Introduction
Deep venous thrombosis (DVT) is a global health
problem that affects 1 in 1,000 individuals according to
epidemiological studies (1). Even
with preventive measures, the incidence of DVT ranges from
2.22–3.29% among patients undergoing major orthopaedic surgeries in
developed countries (2,3). Pulmonary embolism, the most dangerous
compilation of DVT, affects between 500,000 and 700,000 patients,
which may cause mortality in 10–15% of these patients (4). Structural and functional disorders of
venous endothelial cells (ECs) have an initial role in the
pathogenesis and progression of DVT (5). Currently, oxidative stress injury,
which occurs during hypoxia, sepsis and ischaemia-reperfusion
injury, is considered to lead to EC dysfunction and initiation of
the coagulation cascade (6,7).
Excessive levels of reactive oxygen species (ROS) cause oxidative
damage to ECs and subsequently activate platelets and leukocytes,
increasing their adhesion and aggregation ability and triggering
procoagulant reactions (8,9). Therefore, the hypothesis of adopting
antioxidants to prevent vascular embolism diseases has been
proposed and proven effective in several studies (10–12).
Resveratrol (trans-3,4,5-trihydroxystilbene),
a natural, plant-derived polyphenolic compound that is abundant in
grape skin, peanuts, mulberries and red wine, has been proven to
exhibit a wide range of pharmacological activities, including
antioxidant activity, lipoprotein metabolism modulation,
anti-platelet aggregation, and anti-inflammatory and anti-tumour
effects (13). In addition,
cardioprotective activities of resveratrol have previously been
identified, however, studies have primarily focused on cardiac and
arterial diseases (14,15). Whether resveratrol may exhibit
inhibitory effects on venous thrombosis remains largely unknown.
Pathways that have previously been associated with the effects of
resveratrol treatment include AMP-activated protein kinase,
phosphatidylinositol 3-kinase/Akt and sirtuin 1 (16,17).
Extracellular signal-regulated kinase (ERK), c-Jun N-terminal
kinase (c-Jun) and p38 mitogen-activated protein kinase (MAPK) are
conventional MAPK signalling pathway families that are implicated
in various diseases, including tumour growth and haemostasis, among
others. Furthermore, MAPK signalling pathways, which promote
apoptosis and have roles in venous endothelial oxidative stress
injury, have been confirmed as important targets that promote
thrombosis by mediating interactions among venous endothelial
cells, leukocytes and platelets (18). The present study hypothesises that
MAPKs may be involved in mediating the effects of resveratrol on
venous thrombosis. In addition, von Willebrand factor (VWF) and
P-selectin glycoprotein ligand-1 (PSGL-1) are considered thrombotic
risk markers, with increases in these factors indicating a
thrombotic phenotype (19,20). Therefore, the present study
investigated VWF and PSGL-1 levels in HUVECs following different
treatments.
The present study aimed to investigate the effects
of resveratrol on apoptosis, ROS generation and the expression of
thrombosis-associated markers (VWF and PSGL-1) induced by hydrogen
peroxide (H2O2) in addition to the underlying
mechanisms, in vitro. The results of the present study
indicated that resveratrol may exert antithrombotic activity in
vitro by inactivating MAPK signalling pathways.
Materials and methods
Reagents, chemicals and
antibodies
Primary human umbilical vein endothelial cells
(HUVECs) were purchased from CHI Scientific, Inc. (Maynard, MA,
USA). High-glucose Dulbecco's modified Eagle's medium (DMEM) and
fetal bovine serum (FBS) were both purchased from Hyclone (GE
Healthcare Life Sciences, Logan, UT, USA). Resveratrol (purity
>99.0%) was purchased from Tokyo Chemical Industry Co., Ltd.
(Tokyo, Japan). All primary antibodies were purchased from
Proteintech Group, Inc. (Chicago, IL, USA). Phosphorylated
antibodies were purchased from ABclonal, Inc. (Cambridge, MA, USA).
Anisomycin and curcumin were purchased from Beijing TOP Science
Biotechnology Co., Ltd. (Beijing, China). The MTT kit, dimethyl
sulphoxide (DMSO), and 2′,7′-dichlorofluorescin diacetate (DCFH-DA)
were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
The Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide
(PI) double-staining Apoptosis Detection kit was obtained from
Beyotime Institute of Biotechnology (Haimen, China). Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China).
Cell culture and treatments
HUVECs were cultured in DMEM medium supplemented
with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. Cells
were maintained at 37°C with 5% CO2 in a humidified
atmosphere. Cells were digested with 0.25% trypsin, centrifuged and
harvested in a centrifuge (150 × g at room temperature for 5 min)
and resuspended in DMEM medium. The cell concentration was adjusted
to 1×104 cells/ml and cells were inoculated into a
culture flask. The resveratrol solution was prepared in DMSO and
diluted with culture medium immediately prior to use. DMSO was used
as the control group. The cells were pretreated at 37°C with
resveratrol (0, 10, 20 and 30 µM) for 2 h and were then subjected
to H2O2 (200 µM) for 24 h. In addition, in
the MTT assay, one group received treatment with 30 µM resveratrol
without H2O2 treatment. Control cells were
treated with medium alone. After the treatments were performed, the
cells were harvested for further analysis.
Analysis of cell viability
The viability of HUVECs treated with 0, 10, 20 and
30 µM resveratrol for 2 h followed by treatment with or without 200
µM H2O2 for 24 h was assessed with an MTT
assay. After cells were treated and washed with PBS, 10 µl MTT
solution (5 mg/ml) was added to the cells. The samples were
incubated for 4 h at 37°C and 100 µl dimethyl sulfoxide was added
to each well to dissolve the formazan crystals for 4 h at 37°C. The
plates were read at 570 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and the cell viability was
expressed as the optical density (OD) value.
Cellular apoptosis assay
HUVECs that were treated with 0, 10, 20 and 30 µM
resveratrol for 2 h followed by treatment with or without
H2O2 for 24 h were double stained using an
Annexin V-FITC/PI Apoptosis Detection kit, according to the
manufacturer's instructions. Briefly, the cells were washed and
collected in a 1.5 ml tube, 1×105-1×106 cells
were resuspended in 300 µl 1X binding buffer and cells were
transferred to a sterile flow cytometry glass tube. Annexin V-FITC
(5 µl) was added and the tubes were incubated in the dark for 30
min at room temperature. Subsequently, cells were incubated in the
dark with 5 µl PI at room temperature for 15 min and analysed with
a flow cytometer (Sysmex Partec GmbH, Gorlitz, Germany) and
FloMax® software version 2.7 (Sysmex Partec GmbH).
Measurement of intracellular ROS
Intracellular ROS levels were evaluated using the
DCFH-DA fluorescent probe reagent and a flow cytometer, according
to the manufacturer's instructions. Briefly, after cells
(1×105) were seeded in 6-well plates and treated with 0,
10, 20 and 30 µM resveratrol for 2 h followed by treatment with or
without H2O2 for 24 h, the cells were washed
with PBS and incubated with DCFH-DA (10 µM) in DMEM medium without
FBS at 37°C for 20 min. Following incubation, the cellular
2′7′-dichlorofluorescein (DCF) green fluorescence in each well was
subsequently imaged at 488 nm using a fluorescence microscope at
×200 magnification (Olympus Corporation, Tokyo, Japan), and DCF
fluorescence was quantified using Image-Pro Plus 6 software (Media
Cybernetics, Inc., Rockville, MD, USA). Subsequently, flow
cytometry (Sysmex Partec GmbH) and FloMax® software
version 2.7 (Sysmex Partec GmbH) was also performed to quantify DCF
fluorescence and measure ROS generation.
