Introduction
Calcific aortic valve disease (CAVD) involves
gradual thickening of the aortic valve leaflet (aortic sclerosis)
and severe calcification, impairing leaflet motion (aortic
stenosis) (1). CAVD is a prevalent
heart valve disease, present in almost 30% of adults over 65 years,
increasing to around 40–50% in those over 75 years (2–4).
Dysfunctional heart valves frequently require surgical replacement
using mechanical or bioprosthetic valves, however these are prone
to failure over time due to structural or thrombosis-related
problems (5).
Presently, CAVD is considered an actively regulated
and progressive disease (6). The
development of this disease is thought to be initiated by injury,
inflammation and lipid deposition in the valve, followed by a
propagation phase in which factors promoting calcification and
osteogenesis drive disease progression (7,8). The
increased mechanical stress and injury caused by this early
calcification event may then elicit further calcification, leading
to a continuous cycle of valve calcification (9).
Valve interstitial cells (VICs) are the predominant
cell type in the aortic valves, and play a major role in CAVD
progression (7,10). The underlying mechanisms of CAVD
share many similarities with that of physiological bone formation
(11). VICs are thought to acquire
osteoblastic characteristics during the propagation phase of aortic
stenosis, following inflammation (7,9). A
number of studies have established the ability of VICs to undergo
osteogenic trans-differentiation and calcification (12–14).
Despite this knowledge, the pathways underlying the initiation and
progression of CAVD remain unclear, and studies are needed to
elucidate the mechanisms underpinning early disease
pathogenesis.
The in vitro calcification of primary porcine
(15–17), human (14,18,19),
rat (20–22) and bovine (23,24)
VICs is commonly used as models of aortic valve calcification.
However, to date, the application of a cell line to interrogate VIC
function has not been reported. Cell lines offer a valuable
alternative to primary cells isolated directly from animals,
reducing experimental variation and animal use. To our knowledge
this is the first study reporting the generation and evaluation of
the calcification potential of immortalised VIC lines derived from
sheep (SAVIC) and rat (RAVIC).
Materials and methods
Ethics statement
All animal work was approved by The Roslin
Institute's and the University of Edinburgh's Protocols and Ethics
Committees. The animals were maintained in accordance with UK Home
Office guidelines under the regulations of the Animal (Scientific
Procedures) Act 1986.
Establishment of sheep and rat valve
interstitial cell lines
Sheep primary aortic VICs were harvested from a
4-year-old Scottish mule sheep (generated from a Bluefaced
Leicester sire and Scottish Blackface dam cross; Dryden Farm,
Midlothian, UK). Rat aortic VICs were isolated from aortic valve
leaflets dissected from the hearts of eight 5-week-old male Sprague
Dawley rats as previously described (22). Sheep and rat valve leaflets were
digested in 0.6 mg/ml collagenase Type II (Worthington, New Jersey
USA) for 30 min and washed in Hanks' Balanced Salt Solution (HBSS;
Life Technologies, Paisley, UK) to remove valve endothelial cells.
The leaflets were subsequently digested with 0.6 mg/ml collagenase
Type II for a further 1 h to release the VICs. Cells were pelleted
at 300 × g for 5 min, before resuspension in growth medium
consisting of Dulbecco's Modified Eagle Medium: Nutrient Mixture
F-12 (DMEM/F12; Life Technologies) supplemented with 10%
heat-inactivated foetal bovine serum (FBS; Life Technologies) and
1% gentamicin (Life Technologies), and cultured at 37°C in a
humidified atmosphere of 95% air/5% CO2 and grown for
four passages.
Immortalised cell lines were established by Capital
Biosciences (Gaithersburg, Maryland, USA) from the primary sheep
and rat VICs through transduction with recombinant lentivirus
encoding Simian virus (SV40) large and small T antigens (sheep), or
large T antigen only (rat). The non-clonal cell lines were derived
from multiple founder cells. Following continuous culture to 10
passages, transgene expression was confirmed by real-time
quantitative PCR (RT-qPCR) for the expression of SV40 large T
antigen (Capital Biosciences). The resulting cell lines were
designated SAVIC (sheep; SVIC-SVTta) and RAVIC (rat;
RVIC-SV40T).
SAVIC and RAVIC cell culture
SAVIC and RAVIC cells were seeded in growth media in
multi-well plates at a density of 1.11×104
cells/cm2. Calcification was induced as described
previously (22,25). Cells were grown to 80% confluence
(Day 0), before treating with control (1.05 mM Ca/0.95 mM Pi) or
test media: 1.5 to 3.6 mM calcium (Ca) and/or 1.5 to 2.5 mM
phosphate (Pi). CaCl2 and
Na2HPO4/NaH2PO4
(Sigma-Aldrich, Dorset, UK) were used to supplement ionic calcium
and phosphate in the media. To study the effect of calcification
inhibitors and bisphosphonates on VIC calcification, SAVICs were
exposed to inorganic pyrophosphate (PPi) and etidronate (both 0.1
mM; Sigma-Aldrich). Cells were incubated for up to 7 days in a
humidified atmosphere of 95% air/5% CO2, and the medium
was changed every second/third day.
Detection of calcification
Calcium deposition was quantified based on a
previously described method (26,27).
Cells were washed twice with phosphate buffered saline (PBS) and
decalcified with 0.6 M HCl at room temperature for 2 h. Free
calcium was determined colorimetrically by a stable interaction
with phenolsulphonethalein, using a commercially available kit
(Randox Laboratories Ltd., County Antrim, UK), and corrected for
total protein concentration (Bio-Rad Laboratories Ltd., Hemel
Hempstead, UK) following solubilisation with 0.1 M NaOH/0.1% SDS.
