Introduction
Since total joint arthroplasty was applied in the
clinics, it had improved the life qualities for patients who
suffered osteoarthritis and rheumatoid arthritis all through the
world (1). However, with the time
passed by, patients who had received total joint arthroplasty were
increasing, and its complications were now drawing more and more
attentions (2). Aseptic loosening
of the implanted artificial joint was stated to be the most common
complication of total joint arthroplasty, and it was also reported
to be the leading cause of failures for those received total joint
arthroplasty (3). Recently, great
advancements had been made toward the pathogenesis of aseptic
loosening; but few had been transformed to serve the patients in
the clinics (4). Hence, it is of
great urgency to identify the cellular and molecular mechanisms
whereby aseptic loosening occurs so as to reveal novel targets
against the complication.
In the clinical settings, titanium and its alloys
were the preferred material for medical implants dues to the
properties of high strength and ductility, good corrosion
resistance, biological compatibility and long fatigue life
(5). As with other stainless steel
and cobalt-chromium alloy implants, titanium-alloy implants also
produce high levels of particles (6), which function extensively in aseptic
loosening of the implants (4,7,8).
These particles, on the one hand, shatter around the artificial
joints, and been swallowed by the nearby cells, such as
fibroblasts, osteoblasts, macrophages and foreign body giant cells
leading to their over-activation and cytokines secretion. These
factors then function alone and/or in combination to promote
aseptic osteolysis as well as aseptic loosening, and finally the
failure of total joint arthroplasty replacement (9–11).
On the other hand, these particles also interact with cells in the
bone marrow once they permeated through the interfacial pathway
between the prosthesis and marrow cavity (12,13),
which was also said to affect aseptic loosening of the artificial
joints.
Bone marrow mesenchymal stem cell (BMSC) is one of
the most common adult stem cells within the bone marrow and they
were characterized by the capacities of self-renewal and
differentiation (14). Previously,
line of reports indicated that BMSC function to facilitate the
stabilization of the implanted artificial joints. For instance,
Neale SD and colleagues demonstrated that BMSC could enhance the
osseointegration of the peri-prosthetic bone after total hip
arthroplasty (15). Besides, some
other groups reported that BMSC are incline to differentiate into
osteogenic cells (16), which were
capable of stabilizing of the artificial joints. Taken together, we
boldly concluded that deregulated BMSC was a risk factor for
aseptic loosening of the artificial joint. However, there were
still much to know whether titanium and its alloys derived
particles facilitate aseptic loosening of the artificial joint via
affecting the proliferation and apoptosis of BMSC.
In the present study, the pathomechanisms whereby
the titanium-alloys particles contribute to aseptic loosing of
artificial joints were investigated. We found that titanium-alloy
implants derived particles significantly inhibit BMSC
proliferation, and that the particles significantly increased the
apoptosis of BMSC. Since BMSC plays a critical role in the
stabilization of artificial joints, our study might provide a novel
direction for the management of aseptic loosening in the
clinics.
Materials and methods
Particle preparation
Briefly, 20 mg Ti-alloy particles (Polysciences,
Inc., Warrington, PA, USA) were weighed by electronic microbalance
and soaked by 70% alcohol for 48 h at room temperature. The
particles were rinsed by phosphate buffer saline (PBS; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 3 times and
sterilized with epoxyethane gas. Then, they were autoclaved and
suspended in Dulbecco's PBS (D-PBS; Invitrogen; Thermo Fisher
Scientific, Inc.) so as to make stock solution. The final
concentration 0.01, 0.05 and 0.1 mg/ml of the particles could be
obtained when the stock solution was diluted with 20, 4 and 2 times
respectively. All the steps were carried out in thoroughly clean,
heat-sterilized glassware. The characterization of the particles
was conducted under the microscope after their preparation. The
ethic committees of the First Affiliated Hospital Of Nanjing
Medical University approved our study. We also obtained approval
from the animal ethics committees of our hospital.
BMSCs collection and primary
culture
Femur and tibia of Japanese rabbits weighed 2 kg
were used for the isolation and induction of BMSCs. The femurs and
tibias were isolated under sterile condition, a needle was inserted
into the bone, and the primary cells were aspirated, followed by
flushing the bone using a 1 ml syringe filled with culture medium.
The cells were immersed in low glucose Dulbecco's modified Eagle's
medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 2 mmol/l-glutamine (Gibco; Thermo Fisher
Scientific, Inc.) and 100-ug/ml-penicillin streptomycin (Tianjin
Pharmaceutical Group Co., Ltd., Tianjin, China). The cell
suspension was subjected to separation using Fercoll solution as
described previously. The cotton-like cells at the interface were
collected and rinsed in L-DMEM containing 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). Cells were
resuspended in complete medium (90% L-DMEM, 10% FBS, 100 µ/ml
penicillin and streptomycin) and transferred to plastic culture
flask for incubation at 37°C in a humidified atmosphere of 95% air
and 5% CO2. Cells from the third generations were used for
experimental purposes and were detached using trypsin for the
subsequent experiments.
Flow cytometry (FCM) assay
FCM assay was used to confirm the phenotype of the
induced BMSC from the third generation. The protocol had been
described previously (17). The
antibodies used in the assay were listed: Anti-rabbit
CD34/CD90/CD105 (1:100; BD Biosciences, Franklin Lakes, NJ,
USA).