Western blot analysis
Cells were treated at 37°C with 0, 10, 20 and 30 µM
resveratrol for 2 h followed by treatment with or without
H2O2 for 24 h. For experiments including
anisomycin/curcumin treatment, cells were treated with resveratrol
(0 and 30 µM) for 2 h followed by treatment with or without
anisomycin (0.4 µg/ml for 6 h) or curcumin (25 µM for 4 h) prior to
treatment with 200 µM H2O2 for 24 h. Cells
were homogenized in radioimminoprecipitation assay buffer (Beyotime
Institute of Biotechnology). The lysates were centrifuged (10,625 ×
g at room temperature for 5 min) and the protein supernatant was
collected. Protein concentrations were determined using a BCA
Protein assay kit (Beyotime Institute of Biotechnology). The
proteins (30 µg) were separated by 10% SDS-PAGE and transferred to
nitrocellulose membranes. The membranes were blocked with 5% nonfat
dry milk in Tris-buffered saline and 0.1% Tween-20 (TBS-T, pH 7.6)
at room temperature for 2 h and probed with antibodies (at 1:1,000)
against phosphorylated (p)-p38 MAPK (cat. no. AP0297), p38 MAPK
(cat. no. 14064-1-AP), P-c-Jun (cat. no. AP0048), c-Jun (cat. no.
24909-1-AP), P-ERK (cat. no. AP0472), ERK (cat. no. 16443-1-AP),
PSGL-1 (cat. no. 23605-1-AP), VWF (cat. no. 11778-1-AP), caspase-3
(cat. no. 19677-1-AP) and β-actin (cat. no. 60008-1-AP) at 4°C
overnight. The membranes were washed with TBS-T and hybridized with
secondary goat anti-mouse (cat. no. sc-2039) or goat anti-rabbit
(cat. no. sc-2007) horse radish peroxidase antibodies at 1:2,000
dilution (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 2 h
at room temperature and washed again with TBS-T. The protein bands
were developed using an ECL Western Blotting substrate (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and exposed to
autoradiography film (Bio-Rad Laboratories, Inc.) for visualization
of the bands. β-actin was used as a loading control. The results
were quantified using ImageJ software version 1.48 (National
Institutes of Health, Bethesda, MD, USA). The results for the
control group were defined as 100%.
RT-qPCR assay
Cells were treated at 37°C with resveratrol (0 and
30 µM) for 2 h followed by treatment with or without 200 µM
H2O2 for 24 h. For experiments including
anisomycin/curcumin treatment, cellswere treated with resveratrol
(0 and 30 µM) for 2 h followed by treatment with or without
anisomycin (0.4 µg/ml for 6 h) or curcumin (25 µM for 4 h) prior to
treatment with 200 µM H2O2 for 24 h. Total
RNA was isolated from HUVECs using TRIzol reagent (Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's instructions. The integrity of the RNA samples was
evaluated using agarose gel electrophoresis. Total RNA was reverse
transcribed into cDNA using an PrimeScript™ RT Master mix kit,
followed by SYBR® Premix Ex Taq™ II (both from Takara
Biotechnology Co., Ltd.) to detect the expression levels. β-actin
was used as an internal control. The thermocycling conditions were
as follows: Thirty seconds at 95°C for the activation of Taq DNA
polymerase, followed by 40 cycles of amplification at 95°C for 15
sec and 60°C for 1 min. The reactions were performed in triplicate.
The specific primers used for qPCR were as follows: Caspase-3,
5′-GGGCCTGTTGAACTGAAAAA-3′ (forward) and 5′-CCGTCCTTTGAATTTCTCCA-3′
(reverse); PSGL-1, 5′-ATGATTTCCTGCCAGAAACG-3′ (forward) and
5′-GTGGTCAGCTCTGTGACTGC-3′ (reverse); VWF,
5′-TCCTCCTACTCTGCCCCCC-3′ (forward) and 5′-TCCATCCGCTGAATCACCTC-3′
(reverse); β-actin, 5′-ACGGCAAGTTCAACGGCACAG-3′ (forward) and
5′-GACGCCAGTAGACTCCACGACA-3′ (reverse); p38,
5′-GGGGCAGATCTGAACAACAT-3′ (forward) and 5′-CAGGAGCCCTGTACCACCTA-3′
(reverse); ERK, 5′-TGATCACACAGGGTTCCTGA-3′ (forward) and
5′-TGGAAAGATGGGCCTGTTAG-3′ (reverse); and c-Jun,
5′-ACAGAGCATGACCCTGAACC-3′ (forward) and 5′-CCATTFCTGGACTGGATTAT-3′
(reverse). Gene expression values were calculated and normalized to
β-actin expression using the 2−ΔΔCq relative expression
method (21).
Statistical analysis
Data are presented as the mean ± standard deviation.
Group comparisons were assessed using one-way analysis of variance
followed by Tukey's post hoc test using GraphPad Prism 5.0a
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
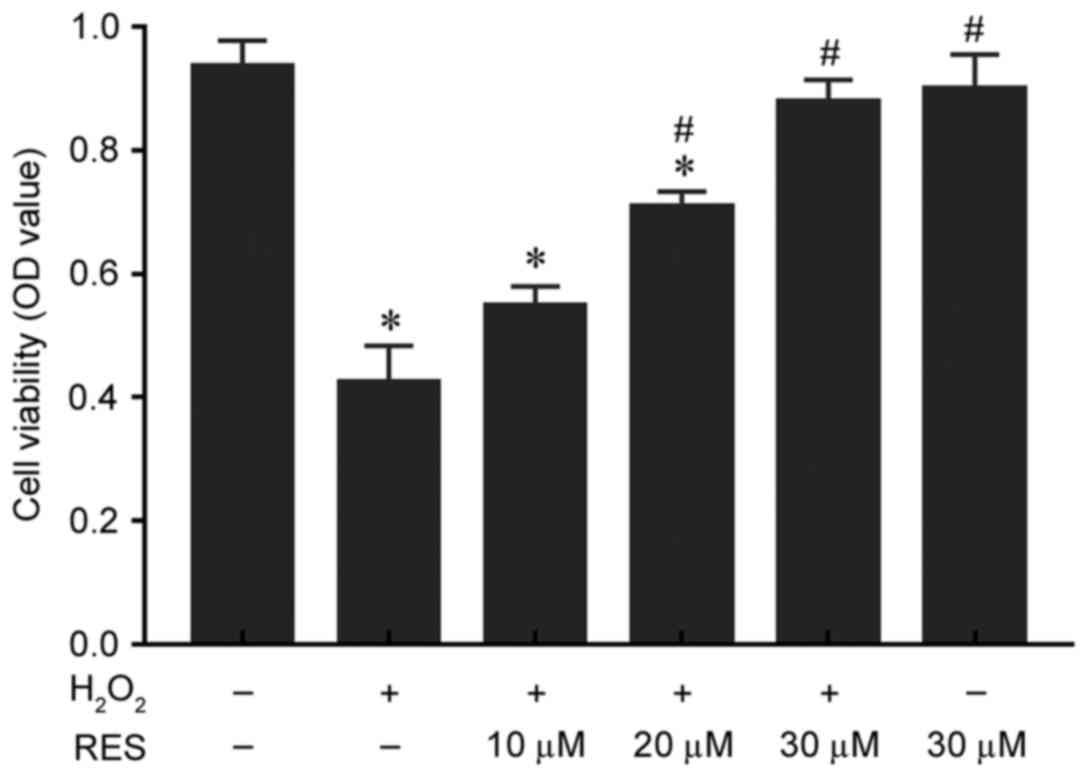
Resveratrol protects HUVECs from
H2O2-induced injury
HUVECs were pretreated with resveratrol for 2 h and
incubated with H2O2 for 24 h, and cell
viability was measured with an MTT assay. The viability of cells
was decreased significantly by treatment with 200 µM
H2O2 for 24 h, compared with the control
group (P<0.05; Fig. 1), while
resveratrol protected cells from H2O2-induced
injury in a concentration-dependent manner (10 µM, 0.55±0.03 OD; 20
µM, 0.71±0.02 OD; and 30 µM, 0.88±0.04 OD; Fig. 1). Resveratrol significantly
enhanced cell viability at 20 and 30 µM compared with the
H2O2 only group (P<0.05), while
resveratrol treatment alone exhibited no cytotoxicity compared with
the control group (P>0.05). These results indicate that
resveratrol may protect HUVECs from
H2O2-induced injury/death.
