|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ruan Y, Hu K and Chen H: Autophagy
inhibition enhances isorhamnetin-induced mitochondria-dependent
apoptosis in non-small cell lung cancer cells. Mol Med Rep.
12:5796–5806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao GF, Huang ZA, Du XK, Yang ML, Huang
DD and Zhang S: Molecular docking studies of traditional chinese
medicinal compounds against known protein targets to treat
non-small cell lung carcinomas. Mol Med Rep. 14:1132–1138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
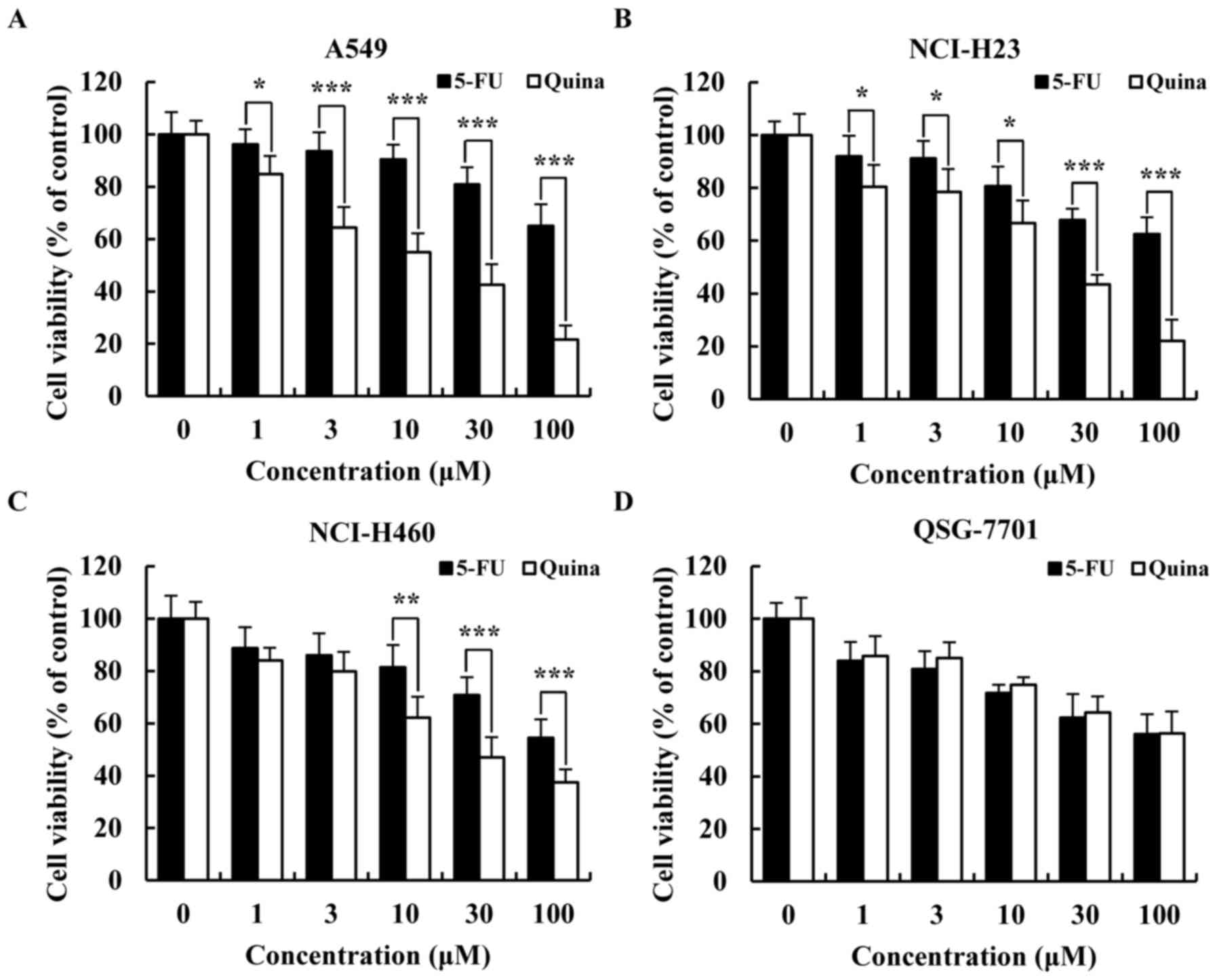
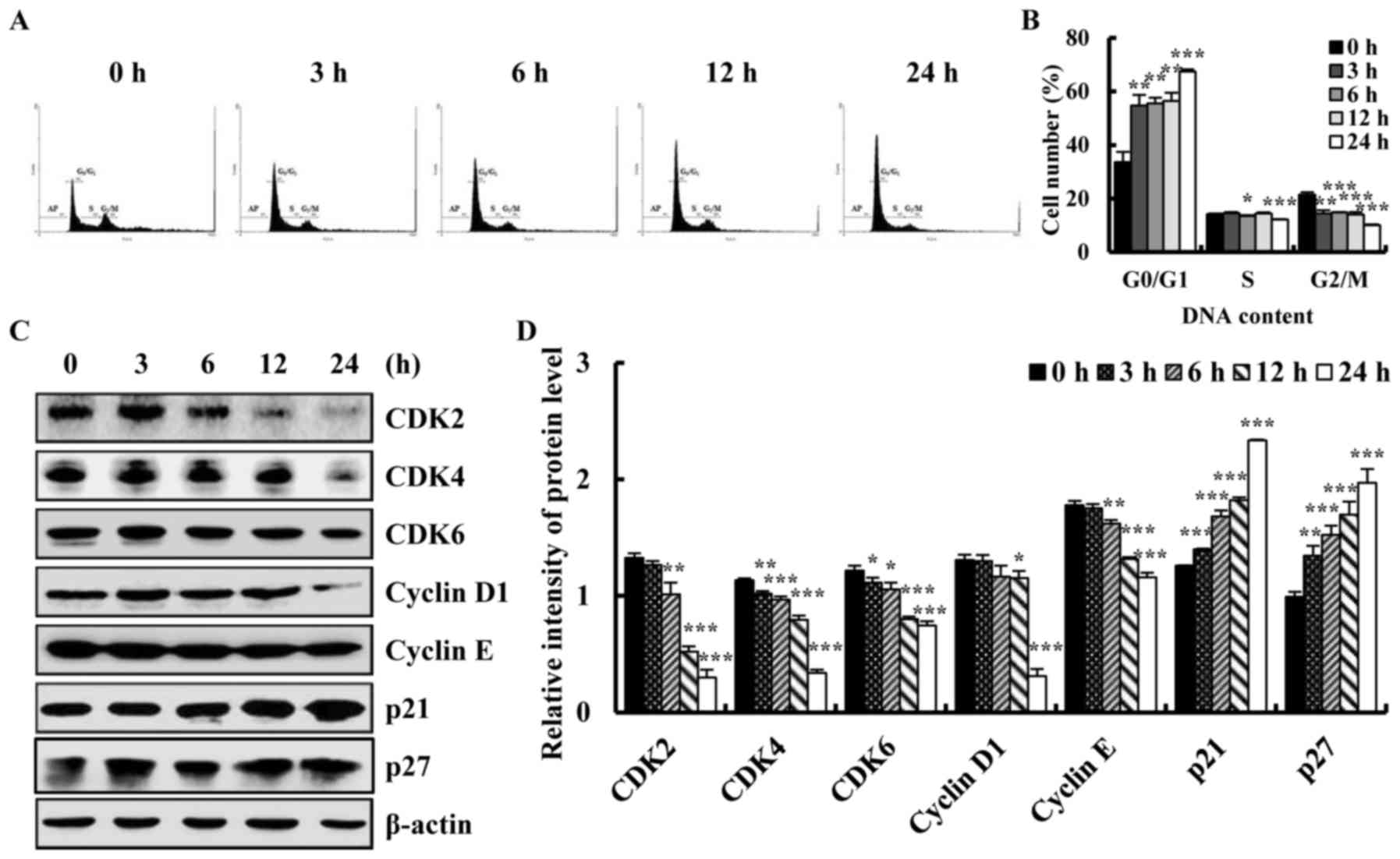
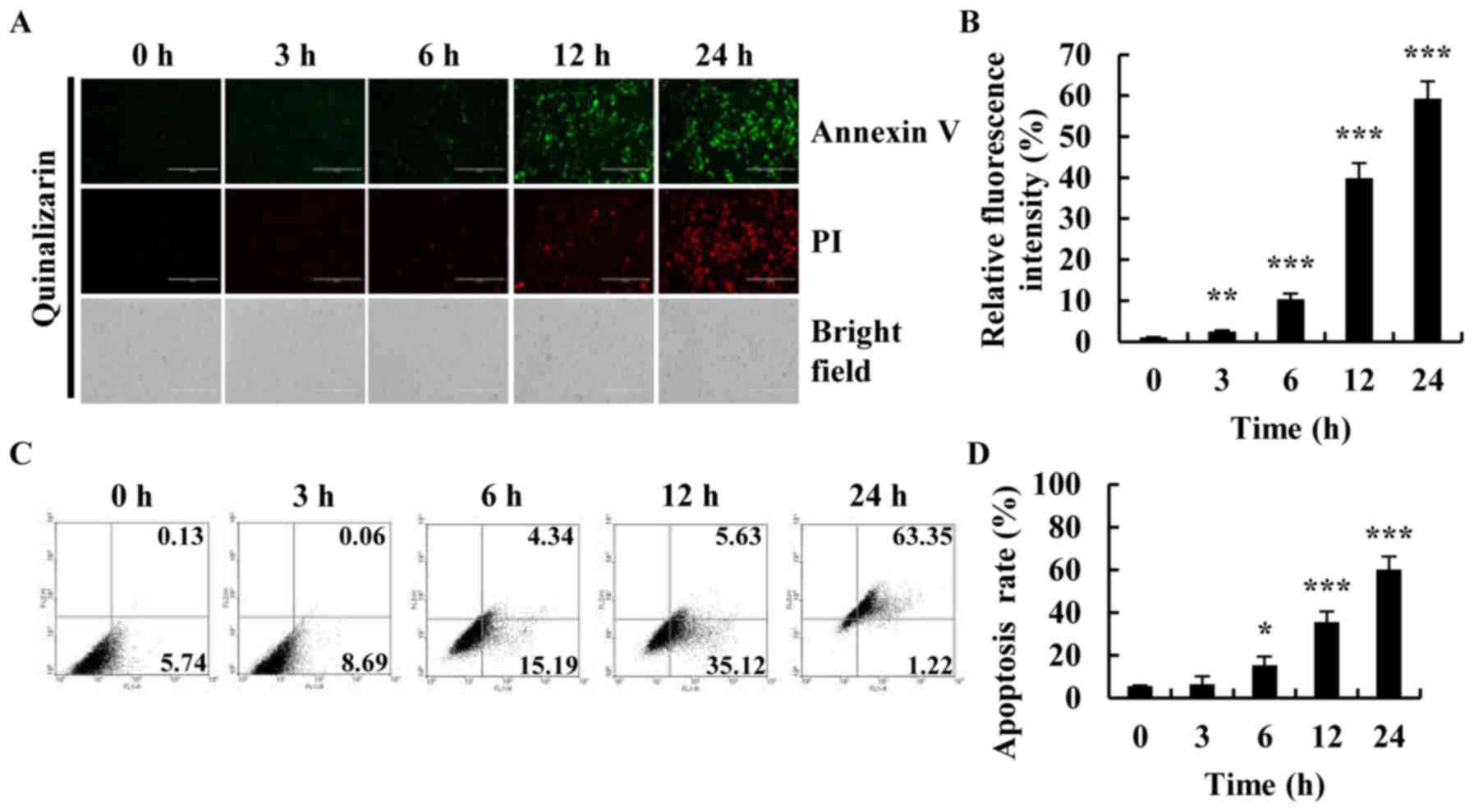