Cell Counting Kit-8 (CCK8) assay
BMSC proliferation was determined using a CCK8
(Dojindo, Kumamoto, Japan) as described (18). BMSC were seeded in 96-well plates,
serum starved and treated with the particles. At the end of the
incubation, CCK8 solution was added for 4 h and the absorbance at
450 nm was measured with a micro plate reader (Sunrise; Tecan Group
Ltd., Männedorf, Switzerland).
FCM assays
BMSCs cells were cultured in 6 well plates (Costar,
Cambridge, MA, USA) at a density of 5×105/per well.
After that, they were exposed to isoquercitrin for 24 h followed by
200 mol/l H2O2 for 4 h. Next, the cells were
stained with Hoechst 33342 solution at 37°C for 10 mins in dark,
followed by staining with PI at 25°C for 15 mins in dark. The
stained cells were observed under a fluorescence microscope (BD
Biosciences). Cells were counted using the Image pro-plus (version
6.0.0; Media Cybernetics, Rockville, MD, USA).
RNA isolation and RT-PCR assay
Total RNA was isolated from the cultured cells using
RNA extraction kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's instructions. Reverse transcription and RT-qPCR kits
(Takara Bio, Inc.) were used to evaluate expression of Bax and
Caspase-3. GAPDH was used to normalize for variance. The PCR
Primers pairs used for each genes were: GAPDH, forward,
5′-CCCCGCTACTCCTCCTCCTAAG-3′, reverse,
5′-TCCACGACCAGTTGTCCATTCC-3′; Bax, forward
5′-CCGGGCGAACAGGGTACGAC-3′, reverse 5′-CGCGTGAAAGGGCGTCAGGT-3′;
Caspase-3, forward 5′-ACGAAACCTCCGTGGACGCAA-3′, reverse
5′-CTCTGTGCCTCGGCAAGCCT-3′. Relative mRNA expression of each gene
was calculated with the comparative threshold cycle (Cq)
(2−ΔΔCq) method.
Western blot analysis
In Brief, cells were washed three times with cold
PBS and lysed on ice in RIPA buffer with the protease inhibitor
PMSF (Beyotime Institute of Biotechnology, Haimen, China). Protein
concentrations were determined by BCA assay (Beyotime Institute of
Biotechnology). A total of 20 µg protein was separated by 10%
SDS-PAGE and electro-blotted onto NC membranes using a semi-dry
blotting apparatus. After blocking in 3% bovine serum albumin
(BSA), the membranes were incubated with primary antibodies
overnight at 4°C. The membranes were washed and incubated with
