Introduction
Pathogen-associated molecular patterns (PAMPs) are
molecules associated with groups of pathogens. PAMPs include
molecules from Gram-positive and -negative bacteria, DNA and RNA
viruses (1). PAMPs can be
recognized by Toll-like receptors (TLRs) and RIG-I-like receptors
(RLRs); two types of pattern recognition receptors (PRRs) in the
innate immune system (2,3). PAMPs recognized by TLRs and RLRs
include lipids, lipoproteins, proteins and nucleic acids derived
from bacteria, viruses, parasites and fungi (4). For example, triacyl lipopeptide and
diacyl lipopeptide are PAMPs that can be recognized by TLR1/2 and
TLR2/6, respectively (5–7). Double-stranded RNA (dsRNA) is a
ligand not only for TLR3, but also for RLRs including melanoma
differentiation-associated gene 5 (MDA5) and retinoic
acid-inducible gene 1 (RIG-I) (8).
MDA5 and RIG-I are cytosolic RNA helicases capable of unwinding
dsRNA molecules (9). Once
recognized by receptors, PAMPs can activate nuclear factor (NF)κB,
activator protein 1 (AP-1), interferon regulatory factor 3 (IRF3),
and IRF7 signaling pathways which induce the expression of
inflammatory cytokines (3,10,11).
Collagen is the main structural protein in the
extracellular space of tissues. Collagen-related diseases can arise
from genetic defects or environmental stresses that affect the
biosynthesis, assembly, secretion or other processes involved in
normal collagen production. Scleroderma results from an
overproduction and accumulation of collagen in tissues (12). Skin aging may result from decreased
synthesis of collagen and/or induced collagen degradation (13).
Poly(I:C) is a synthetic dsRNA that has frequently
been used as a representative dsRNA ligand in numerous studies
(14,15). Upon binding to receptors, poly(I:C)
is able to selectively activate NF-κB, AP-1 and IRF3 signaling
pathways depending on different experiment conditions (8,16,17).
It has been suggested that poly(I:C) treatment inhibited
procollagen expression by autocrine interferon signaling in skin
fibroblasts (18). However, the
signaling pathways involved in poly(I:C)-induced procollagen
reduction have yet to be fully elucidated.
The present study identified that treatment of
poly(I:C), but not another PAMP, Pam3CSK4, inhibited procollagen
expression in cultured human skin fibroblasts. Although treatment
of poly(I:C) and Pam3CSK4 induced activations of the
mitogen-activated protein kinases (MAPK) and the NF-κB pathways,
only poly(I:C), not Pam3CSK4, induced the activation of IRF3
pathway. By using two different specific inhibitors, it was
identified that inhibition of IRF3 signaling pathway relieved
poly(I:C)-induced procollagen reduction in skin fibroblasts.
Materials and methods
Reagents
Pam3CSK4 and poly(I:C) were purchased from InvivoGen
(San Diego, CA, USA) and Tank binding kinase 1 (TBK1) inhibitor
BX795 from Calbiochem (EMD Millipore, Billerica, MA, USA). Another
TBK1 inhibitor, SU6668, was purchased from Tocris Bioscience
(Bristol, UK). For detecting procollagen protein, monoclonal
anti-type I procollagen aminoterminal extension peptide antibody
(clone SP1.D8; Developmental Studies Hybridoma Bank, Iowa City, IA,
USA) was diluted 1:10 in TBST to be used. Antibody for matrix
metalloproteinase-1 (MMP-1) was made by Lab Frontier Co., Ltd.
(Seoul, Korea). Antibody for β-actin (cat. no. sc-1616) was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Antibodies for phosphorylated-extracellular signal-regulated kinase
(p-ERK)1/2 (cat. no. 9101), p-c-Jun N-terminal kinases (p-JNK)
(cat. no. 9251), p-p38 (cat. no. 9211), p-nuclear factor of κ light
polypeptide gene enhancer in B-cells inhibitor, α (p-IκB-α) (cat.
no. 9246), and p-IRF3 (cat. no. 4947) were purchased from Cell
Signaling Technology Inc. (Danvers, MA, USA). Horseradish
peroxidase-conjugated anti-mouse (cat. no. sc-2031), anti-rabbit
(cat. no. sc-2030) or anti-goat (cat. no. sc-2020) IgG (Santa Cruz
Biotechnology, Inc.) were used as secondary antibodies. Primary
antibodies other than type I procollagen antibody were diluted
1:1,000 and secondary antibodies were diluted 1:10,000 in TBST for
western blotting.