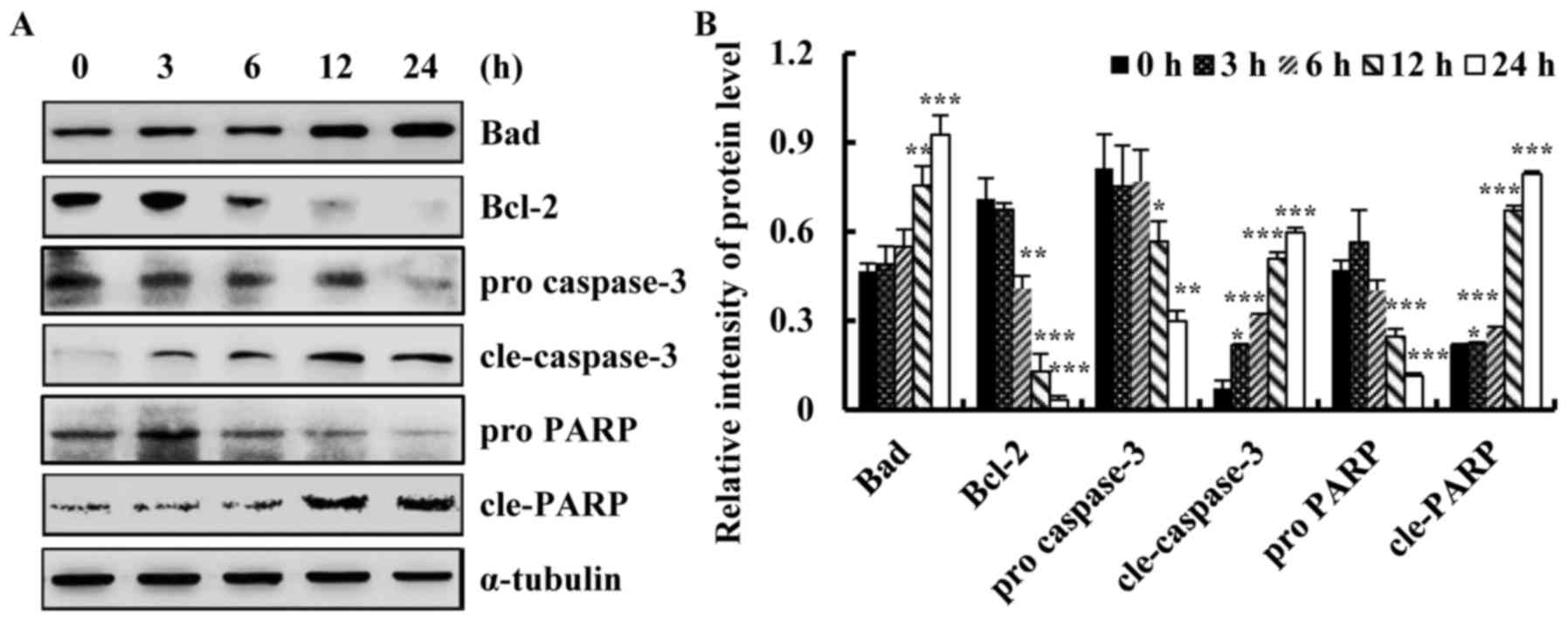
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dai GH, Meng GM, Tong YL, Chen X, Ren ZM,
Wang K and Yang F: Growth-inhibiting and apoptosis-inducing
activities of Myricanol from the bark of Myrica rubra in human lung
adenocarcinoma A549 cells. Phytomedicine. 21:1490–1496. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu K, Liu B and Liu Y: Impact of Brachyury
on epithelial-mesenchymal transitions and chemosensitivity in
non-small cell lung cancer. Mol Med Rep. 12:995–1001. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo W, Xie L, Zhao L and Zhao Y: mRNA and
microRNA expression profiles of radioresistant NCI-H520 non-small