secondary antibodies for 1 h at 25°C on a shaker. The protein bands
were visualized using a commercially available enhanced
chemiluminesence kit (Thermo Fisher Scientific, Inc.). GAPDH and
β-Actin were used as control. The primary antibodies used in this
study include Caspase-2, Bax, β-Actin and GAPDH (Cell Signaling
Technology, Inc., Danvers, MA, USA). The quantitation of the blots
using image J as described previously (19).
Statistical analysis
Data are presented as mean ± standard deviation.
Values and percentages between groups were compared using ANOVAs
followed by appropriate post hoc tests. All statistical analysis
was performed with SPSS statistical software (version 21.0; SPSS,
Inc., Chicago, IL, USA). All statistics were two-sided, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
The preparation of titanium alloy
particles and BMSC
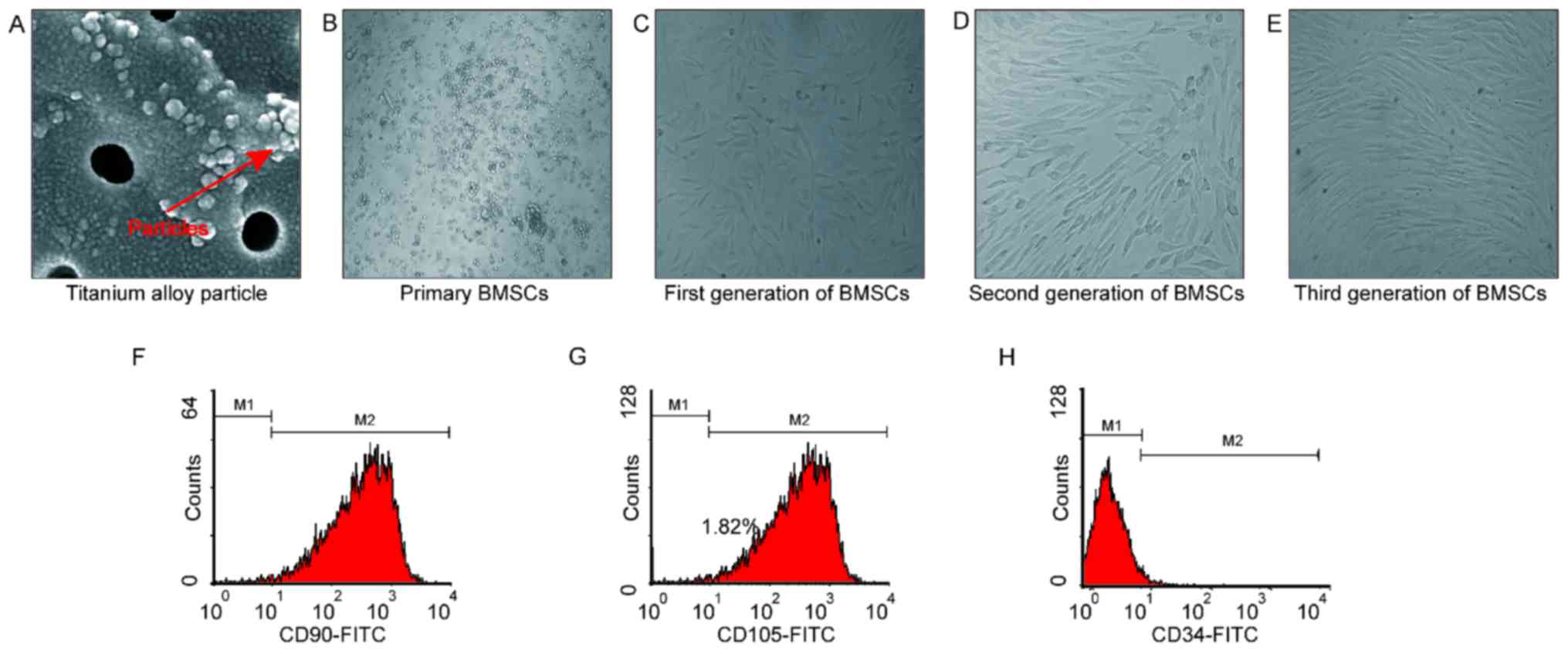
To begin with, we prepared the Titanium alloy
particles as instructed. As shown in Fig. 1, 90% of the particles were ball in
shape with a diameter of less than 100 nm (Fig. 1A). Meanwhile, BMSC were also
prepared accordingly. We found that the primary BMSC (Fig. 1B) adhered within 36 h. After grown
for 12 days, they were subjected to passage. Generally, cells from
the first generation adhered within 24 h, and they were ribbed in
shape (Fig. 1C). Cells from the
second and third generation were uniformly and orderly arranged
(Fig. 1D and E). Cells from the
third generation were subjected to FCM inspections so as to examine
their phenotypes. We found that the CD90, CD105 and CD34 positive
cell rate were 99.5, 99.9 and 2.0% (Fig. 1F-H), which suggested that these
cells were suitable for the subsequent experiments.
Morphology change of BMSC when
cultured with titanium-alloy particles
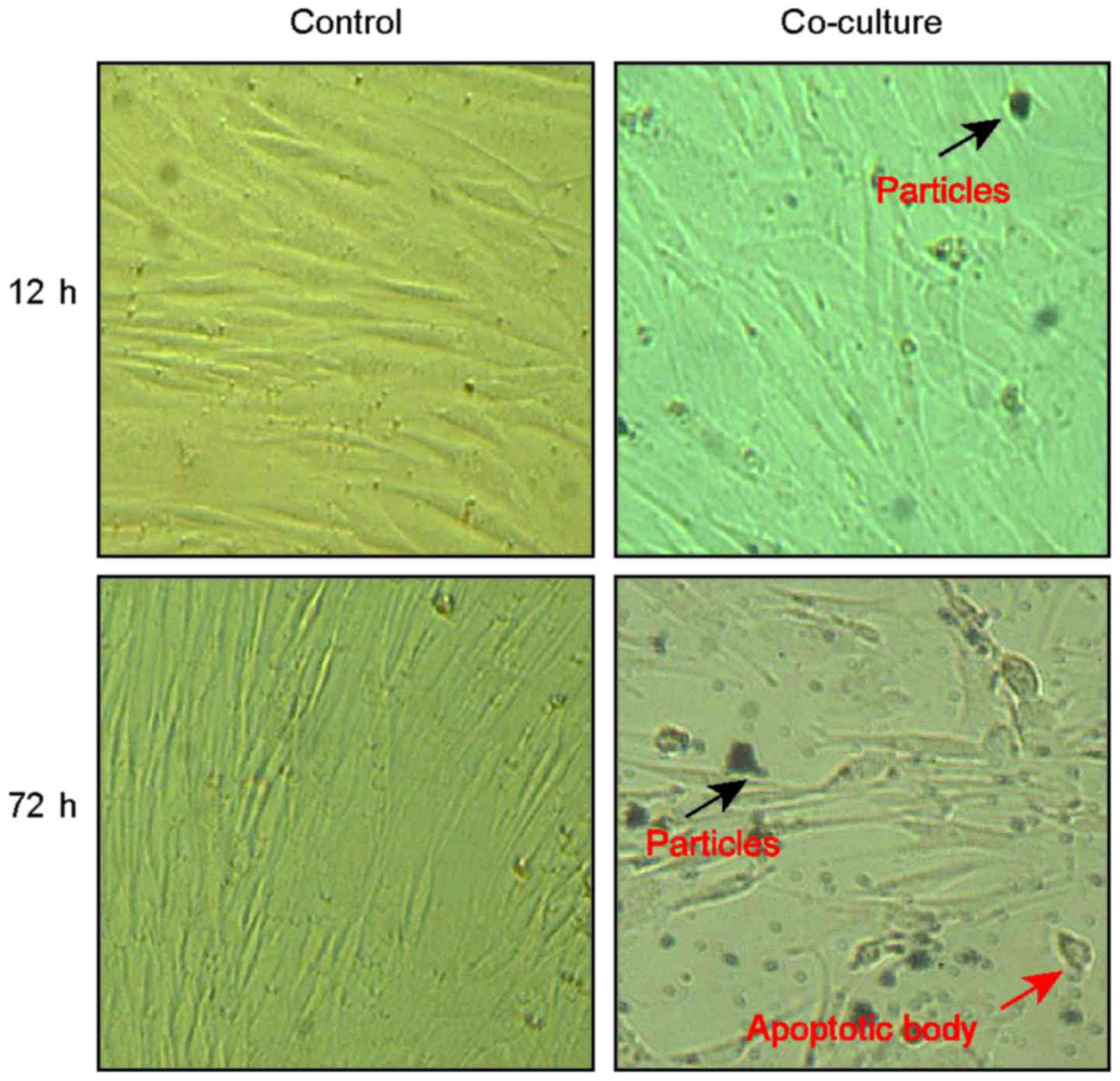
We then investigated the effects of Nano-sized
titanium-alloy particles on BMSC. Initially, the morphology changes
of BMSC were observed. As we see, the cells exhibited no obvious
change within 6 h compared to the normal control (Fig. 2). However, with time passed by, the
treated cells become smaller in shape with the cytoplasm and
unclear concentrated at 72 h (Fig.