Cell culture
From December 2013 to January 2014, three young
healthy volunteers provided foreskin samples at the Department of
Dermatology, Seoul National University Hospital (Seoul, Korea).
Human foreskin fibroblasts from young healthy volunteers were
cultured in Dulbecco's modified Eagle's medium (DMEM; Welgene,
Geyongsan, Korea) supplemented with glutamine (2 mM), penicillin
(400 U/ml), streptomycin (50 mg/ml), and 10% fetal bovine serum
(FBS; Welgene) in a humidified 5% CO2 atmosphere at
37°C. Skin fibroblasts were used for the experiments at passages
6–8. For chemical treatment, skin fibroblasts were serum-starved
for 24 h in DMEM containing 0.1% FBS. This study was approved by
the Institutional Review Board at Seoul National University
Hospital and conducted according to the Declaration of Helsinki
(IRB no. 1101-116-353).
Western blotting
The amounts of procollagen and MMP-1 proteins
secreted into culture media were analyzed. β-actin was detected
from equal volume of cell lysate as a loading control for
procollagen and MMP-1. For the detection of p-ERK1/2, JNK, p38,
IκBα and IRF3 in cell lysates, cells were washed twice with
ice-cold phosphate buffered saline, and then lysed in
radioimmunoprecipitation assay (RIPA) buffer (EMD Millipore). Cell
lysates were incubated in RIPA buffer for 30 min at 4°C,
centrifuged for 15 min at 12,000 × g, 4°C, and then supernatants
were collected. Protein concentrations were measured by Bradford
protein assay using the Bio-Rad protein assay (Bio-Rad
Laboratories, Mississauga, ON, Canada). Protein (30 µg) was
separated by 10% SDS-PAGE. Separated proteins were transferred onto
PVDF membranes, which were then incubated in blocking buffer
consisting of 5% skim milk in TBST at room temperaature for 30 min.
Then, membranes were incubated with the appropriate primary
antibodies at 4°C for 16 h and secondary antibodies at room
