Introduction
Membranous nephropathy (MN), the most common cause
of adult nephrosis, is an antibody-mediated glomerular illness
characterized by subepithelial immune complex deposits, including
antigen and complement components (1,2). The
clinical symptoms of MN are usually benign or painless; however,
30–40% of patients develop progressive nephrotic impairments that
result in end-stage renal failure within 5–15 years (3). The various types of MN make it
difficult to determine the best treatment method for each patient
(4,5). MN is an immune complex-mediated
nephritogenic immunoreaction. The pathogenesis of MN has not been
clarified; therefore, finding biomarkers for the early diagnosis
and classification of MN severity is necessary (6).
IL-33 is a member of the IL-1 cytokine family, which
includes IL-1a, IL-1b, IL-1 receptor antagonists; in addition,
IL-33 and can be produced by epithelial and vascular endothelial
cells (7). IL-33 activates various
immune cells through binding to the suppression of tumorigenicity 2
(ST2) receptor. This action leads to the production of various
molecules and pro-inflammatory cytokines. Once bound to the ST2
acceptor, IL-33 can activate the MyD88 and NF-κB signaling pathways
(7,8). ST2, an IL-33 receptor and IL-1
receptor family member, is located on Th2 cells, mast cells and
epithelial cells, but not Th1 cells, and contributes to Th2
activity (7,9). ST2 exists in a transmembrane form
(ST2L) and an excretable soluble form of ST2 (sST2). sST2 acts as a
decoy receptor by binding to free IL-33 and preventing its
signaling via ST2L; however, ST2 plays a role as an intermediary of
IL-33 biological activity (10).
In fact, the IL-33/ST2 axis plays a vital role in several chronic
immune inflammatory disorders, including asthma (7), rheumatoid arthritis (11), and chronic intestinal inflammation
(12). Levels of sST2 have been
associated with the activity and severity of these diseases
(13). Nonetheless, there are few
studies of whether the IL-33/ST2 axis could be involved in the
pathogenesis of membranous nephropathy.
A recent study showed that urinary concentrations of
IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ and TNF-α were considerably
higher in MN patients than in healthy controls (HCs) (14). Another study reported no
differences in IFN-γ and IL-4 mRNA levels in peripheral blood
mononuclear cells (PBMCs) in MN and HC groups. In contrast, IL-10
mRNA levels in PBMCs were considerably higher in the MN patients
than in the HCs (15).
Unfortunately, there is little information on variations in serum
cytokine levels in MN. Therefore, it is imperative that this area
be studied.
To determine the underlying function of IL-33 and
sST2 in the pathogenesis of MN, we measured their serum
concentrations in MN patients and HCs. Furthermore, we investigated
the relation between serum sST2 levels and disease severity. Our
data provide new insight into the role of the ST2/IL-33 system in
the pathogenesis of MN.
Materials and methods
Patients
A total of 93 consecutive MN patients from the
inpatient service of the First Hospital of Jilin University
(Changchun, China) from July 2012 to August 2015 were included in
our study. Written informed consent was obtained from all
participators. The protocol was established according to the
guidelines of the Declaration of Helsinki and was approved by the
Human Ethics Committee of the First Hospital of Jilin University
(no. 2012-102). Individual patients with MN were diagnosed
according to the World Health Organization (WHO) histological
classification standards as follows: stage I, most glomerular
basement membranes (GBMs) are normal. Only GBM vacuolar
degeneration and a small amount of homophilic protein deposited in
the epithelium are visible by light microscopy. Electron-dense
deposits can be observed by using an electron microscope. Stage II,
the GBMs are incrassate. Increased electron density and GBM
hyperplasia are visible by electron microscopy. Stage III, the GBMs
are visibly incrassate. Electron-dense deposits appear as
double-track or chain-like structures. Stage IV, GBM are severely
incrassate. The lumen has shrunk, and the renal tubules are
sclerous. Electron microscopy show that the electron-dense deposits
look like round holes after being dissolved and absorbed. Stage V,
the incrassate GBM is gradually restored. Immunopathology shows
that immunoglobulin IgG and complement C3 are deposited along the
lateral basolateral membrane with granular and high intensity.
Later, this deposition is decreased. Some patients with MN were
diagnosed with nephrotic syndrome on the basis of diagnostic
standards, including proteinuria (>3.5 g/day), hypoalbuminemia
(albumin <30 g/l), dropsy and hyperlipidemia. No patients were
treated with hormone medication or other immunosuppressants prior
to our research. The levels of ANA, anti-Sm antibody,
antineutrophil cytoplasmic antibody (ANCA), viral serology, alexin
C3 and C4, rheumatoid factor, and blood glucose were measured in
each patient, and each patient was subjected to an ophthalmological
review or an echocardiogram. Exclusion criteria included: i) MN
with a fast progression (with a rapid decline in kidney function)
and secondary types of MN, such as lupus nephritis,
Henoch-Schonlein purpura, and diabetes mellitus; and ii) neoplasia,
pregnancy, other autoimmune diseases, kinetic peptic ulcer illness
or short-term infections. A total of 34 subjects matched for sex,
age and race were included as HCs. Their demographic and clinical
features were recorded and analyzed (Table I).
 | Table I.Demographic and clinical
characteristics of the participants. |
Table I.