Resveratrol attenuates
H2O2-induced ROS production in HUVECs
ROS are considered indicators of the cellular
oxidative stress state. Therefore, intracellular ROS accumulation
was determined by measuring DCF-derived fluorescence with the
DCFH-DA probe. The results demonstrated that
H2O2 significantly increased ROS levels
compared with the control group (P<0.05) and that resveratrol
significantly decreased ROS levels in a concentration-dependent
manner compared with the H2O2 only group
(Fig. 2A-F). The DCF fluorescence
quantification results obtained by flow cytometry and presented in
Fig. 2G-L were consistent with the
micrographs. These results indicate that resveratrol may attenuate
H2O2-induced ROS production and oxidative
stress in HUVECs.
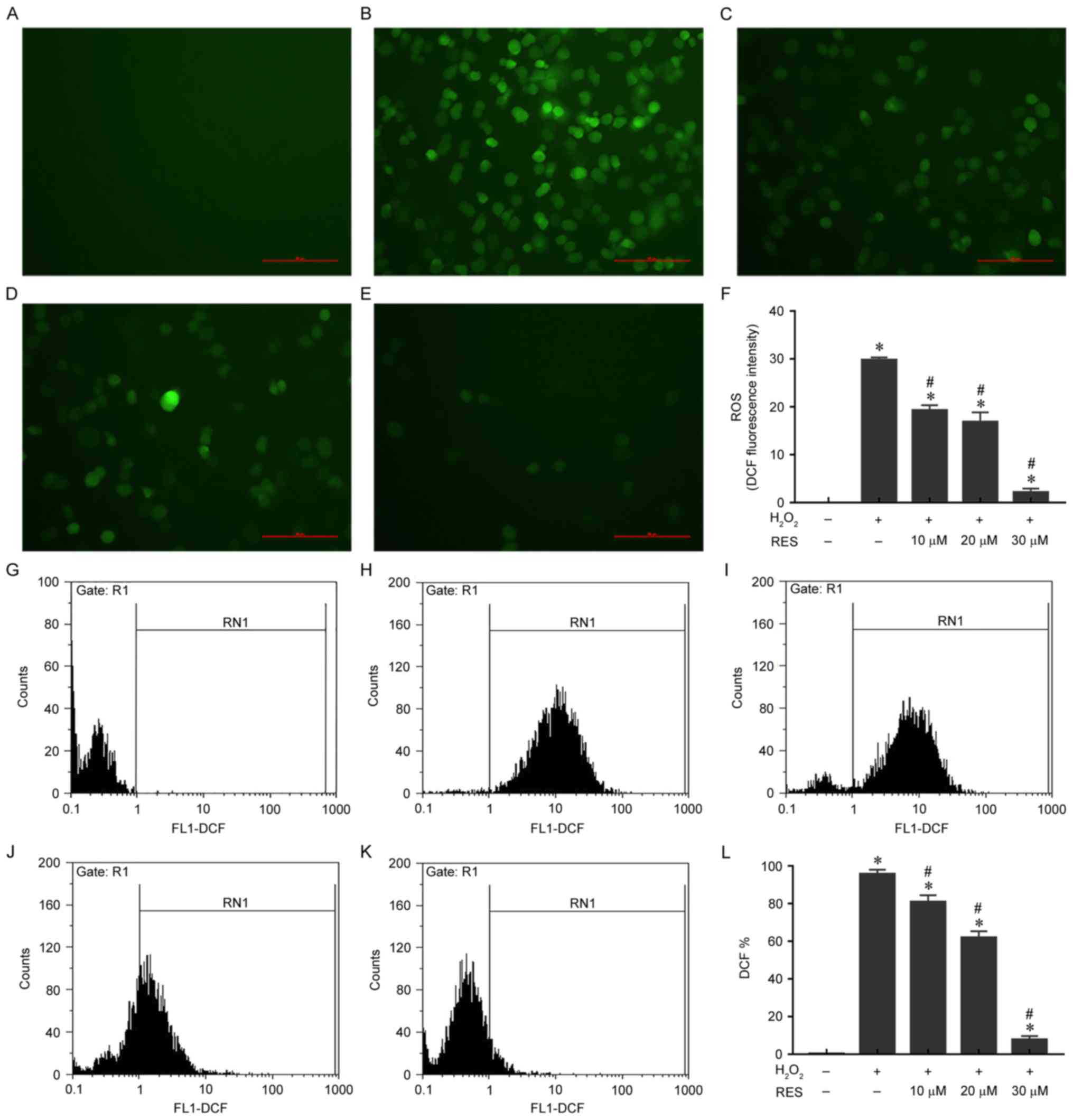 | Figure 2.Resveratrol ameliorates
H2O2-induced ROS generation in HUVECs.
Representative micrographs of HUVECs stained with DCF in (A)
control, (B) 200 µM H2O2, (C) 200 µM
H2O2 + 10 µM resveratrol, (D) 200 µM
H2O2 + 20 µM resveratrol and (E) 200 µM
H2O2 + 30 µM resveratrol groups, as
visualized under a fluorescence microscope at ×200 magnification.
Scale bar, 100 µm. (F) Quantification of DCF fluorescence in
micrographs using Image-Pro Plus 6.0 software. DCF fluorescence was
also quantified by flow cytometry. Representative flow cytometry
plots for HUVECs stained with DCF in (G) control, (H) 200 µM
H2O2, (I) 200 µM H2O2 +
10 µM resveratrol, (J) 200 µM H2O2 + 20 µM
resveratrol and (K) 200 µM H2O2 + 30 µM
resveratrol groups. (L) Statistical results of flow cytometry.
*P<0.05 vs. control; #P<0.05 vs.