cell lung cancer cells. Mol Med Rep. 12:1857–1867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Yang Z, Sun R, Mo Z, Jin G, Wei F,
Hu J, Guan W and Zhong N: Preparation of lung-targeting,
emodin-loaded polylactic acid microspheres and their properties.
Int J Mol Sci. 15:6241–6251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Guo G, Song J, Cai Z, Yang J, Chen
Z, Wang Y, Huang Y and Gao Q: B7-H3 promotes the migration and
invasion of human bladder cancer cells via the PI3K/Akt/STAT3
signaling pathway. J Cancer. 8:816–824. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu S, Huang L, Meng L, Sun H, Zhang W and
Xu Y: Isorhamnetin inhibits cell proliferation and induces
apoptosis in breast cancer via Akt and mitogen-activated protein
kinase kinase signaling pathways. Mol Med Rep. 12:6745–6751. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang G, Wang C, Sun M, Li J, Wang B, Jin
C, Hua P, Song G, Zhang Y, Nguyen LL, et al: Cinobufagin inhibits
tumor growth by inducing intrinsic apoptosis through AKT signaling
pathway in human nonsmall cell lung cancer cells. Oncotarget.
7:28935–28946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang D, Chen B, Zhou J, Zhou L, Li Q, Liu
F, Chou KY, Tao L and Lu LM: Low concentrations of trichosanthin
induce apoptosis and cell cycle arrest via c-Jun N-terminal protein
kinase/mitogen-activated protein kinase activation. Mol Med Rep.