Demographic and clinical
characteristics of the participants.
|
|
| MN patients
(n=93) |
|---|
|
|
|
|
|---|
| Characteristic | HC (n=34) | Stage I (n=18) | Stage I–II
(n=38) | Stage II
(n=26) | Stage III
(n=11) |
|---|
| Age, years | 43 (18–76) | 46 (22–68) | 51 (19–81) | 44 (21–74) | 53 (32–63) |
| Sex,
female/male | 15/19 | 10/8 | 16/22 | 16/10 | 4/7 |
| Lymphocytes,
109/l | 1.72
(0.84–2.87) | 2.12
(0.86–3.14) | 2.31
(0.87–3.51) | 2.36
(0.84–3.65) | 2.23
(1.72–2.84) |
| Serum albumin,
g/l | 43.2
(40.8–50.6) | 29.3
(11.7–41.4)a | 28.4
(15.3–36.8)a | 27.7
(14.6–37.4)a | 24.6
(19.4–36.2)a |
| Serum uric acid,
µmol/l | 305 (245–416) | 346 (247–618) | 389 (259–602) | 398 (268–574) | 366 (274–524) |
| Triglycerides,
mmol/l | 1.17
(0.51–1.57) | 2.43
(1.04–5.56) | 2.52
(1.24–4.73) | 2.61
(1.18–5.85) | 2.36
(2.05–8.94) |
| Cholesterol,
mmol/l | 4.07
(2.86–5.47) | 7.42
(5.4–12.45) | 7.25
(5.05–11.6) | 7.51
(5.2–10.46) | 6.37
(5.86–12.7) |
| Urinary proteins,
g/24 h | 0.048 (0–0.12) | 4.15
(1.63–6.85)a | 5.28
(1.26–11.2)a | 5.77
(1.71–12.6)a | 3.16
(2.38–6.45)a |
| Urea nitrogen,
mmol/l | 5.14
(3.62–6.68) | 5.68
(3.03–12.41) | 5.47
(3.37–11.6) | 5.8
(3.28–11.43) | 5.37
(4.13–8.52) |
| eGFR, ml/min/1.73
m2 | 104.5
(88.5–112.4) | 86.6
(64.4–114.2)a | 81.4
(37.4–116.6)a | 74.5
(28.6–92.5)a | 62.3
(22.3–84.3)a |
| Microscopic
hematuria, rbc/hpf | 1.1 (0–2.4) | 6.4
(1.4–15.3)a | 7.3
(0.8–40.5)a | 9.6
(3.2–35.7)a | 8.4
(4.9–72.4)a |
| Serum calcium,
mmol/l | 2.38
(1.91–2.62) | 1.68
(1.23–3.16)a | 2.04
(0.57–2.85)a | 1.97
(1.35–2.87)a | 1.94
(1.44–2.76)a |
| Serum phosphorus,
mmol/l | 1.15
(0.74–1.49) | 1.46
(0.74–3.25)a | 1.72
(0.64–3.18)a | 1.59
(0.61–2.64)a | 1.74
(1.03–3.55)a |
Therapy and follow-up study
After being admitted, individual patients were
treated with 1 mg/kg/day prednisolone (PDN; Tianyao
Pharmaceuticals, Tianjin, China) for the first two months.
Afterwards, the dose was gradually reduced to 10 mg/day for
maintenance and continued for the next 6 months. The patients were
also treated with cyclosporine. Patient follow-ups were conducted
for 8–12 weeks. Altogether, 19 patients completed their follow-up,
but the other 74 patients did not. Of these 19 patients, 4 were in
stage I, 6 were in stage I–II, 8 were in stage II, and 1 was in
stage III. After 8–12 weeks of treatment, serum samples were
collected during kidney biopsy.
Measurement of serum IL-33 and sST2
levels by enzyme-linked immunosorbent serologic assay (ELISA)
Serum IL-33 and sST2 concentrations in MN patients
and HCs were measured by human IL-33 (Affymetrix, Santa Clara, CA,
USA; eBioscience, San Diego, CA, USA) and sST2 (ImmunoWay, Plano,
TX, USA) ELISA kits according to the manufacturer's instructions.
Serum IL-33 and sST2 levels in the specimens were calculated using
standard curves established from the recombinant IL-33 and sST2
supplied in the kits. The limits of detection for human IL-33 and
sST2 were 0.2 and 0.25 pg/ml, respectively.
Cytometric bead array (CBA) analysis
of serum cytokines
Serum IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL-10 and
IL-17A levels were measured by CBA (BD Biosciences) (16) according to the manufacturer's
instructions with minor modifications. Individual serum samples (25
µl) were analyzed in duplicate using a FACSCalibur flow cytometry
system (BD Biosciences, San Jose, CA, USA) (17), and serum cytokine levels were
calculated using CellQuest Pro and CBA software (Becton-Dickinson,
San Jose, CA, USA) and a BD FACSAria II system.
Statistical analysis
Data are expressed as the median and range unless
specified. Differences between two groups were analyzed by
Mann-Whitney U nonparametric tests. Relationships between variables
were evaluated by Spearman's rank correlation tests. All
statistical analyses were performed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA) software. Two-sided P-values <0.05 were
considered to indicate a statistically significant difference.
Results
Characteristics of MN patients
To determine the potential role of serum IL-33 and
sST2 concentrations in the pathogenesis of MN, 93 Chinese patients
with newly diagnosed MN and 34 HCs were recruited for this study.
There were no significant differences in the age distribution;
serum uric acid, triglyceride, cholesterol or urea nitrogen levels
or lymphocyte counts between the groups (Table I). As expected, the MN patients had
significantly higher 24-h urine protein and serum phosphorus levels
than the HCs. However, the estimated glomerular filtration rates
(eGFRs) and the serum calcium and albumin levels were considerably
lower in the patients than in the HCs. These data suggest that the
MN patients had renal dysfunction.