H2O2 only group. H2O2,
hydrogen peroxide; ROS, reactive oxygen species; HUVECs, human
umbilical vein endothelial cells; DCF, dichlorofluorescein; RES,
resveratrol. |
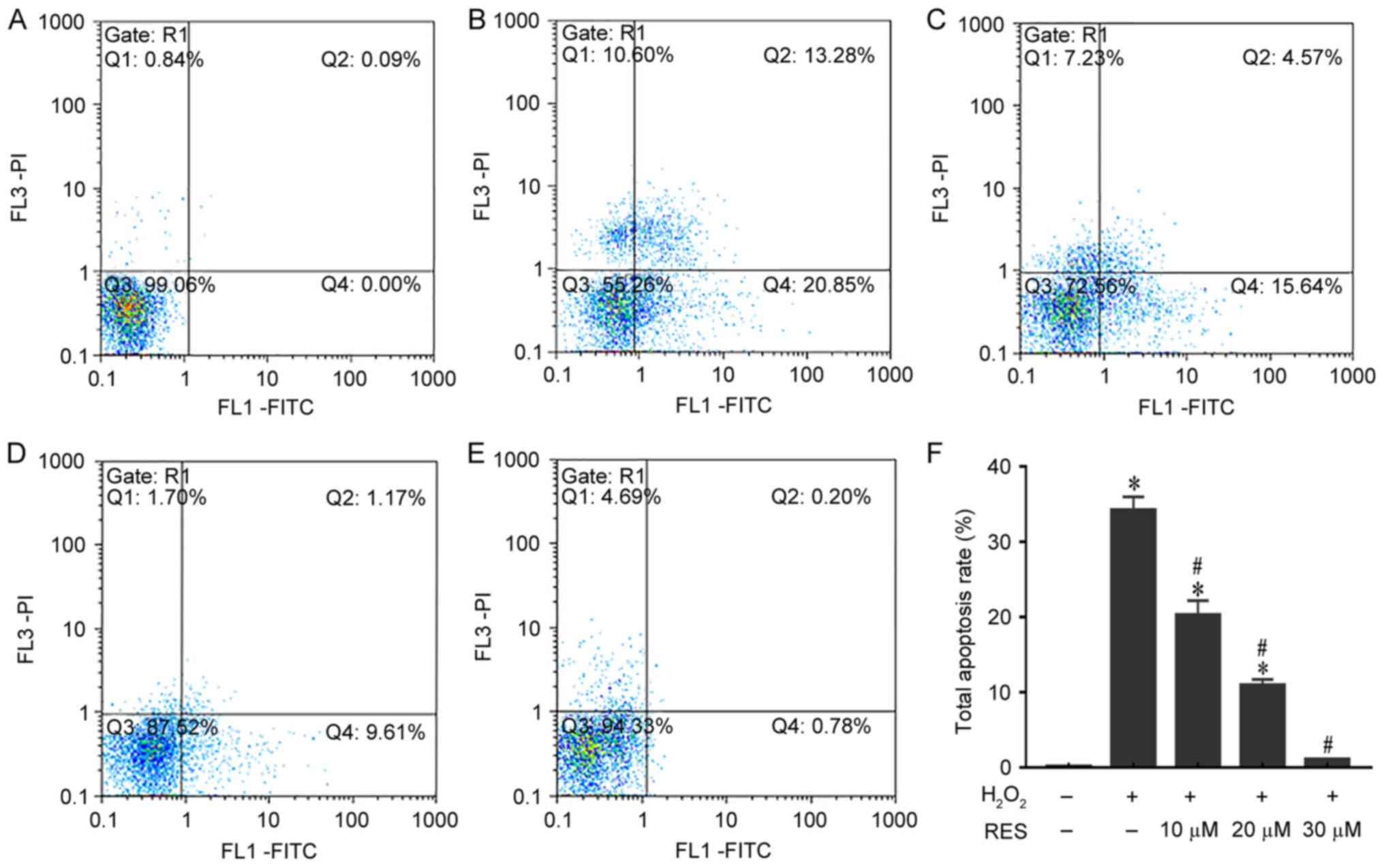
Resveratrol reduces
H2O2-induced apoptosis in HUVECs
To quantitatively investigate the anti-apoptotic
effects of resveratrol in H2O2-treated
HUVECs, cell apoptosis was measured by Annexin V/PI staining.
Quadrants 2 and 4 were considered to indicate apoptotic cells. As
demonstrated in Fig. 3, HUVEC
apoptosis increased to 34.19±1.8% (P<0.05 vs. control group)
when exposed to H2O2. By contrast, increasing
doses of resveratrol clearly reduced the apoptosis of HUVECs to
20.24±1.95, 10.93±0.77 and 1.03±0.05% (P<0.05 vs.
H2O2 only group). These results indicate that
resveratrol may prevent apoptosis induced by
H2O2.
Resveratrol inhibits the activation of
caspase-3, PSGL-1 and VWF induced by
H2O2
Caspase-3 is a key mediator of cell death and an
executioner for the death programme in cells in response to
H2O2. PSGL-1 and VWF are markers of a
prothrombotic status and predict the occurrence of thrombosis. As
demonstrated in Fig. 4A and B by
western blotting results, the protein expression of caspase-3 was
significantly increased by H2O2 treatment
compared with the control group (P<0.05). However, when cells
were pretreated with resveratrol for 2 h prior to incubation with
H2O2, caspase-3 protein expression was
significantly decreased in a concentration-dependent manner
compared with the H2O2 only group
(P<0.05). The protein levels of PSGL-1 and VWF were also
significantly increased by H2O2 compared with
the control group (P<0.05), while PSGL-1 and VWF protein
expression was significantly decreased by resveratrol pretreatment
in a concentration-dependent manner compared with the
H2O2 only group (P<0.05; Fig. 4A and B). The results for the mRNA
levels of caspase-3, PSGL-1 and VWF were similar to those observed
for protein levels, although only a resveratrol concentration of 30
µM was used (Fig. 4C).