2). In addition, we also observed shattered apoptosis body
within the cells when they were cultured with the particles for 72
h (Fig. 2).
Cell proliferation of BMSC when
co-cultured with titanium-alloy particles
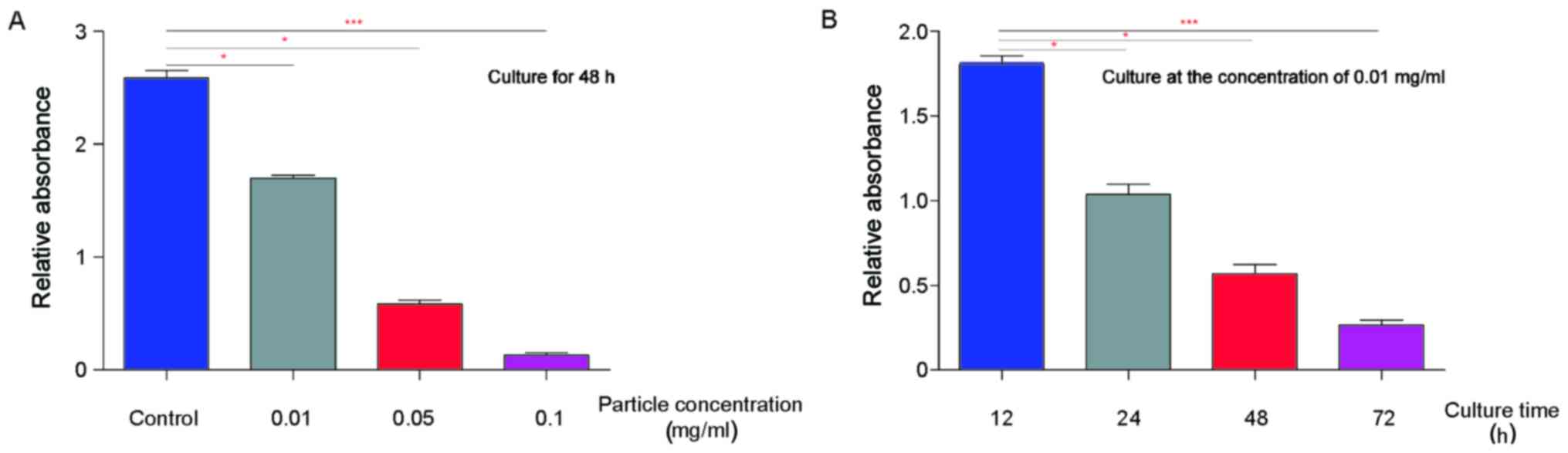
Then, we investigated the proliferation of BMSC when
they were cultured with the particles. As shown in Fig. 3A, we observed that the
proliferation of BMSC decreased significantly (P=0.035) when they
were cultured with low concentration particles (0.01 mg/ml). Of
note, with the concentration grows, BMSC proliferation decreased.
Statistically, our data showed that the particles inhibit the
proliferation of BMSC in a manner dependent on the concentration
(Fig. 3A). Meanwhile, we also
examined whether co-culture time affects the proliferation of
BMSCs. We found that the particles inhibit BMSCs proliferation in a
manner dependent on culture time (Fig.
3B).