Serum levels of IL-33 and sST2
Serum levels of IL-33 and sST2 were measured in 93
patients and 34 age- and sex-matched HCs. Serum cytokine analyses
showed no evident differences in the serum levels of IL-33 between
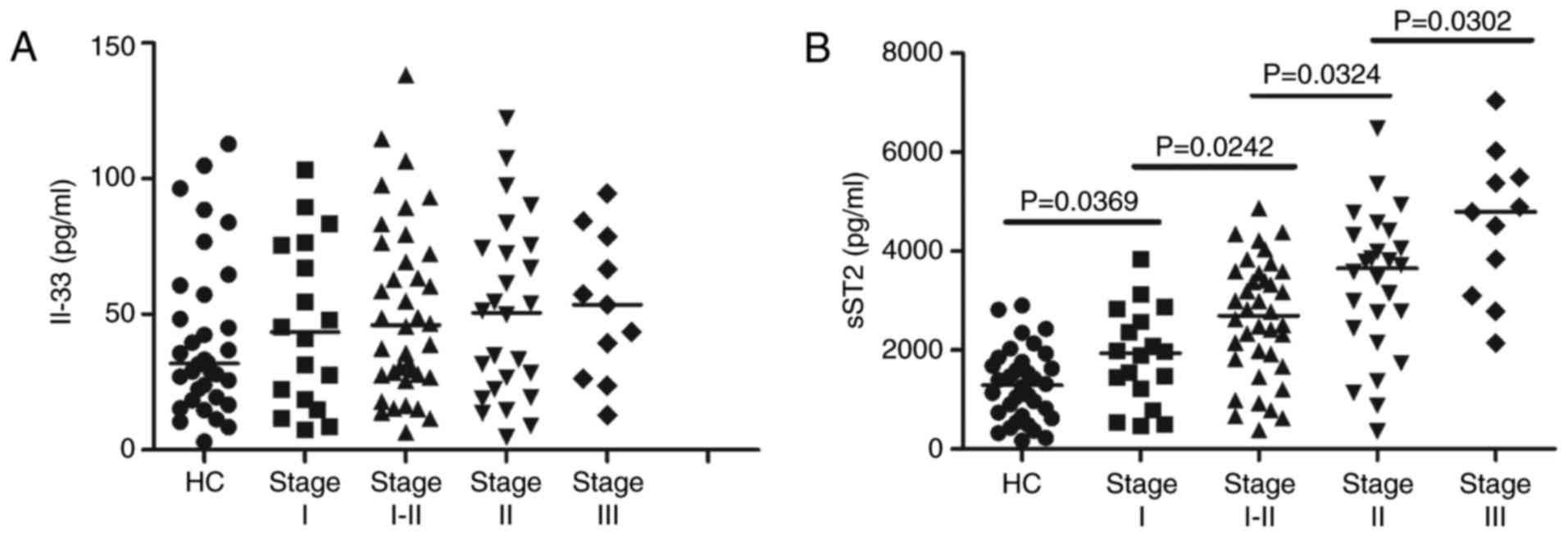
the MN patients at different stages and the HCs (Fig. 1A). In addition, there were no
marked differences in the serum levels of IL-33 among MN patients
at different stages (data not shown). However, the serum sST2
concentrations were higher in MN patients at all stages than in the
HCs (P<0.05; Fig. 1B).
Furthermore, the serum sST2 levels were positively correlated with
disease severity in the MN patients (r=0.503, P<0.001; Fig. 1B).
Relationship between the serum levels
of sST2 and the clinical features of MN patients
To understand the role of sST2 in the pathogenesis
of MN, we evaluated the relationship between the serum sST2 levels
and the clinical features of MN patients. In these patients, serum
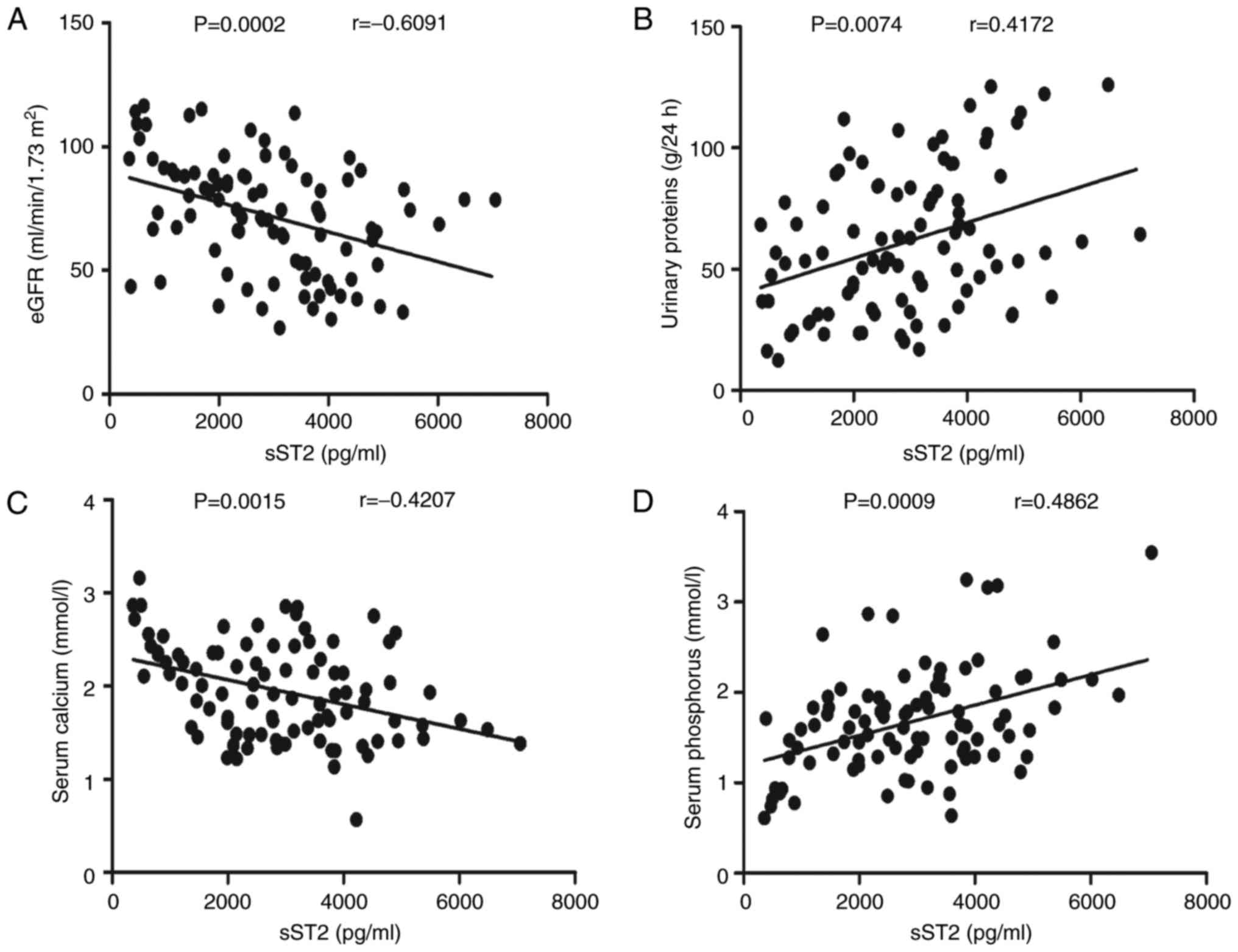
sST2 levels were negatively correlated with the eGFRs (r=−0.6091,
P<0.001; Fig. 2A) but
positively correlated with the 24-h urinary protein (r=0.4172,
P=0.0074; Fig. 2B). Serum sST2
levels were negatively correlated with serum calcium levels
(r=−0.4207, P=0.0015; Fig. 2C) but
positively correlated with serum phosphorus (r=0.4862, P<0.001;
Fig. 2D) levels. In contrast, the
clinical features and IL-2, IL-4, IL-10, IL-17A, IFN-γ and IL-33
levels were not correlated (data not shown). Taken together, our
research reveals that sST2 may participate in the pathogenesis of
MN and play a pivotal role in bone metabolism disorders in MN
patients.