temperature for 1 h. The signals were developed by enhanced
chemiluminescence (GE Healthcare Life Sciences, Chalfont, UK).
After being probed for procollagen and MMP-1, the same membrane was
washed and stained with Coomassie Blue as a loading control. For
certain experiments, the relative level of protein bands was
quantified by densitometric analysis (ImageJ v1.47; National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Experiments were carried out in triplicate. Data are
expressed as mean values ± standard error of the mean. Statistical
analysis was performed using the Student's t-test (Microsoft Office
Excel 2013; Microsoft Corporation, Redmond, WA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
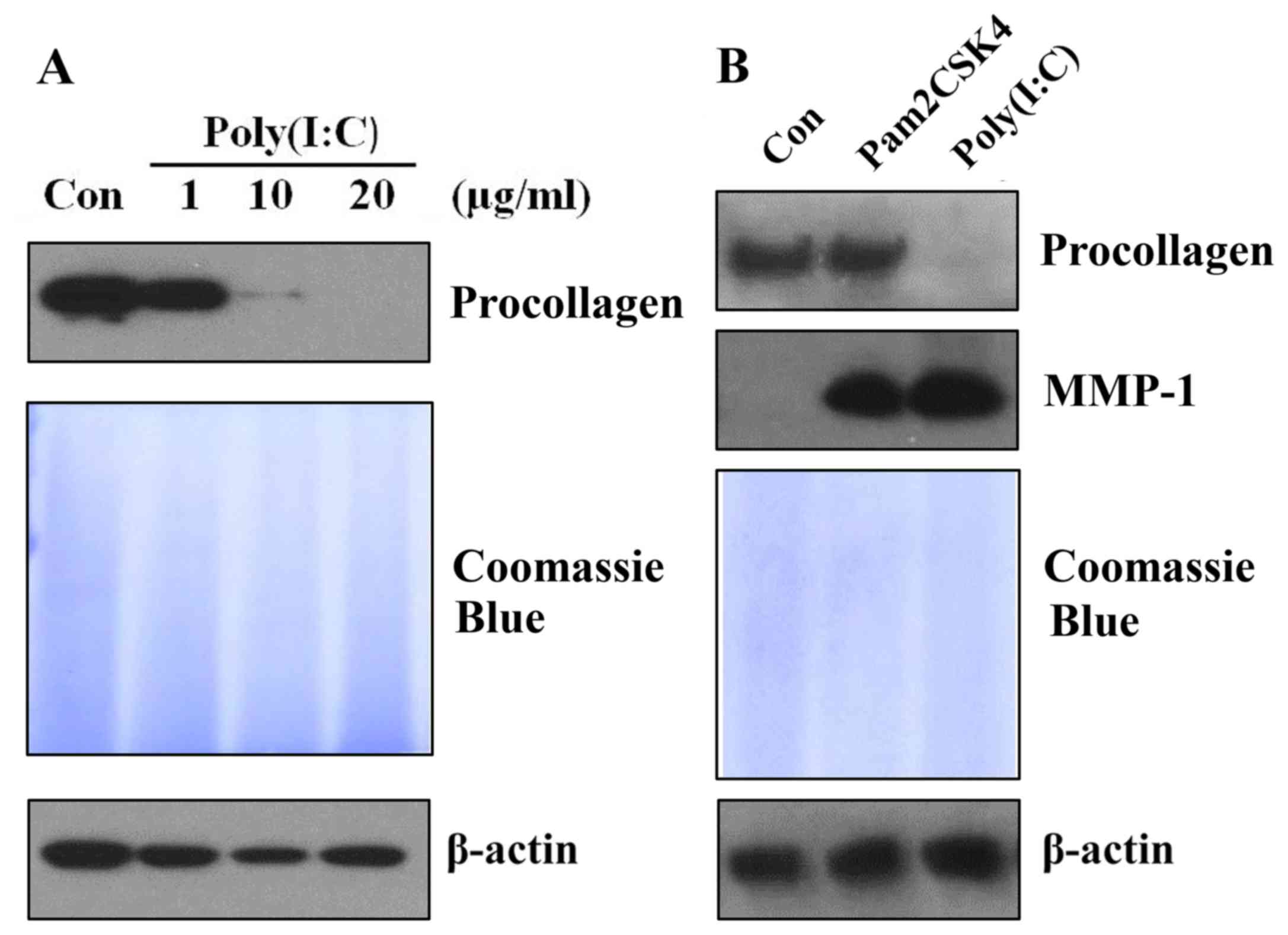
Treatment of poly(I:C), but not
Pam3CSK4, reduces procollagen expression in skin fibroblasts
Poly(I:C) is commonly known as a viral PAMP mimic
(19). It has been demonstrated
that poly(I:C) inhibits procollagen expression in skin fibroblasts
(18). To confirm this result in
the present study, various doses of poly(I:C) were administered to
cultured skin fibroblasts. Following 48 h of treatment, the protein
expression of procollagen was analyzed. It was identified that
poly(I:C) reduced procollagen expression dose-dependently in skin
fibroblasts (Fig. 1A). Next, in
order to investigate whether other PAMPs or PAMP mimics can also
inhibit procollagen expression levels in skin fibroblasts, skin
fibroblasts were treated with a bacterial PAMP mimic, Pam3CSK4,
which is commonly used as a ligand for TLR1/2 (16,20).
It was observed that treatment with 5 µg/ml Pam3CSK4 and 20 µg/ml
poly(I:C) induced MMP-1 expression to a similar level. However,
only poly(I:C), but not Pam3CSK4, reduced procollagen expression in
skin fibroblasts (Fig. 1B). Thus,
the data indicated that Pam3CSK4 has no effect on procollagen
expression in skin fibroblasts.