Absorbances were measured using a Synergy HT microplate reader
(BioTek, Swindon UK) at 570 nm (calcium) and at 690 nm
(protein).
Fluorescent immunocytochemical
staining
To confirm retention of mesenchymal phenotype, cell
monolayers cultured on glass coverslips were fixed with 4%
paraformaldehyde (PFA) and washed with phosphate-buffered saline
(PBS). Fixed cells were permeabilised with 0.3% Triton X-100
(Sigma-Aldrich) and incubated with rabbit polyclonal anti-α-smooth
muscle actin (α-SMA; catalogue #ab5694; 1:100; Abcam, Cambridge,
UK), mouse monoclonal anti-vimentin (catalogue #V6384; 1:900;
Sigma-Aldrich) or rabbit polyclonal anti-cluster of differentiation
31 (CD31; 1:900; Abcam, Cambridge, UK) overnight at 4°C. After
washing, cells were incubated with Alexa Fluor® 488
donkey-anti-rabbit antibody (catalogue #A-21,206; 1:250;
ThermoFisher Scientific) or Alexa Fluor® 594
goat-anti-mouse antibody (catalogue #A-110,055; 1:250; ThermoFisher
Scientific) for 1 h in the dark. Glass coverslips were mounted onto
slides with Prolong Gold Anti-Fade Reagent containing DAPI (Life
Technologies). Slides were examined using a Leica DMLB fluorescence
microscope (Leica Geosystems, Milton Keynes, UK). In place of the
primary antibody, control cells were incubated with non-immune
mouse or rabbit IgG (2 µg IgG/ml, Sigma-Aldrich).
Real-time quantitative PCR
RNA extraction was performed using the RNeasy Mini
kit (Qiagen, West Sussex, UK), according to the manufacturer's
instructions. RNA abundance was quantified, RNA was reverse
transcribed, and the expression of selected genes were quantified
via RT-qPCR employing the SYBR green detection method
(PrecisionPLUS mastermix, Primerdesign Ltd, Southampton, UK),
measured on a Stratagene Mx3000P (Agilent Technologies, Stockport,
UK), as previously reported (28,29).
Sheep primers for Runt-related transcription factor 2
(RUNX2; Forward 5′-CTCCTCCATCCATCCACTCC-3′; Reverse
5′-CAGAGGCAGAAGTCAGAGGT-3′) and Matrix Gla protein (MGP;
Forward 5′-ACAACAGAGATGGAGAGCGA-3′; Reverse
5′-CGGAAATAACGGTCGTAGGC-3′) were designed via Primer3 (http://primer3.ut.ee/) to span exon-exon junctions,
and obtained from Invitrogen (Paisley, UK). Primers for sheep
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; sequences
not disclosed), tyrosine 3-monooxygenase (YWHAZ; sequences
not disclosed), and sodium-dependent phosphate transporter 1
(PiT1; also known as SLC20A1; Forward
5′-ACATCTTGAACGCCGCTA-3′; Reverse 5′-AGTAGCAGCAATAGCAGTGGTA-3′)
were obtained from Primerdesign Ltd. Sheep expression data were
normalised against the geometric mean of GAPDH and
YWHAZ. Rat primers for Gapdh, Mgp, and
Runx2 were obtained from Qiagen (sequences not disclosed;
QuantiTect primers, Qiagen). Rat Pit1 primers were acquired
from Primerdesign Ltd. (sequences not disclosed). Rat expression
data were normalised against Gapdh. The ΔΔCq method was used
to analyse relative gene expression (30).
Statistical analysis
All statistical analyses were performed using
Minitab 17 (Minitab Inc., Coventry, UK). General Linear Model (GLM)
analysis incorporating pairwise comparisons and the Student's
t-test were used to assess the data. Data are presented as mean ±
standard error of the mean (SEM). P<0.05 was considered to be
statistically significant, and P-values are represented as:
*P<0.05; **P<0.01; ***P<0.001.