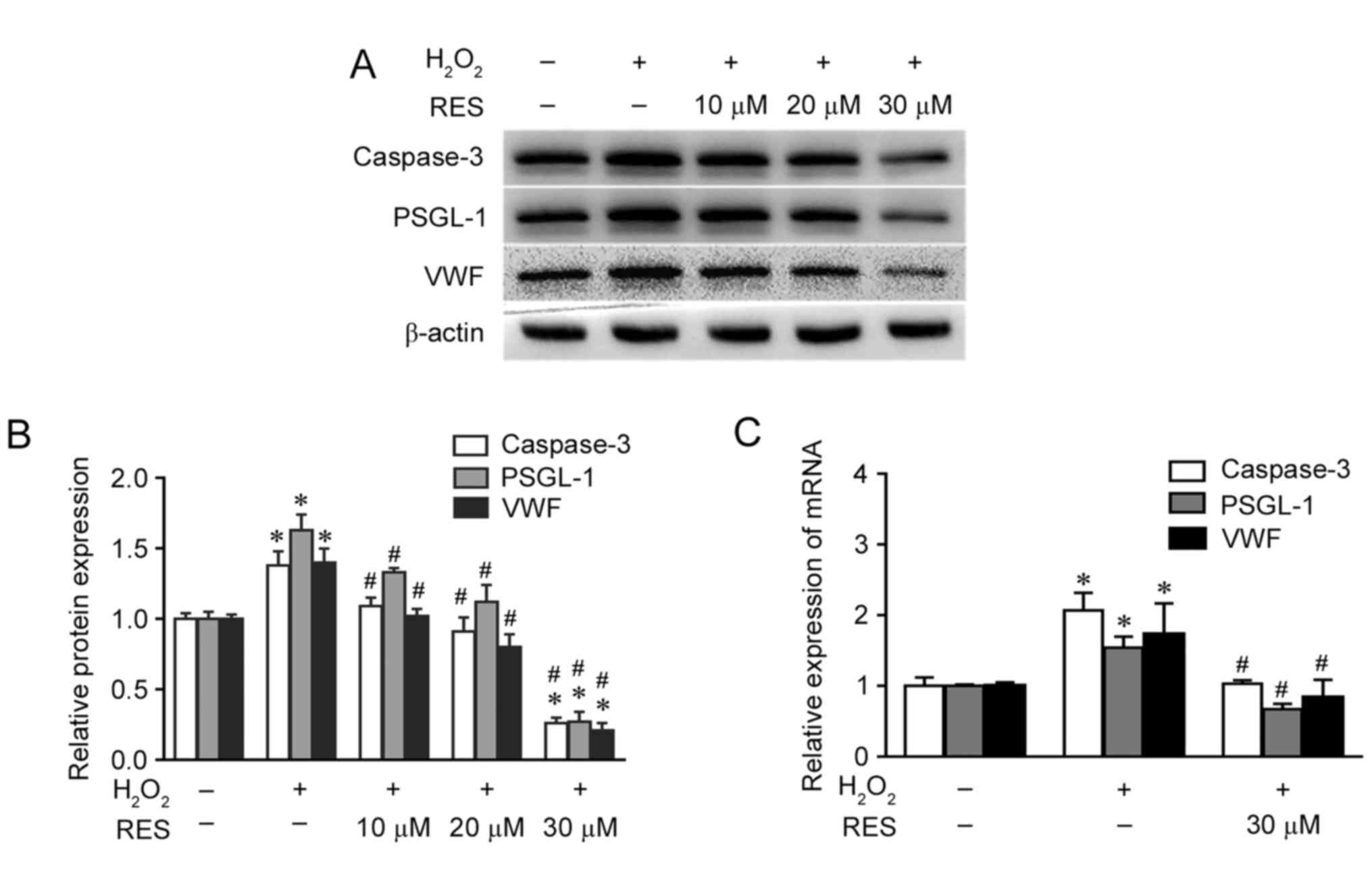 | Figure 4.Resveratrol inhibits the protein and
mRNA expression of caspase-3, VWF and PSGL-1 induced by
H2O2 in HUVECs. (A) Protein expression levels
of caspase-3, VWF and PSGL-1 in HUVECs exposed to 0, 10, 20 and 30
µM resveratrol for 2 h prior to treatment with or without 200 µM
H2O2 for 24 h, as measured by western blot
analysis. β-actin was used as the loading control. (B) Relative
expression ratios of caspase-3, VWF and PSGL-1 were determined by
densitometric analysis. (C) mRNA expression of caspase-3, PSGL-1
and VWF in the different groups was investigated by reverse
transcription-quantitative polymerase chain reaction. *P<0.05
vs. control; #P<0.05 vs. H2O2
only group. VWF, von Willebrand factor; PSGL-1, P-selectin
glycoprotein ligand-1; H2O2, hydrogen
peroxide; HUVECs, human umbilical vein endothelial cells; RES,
resveratrol. |
Resveratrol suppresses the activation
of PSGL-1 and VWF via MAPK signalling pathways
To investigate the underlying mechanisms of the
effects of resveratrol on thrombosis-associated markers, the
present study also detected the expression of MAPK signalling
pathway-associated proteins and mRNA by western blotting and
RT-qPCR, respectively. The mRNA levels of p38, ERK and c-Jun, and
the protein levels of p-p38, P-ERK and P-c-Jun, were significantly
increased by H2O2 compared with the control
group (P<0.05; Figs. 5 and
6). However, these increases were
significantly decreased by pretreatment with 30 µM resveratrol
(P<0.05; Figs. 5 and 6). When HUVECs were exposed to anisomycin
(0.4 µg/ml for 6 h), an activator of p38 and c-Jun, or curcumin (25
µM for 4 h), an activator of ERK, following treatment with
resveratrol, the suppressive effects of resveratrol on PSGL-1 and
VWF protein and mRNA expression were attenuated (P<0.05 vs. the
H2O2 + resveratrol group; Figs. 5 and 6). In addition, anisomycin or curcumin
treatment also significantly attenuated the effects of resveratrol
treatment on the mRNA expression of p38, ERK and c-Jun, and the
protein levels of p-p38, p-ERK and p-c-Jun (P<0.05 vs. the
H2O2 + resveratrol group; Figs. 5 and 6). However, no significant differences
were observed in the expression of total p38, ERK and c-Jun protein