The apoptosis of BMSCs when
co-cultured with titanium-alloy particles
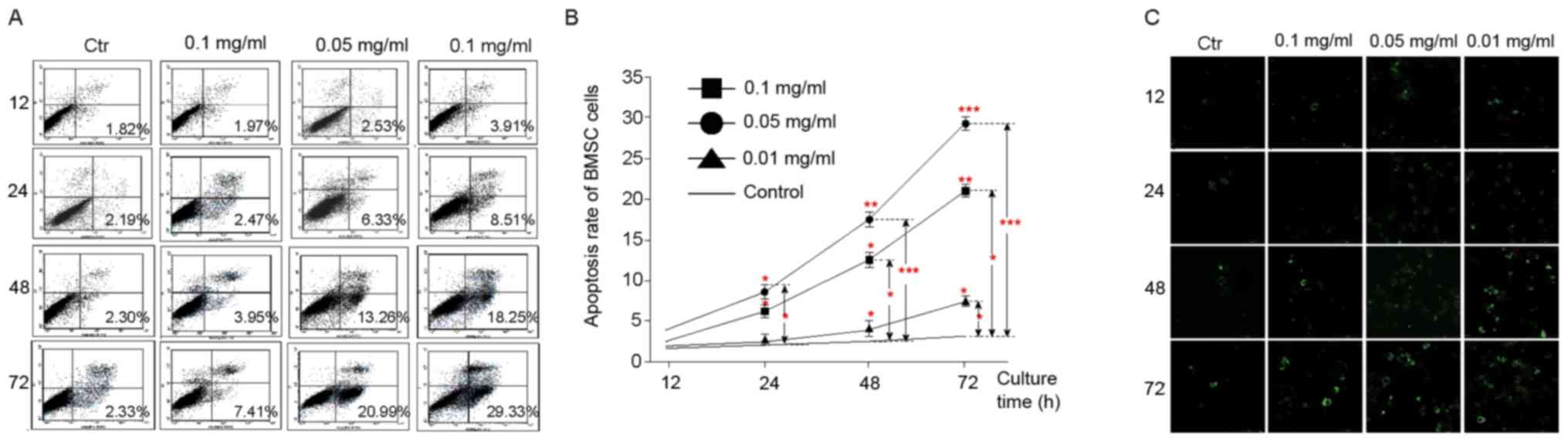
Furthermore, we examined whether the particles
facilitate the apoptosis of BMSC using co-culture strategy. We
found under the microscope that low-density particles (0.01 mg/ml)
had a slight effect on BMSC apoptosis (1.97%). However, with the
concentration grows, their effect on BMSC apoptosis become more and
more evident, exhibiting a concentration-dependent manner (Fig. 4A). Consistently, we observed that
these particles confer the apoptosis of BMSC in a time-dependent
manner (Fig. 4A). Correspondingly,
FCM inspection also showed that the particles facilitate BMSC
apoptosis in a manner dependent on time and concentrations
(Fig. 4B).
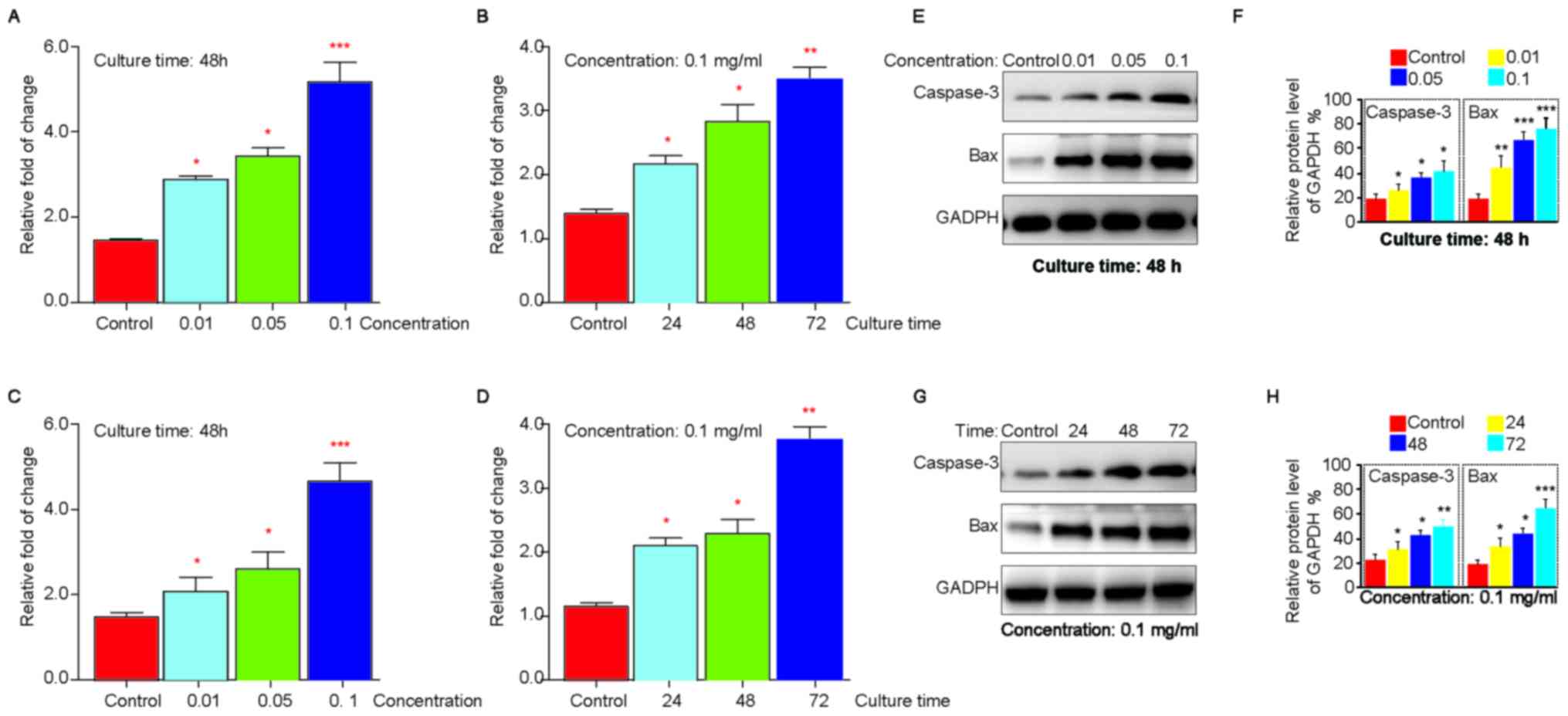
To further confirm the role of titanium-alloy
particles on apoptosis of BMSC, we analyzed specific apoptosis
markers of the particles treated-cells using RT-PCR. We found that
both Bax and Caspase-3 elevated in a time and concentration manner
(Fig. 5A-D). Consistently, we also
revealed that Bax and Caspase-3 expression decreased in protein
level in a manner dependent on time and concentration (Fig. 5E and F). Taken together, our data
suggested that Nano-sized titanium-alloy particles could facilitate
the apoptosis of BMSC.