Treatment of poly(I:C), but not
Pam3CSK4, induces activation of IRF3 in skin fibroblasts
Since only poly(I:C), but not Pam3CSK4, reduced
procollagen expression, it was hypothesized that specific signaling
pathway(s) could be involved in poly(I:C)-induced procollagen
reduction. Thus, the activation of several signaling pathways was
checked and compared following treatment of poly(I:C) and Pam3CSK4.
At 2 h following treatment, it was observed that Pam3CSK4 and
poly(I:C) induced phosphorylation of ERK1/2, JNK, p38 and IκB-α.
However, only poly(I:C), not Pam3CSK4, induced the phosphorylation
of IRF3 (Fig. 2).
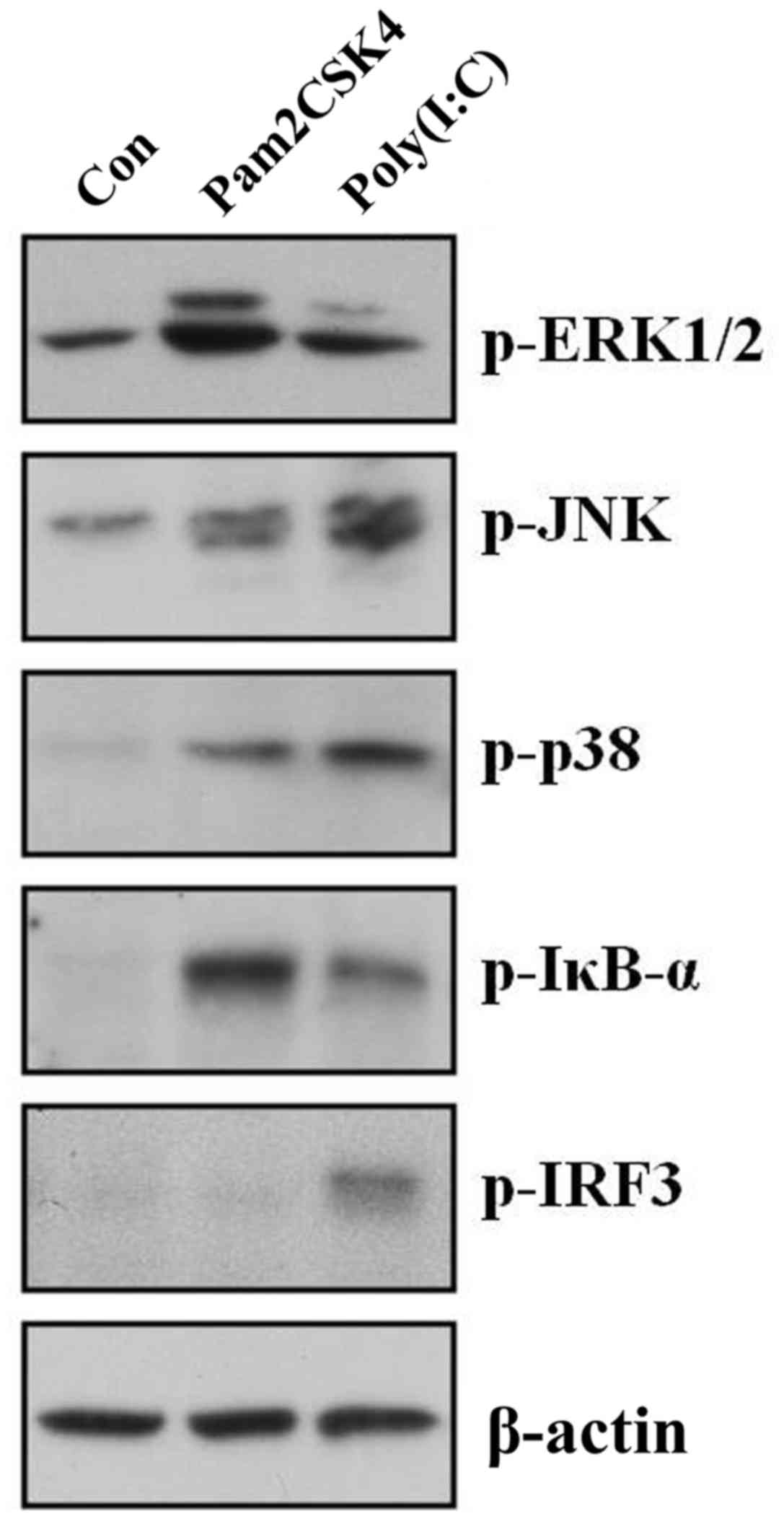 | Figure 2.Treatment of poly(I:C), but not
Pam3CSK4, induces activation of IRF3 in skin fibroblasts. Cultured
skin fibroblasts were stimulated with 5 µg/ml Pam3CSK4 and 20 µg/ml
poly(I:C) for 2 h. Cell lysates were fractionated by SDS-PAGE and
then protein levels of MAPKs (p-ERK1/2, p-JNK, and p-p38), p-IκB-α
and p-IRF3 were analyzed by western blotting. A single
representative experiment is presented from three different
experiments. Con, control; p, phosphorylated; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; IκB-α,
nuclear factor of κ light polypeptide gene enhancer in B-cells
inhibitor, α; IRF3, interferon regulatory factor 3. |
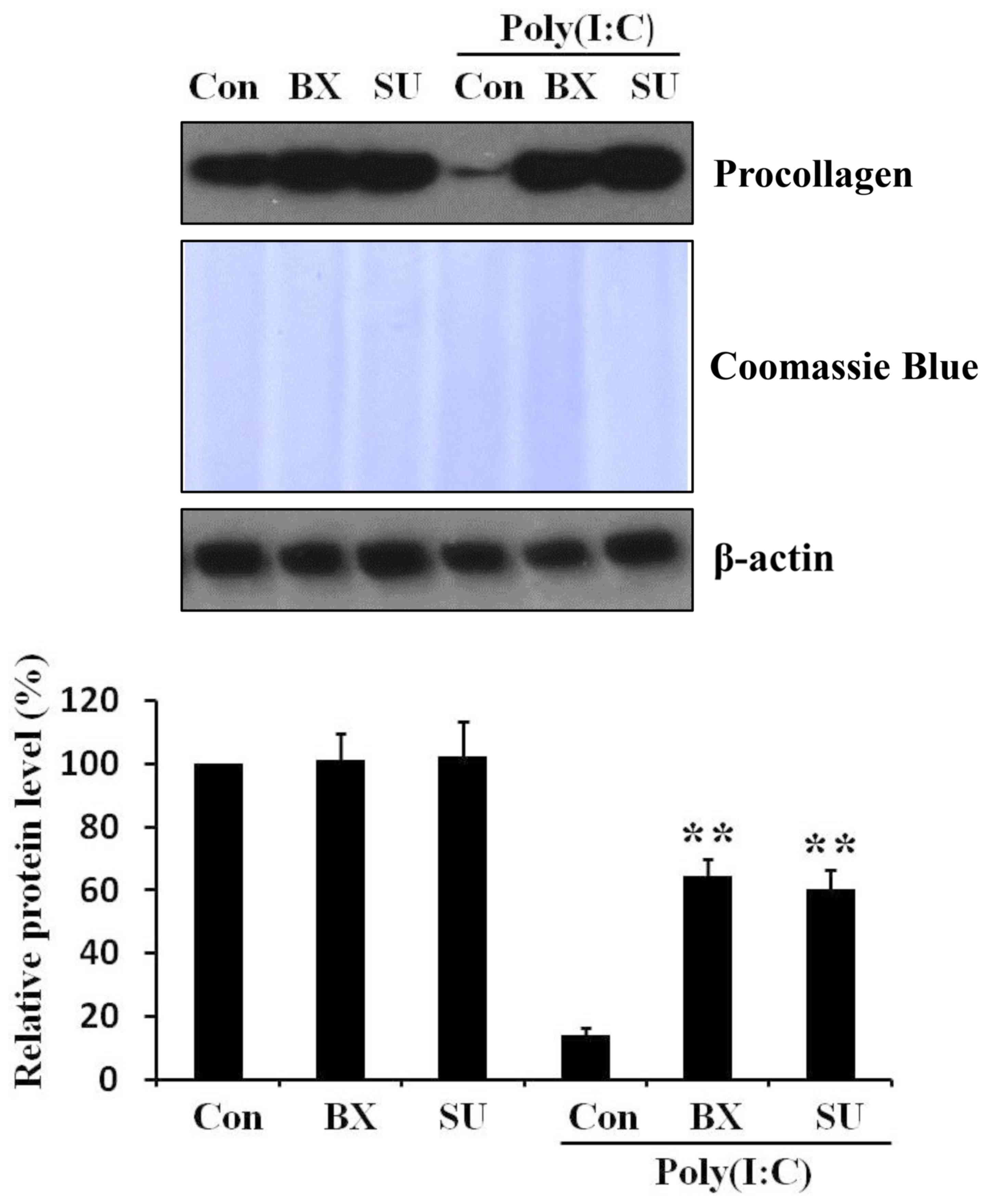
Inhibition of IRF3 signaling pathway
relieves poly(I:C)-induced procollagen reduction in skin
fibroblasts
To investigate whether IRF3 signaling pathway is