Increased serum concentrations of
IL-2, IL-4, IL-10, IL-17A and IFN-γ in MN patients before
treatment
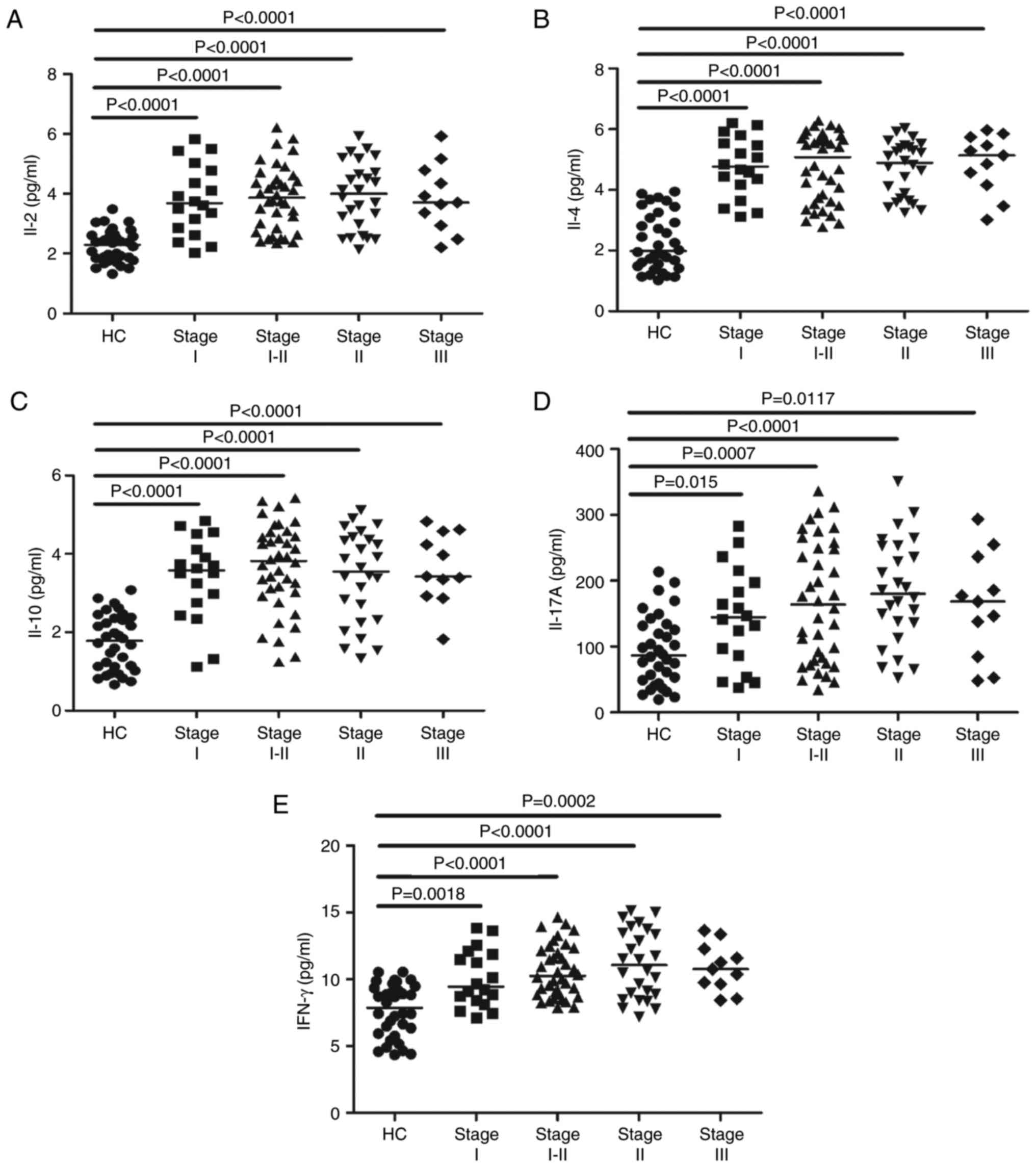
To determine the potential effects of IL-2, IL-4,
IL-10, IL-17A and IFN-γ on MN, we measured their serum levels by
CBA analysis. The serum levels of IL-2, IL-4, IL-10, IL-17A and
IFN-γ were considerably higher in the MN patients than in the HCs
(P<0.05; Fig. 3A-E). However,
the concentrations of those cytokines were not obviously different
among MN patients at different stages (data not shown). These data
indicate that these cytokines may be involved in the mechanism of
MN.
Correlation analyses of the serum
levels of sST2 with IL-2, IL-4, IL-10, IL-17A, IFN-γ and IL-33 in
MN patients
To understand the importance of sST2 in MN, we
evaluated the relationship between serum sST2 levels and IL-2,
IL-4, IL-10, IL-17A, IFN-γ and IL-33 levels in MN patients. Serum
sST2 levels were negatively correlated with IL-4 levels (r=−0.4337,
P<0.001; Fig. 4A). In contrast,
there were no significant correlations between serum sST2 levels
and IL-2, IL-10, IL-17A, IFN-γ or IL-33 levels in MN patients
(Fig. 4B-F).
