, Kang KS, Koo HC, Park JR, Choi EW
and Park YH: Soluble factors-mediated immunomodulatory effects of
canine adipose tissue-derived mesenchymal stem cells. Stem Cells
Dev. 17:681–693. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kingham PJ, Kalbermatten DF, Mahay D,
Armstronga SJ, Wibergb M and Terenghia G: Adipose-derived stem
cells differentiate into a Schwann cell phenotype and promote
neurite outgrowth in vitro. Exp Neurol. 207:267–274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Y, Liu L, Li Y, Zhou C, Xiong F, Liu Z,
Gu R, Hou X and Zhang C: Myelin-forming ability of Schwann
cell-like cells induced from rat adipose-derived stem cells in
vitro. Brain Res. 1239:49–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chi GF, Kim MR, Kim DW, Jiang MH and Son
Y: Schwann cells differentiated from spheroid-forming cells of rat
subcutaneous fat tissue myelinate axons in the spinal cord injury.
Exp Neurol. 222:304–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
di Summa PG, Kalbermatten DF, Pralong E,
Raffoula W, Kinghamb PJ and Terenghi G: Long-term in vivo
regeneration of peripheral nerves through bioengineered nerve
grafts. Neuroscience. 181:278–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Ugarte DA, Morizono K, Elbarbary A,
Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim
P, et al: Comparison of multi-lineage cells from human adipose
tissue and bone marrow. Cells Tissues Organs. 174:101–109. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
LetoBarone AA, Khalifian S, Lee WP and
Brandacher G: Immunomodulatory effects of adipose-derived stem
cells: Fact or fiction? Biomed Res Int 2013. 3836852013.
|
|
21
|
Arnalich-Montiel F, Pastor S,
Blazquez-Martinez A, Fernandez-Delgado J, Nistal M, Alio JL and De
Miguel MP: Adipose-derived stem cells are a source for cell therapy
of the corneal stroma. Stem Cells. 26:570–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mead B, Logan A, Berry M, Leadbeater W and
Scheven BA: Intravitreally transplanted dental pulp stem cells
promote neuroprotection and axon regeneration of retinal ganglion
cells after optic nerve injury. Invest Ophthalmol Vis Sci 2013.
54:7544–7556. 2013.
|