involved in the reduction of procollagen by poly(I:C) in skin
fibroblasts, cells were pretreated for 1 h with two different TBK1
(an upstream molecule of IRF3) inhibitors: BX795 (21) and SU6668 (8). Then poly(I:C) was further added to
the cell cultured medium. After 48 h, it was observed that
pretreatment of IRF3 pathway inhibitors mitigated poly(I:C)-induced
procollagen reduction (Fig. 3).
Thus, the results indicated that poly(I:C)-induced procollagen
reduction is regulated by IRF3 signaling pathways in skin
fibroblasts.
Discussion
dsRNA can act as a form of genetic information
carried by certain viruses including retroviruses (22). dsRNA can also exist as an
endogenous TLR3 ligand. For example, it was identified that
UV-damaged self-noncoding RNA can be detected by TLR3 (23). Poly(I:C) is a synthetic dsRNA which
has frequently been used as a representative dsRNA ligand in a
number of studies (14,15). The effects of poly(I:C) on collagen
expression have been extensively investigated in a number of
studies (15,18) and it has been demonstrated that
subcutaneous poly(I:C) delivery by osmotic pumps induces epidermal
hyperplasia and increased matrix deposition in mice. TGF-β-related
genes were elevated in lesional mouse skin and Farina et al
(14) concluded that chronic TLR3
stimulation can induce cutaneous fibrosis in mice. However, other
studies have provided evidence of the anti-fibrosis effects of
poly(I:C) in mice. For example, studies have identified that