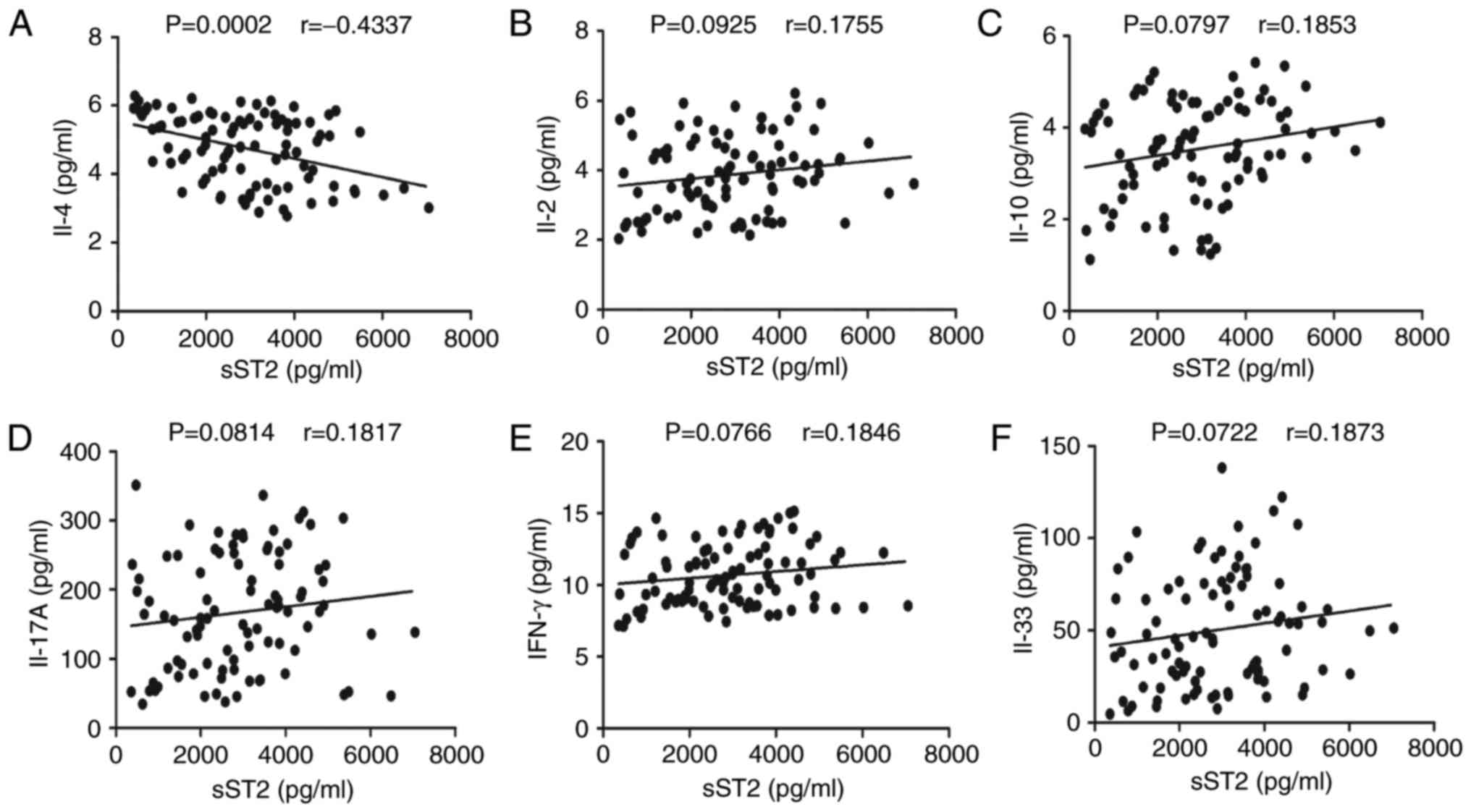 | Figure 4.Correlation analyses of serum sST2
levels with IL-2, IL-4, IL-10, IL-17A, IFN-γ and IL-33 levels in MN
patients. The potential correlations between serum sST2 levels and
IL-2, IL-4, IL-10, IL-17A, IFN-γ and IL-33 levels were determined
by using Spearman correlation tests. The data shown are the
averages of individual specimens from two independent tests. (A)
Serum sST2 levels were negatively correlated with IL-4 levels.
(B-F) No apparent differences were discovered between serum sST2
levels and IL-2, IL-10, IL-17A, IFN-γ or IL-33 levels in MN
patients. |
Correlation analyses of serum sST2
levels with IL-4 levels in MN patients at different stages
As shown in Fig.
4A, serum sST2 levels were negatively correlated with IL-4
levels (r=−0.4337, P=0.0002). We next analyzed the correlations
between serum sST2 levels and IL-4 levels in MN patients at
different stages. We found that serum sST2 levels were negatively
correlated with IL-4 levels in stage I (r=−0.6409, P=0.0042;
Fig. 5A), stage I–II (r=−0.4183,
P=0.009; Fig. 5B), stage II
(r=−0.4719, P=0.0149; Fig. 5C) and
stage III (r=−0.6364, P=0.0353; Fig.
5D) MN patients.