expression among the different groups (Fig. 5). These results indicate that
resveratrol may suppress the activation of PSGL-1 and VWF induced
by H2O2 via MAPK signalling pathways.
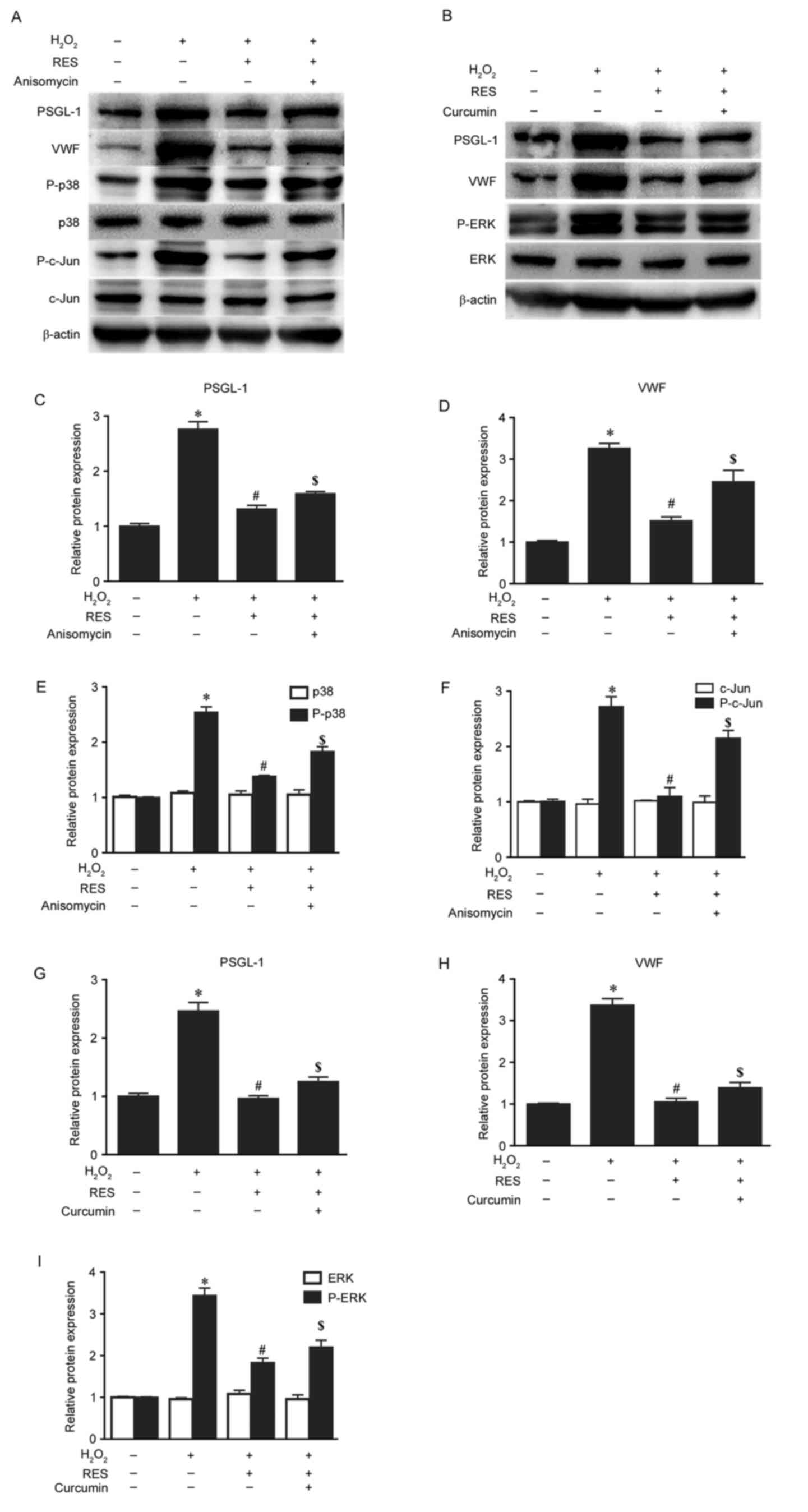 | Figure 5.Resveratrol suppresses the protein
expression of PSGL-1 and VWF induced by H2O2
via the mitogen-activated protein kinase signalling pathway. (A)
Protein expression levels of PSGL-1, VWF, p-p38, p38, p-c-Jun and
c-Jun in HUVECs exposed to 30 µM resveratrol for 2 h and 0.4 µg/ml
anisomycin for 6 h prior to treatment with 200 µM
H2O2 for 24 h were measured by western blot
analysis. (B) Protein expression levels of PSGL-1, VWF, p-ERK and
ERK in HUVECs exposed to 30 µM resveratrol for 2 h and 25 µM
curcumin for 4 h prior to treatment with 200 µM
H2O2 for 24 h were measured by western blot
analysis. β-actin was used as the loading control. The relative
expression ratios of (C) PSGL-1, (D) VWF, (E) p-p38 and p-38, and
(F) p-c-Jun and c-Jun, in cells treated with
H2O2 with or without pretreatment with
resveratrol and anisomycin were determined by densitometric
analysis. The relative expression ratios of (G) PSGL-1, (H) VWF,
and (I) P-ERK and ERK, in cells treated with
H2O2 with or without pretreatment with
resveratrol and curcumin were determined by densitometric analysis.
*P<0.05 vs. control; #P<0.05 vs.
H2O2 only group; $P<0.05 vs.
H2O2 + RES group. PSGL-1, P-selectin
glycoprotein ligand-1; VWF, von Willebrand factor;
H2O2, hydrogen peroxide; p, phosphorylated;
c-Jun, c-Jun N-terminal kinase; HUVECs, human umbilical vein
endothelial cells; ERK, extracellular signal-regulated kinase; RES,
resveratrol. |
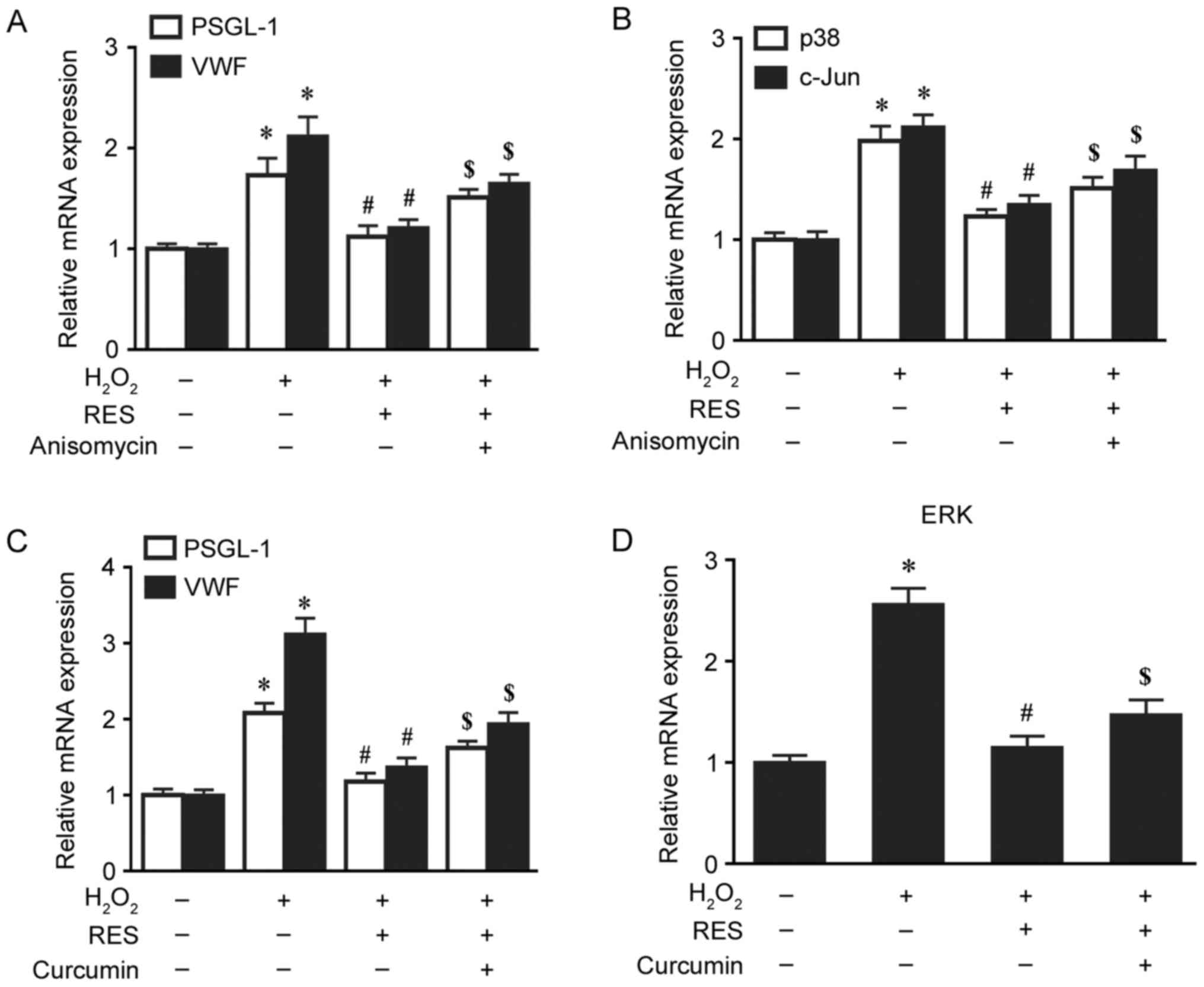 | Figure 6.Resveratrol suppresses the mRNA
upregulation of PSGL-1 and VWF induced by
H2O2 via the mitogen-activated protein kinase
signalling pathway. The relative mRNA expression levels of (A)
PSGL-1 and VWF, and (B) p38 and c-Jun, in HUVECs exposed to 30 µM
resveratrol for 2 h and 0.4 µg/ml anisomycin for 6 h prior to
treatment with 200 µM H2O2 for 24 h were
measured by RT-qPCR. The relative mRNA expression levels of (C)
PSGL-1 and VWF, and (D) ERK, in HUVECs exposed to 30 µM resveratrol
for 2 h and 25 µM curcumin for 4 h prior to treatment with 200 µM
H2O2 for 24 h were measured by RT-qPCR.