injection of poly(I:C) ameliorated lung and liver fibrosis in mice
(24,25). Conflicting results of poly(I:C) on
collagen expression were also identified in several in vitro
studies (15,18). Sugiura et al (15) identified that activation of TLR3 by
poly(I:C) augments collagen production in cultured human fetal lung
fibroblasts. A NF-κB-TGF-β1-dependent pathway was identified to be
involved in the processes. It has been suggested (15) that IRF3 signaling pathway is not
associated with collagen production by poly(I:C) in fetal lung
fibroblasts. Another study (18)
demonstrated that poly(I:C) reduces TGF-β-induced collagen
expression in cultured skin fibroblasts. It was suggested that
poly(I:C) upregulates the expression of Smad7 which inhibits
TGF-β-induced collagen production.
The present study identified that treatment with
poly(I:C), but not another PAMP, Pam3CSK4, inhibited procollagen
expression in skin fibroblasts (Fig.
1). It was hypothesized that poly(I:C) could activate specific
factor(s) that mediate(s) procollagen reduction in skin
fibroblasts. Indeed, poly(I:C), but not Pam3CSK4, induced
activation of IRF3 (Fig. 2). Apart
from the IRF3 pathway, the other signaling pathways, including
MAPKs and NF-κB pathways, were activated by treatment with the two
PAMPs (Fig. 2). To understand the
role of IRF3 in poly(I:C)-induced collagen reduction, two
inhibitors were used for the IRF3 pathway (Fig. 3). The data indicated that
poly(I:C)-induced procollagen reduction is regulated by IRF3
pathway in skin fibroblasts (Fig.