Clinical parameters and serum cytokine
levels in MN patients after treatment
To better understand the role of the IL-33/ST2 axis
in MN development, we analyzed the clinical features and serum
cytokine concentrations of patients who were followed up for 8–12
weeks. In total, 19 patients had complete records, whereas the
other 74 patients failed to complete the follow-up. The 24-h urine
protein, microscopic hematuria and serum phosphorus levels were
obviously lower in the MN patients than in the HCs, but the eGFRs
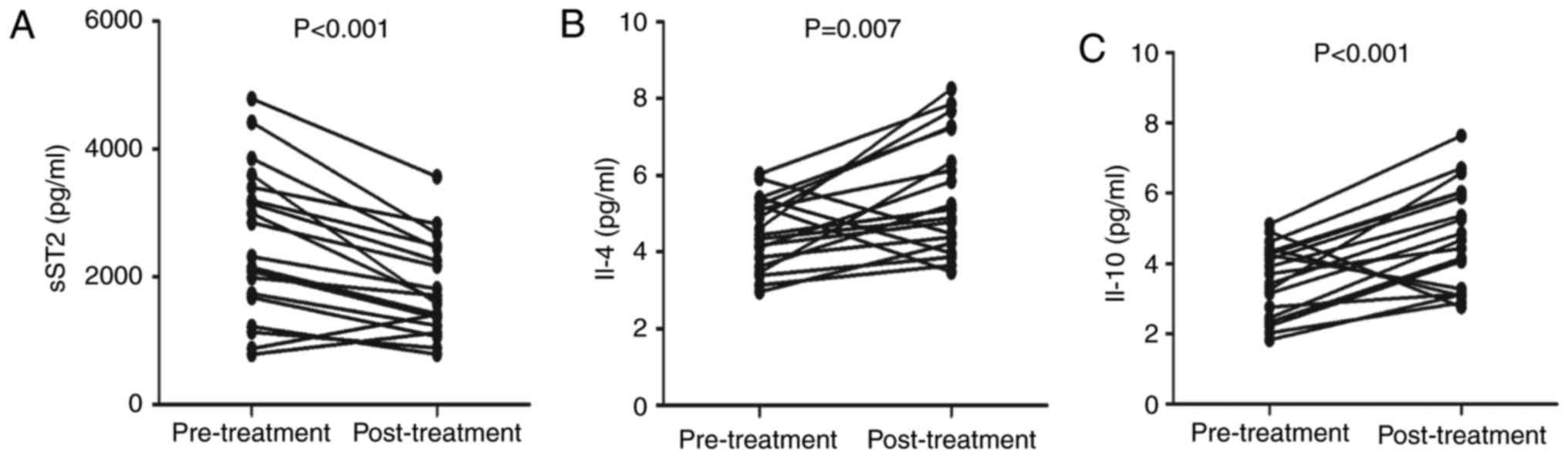
and serum calcium levels were significantly higher (Table II). In addition, the serum sST2
levels were significantly lower than the pretreatment levels
(P<0.001; Fig. 6A).
Furthermore, serum IL-4 (P=0.007; Fig.
6B) and IL-10 (P<0.001; Fig.
6C) levels were higher after treatment than before treatment.
No marked differences were found in the concentrations of other
serum cytokines before and after treatment (data not shown).
Collectively, MN treatment obviously improved renal function by
decreasing the sST2 serum levels and increasing the IL-4 and IL-10
levels in MN patients.
 | Table II.Effects of treatment on the clinical
measurements in the MN patients at follow-up. |
Table II.
Effects of treatment on the clinical
measurements in the MN patients at follow-up.
| Characteristic | Before
treatment | After
treatment |
|---|
| Age, years | 48 (29–68) | 48 (29–68) |
| Sex,
female/male | 10/9 | 10/9 |
| Lymphocytes,
109/l | 2.34
(1.14–3.25) | 2.28
(1.10–3.11) |
| Serum albumin,
g/l | 28.6
(16.8–34.6) | 37.2
(26.8–46.6) |
| Serum uric acid,
µmol/l | 376 (253–564) | 348 (232–523) |
| Triglycerides,
mmol/l | 2.73
(1.34–5.42) | 2.57
(1.18–5.16) |
| Cholesterol,
mmol/l | 7.22
(5.28–11.26) | 6.27
(4.81–10.13) |
| Urinary proteins,
g/24 h | 5.31
(1.44–8.26) | 3.45
(1.78–6.12)a |
| Urea nitrogen,
mmol/l | 5.74
(3.87–10.85) | 4.42
(2.75–9.18) |
| eGFR, ml/min/1.73
m2 | 82.36
(46.74–102.85) | 98.63 (62.26
−115.28)a |
| Microscopic
hematuria, rbc/hpf | 8.65
(1.45–67.35) | 3.34
(0.31–42.23)a |
| Serum calcium,
mmol/l | 1.96
(1.34–2.43) | 2.34
(2.05–2.54)a |
| Serum phosphorus,
mmol/l | 1.76
(0.93–2.03) | 1.17
(0.78–1.35)a |
Discussion
IL-33 is a multipurpose cytokine that participates
in several illnesses (13,18–20).
Furthermore, IL-33 can activate various immune cells, MAP kinases
and NF-κB signaling pathways and facilitate Th2 reactions through
its receptor complex, which comprises ST2 and IL-1RaP (7). When activated by IL-33, structures
containing endothelial cells, epithelial cells and fibroblasts
produce mediators consistent with the respective cell types. This
action exacerbates allergic inflammation. Recently, the IL-33/ST2
axis has been shown to play an important part in several chronic
immune inflammatory disorders, including systemic lupus
erythematosus (SLE) and rheumatoid arthritis (RA) (21). Serum IL-33 levels are increased in
SLE and RA patients and are correlated with the serum erythrocyte
sedimentation rate (ESR) and C-reactive protein (CRP) levels; these
data indicate that IL-33 may take part in the acute phase of SLE
(21). However, the function of
the IL-33/ST2 axis in MN patients remains unclear. Therefore, it is
of clinical importance to determine the functions and mechanisms of
IL-33 and sST2 in MN patients. This study is the first to measure
serum concentrations of IL-33 and sST2 and explore the relationship
between the IL-33/ST2 axis and disease severity in MN patients.
Recent studies have revealed that sST2 is a negative modulator and
competitive decoy receptor for IL-33 (22,23).
However, in our research, serum IL-33 levels were not increased in
MN patients, and IL-33 levels were not correlated with disease
severity. These data indicate that IL-33 may not participate in the
pathogenesis of MN or may be inversely downregulated by sST2.
ST2 is selectively expressed by some Th2 cells, but
not Th1 cells, and is involved in the Th2 response (9). Importantly, sST2 is a negative
modulator of IL-33 and may participate in the pathogenesis of many
immune-mediated diseases (22–26).