11:349–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng Y, McFarland BC, Drygin D, Yu H,
Bellis SL, Kim H, Bredel M and Benveniste EN: Targeting protein
kinase CK2 suppresses prosurvival signaling pathways and growth of
glioblastoma. Clin Cancer Res. 19:6484–6494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan B, Zhong W, Deng Z, Lai C, Chu J, Jiao
G, Liu J and Zhou Q: Inhibition of prostate cancer growth by
solanine requires the suppression of cell cycle proteins and the
activation of ROS/P38 signaling pathway. Cancer Med. 5:3214–3222.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhai H, Hu S, Liu T, Wang F, Wang X, Wu G,
Zhang Y, Sui M, Liu H and Jiang L: Nitidine chloride inhibits
proliferation and induces apoptosis in colorectal cancer cells by
suppressing the ERK signaling pathway. Mol Med Rep. 13:2536–2542.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park KR, Yun HM, Quang TH, Oh H, Lee DS,
Auh QS and Kim EC: 4-Methoxydalbergione suppresses growth and
induces apoptosis in human osteosarcoma cells in vitro and in vivo
xenograft model through down-regulation of the JAK2/STAT3 pathway.
Oncotarget. 7:6960–6971. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai WC, Bai LY, Chen YJ, Chu PC, Hsu YW,
Sargeant AM and Weng JR: OSU-A9 inhibits pancreatic cancer cell
lines by modulating p38-JAK-STAT3 signaling. Oncotarget.
8:29233–29246. 2017.PubMed/NCBI
|
|
18
|
Miao D and Zhang L: Leptin modulates the
expression of catabolic genes in rat nucleus pulposus cells through
the mitogen-activated protein kinase and Janus kinase 2/signal
transducer and activator of transcription 3 pathways. Mol Med Rep.
12:1761–1768. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Principe M, Borgoni S, Cascione M,
Chattaragada MS, Ferri-Borgogno S, Capello M, Bulfamante S,
Chapelle J, Di Modugno F, Defilippi P, et al: Alpha-enolase (ENO1)
controls alpha v/beta 3 integrin expression and regulates
pancreatic cancer adhesion, invasion, and metastasis. J Hematol
Oncol. 10:162017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi XY, Xiong LX, Xiao L, Meng C, Qi GY
and Li WL: Downregulation of caveolin-1 upregulates the expression
of growth factors and regulators in co-culture of fbroblasts with
cancer cells. Mol Med Rep. 13:744–752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan M, Khan M, Al-Marri AH, Al-Warthan A,
Alkhathlan HZ, Siddiqui MR, Nayak VL, Kamal A and Adil SF:
Apoptosis inducing ability of silver decorated highly reduced
graphene oxide nanocomposites in A549 lung cancer. Int J
Nanomedicine. 11:873–883. 2016.PubMed/NCBI
|
|
22
|
Chen Z, Teo AE and McCarty N: ROS induced
CXCR4 signaling regulates mantle cell lymphoma (MCL) cell survival
and drug resistance in the bone marrow microenvironment via
autophagy. Clin Cancer Res. 22:187–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Zhang Q, Jiang Y, Li F and Xin H:
Effects of ophiopogonin B on the proliferation and apoptosis of
SGC-7901 human gastric cancer cells. Mol Med Rep. 13:4981–4986.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Liu JM, Xiong XX, Qiu XY, Pan F,
Liu D, Lan SJ, Jin S, Yu SB and Chen XQ: Piperlongumine selectively
kills hepatocellular carcinoma cells and preferentially inhibits
their invasion via ROS-ER-MAPKs-CHOP. Oncotarget. 6:6406–6421.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cozza G, Mazzorana M, Papinutto E, Bain J,
Elliott M, di Maira G, Gianoncelli A, Pagano MA, Sarno S, Ruzzene
M, et al: Quinalizarin as a potent, selective and cell-permeable
inhibitor of protein kinase CK2. Biochem J. 421:387–395. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song C, Gowda C, Pan X, Ding Y, Tong Y,
Tan BH, Wang H, Muthusami S, Ge Z, Sachdev M, et al: Targeting
casein kinase II restores Ikaros tumor suppressor activity and
demonstrates therapeutic efficacy in high-risk leukemia. Blood.