Results
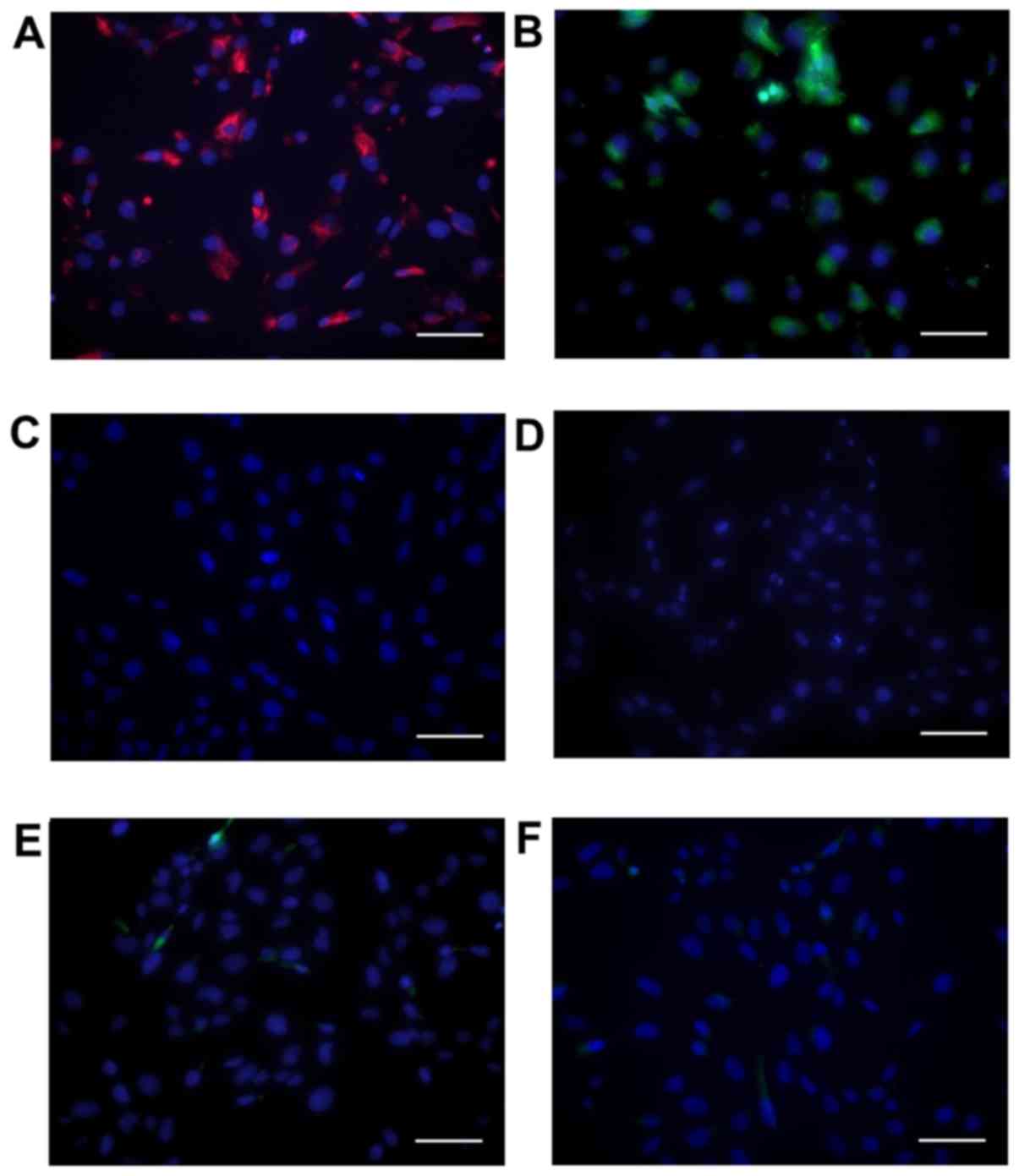
SAVICs express VIC markers
Cells showed positive immunohistochemical staining
for vimentin (Fig. 1A) and α-SMA
(Fig. 1B), in agreement with
previous reports of primary VIC cultures (31,32).
Cells were negative for the endothelial marker CD31 (Fig. 1C).
Calcification of SAVICs
Initial studies were undertaken to determine whether
the calcification of SAVICs could be induced when cultured in the
presence of calcifying medium containing Ca and/or Pi (Fig. 2A). Ca potently induced the
calcification of SAVICs from 2.7 mM (1.9 fold; P<0.001; n=6;
Fig. 2A) whereas Pi treatment
alone had no effect (Fig. 2A). The
treatment of VICs with Ca and Pi together had a synergistic effect
on VIC calcification from 2.7 mM Ca/2.0 mM Pi (22.2 fold;
P<0.001; n=6; Fig. 2B).
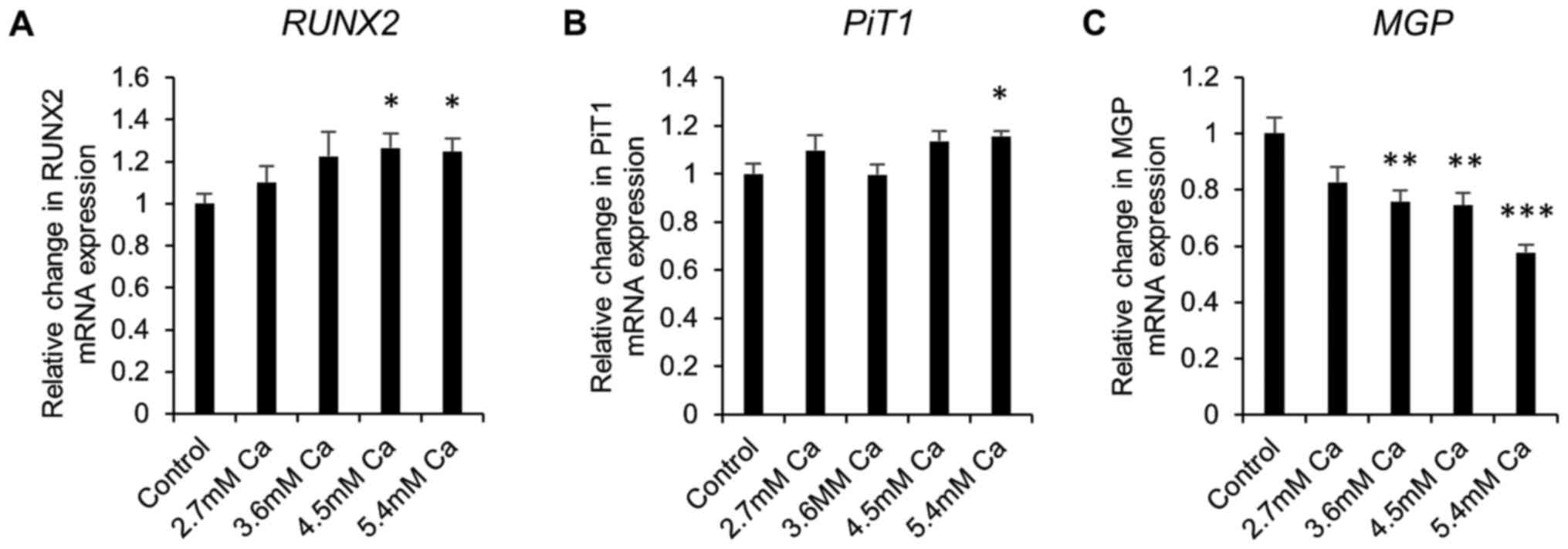
Gene expression in early calcification
in vitro
Next, gene expression studies were undertaken in
calcifying SAVICs to investigate the expression profile of key
genes associated with vascular calcification. Expression of the
gene encoding the master osteoblastic transcription factor,
RUNX2, was significantly increased from 4.5 mM Ca (1.3 fold;
P<0.05; n=6; Fig. 3A) compared
to control culture conditions. Expression of sodium-dependent
phosphate transporter 1 gene (PiT1) was significantly
increased at 5.4 mM Ca (1.2 fold; P<0.05; n=6; Fig. 3B). In contrast, a decrease in the
expression of the calcification inhibitor Matrix Gla Protein gene
(MGP) was noted from 3.6 mM Ca (1.3 fold; P<0.01; n=6;
Fig. 3C).