*P<0.05 vs. control; #P<0.05 vs.
H2O2 only group; $P<0.05 vs.
H2O2 + RES group. PSGL-1, P-selectin
glycoprotein ligand-1; VWF, von Willebrand factor;
H2O2, hydrogen peroxide; c-Jun, c-Jun
N-terminal kinase; HUVECs, human umbilical vein endothelial cells;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; ERK, extracellular signal-regulated kinase; RES,
resveratrol. |
Discussion
DVT is a common postoperative complication following
high-energy trauma that leads to localized clot (thrombus)
formation within a femoral or popliteal vein. Venous ECs, platelets
and leukocytes have crucial roles in regulating the local
microenvironmental balance between coagulation and anticoagulation.
Under normal physiological conditions, ECs maintain a vasodilatory
and local fibrinolytic state, in which the coagulation, adhesion
and activation of platelets and leukocytes is suppressed (22). Oxidative stress, defined as the
excessive production of ROS and reactive nitrogen species, has been
implicated in numerous diseases, including venous thrombosis. Under
pathological conditions, excess ROS production is induced by venous
stasis and hypoxia, which destroys endothelial structure and
function, promotes procoagulation reactions by upregulating cell
adhesion molecules (VWF and PSGL-1), inflammatory cytokines and
antifibrinolytic substances (plasminogen activator inhibitor-1),
and downregulates anticoagulants and fibrinolytic substances
(23,24).
Resveratrol, a potent antioxidant, was recently
determined to suppress oxidative stress and decrease ROS
generation; the study by Pan et al (25) demonstrated that resveratrol
suppressed tumour necrosis factor-α-triggered ROS generation in
HUVECs. Additionally, Zhou et al (26) reported that resveratrol
pretreatment significantly attenuated the detrimental effect of
tert-butyl hydroperoxide on cell viability and apoptosis by
inhibiting mitochondrial ROS generation in HUVECs. In addition,
Zhang et al (27) reported
that the effects of vinorelbine on cell apoptosis, intracellular
ROS generation and intracellular superoxide dismutase activity were
attenuated when ECV-304 human vascular endothelial cells were
pretreated with resveratrol. To confirm these studies, in the
present study, HUVECs were pretreated with resveratrol for 2 h and
incubated with H2O2 for 24 h. HUVEC viability
was decreased significantly by H2O2, one of
the major components of ROS. In addition, resveratrol protected
cells from H2O2-induced cytotoxicity in a
concentration-dependent manner, as demonstrated in Fig. 1. Furthermore, resveratrol
significantly decreased ROS levels and clearly attenuated
H2O2-induced apoptosis, as presented in
Figs. 2 and 3. As sufficient evidence indicates that
resveratrol may suppress oxidative stress and decrease ROS
generation, resveratrol may have potential in the prevention of
DVT.
VWF, which is produced by megakaryocytes and ECs, is
a multimeric glycoprotein that is expressed in the subendothelial
matrix, blood plasma, platelets and endothelium (28). VWF binds to exposed subendothelial
collagen rapidly upon damage to the vascular surface and triggers a
complex pathway, including platelet activation, adhesion and
aggregation (29). PSGL-1 is a
glycoprotein that was originally identified in leukocytes as the
ligand of P-selectin and has an important role in the selective
recruitment of leukocytes and activated platelets into the venous
wall, which amplifies the coagulation cascade and results in
increased thrombin generation (30,31).
Consequently, VWF and PSGL-1 are referred to as
thrombosis-associated markers, and increased levels of these
markers in the plasma predicts the occurrence of thrombosis
(20,32). To validate the inhibitory effect of
resveratrol on DVT in vitro, the present study investigated
the effect of resveratrol on VWF and PSGL-1 in HUVECs. The results
demonstrated that VWF and PSGL-1 secretion from the HUVECs was
stimulated by H2O2, while resveratrol
markedly decreased VWF and PSGL-1 elevation in a
concentration-dependent manner, as demonstrated in Fig. 4. To the best of our knowledge, the
present study is the first to investigate the effect of resveratrol
on thrombosis-associated markers, and the results indicate that
resveratrol may inhibit venous thrombosis by suppressing VWF and
PSGL-1. However, it should be noted that this conclusion is only
based on in vitro experiments and at the molecular level.
Follow-up animal studies are required to confirm this
hypothesis.
Various studies have reported that MAPK pathways
contribute to vascular endothelial oxidative stress and venous
thrombosis. The expression and secretion of VWF via the activation
of ERK was blocked by inhibitors of ERK in HUVECs (33). Venous thrombosis triggered by
PSGL-1 and P-selectin was reported to depend on MAPK signalling
pathways (34). In the present
study, the results demonstrated that H2O2
(200 µM) induced upregulation of mRNA and protein phosphorylation
levels of p38, ERK and c-Jun in HUVECs, and resveratrol
downregulated these effects at the mRNA and protein phosphorylation
levels. When HUVECs were pretreated with the MAPK activators
anisomycin and curcumin, the suppressive effects of resveratrol on
PSGL-1 and VWF were attenuated, as demonstrated in Figs. 5 and 6. A studies reported that MAPKs cascades
were maximally activated within 10–20 min after treatment with
numerous stimuli (35). To
investigate the simultaneous alterations in MAPKs with PSGL-1 and
VWF, levels of p-p38, P-ERK and p-c-Jun were assayed after
treatment with H2O2 for 24 h. Due to this
discrepancy and limitation of the present study, MAPK pathways
should be assayed within 20 min to validate the results of the
present study.
The results of the present study indicated that
resveratrol may inhibit the activation of VWF and PSGL-1 via MAPK
pathways. However, regarding the development of venous thrombosis,
the underlying molecular mechanisms regulating the expression of
VWF and PSGL-1 is complex and requires further investigation. In
conclusion, these findings demonstrate that resveratrol may protect
HUVECs from oxidative stress and apoptosis, and suppress the
expression of thrombosis-associated markers, by inactivating MAPK
signalling pathways.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81160236) and the Health
Science and Technology Project of Yunnan province (grant nos.
2014NS142 and 2017NS022).
Glossary
Abbreviations
Abbreviations:
|
DVT
|
deep venous thrombosis
|
|
ROS
|
reactive oxygen species
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
ECs
|
endothelial cells
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
c-Jun
|
c-Jun N-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
VWF
|
von Willebrand factor
|
|
PSGL-1
|
P-selectin glycoprotein ligand-1
|
References
|
1
|
Ashrani AA and Heit JA: Incidence and cost
burden of post-thrombotic syndrome. J Thromb Thrombolysis.
28:465–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dixon J, Ahn E, Zhou L, Lim R, Simpson D
and Merriman EG: Venous thromboembolism rates in patients
undergoing major hip and knee joint surgery at waitemata district
health board: A retrospective audit. Intern Med J. 45:416–422.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cha SI, Lee SY, Kim CH, Park JY, Jung TH,
Yi JH, Lee J, Huh S, Lee HJ and Kim SY: Venous thromboembolism in
Korean patients undergoing major orthopedic surgery: A prospective
observational study using computed tomographic (CT) pulmonary
angiography and indirect CT venography. J Korean Med Sci. 25:28–34.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ho KM, Burrell M, Rao S and Baker R:
Incidence and risk factors for fatal pulmonary embolism after major
trauma: A nested cohort study. Br J Anaesth. 105:596–602. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
López JA and Chen J: Pathophysiology of
venous thrombosis. Thromb Res. 123 Suppl 4:S30–S34. 2009.