Discussion
It is widely accepted that postoperative aseptic
loosening of the artificial joints was one of the most common
complications for patients who received the management (7). Recent advancements had indicated that
crosstalk between artificial joints derived particles and BMSC
involve extensively in the process (20,21),
suggesting that deregulated BMSC was a risk factor for aseptic
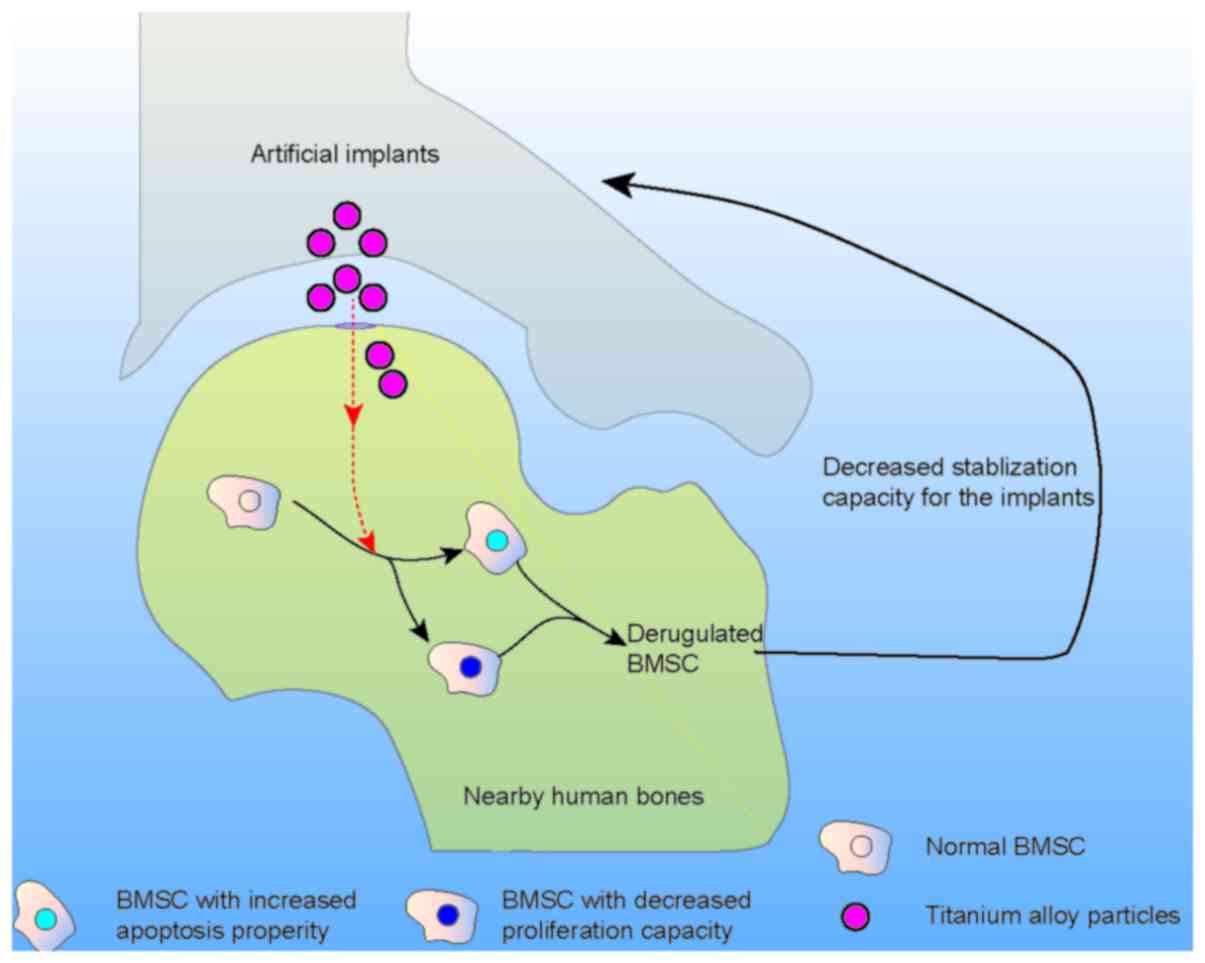
loosening of the artificial joints. In the present study, we
proposed that titanium-alloy particles that permeated into the bone
marrow significantly inhibit the proliferation and promote the
apoptosis of BMSC, which were critical in the stabilization of
implanted artificial joints (Fig.