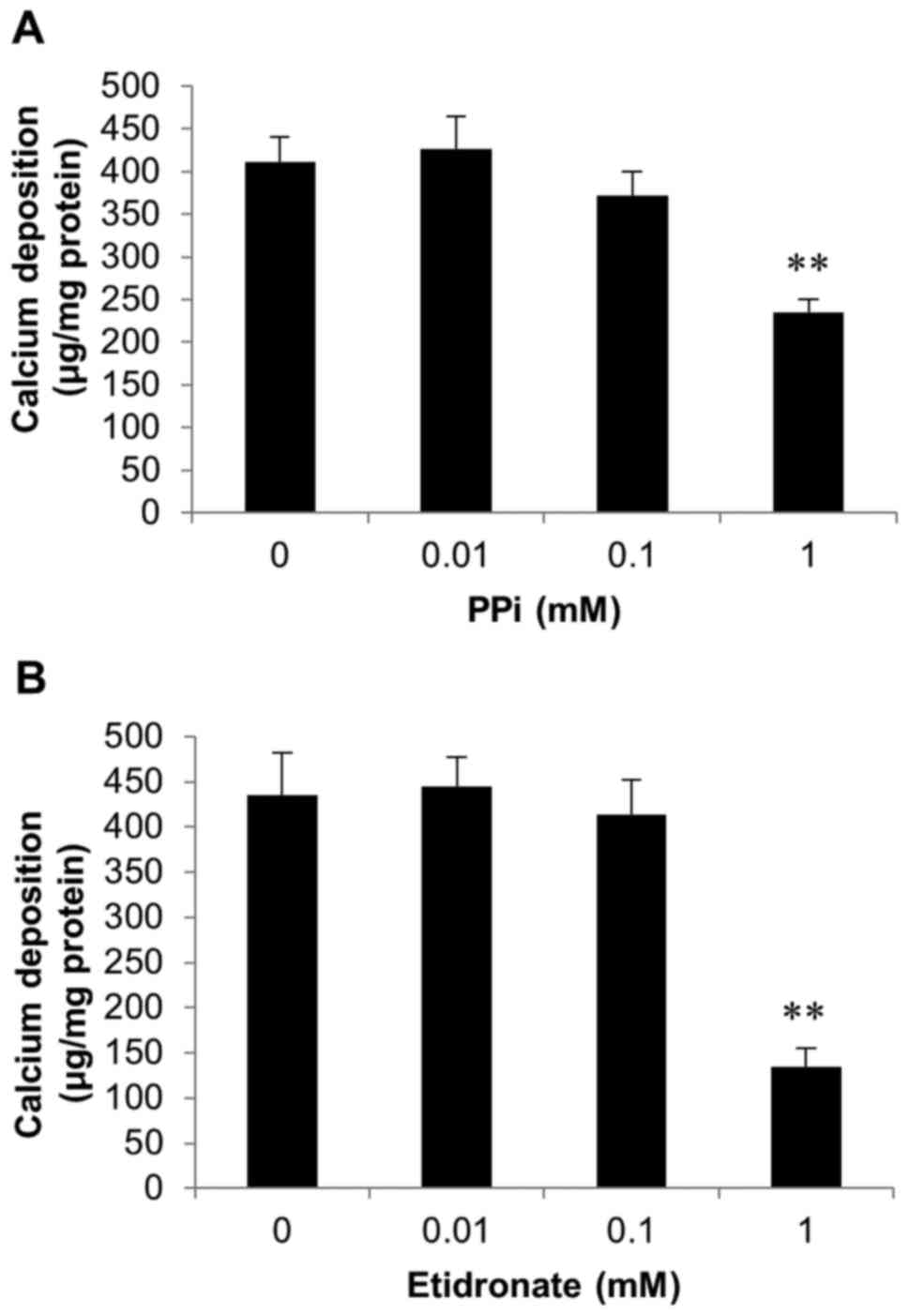
Inhibition of SAVICs calcification by
pyrophosphate and etidronate
Further experiments investigated whether the
calcification of SAVICs could be reduced using recognised
inhibitors of calcification.