View Article : Google Scholar
|
|
6
|
Kim YW and Byzova TV: Oxidative stress in
angiogenesis and vascular disease. Blood. 123:625–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ekim M, Sekeroglu MR, Balahoroglu R, Ozkol
H and Ekim H: Roles of the oxidative stress and ADMA in the
development of deep venous thrombosis. Biochem Res Int.
2014:7031282014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan LM, Douglas G, Bendall JK, McNeill E,
Crabtree MJ, Hale AB, Mai A, Li JM, McAteer MA, Schneider JE, et
al: Endothelial cell-specific reactive oxygen species production
increases susceptibility to aortic dissection. Circulation.
129:2661–2672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng M, Wei X, Wu Z, Li W, Li B, Fei Y, He
Y, Chen J, Wang P and Liu X: Reactive oxygen species contribute to
simulated ischemia/reperfusion-induced autophagic cell death in
human umbilical vein endothelial cells. Med Sci Monit.
20:1017–1023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Duan W, Liang Z, Yi W, Yan J, Wang
N, Li Y, Chen W, Yu S, Jin Z and Yi D: Curcumin attenuates
endothelial cell oxidative stress injury through Notch signaling
inhibition. Cell Signal. 25:615–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kostyuk VA, Potapovich AI, Suhan TO, de
Luca C and Korkina LG: Antioxidant and signal modulation properties
of plant polyphenols in controlling vascular inflammation. Eur J
Pharmacol. 658:248–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Csiszár A, Csiszar A, Pinto JT, Gautam T,
Kleusch C, Hoffmann B, Tucsek Z, Toth P, Sonntag WE and Ungvari Z:
Resveratrol encapsulated in novel fusogenic liposomes activates
Nrf2 and attenuates oxidative stress in cerebromicrovascular
endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci.
70:303–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brisdelli F, D'Andrea G and Bozzi A:
Resveratrol: A natural polyphenol with multiple chemopreventive
properties. Curr Drug Metab. 10:530–546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo H, Chen Y, Liao L and Wu W:
Resveratrol protects HUVECs from oxidized-LDL induced oxidative
damage by autophagy upregulation via the AMPK/SIRT1 pathway.
Cardiovasc Drugs Ther. 27:189–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu YX, Cui H, Fan L, Pan XJ, Wu JH, Shi
SZ, Cui SY, Wei ZM and Liu L: Resveratrol attenuates left
ventricular remodeling in old rats with COPD induced by cigarette
smoke exposure and LPS instillation. Can J Physiol Pharmacol.
91:1044–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lan F, Weikel KA, Cacicedo JM and Ido Y:
Resveratrol-induced AMP-Activated protein kinase activation is
cell-type dependent: Lessons from basic research for clinical
application. Nutrients. 9:E7512017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdel-Aleem GA, Khaleel EF, Mostafa DG and
Elberier LK: Neuroprotective effect of resveratrol against brain
ischemia reperfusion injury in rats entails reduction of DJ-1
protein expression and activation of PI3K/Akt/gsk3b survival
pathway. Arh Physiol Biochem. 122:200–213. 2016. View Article : Google Scholar
|
|
18
|
Adam F, Kauskot A, Rosa JP and Bryckaert
M: Mitogen-activated protein kinases in hemostasis and thrombosis.
J Thromb Haemost. 6:2007–2016. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lazzari MA, Sanchez-Luceros A, Woods AI,
Alberto MF and Meschengieser SS: Von Willebrand factor (VWF) as a
risk factor for bleeding and thrombosis. Hematology. 17 Suppl
1:S150–S152. 2012.PubMed/NCBI
|
|
20
|
Miszti-Blasius K, Debreceni IB, Felszeghy
S, Dezso B and Kappelmayer J: Lack of P-selectin glycoprotein
ligand-1 protects mice from thrombosis after collagen/epinephrine
challenge. Thromb Res. 127:228–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bertina RM: The role of procoagulants and
anticoagulants in the development of venous thromboembolism. Thromb
Res. 123 Suppl 4:S41–S45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Violi F and Pignatelli P: Platelet
oxidative stress and thrombosis. Thromb Res. 129:378–381. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wakefield TW, Myers DD and Henke PK: Role
of selectins and fibrinolysis in VTE. Thromb Res. 123 Suppl
4:S35–S40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan W, Yu H, Huang S and Zhu P:
Resveratrol protects against TNF-α-induced injury in human
umbilical endothelial cells through promoting sirtuin-1-induced
repression of NF-KB and p38 MAPK. PLoS One. 11:e01470342016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou X, Chen M, Zeng X, Yang J, Deng H, Yi
L and Mi MT: Resveratrol regulates mitochondrial reactive oxygen
species homeostasis through Sirt3 signaling pathway in human
vascular endothelial cells. Cell Death Dis. 5:e15762014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Tong N, Chen Y, Li P, Yang S and
Zhao X: Resveratrol protects against vinorelbine-induced vascular
endothelial cell injury. Toxicol Mech Methods. 23:665–671. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsui T and Hamako J: von Willebrand
factor and von Willebrand disease. Rinsho Ketsueki. 57:2113–2123.
2016.PubMed/NCBI
|
|
29
|
Castaman G and Linari S: Human von
Willebrand factor/factor VIII concentrates in the management of
pediatric patients with von Willebrand disease/hemophilia A. Ther
Clin Risk Manag. 12:1029–1037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cerletti C, Tamburrelli C, Izzi B,
Gianfagna F and de Gaetano G: Platelet-leukocyte interactions in
thrombosis. Thromb Res. 129:263–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hubert L, Darbousset R, Panicot-Dubois L,
Robert S, Sabatier F, Fallague K, Dignat-George F and Dubois C:
Neutrophils recruit and activate human endothelial colony-forming
cells at the site of vessel injury via P-selectin glycoprotein
ligand-1 and L-selectin. J Thromb Haemost. 12:1170–1181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lazzari MA, Sanchez-Luceros A, Woods AI,
Alberto MF and Meschengieser SS: Von Willebrand factor (VWF) as a
risk factor for bleeding and thrombosis. Hematology. 17 Suppl
1:S150–S152. 2012.PubMed/NCBI
|
|
33
|
Wang J, Li H, He J, Li B, Bao Q, Zhang X,
Lv Z, Zhang Y, Han J, Ai D and Zhu Y: 20-Hydroxyeicosatetraenoic
acid involved in endothelial activation and thrombosis. Am J
Physiol Heart Circ Physiol. 308:H1359–H1367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zuchtriegel G, Uhl B, Puhr-Westerheide D,
Pörnbacher M, Lauber K, Krombach F and Reichel CA: Platelets guide
leukocytes to their sites of extravasation. PLoS Biol.
14:e10024592016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao H, Liu L, Zhao Y, Hara H, Chen P, Xu
J, Tang J, Wei L, Li Z, Cooper DK, et al: Human IL-6, IL-7, IL-1β,
and TNF-α differently regulate the expression of pro-inflammatary
related genes, tissue factor, and swine leukocyte antigen class I
in porcine aortic endothelial cells. Xenotransplantation.
24:e122912017. View Article : Google Scholar
|