6). Taken together, our data revealed a novel mechanism whereby
titanium-alloy implants derived particles facilitate aseptic
loosening of artificial joints, suggesting that blocking
titanium-alloy particles permeating into the bone marrow might be a
novel direction to avoid septic loosening of artificial joints.
In general, the stabilization of newly implanted
artificial joints was a complex process with the involvement of
osteoblasts, osteoclasts as well as some side cells (21,22).
These cells function synergistically to promote the stabilization
of the artificial joints via cytokines and chemokine secretion. It
is reasonable that deregulated functions of these cells were risky
for the stabilization of artificial joints. In the clinics,
revealing the dysfunctions of BMSC after implants replacement was
becoming a hotpot. For one thing, the artificial joints-derived
particles had been well stated to involve in septic loosening. For
instance, Yao et al found that titanium derived particles
significantly suppress the osteogenic function of osteoblast-like
cells (23), which then induce
osteoblast apoptosis directly or/and indirectly (24). For another thing, mounting studies
suggested that deregulated cells nearby the implants were effective
in aseptic loosening. As showed by Yrjo T Konttinen and colleagues,
macrophages together with their bioactive factors play a critical
role in the corrosion of the implants (25). Our study added the wealth
knowledge, which showed that titanium particles facilitate aseptic
loosening of the artificial joints by promoting apoptosis and
inhibiting proliferation of BMSC. Our data shared a lot in common
with the previous studies. For instance, Hou and colleagues also
showed that Ti-alloy particles were effective in decreasing the
proliferation of BMSC (26).
However, there were also huge differences between the two studies.
At first, the study by Hou learned the effect of Ti-alloy particles
on BMSC on three levels (14, 108 and 196 nm), while our study only
focused on particles particle with the diameter of 100 nm.
Secondly, they investigated the role of particles on the adhesion,
migration and differentiation of BMSC, while we focused on the
proliferation and apoptosis of BMSC. Thirdly, we showed that the
particles facilitate the proliferation and apoptosis of BMSC by
decreasing proliferation and elevating apoptosis related factors.
Despite these differences, both studies all concluded that the
particle was a risk factor for dysregulated BMSC in
vitro.
To be honest, there were also limitations of our
study. For one thing, we give no clarifications on how the
particles influence BMSC proliferation and apoptosis. Systematical
literature review suggested that BMSCs usually differentiate into
osteoblasts, which function as effector cells in the stabilization
of artificial joint (27). These
data provided the basis for our study as they pointed the critical
role of BMSC in the stabilization of artificial joints. Meanwhile,
other reports indicated that silicon ions decreased the
proliferation of BMSC with the involvement of WNT and SHH signaling
pathways (28). These studies
indicated that PI3K/Akt, ERK1/2 and WNT signaling might also
involve in particles resultant abnormal proliferation and apoptosis
of BMSC. For another thing, our data was based on in vitro
studies, and cells used in the study were from rabbits. We could
not tell whether the particles affect human BMSC proliferation and
apoptosis in the same manner. Meanwhile, the exact particle
concentration within individuals for who received implants is not
known, and these results will need to be validated in vivo
as our study also did not tell whether the particles function
similarly to the implant-derived particles.
In conclusion, we showed in our study that
Nano-sized titanium particles suppress the proliferation of BMSCs
in a manner dependent on time and concentration, and that the
particles could promote BMSC apoptosis in the same manner. Since
BMSCs plays a positive role in the stabilization of artificial
joints, further studies are urgently needed in the near further to
reveal the detailed crosstalk between the particles and BMSC so as
to provide novel targets for aseptic loosening in the clinics.
References
|
1
|
Belatti DA, Pugely AJ, Phisitkul P,
Amendola A and Callaghan JJ: Total joint arthroplasty: Trends in
medicare reimbursement and implant prices. J Arthroplasty.
29:1539–1544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nwachukwu BU, Dy CJ, Burket JC, Padgett DE
and Lyman S: Risk for complication after total joint arthroplasty
at a center of excellence: The impact of patient travel distance. J
Arthroplasty. 30:1058–1061. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ribera A, Morata L, Moranas J, Agulló JL,
Martínez JC, López Y, García D, Cabo J, García-Ramiro S, Soriano A
and Murillo O: Clinical and microbiological findings in prosthetic
joint replacement due to aseptic loosening. J Infect. 69:235–243.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drees P, Eckardt A, Gay RE, Gay S and
Huber LC: Mechanisms of disease: Molecular insights into aseptic
loosening of orthopedic implants. Nat Clin Pract Rheumatol.
3:165–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Long M and Rack HJ: Titanium alloys in
total joint replacement-a materials science perspective.
Biomaterials. 19:1621–1639. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salvati EA, Betts F and Doty SB:
Particulate metallic debris in cemented total hip arthroplasty.