As CAVD is a consequence of hydroxyapatite formation
and deposition in the aortic valve, we examined the effects of PPi
(0.01-1 mM; Fig. 4A) and the
bisphosphonate etidronate (0.01-1 mM; Fig. 4B), both established inhibitors of
hydroxyapatite formation (33–35)
on SAVIC calcification. A significant decrease in calcium
deposition was observed at 1 mM PPi (1.8 fold; P<0.01; n=6;
Fig. 4A). Additionally, following
exposure to 1 mM etidronate, a significant reduction in calcium
deposition (3.2 fold; n=6; P<0.01) was observed, confirming the
inhibitory effect of this bisphosphonate on valve calcification
in vitro (Fig. 4B).
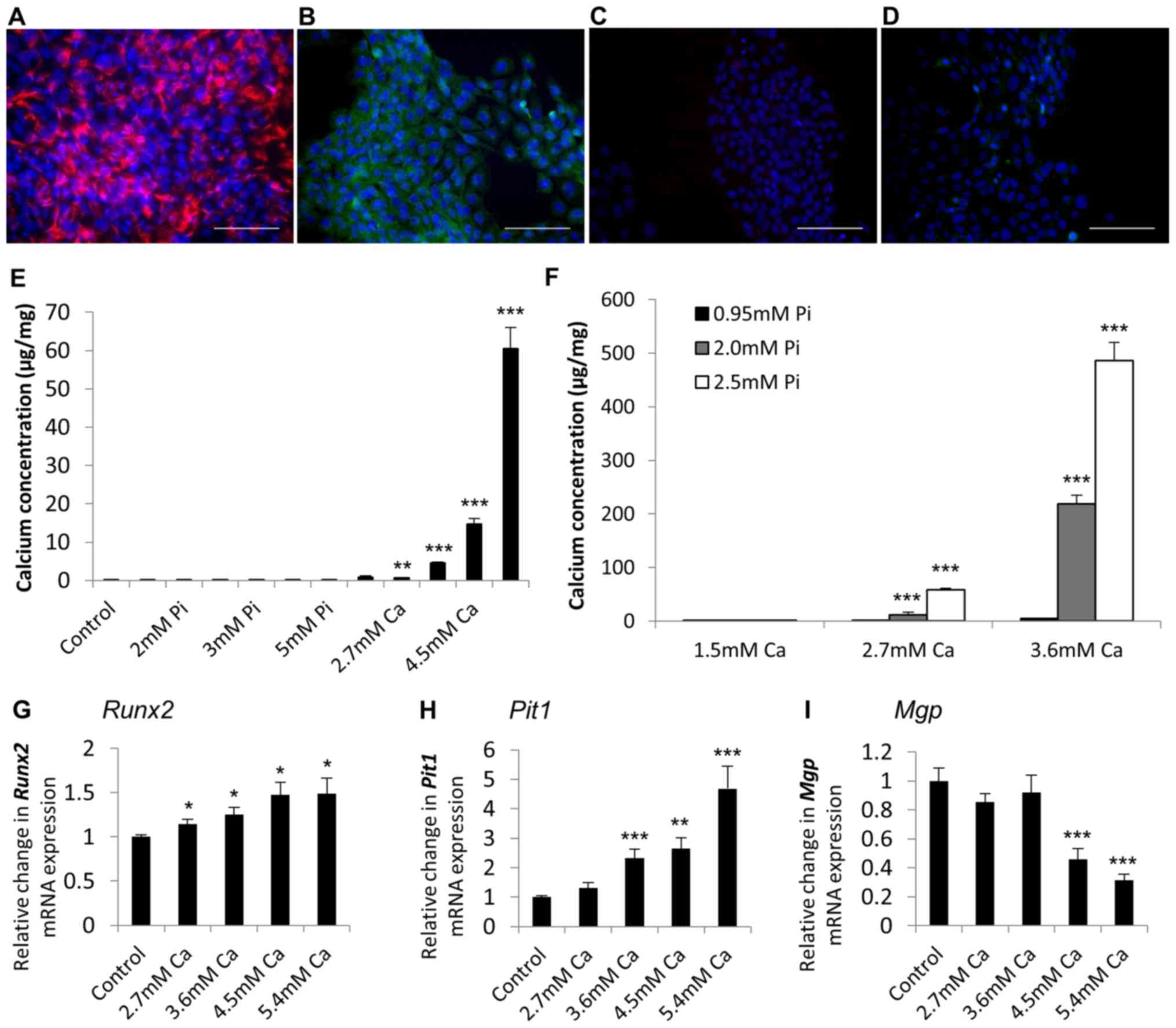
Additional RAVIC studies
We further corroborated the application of
immortalised VICs as an in vitro model of aortic valve
calcification through the generation of a rat immortalised VIC cell
line, RAVIC. A presented in Fig.
5, RAVICs also showed positive immunohistochemical staining for
vimentin (Fig. 5A) and α-SMA
(Fig. 5B), and were negative for
the endothelial marker CD31 (data not shown).
Analogous to our findings with the SAVICs, Ca
markedly induced the calcification of RAVICs from 2.7 mM (4.6 fold;
P<0.01; n=6; Fig. 5E) and Pi
treatment alone had no effect (Fig.