3). At present, only a small number of studies have
investigated the relationship between IRF3 and collagen. It has
been suggested that the IRF3 pathway is not associated with
increased collagen production by poly(I:C) in cultured fetal lung
fibroblasts (15). Recently
(26), it was demonstrated that
inhibition of IRF3 significantly decreases the expression of type I
collagen in human hepatic stellate cells: Indeed, overexpression of
IRF3 increases collagen expression. The data appears to conflict
with the data of the present study, which demonstrated that
activation of IRF3 decreased collagen expression. However, another
study by Xu et al (27)
demonstrated that poly(I:C) suppresses TGF-β-induced Smad3
signaling through activation of IRF3 in human HepG2 hepatoma cells.
This result may imply that poly(I:C) can suppress TGF-β-induced
collagen expression through IRF3 in human HepG2 hepatoma cells,
although the direct evidence was not revealed (27). Thus, the data from the present
study and the published results may indicate that IRF3 pathway can
be either a suppressor or inducer of collagen production depending
on experiment conditions.
In conclusion, it has been demonstrated that the
IRF3 signaling pathway is involved in poly(I:C)-induced procollagen
reduction in skin fibroblasts. In particular, it is for the first
time, to the best of the authors' knowledge, demonstrated that
activation of the IRF3 signaling pathway is involved in procollagen
reduction in skin fibroblasts. Understanding the relation between
IRF3 and collagen may aid the treatment of fibrotic diseases,
including scleroderma and liver fibrosis.
Acknowledgements
The present study was supported by a grant from the
Korea Health Technology R&D Project through the Korea Health
Industry Development Institute, funded by the Ministry of Health
and Welfare, Republic of Korea (grant no. HI14C1277) and by a grant
from the National Research Foundation of Korea funded by the
Ministry of Science, ICT and Future Planning (grant no.
2014M3C9A2064536).
References
|
1
|
Chen K, Huang J, Gong W, Iribarren P,
Dunlop NM and Wang JM: Toll-like receptors in inflammation,
infection and cancer. Int Immunopharmacol. 7:1271–1285. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ermertcan AT, Öztürk F and Gündüz K:
Toll-like receptors and skin. J Eur Acad Dermatol Venereol.
25:997–1006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Medzhitov R: Toll-like receptors and
innate immunity. Nat Rev Immunol. 1:135–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller LS and Modlin RL: Toll-like
receptors in the skin. Semin Immunopathol. 29:15–26. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeuchi O, Kaufmann A, Grote K, Kawai T,
Hoshino K, Morr M, Mühlradt PF and Akira S: Cutting edge:
Preferentially the R-stereoisomer of the mycoplasmal lipopeptide
macrophage-activating lipopeptide-2 activates immune cells through
a toll-like receptor 2- and MyD88-dependent signaling pathway. J
Immunol. 164:554–557. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takeuchi O, Sato S, Horiuchi T, Hoshino K,
Takeda K, Dong Z, Modlin RL and Akira S: Cutting edge: Role of
Toll-like receptor 1 in mediating immune response to microbial
lipoproteins. J Immunol. 169:10–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeuchi O, Kawai T, Mühlradt PF, Morr M,
Radolf JD, Zychlinsky A, Takeda K and Akira S: Discrimination of
bacterial lipoproteins by Toll-like receptor 6. Int Immunol.
13:933–940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalali BN, Köllisch G, Mages J, Müller T,
Bauer S, Wagner H, Ring J, Lang R, Mempel M and Ollert M:
Double-stranded RNA induces an antiviral defense status in
epidermal keratinocytes through TLR3-, PKR-, and
MDA5/RIG-I-mediated differential signaling. J Immunol.
181:2694–2704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanner NK and Linder P: DExD/H box RNA
helicases: From generic motors to specific dissociation functions.