126:1813–1822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hung MS, Xu Z, Chen Y, Smith E, Mao JH,
Hsieh D, Lin YC, Yang CT, Jablons DM and You L: Hematein, a casein
kinase II inhibitor, inhibits lung cancer tumor growth in a murine
xenograft model. Int J Oncol. 43:1517–1522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou Y, Li K, Zhang S, Li Q, Li Z, Zhou F,
Dong X, Liu L, Wu G and Meng R: Quinalizarin, a specific CK2
inhibitor, reduces cell viability and suppresses migration and
accelerates apoptosis in different human lung cancer cell lines.
Indian J Cancer. 2 Suppl 52:e119–e124. 2015.
|
|
29
|
Kim J, Choi WJ, Moon SH, Jung J, Park JK,
Kim SH and Lee JO: Micropillar arrays as potential drug screens:
Inhibition of micropillar-mediated activation of the
FAK-Src-paxillin signaling pathway by the CK2 inhibitor CX-4945.
Acta Biomater. 27:13–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang NH, Shin HC, Oh S, Lee KH, Lee YB and
Choi KC: Soy milk digestion extract inhibits progression of
prostate cancer cell growth via regulation of prostate
cancer-specific antigen and cell cycle-regulatory genes in human
LNCaP cancer cells. Mol Med Rep. 14:1809–1816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Wang D, Li L, Ding X and Ma H:
Dehydroepiandrosterone inhibits cell proliferation and improves
viability by regulating S phase and mitochondrial permeability in
primary rat Leydig cells. Mol Med Rep. 14:705–714. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Tian H, Li M, Ge C, Zhao F, Zhang
L, Li H, Liu J, Wang T, Yao M and Li J: Derivate isocorydine
inhibits cell proliferation in hepatocellular carcinoma cell lines
by inducing G2/M cell cycle arrest and apoptosis. Tumour Biol.
37:5951–5961. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schneider CC, Götz C, Hessenauer A,
Günther J, Kartarius S and Montenarh M: Down-regulation of CK2
activity results in a decrease in the level of cdc25C phosphatase
in different prostate cancer cell lines. Mol Cell Biochem.
356:177–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi W, Deng J, Tong R, Yang Y, He X, Lv J,
Wang H, Deng S, Qi P, Zhang D and Wang Y: Molecular mechanisms
underlying mangiferin-induced apoptosis and cell cycle arrest in
A549 human lung carcinoma cells. Mol Med Rep. 13:3423–3432. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Woo SM, Choi YK, Kim AJ, Cho SG and Ko SG:
p53 causes butein-mediated apoptosis of chronic myeloid leukemia
cells. Mol Med Rep. 13:1091–1096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu Y, Chen Y, Wu Q, Jia L and Du X:
Minocycline inhibits PARP-1 expression and decreases apoptosis in
diabetic retinopathy. Mol Med Rep. 12:4887–4894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zeng J, Chen S, Li N, Chen L, Su J, Niu G,
Zhu S and Liang Y: Sasanquasaponin from Camellia oleifera Abel.
induces apoptosis via Bcl-2, Bax and caspase-3 activation in HepG2
cells. Mol Med Rep. 12:1997–2002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin J, Lin G, Huang H, Xu D, Yu H, Ma X,
Zhu L, Ma D and Jiang H: Capsaicin mediates cell cycle arrest and
apoptosis in human colon cancer cells via stabilizing and
activating p53. Int J Biol Sci. 10:285–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Conway GE, Casey A, Milosavljevic V, Liu
Y, Howe O, Cullen PJ and Curtin JF: Non-thermal atmospheric plasma
induces ROS-independent cell death in U373MG glioma cells and
augments the cytotoxicity of temozolomide. Br J Cancer.
114:435–443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao W, Lu M and Zhang Q: Chloride
intracellular channel 1 regulates migration and invasion in gastric
cancer by triggering the ROS-mediated p38 MAPK signaling pathway.
Mol Med Rep. 12:8041–8047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aredia F, Czaplinski S, Fulda S and
Scovassi AI: Molecular features of the cytotoxicity of an NHE
inhibitor: Evidence of mitochondrial alterations, ROS
overproduction and DNA damage. BMC Cancer. 16:8512016. View Article : Google Scholar : PubMed/NCBI
|