5E). Furthermore, the treatment of RAVICs with Ca and Pi
together had a synergistic effect on cell calcification (2.7 mM
Ca/2.0 mM Pi; 82.2 fold; P<0.001; n=6; Fig. 5F). 5.4 mM Ca treatment induced a
significant increase in the mRNA expression of Runx2 and
Pit1 (1.5 fold; P<0.01, and 4.7 fold; P<0.001,
respectively; Fig. 5G and H), with
a concomitant reduction in Mgp expression (3.2 fold;
P<0.001; Fig. 5I).
Discussion
The calcification of primary porcine (15–17),
human (14,18,19),
rat (20–22) and bovine (23,24)
VICs in 10% serum, inducing an activated phenotype, is frequently
employed to produce in vitro models of aortic valve
calcification. Compared to cell lines, primary cultures exhibit
slow growth and cannot be used beyond a limited number of passages
due to senescence and phenotypic changes that occur during culture.
Furthermore, the use of primary cells requires animal sacrifice and
is labour intensive, costly, and time consuming (36). A number of studies have employed
the mouse vascular smooth muscle cell line, MOVAS-1, to investigate
arterial calcification in vitro (28,37,38).
As yet, however, no published studies have employed immortalized
VIC cell lines to investigate aortic valve calcification. In the
present study, we have characterized the in vitro
calcification potential of immortalised sheep (SAVIC) and rat
(RAVIC) VIC cell lines.
Our data have established that calcification of
SAVICs and RAVICs can be induced in the presence of calcifying
medium containing high concentrations of calcium and phosphate.
This was demonstrated through standard assays of in vitro
vascular calcification (28,39,40),
verifying these cell lines as a feasible and relevant in
vitro model of aortic valve calcification.
Calcified SAVICs and RAVICs showed increased gene
expression of RUNX2 and PiT1, recognized markers of
vascular calcification, with a concomitant reduction in the
expression of MGP, an established calcification inhibitor.
RUNX2 is an early marker of vascular calcification, initiating
osteoblastic differentiation via the upregulation of mineralization
proteins, including osteopontin and osteocalcin (10,41).
Increased PiT1 expression leads to elevated intracellular
phosphate and induces the osteogenic conversion of vascular smooth
muscle cells (VSMCs) (42).
Conversely, down-regulation of PiT1 gene expression by siRNA
silencing has been shown to reduce phosphate uptake and inhibit
phosphate-induced VSMC phenotypic transition and calcification
(42). MGP is a γ-carboxyglutamic
acid-rich and vitamin K-dependent protein, and is proposed to block
calcification by antagonising bone morphogenetic protein signaling
(10,43). Mouse models that lack MGP develop
arterial calcification that results in blood vessel rupture, as
well as ectopic cartilage calcification (44). Additionally, circulating MGP levels
have been shown to be reduced in aortic valve disease patients
(45). Taken together, our data
suggests that the culture of SAVICs and RAVICs in calcifying medium
is an appropriate in vitro model with which to study the
processes leading to aortic valve calcification.
Using known molecular inhibitors, we have also shown
that functional studies can be performed in SAVICs. Pyrophosphate
is a potent inhibitor of the calcification of primary VSMCs
(46) and VICs (23). The bisphosphonate etidronate, an
inhibitor of hydroxyapatite formation and a non-hydrolysable
analogue of PPi, has also been reported to inhibit the
calcification of MOVAS-1 cells and NIH3T3 cells (28,47).
The reduction in calcification observed in SAVICs following
treatment with PPi and etidronate therefore establishes this cell
line as highly appropriate for modeling aortic valve
calcification.
The severe clinical implications of CAVD are widely
recognized. Nonetheless, the underlying mechanisms have not been
fully determined and effective therapeutic strategies that may
prevent or possibly reverse aortic valve calcification have yet to
be developed. The SAVIC and RAVIC lines are convenient and
inexpensive models in which to investigate aortic valve
calcification in vitro, and will make a valuable
contribution to expanding our knowledge of this pathological
process.
Acknowledgements
This study was supported by funding from the
Biotechnology and Biological Sciences Research Council (BBSRC) in
the form of an Institute Strategic Programme Grant (BB/J004316/1;
BBS/E/D/20,221,657) (VEM, KMS and CF), a CASE Studentship
BB/K011618/1 (LC) and an East of Scotland Bioscience Doctoral
Training Partnership (EASTBIO DTP) Studentship BB/J01446X/1
(HGT).
Glossary
Abbreviations
Abbreviations:
|
CAVD
|
calcific aortic valve disease
|
|
VIC
|
valve interstitial cell
|
|
SAVIC
|
sheep aortic valve interstitial cell
line
|
|
RAVIC
|
rat aortic valve interstitial cell
line
|
|
RUNX2/Runx2
|
Runt-related transcription factor
2
|
|
PiT1/Pit1
|
sodium-dependent phosphate transporter
1
|
|
MGP/Mgp
|
matrix Gla protein
|
|
PPi
|
pyrophosphate
|
|
α-SMA
|
alpha-smooth muscle actin
|
|
CD31
|
cluster of differentiation 31
|
|
VEC
|
valve endothelial cell
|
|
DMEM
|
Dulbecco's modified eagles media
|
|
FBS
|
foetal bovine serum
|
|
PFA
|
paraformaldehyde
|
|
Ca
|
calcium
|
|
Pi
|
phosphate
|
References
|
1
|
Freeman RV and Otto CM: Spectrum of
calcific aortic valve disease: Pathogenesis, disease progression
and treatment strategies. Circulation. 111:3316–3326. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart BF, Siscovick D, Lind BK, Gardin
JM, Gottdiener JS, Smith VE, Kitzman DW and Otto CM: Clinical
factors associated with calcific aortic valve disease.