Clin Orthop Relat Res. 293:160–173. 1993.
|
|
7
|
Jiang Y, Jia T, Wooley PH and Yang SY:
Current research in the pathogenesis of aseptic implant loosening
associated with particulate wear debris. Acta Orthop Belg. 79:1–9.
2013.PubMed/NCBI
|
|
8
|
Abu-Amer Y, Darwech I and Clohisy JC:
Aseptic loosening of total joint replacements: Mechanisms
underlying osteolysis and potential therapies. Arthritis Res Ther.
9 Suppl 1:S62007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hallab NJ and Jacobs JJ: Biologic effects
of implant debris. Bull NYU Hosp Jt Dis. 67:182–188.
2009.PubMed/NCBI
|
|
10
|
Cooper RA, McAllister CM, Borden LS and
Bauer TW: Polyethylene debris-induced osteolysis and loosening in
uncemented total hip arthroplasty: A cause of late failure. J
Arthroplasty. 7:285–290. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmalzried TP, Jasty M and Harris WH:
Periprosthetic bone loss in total hip arthroplasty. Polyethylene
wear debris and the concept of the effective joint space. J Bone
Joint Surg Am. 74:849–863. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Granchi D, Amato I, Battistelli L,
Ciapetti G, Pagani S, Avnet S, Baldini N and Giunti A: Molecular
basis of osteoclastogenesis induced by osteoblasts exposed to wear
particles. Biomaterials. 26:2371–2379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jacobs JJ, Roebuck KA, Archibeck M, Hallab
NJ and Glant TT: Osteolysis: Basic science. Clin Orthop Relat Res.
1–77. 2001.
|
|
14
|
Bruder SP, Fink DJ and Caplan AI:
Mesenchymal stem cells in bone development, bone repair, and
skeletal regeneration therapy. J Cell Biochem. 56:283–294. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang ML, Sharkey PF and Tuan RS: Particle
bioreactivity and wear-mediated osteolysis. J Arthroplasty.
19:1028–1038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao R, Li Y, Lin Z, Wan J, Xu C, Zeng Y
and Zhu Y: miR-199b-5p modulates BMSC osteogenesis via suppressing
GSK-3β/β-catenin signaling pathway. Biochem Biophys Res Commun.
477:749–754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bae IH, Park MJ, Yoon SH, Kang SW, Lee SS,
Choi KM and Um HD: Bcl-w promotes gastric cancer cell invasion by
inducing matrix metalloproteinase-2 expression via phosphoinositide
3-kinase, Akt, and Sp1. Cancer Res. 66:4991–4995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu J, Wang X, Wei SM, Tang YH, Zhou Q and
Huang CX: Activin A stimulates the proliferation and
differentiation of cardiac fibroblasts via the ERK1/2 and p38-MAPK
pathways. Eur J Pharmacol. 789:319–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang JG, Li XY, Wang YZ, Zhang QD, Gu SY,
Wu X, Zhu GH, Li Q and Liu GL: ROCK is involved in vasculogenic
mimicry formation in hepatocellular carcinoma cell line. PLoS One.
9:e1076612014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Y, Jia T, Gong W, Wooley PH and Yang
S: Titanium particle-challenged osteoblasts promote
osteoclastogenesis and osteolysis in a murine model of
periprosthestic osteolysis. Acta Biomater. 9:7564–7572. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Otto M, Kriegsmann J, Gehrke T and Bertz
S: Wear particles: Key to aseptic prosthetic loosening? Pathologe.
27:447–460. 2006.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miller MD, Peters CL and Allen B: Early
aseptic loosening of a total knee arthroplasty due to Gore-Tex
particle-induced osteolysis. J Arthroplasty. 21:765–770. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao J, Cs-Szabó G, Jacobs JJ, Kuettner KE
and Glant TT: Suppression of osteoblast function by titanium
particles. J Bone Joint Surg Am. 79:107–112. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pioletti DP, Leoni L, Genini D, Takei H,
Du P and Corbeil J: Gene expression analysis of osteoblastic cells
contacted by orthopedic implant particles. J Biomed Mater Res.
61:408–420. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jämsen E, Kouri VP, Olkkonen J, Cör A,
Goodman SB, Konttinen YT and Pajarinen J: Characterization of
macrophage polarizing cytokines in the aseptic loosening of total
hip replacements. J Orthop Res. 32:1241–1246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou Y, Cai K, Li J, Chen X, Lai M, Hu Y,
Luo Z, Ding X and Xu D: Effects of titanium nanoparticles on
adhesion, migration, proliferation, and differentiation of
mesenchymal stem cells. Int J Nanomedicine. 8:3619–3630.
2013.PubMed/NCBI
|
|
27
|
von Knoch F, Jaquiery C, Kowalsky M,
Schaeren S, Alabre C, Martin I, Rubash HE and Shanbhag AS: Effects
of bisphosphonates on proliferation and osteoblast differentiation
of human bone marrow stromal cells. Biomaterials. 26:6941–6949.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han P, Wu C and Xiao Y: The effect of
silicate ions on proliferation, osteogenic differentiation and cell
signalling pathways (Wnt and Shh) of bone marrow stromal cells.
Biomater Sci. 1:379–392. 2013. View Article : Google Scholar
|