Mol Cell. 8:251–262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sloane JA, Blitz D, Margolin Z and
Vartanian T: A clear and present danger: Endogenous ligands of
Toll-like receptors. Neuromolecular Med. 12:149–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lester SN and Li K: Toll-like receptors in
antiviral innate immunity. J Mol Biol. 426:1246–1264. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pattanaik D, Brown M, Postlethwaite BC and
Postlethwaite AE: Pathogenesis of systemic sclerosis. Front
Immunol. 6:2722015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rittié L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farina GA, York MR, Di Marzio M, Collins
CA, Meller S, Homey B, Rifkin IR, Marshak-Rothstein A, Radstake TR
and Lafyatis R: Poly(I:C) drives type I IFN- and TGFβ-mediated
inflammation and dermal fibrosis simulating altered gene expression
in systemic sclerosis. J Invest Dermatol. 130:2583–2593. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugiura H, Ichikawa T, Koarai A,
Yanagisawa S, Minakata Y, Matsunaga K, Hirano T, Akamatsu K and
Ichinose M: Activation of Toll-like receptor 3 augments
myofibroblast differentiation. Am J Respir Cell Mol Biol.
40:654–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee Y, Kim H, Kim S, Kim KH and Chung JH:
Activation of toll-like receptors 2, 3 or 5 induces matrix
metalloproteinase-1 and −9 expression with the involvement of MAPKs
and NF-kappaB in human epidermal keratinocytes. Exp Dermatol.
19:e44–e49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reimer T, Schweizer M and Jungi TW:
Stimulation-specific contribution of p38 and JNK to IFN-beta gene
expression in human macrophages. J Interferon Cytokine Res.
27:751–755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang F, Ooka K, Sun X, Shah R,
Bhattacharyya S, Wei J and Varga J: A synthetic TLR3 ligand
mitigates profibrotic fibroblast responses by inducing autocrine
IFN signaling. J Immunol. 191:2956–2966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kinnier CV, Martinu T, Gowdy KM, Nugent
JL, Kelly FL and Palmer SM: Innate immune activation by the viral
PAMP poly I:C potentiates pulmonary graft-versus-host disease after
allogeneic hematopoietic cell transplant. Transpl Immunol.
24:83–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao C, Oh JH, Lee DH, Bae JS, Jin CL, Park
CH and Chung JH: Toll-like receptor family members in skin
fibroblasts are functional and have a higher expression compared to
skin keratinocytes. Int J Mol Med. 35:1443–1450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao C, Lee DH, Oh JH, Kim MK, Kim KH, Park
CH and Chung JH: Poly(I:C) induces expressions of MMP-1, −2, and −3
through various signaling pathways including IRF3 in human skin
fibroblasts. J Dermatol Sci. 80:54–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiappinelli KB, Strissel PL, Desrichard
A, Li H, Henke C, Akman B, Hein A, Rote NS, Cope LM, Snyder A, et
al: Inhibiting DNA methylation causes an interferon response in
cancer via dsRNA including endogenous retroviruses. Cell.
169:3612017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bernard JJ, Cowing-Zitron C, Nakatsuji T,
Muehleisen B, Muto J, Borkowski AW, Martinez L, Greidinger EL, Yu
BD and Gallo RL: Ultraviolet radiation damages self noncoding RNA
and is detected by TLR3. Nat Med. 18:1286–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hyde DM and Giri SN:
Polyinosinic-polycytidylic acid, an interferon inducer, ameliorates
bleomycin-induced lung fibrosis in mice. Exp Lung Res. 16:533–546.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou X, Yu F, Man S, Huang D, Zhang Y, Liu
M, Ren C and Shen J: Polyinosinic-polycytidylic acid attenuates
hepatic fibrosis in C57BL/6 mice with Schistosoma japonicum
infection. Acta Trop. 121:99–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ni MM, Xu T, Wang YR, He YH, Zhou Q, Huang
C, Meng XM and Li J: Inhibition of IRF3 expression reduces
TGF-β1-induced proliferation of hepatic stellate cells. J Physiol
Biochem. 72:9–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu P, Bailey-Bucktrout S, Xi Y, Xu D, Du
D, Zhang Q, Xiang W, Liu J, Melton A, Sheppard D, et al: Innate
antiviral host defense attenuates TGF-β function through
IRF3-mediated suppression of Smad signaling. Mol Cell. 56:723–737.
2014. View Article : Google Scholar : PubMed/NCBI
|