Cardiovascular Health Study. J Am Coll Cardiol. 29:630–634. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coffey S, Cox B and Williams MJ: The
prevalence, incidence, progression and risks of aortic valve
sclerosis: A systematic review and meta-analysis. J Am Coll
Cardiol. 63:2852–2861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lindman BR, Clavel MA, Mathieu P, Gardin
JM, Gottdiener JS, Smith VE, Kitzman DW and Otto CM: Calcific
aortic stenosis. Nat Rev Dis Primers. 2:160062016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balaoing LR, Post AD, Liu H, Minn KT and
Grande-Allen KJ: Age-related changes in aortic valve hemostatic
protein regulation. Arterioscler Thromb Vasc Biol. 34:72–80. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yutzey KE, Demer LL, Body SC, Huggins GS,
Towler DA, Giachelli CM, Hofmann-Bowman MA, Mortlock DP, Rogers MB,
Sadeghi MM and Aikawa E: Calcific aortic valve disease: A consensus
summary from the alliance of investigators on calcific aortic valve
disease. Arterioscler Thromb Vasc Biol. 34:2387–2393. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pawade TA, Newby DE and Dweck MR:
Calcification in aortic stenosis: The skeleton key. J Am Coll
Cardiol. 66:561–577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
New SE and Aikawa E: Molecular imaging
insights into early inflammatory stages of arterial and aortic
valve calcification. Circ Res. 108:1381–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dweck MR, Pawade TA and Newby DE: Aortic
stenosis begets aortic stenosis: Between a rock and a hard place?
Heart. 101:919–920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leopold JA: Cellular mechanisms of aortic
valve calcification. Circ Cardiovasc Interv. 5:605–614. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohler ER III, Gannon F, Reynolds C,
Zimmerman R, Keane MG and Kaplan FS: Bone formation and
inflammation in cardiac valves. Circulation. 103:1522–1528. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poggio P, Sainger R, Branchetti E, Grau
JB, Lai EK, Gorman RC, Sacks MS, Parolari A, Bavaria JE and Ferrari
G: Noggin attenuates the osteogenic activation of human valve
interstitial cells in aortic valve sclerosis. Cardiovasc Res.
98:402–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monzack EL and Masters KS: Can valvular
interstitial cells become true osteoblasts? A side-by-side
comparison. J Heart Valve Dis. 20:449–463. 2011.PubMed/NCBI
|
|
14
|
Osman L, Yacoub MH, Latif N, Amrani M and
Chester AH: Role of human valve interstitial cells in valve
calcification and their response to atorvastatin. Circulation.
114:I547–I552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yip CY, Chen JH, Zhao R and Simmons CA:
Calcification by valve interstitial cells is regulated by the
stiffness of the extracellular matrix. Arterioscler Thromb Vasc
Biol. 29:936–942. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cloyd KL, El-Hamamsy I, Boonrungsiman S,
Grau JB, Lai EK, Gorman RC, Sacks MS, Parolari A, Bavaria JE and
Ferrari G: Characterization of porcine aortic valvular interstitial
cell ‘calcified’ nodules. PLoS One. 7:e481542012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomez-Stallons MV, Wirrig-Schwendeman EE,
Hassel KR, Conway SJ and Yutzey KE: Bone morphogenetic protein
signaling is required for aortic valve calcification. Arterioscler
Thromb Vasc Biol. 36:1398–1405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Côté N, El Husseini D, Pépin A,
Guauque-Olarte S, Ducharme V, Bouchard-Cannon P, Audet A, Fournier
D, Gaudreault N, Derbali H, et al: ATP acts as a survival signal
and prevents the mineralization of aortic valve. J Mol Cell
Cardiol. 52:1191–1202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El Husseini D, Boulanger MC, Fournier D,
Mahmut A, Bossé Y, Pibarot P and Mathieu P: High expression of the
Pi-transporter SLC20A1/Pit1 in calcific aortic valve disease
promotes mineralization through regulation of Akt-1. PLoS One.
8:e533932013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seya K, Yu Z, Kanemaru K, Daitoku K,
Akemoto Y, Shibuya H, Fukuda I, Okumura K, Motomura S and Furukawa
K: Contribution of bone morphogenetic protein-2 to aortic valve
calcification in aged rat. J Pharmacol Sci. 115:8–14. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Acharya A, Hans CP, Koenig SN, Nichols HA,
Galindo CL, Garner HR, Merrill WH, Hinton RB and Garg V: Inhibitory
role of Notch1 in calcific aortic valve disease. PLoS One.