MN is a non-inflammatory organ-specific autoimmune illness that
affects the glomerulus and is characterized by the formation of
subepithelial immune deposits. When the levels of inflammatory
markers increase, kidney function will deteriorate. Our study shows
markedly higher sST2 concentrations in MN patients than HCs and
indicates that serum sST2 levels are positively correlated with MN
disease severity. Following treatment, the serum concentrations of
sST2 were obviously lower than those at pretreatment. Furthermore,
we discovered that serum sST2 concentrations were positively
correlated with the 24-h urinary protein levels but negatively
correlated with the eGFRs in MN patients. Our study suggests that
increased levels of sST2 may be associated with an increased risk
of MN progression and that serum sST2 levels could be used as a
biomarker for assessing MN disease development and appropriate
therapeutic interventions.
Th cells are classified as Th1, Th2, Th17 and Treg
cells. Th1 cells produce IFN-γ and IL-2 and facilitate
cell-mediated immunity. In contrast, Th2 cells secrete IL-4 and
IL-10 and induce antibody production. IL-17 is produced by Th7
cells, mast cells, neutrophils and macrophages. Studies have
suggested that IL-17A plays a key role in the mechanisms of host
defense, autoimmunity reactions and chronic inflammatory diseases
(27). Our study reveals that the
serum levels of IL-2, IL-4, IL-10, IL-17A and IFN-γ were
considerably higher in MN patients than in HCs. Moreover, MN
treatment increased the serum levels of IL-4 and IL-10 but did not
affect the serum levels of IL-2, IL-17A and IFN-γ. Our study
indicates that pro-inflammatory Th1 and Th17 responses may take
part in the pathogenesis of MN and stimulate anti-inflammatory Th2
and Tregs that downregulate pro-inflammatory responses during the
pathogenesis of MN.
ST2 expression by Th cells relies on GATA3
signaling, and high levels of sST2 can downregulate the expression
of IL-4 but not type 1 cytokines (28). One study reported that the
inhibition of IL-33 signaling by sST2 can effectively inhibit the
production of IL-4 following allergen exposure in an experimental
asthma setting (29). Another
study found that sST2 strikingly reduced the levels of IL-4 in mice
with airway inflammation compared with those in control mice
(30). Furthermore, we found that
serum sST2 levels were negatively correlated with IL-4 levels in MN
patients. These data indicate that serum sST2 may downregulate IL-4
in MN patients.
Some researchers have found that IL-4 enhances the
production of IgG4 by B cells in idiopathic MN in vitro
(15). IgG4 is deposited in the
glomeruli, which are involved in the pathogenesis of MN (31). Therefore, decreased IL-4 levels may
alleviate the course of MN.
Our study shows that the serum calcium levels were
lower and that the serum phosphorus levels were higher in MN
patients before treatment than in the HCs. Furthermore, serum sST2
levels were positively correlated with serum phosphorus levels but
negatively correlated with serum calcium levels. Following
treatment, the serum calcium levels were increased considerably,
and the serum phosphorus levels were decreased considerably. Study
has indicated that fibroblast growth factor 23 (FGF23) plays a role
in reducing parathyroid hormone (PTH) secretion from the
parathyroid glands; however, high PTH and FGF23 serum
concentrations, in addition to FGF23 resistance in the parathyroid
glands, contribute to chronic kidney disease (CKD) (32). FGF23 can act on the nephridium to
promote phosphate secretion while inhibiting 25(OH)-vitamin D
1α-(OH) activity and intestinal calcium and phosphorus absorption
(33). Importantly, it has been
shown that sST2 may be a regulatory target of PTH that affects bone
metabolism (34). One study even
showed that CKD patients with elevated PTH levels had higher sST2
levels than HCs (35). MN is the
main cause of nephrotic syndrome in adults. Calcium and phosphorus
are maintained at relatively constant levels in the blood of
healthy subjects, and when phosphorus levels increase, serum
calcium levels decrease. In addition, PTH can increase blood
calcium levels and reduce blood phosphorus levels. Together, these
data suggest that sST2 plays a pivotal role in bone metabolism
disorders in MN patients.
In conclusion, our results show that the serum
levels of sST2 are considerably higher in MN patients than in HCs.
These data suggest that sST2 plays a critical role in the
pathogenesis of MN and can be used as a biomarker for assessing
illness seriousness. We admit that our research is limited by its
small number of patients and single time-point data for some
patients; other limitations include the limited amount of
information regarding renal system damage and the lack of data
regarding sST2 levels after treatment. Although further study is
required to more clearly define the role and mechanism of sST2 in
the pathogenesis of MN, our novel research provides new data for
understanding this disease.
References
|
1
|
Glassock RJ: The pathogenesis of
idiopathic membranous nephropathy: A 50-year odyssey. Am J Kidney
Dis. 56:157–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ronco P and Debiec H: Pathogenesis of
membranous nephropathy: Recent advances and future challenges. Nat
Rev Nephrol. 8:203–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cattran D: Management of membranous
nephropathy: When and what for treatment. J Am Soc Nephrol.
16:1188–1194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cattran DC: Idiopathic membranous
glomerulonephritis. Kidney Int. 59:1983–1994. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Honkanen E, Törnroth T and Grönhagen-Riska
C: Natural history, clinical course and morphological evolution of
membranous nephropathy. Nephrol Dial Transplant. 7 Suppl 1:S35–S41.
1992.
|
|
6
|
Glassock RJ: The treatment of idiopathic
membranous nephropathy: A dilemma or a conundrum? Am J Kidney Dis.
44:562–566. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptorrelated protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alves-Filho JC, Sônego F, Souto FO,
Freitas A, Verri WA Jr, Auxiliadora-Martins M, Basile-Filho A,
McKenzie AN, Xu D, Cunha FQ and Liew FY: Interleukin-33 attenuates
sepsis by enhancing neutrophil influx to the site of infection. Nat
Med. 16:708–712. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Löhning M, Stroehmann A, Coyle AJ, Grogan
JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A and Kamradt
T: T1/ST2 is preferentially expressed on murine Th2 cells,
independent of interleukin 4, interleukin 5, and interleukin 10,
and important for Th2 effector function. Proc Natl Acad Sci USA.
95:pp. 6930–6935. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanada S, Hakuno D, Higgins LJ, Schreiter
ER, McKenzie AN and Lee RT: IL-33 and ST2 comprise a critical
biomechanically induced and cardioprotective signaling system. J
Clin Invest. 117:1538–1549. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palmer G, Talabot-Ayer D, Lamacchia C, Toy
D, Seemayer CA, Viatte S, Finckh A, Smith DE and Gabay C:
Inhibition of interleukin-33 signaling attenuates the severity of
experimental arthritis. Arthritis Rheum. 60:738–749. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pastorelli L, De Salvo C, Cominelli MA,
Vecchi M and Pizarro TT: Novel cytokine signaling pathways in
inflammatory bowel disease: Insight into the dichotomous functions
of IL-33 during chronic intestinal inflammation. Therap Adv
Gastroenterol. 4:311–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mok MY, Huang FP, Ip WK, Lo Y, Wong FY,
Chan EY, Lam KF and Xu D: Serum levels of IL-33 and soluble ST2 and
their association with disease activity in systemic lupus
erythematosus. Rheumatology (Oxford). 49:520–527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalavrizioti D, Gerolymos M, Rodi M,
Kalliakmani P, Provatopoulou S, Eleftheriadis T, Mouzaki A and
Goumenos DS: T helper (Th)-cytokines in the urine of patients with
primary glomerulonephritis treated with immunosuppressive drugs:
Can they predict outcome? Cytokine. 76:260–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuroki A, Iyoda M, Shibata T and Sugisaki
T: Th2 cytokines increase and stimulate B cells to produce IgG4 in
idiopathic membranous nephropathy. Kidney Int. 68:302–310. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morgan E, Varro R, Sepulveda H, Ember JA,
Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, et al:
Cytometric bead array: A multiplexed assay platform with
applications in various areas of biology. Clin Immunol.
110:252–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tárnok A, Hambsch J, Chen R and Varro R:
Cytometric bead array to measure six cytokines in twenty-five
microliters of serum. Clin Chem. 49:1000–1002. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barksby HE, Lea SR, Preshaw PM and Taylor
JJ: The expanding family of interleukin-1 cytokines and their role
in destructive inflammatory disorders. Clin Exp Immunol.
149:217–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liew FY, Pitman NI and McInnes IB:
Disease-associated functions of IL-33: The new kid in the IL-1
family. Nat Rev Immunol. 10:103–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Cai Y, Ji H, Feng J, Ayana DA, Niu
J and Jiang Y: Serum IL-33 levels are associated with liver damage
in patients with chronic hepatitis B. J Interferon Cytokine Res.
32:248–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Z, Liang Y, Xi W, Li C and Zhong R:
Association of increased serum IL-33 levels with clinical and
laboratory characteristics of systemic lupus erythematosus in
Chinese population. Clin Exp Med. 11:75–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayakawa H, Hayakawa M, Kume A and
Tominaga S: Soluble ST2 blocks interleukin-33 signaling in allergic
airway inflammation. J Biol Chem. 282:26369–26380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dinarello CA: An IL-1 family member
requires caspase-1 processing and signals through the ST2 receptor.
Immunity. 23:461–462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brunner M, Krenn C, Roth G, Moser B,
Dworschak M, Jensen-Jarolim E, Spittler A, Sautner T, Bonaros N,
Wolner E, et al: Increased levels of soluble ST2 protein and IgG1
production in patients with sepsis and trauma. Intensive Care Med.
30:1468–1473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimpo M, Morrow DA, Weinberg EO, Sabatine
MS, Murphy SA, Antman EM and Lee RT: Serum levels of the
interleukin-1 receptor family member ST2 predict mortality and
clinical outcome in acute myocardial infarction. Circulation.
109:2186–2190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mueller T, Dieplinger B, Gegenhuber A,
Poelz W, Pacher R and Haltmayer M: Increased plasma concentrations
of soluble ST2 are predictive for 1-year mortality in patients with
acute destabilized heart failure. Clin Chem. 54:752–756. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodrigues-Díez R, Aroeira LS, Orejudo M,
Bajo MA, Heffernan JJ, Rodrigues-Díez RR, Rayego-Mateos S, Ortiz A,
Gonzalez-Mateo G, López-Cabrera M, et al: IL-17A is a novel player
in dialysis-induced peritoneal damage. Kindey Int. 86:303–315.
2014. View Article : Google Scholar
|
|
28
|
Griesenauer B and Paczesny S: The
ST2/IL-33 axis in immune cells during inflammatory diseases. Front
Immunol. 8:4752017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hayakawa H, Hayakawa M, Kume A and
Tominaga S: Soluble ST2 blocks IL-33 signaling in allergic airway
inflammation. J Biol Chem. 282:26369–26380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin H, Li XY, Liu T, Yuan BH, Zhang BB, Hu
SL, Gu HB, Jin XB and Zhu JY: Adenovirus-mediated delivery of
soluble ST2 attenuates ovalbumin-induced allergic asthma in mice.
Clin Exp Immunol. 170:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prunotto M, Carnevali ML, Candiano G,
Murtas C, Bruschi M, Corradini E, Trivelli A, Magnasco A, Petretto
A, Santucci L, et al: Autoimmunity in membranous nephropathy
targets aldose reductase and SOD2. J Am Soc Nephrol. 21:507–519.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lavi-Moshayoff V, Wasserman G, Meir T,
Silver J and Naveh-Many T: PTH increases FGF23 gene expression and
mediates the high-FGF23 levels of experimental kidney failure: A
bone parathyroid feedback loop. Am J Physiol Renal Physiol.
299:F882–F889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shimada T, Hasegawa H, Yamazaki Y, Muto T,
Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S and Yamashita
T: FGF-23 is a potent regulator of vitamin D metabolism and
phosphate homeostasis. J Bone Miner Res. 19:429–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Torres VE: Treatment strategies and
clinical trial design in ADPKD. Adv Chronic Kidney Dis. 17:190–204.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bao YS, Na SP, Zhang P, Jia XB, Liu RC, Yu
CY, Mu SH and Xie RJ: Characterization of interleukin-33 and
Soluble ST2 in serum and their association with disease severity in
patients with chronic kidney disease. J Clin Immunol. 32:587–594.
2012. View Article : Google Scholar : PubMed/NCBI
|