6:e277432011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui L, Rashdan NA, Zhu D, Milne EM, Ajuh
P, Milne G, Helfrich MH, Lim K, Prasad S, Lerman DA, et al: End
stage renal disease-induced hypercalcemia may promotes aortic valve
calcification via Annexin VI enrichment of valve interstitial
cell-derived matrix vesicles. J Cell Physiol. 232:2985–2995. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rattazzi M, Bertacco E, Iop L, D'Andrea S,
Puato M, Buso G, Causin V, Gerosa G, Faggin E and Pauletto P:
Extracellular pyrophosphate is reduced in aortic interstitial valve
cells acquiring a calcifying profile: Implications for aortic valve
calcification. Atherosclerosis. 237:568–576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rattazzi M, Iop L, Faggin E, Bertacco E,
Zoppellaro G, Baesso I, Puato M, Torregrossa G, Fadini GP, Agostini
C, et al: Clones of interstitial cells from bovine aortic valve
exhibit different calcifying potential when exposed to endotoxin
and phosphate. Arterioscler Thromb Vasc Biol. 28:2165–2172. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reynolds JL, Joannides AJ, Skepper JN,
McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL
and Shanahan CM: Human vascular smooth muscle cells undergo
vesicle-mediated calcification in response to changes in
extracellular calcium and phosphate concentrations: A potential
mechanism for accelerated vascular calcification in ESRD. J Am Soc
Nephrol. 15:2857–2867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu D, Mackenzie NC, Millan JL,
Farquharson C and Macrae VE: Upregulation of IGF2 expression during
vascular calcification. J Mol Endocrinol. 52:77–85. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu D, Mackenzie NC, Shanahan CM, Shroff
RC, Farquharson C and MacRae VE: BMP-9 regulates the osteoblastic
differentiation and calcification of vascular smooth muscle cells
through an ALK1 mediated pathway. J Cell Mol Med. 19:165–174. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mackenzie NC, Zhu D, Longley L, Patterson
CS, Kommareddy S and MacRae VE: MOVAS-1 cell line: A new in vitro
model of vascular calcification. Int J Mol Med. 27:663–668.
2011.PubMed/NCBI
|
|
29
|
Staines KA, Zhu D, Farquharson C and
MacRae VE: Identification of novel regulators of osteoblast matrix
mineralization by time series transcriptional profiling. J Bone
Miner Metab. 32:240–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu AC, Joag VR and Gotlieb AI: The
emerging role of valve interstitial cell phenotypes in regulating
heart valve pathobiology. Am J Pathol. 171:1407–1418. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Latif N, Quillon A, Sarathchandra P,
McCormack A, Lozanoski A, Yacoub MH and Chester AH: Modulation of
human valve interstitial cell phenotype and function using a
fibroblast growth factor 2 formulation. PLoS One. 10:e01278442015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Register TC and Wuthier RE: Effect of
pyrophosphate and two diphosphonates on 45Ca and 32Pi uptake and
mineralization by matrix vesicle-enriched fractions and by
hydroxyapatite. Bone. 6:307–312. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lomashvili KA, Garg P, Narisawa S, Millan
JL and O'Neill WC: Upregulation of alkaline phosphatase and
pyrophosphate hydrolysis: Potential mechanism for uremic vascular
calcification. Kidney Int. 73:1024–1030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lomashvili KA, Monier-Faugere MC, Wang X,
Malluche HH and O'Neill WC: Effect of bisphosphonates on vascular
calcification and bone metabolism in experimental renal failure.
Kidney Int. 75:617–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Afroze T, Yang LL, Wang C, Gros R, Kalair
W, Hoque AN, Mungrue IN, Zhu Z and Husain M:
Calcineurin-independent regulation of plasma membrane
Ca2+ ATPase-4 in the vascular smooth muscle cell cycle.
Am J Physiol Cell Physiol. 285:C88–C95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Idelevich A, Rais Y and Monsonego-Ornan E:
Bone Gla protein increases HIF-1alpha-dependent glucose metabolism
and induces cartilage and vascular calcification. Arterioscler
Thromb Vasc Biol. 31:e55–e71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kelynack KJ and Holt SG: An in vitro
murine model of vascular smooth muscle cell mineralization. Methods
Mol Biol. 1397:209–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu D, Rashdan NA, Chapman KE, Hadoke PW
and MacRae VE: A novel role for the mineralocorticoid receptor in
glucocorticoid driven vascular calcification. Vascul Pharmacol.
86:87–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu D, Hadoke PW, Wu J, Vesey AT, Lerman
DA, Dweck MR, Newby DE, Smith LB and MacRae VE: Ablation of the
androgen receptor from vascular smooth muscle cells demonstrates a
role for testosterone in vascular calcification. Sci Rep.
6:248072016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Johnson RC, Leopold JA and Loscalzo J:
Vascular calcification: Pathobiological mechanisms and clinical
implications. Circ Res. 99:1044–1059. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Yang HY and Giachelli CM: Role of
the sodium-dependent phosphate cotransporter, Pit-1, in vascular
smooth muscle cell calcification. Circ Res. 98:905–912. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yao Y, Bennett BJ, Wang X, Rosenfeld ME,
Giachelli C, Lusis AJ and Boström KI: Inhibition of bone
morphogenetic proteins protects against atherosclerosis and
vascular calcification. Circ Res. 107:485–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luo G, Ducy P, McKee MD, Pinero GJ, Loyer
E, Behringer RR and Karsenty G: Spontaneous calcification of
arteries and cartilage in mice lacking matrix GLA protein. Nature.
386:78–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koos R, Krueger T, Westenfeld R, Kühl HP,
Brandenburg V, Mahnken AH, Stanzel S, Vermeer C, Cranenburg EC,
Floege J, et al: Relation of circulating Matrix Gla-Protein and
anticoagulation status in patients with aortic valve calcification.
Thromb Haemost. 101:706–713. 2009.PubMed/NCBI
|
|
46
|
Villa-Bellosta R, Wang X, Millan JL,
Dubyak GR and O'Neill WC: Extracellular pyrophosphate metabolism
and calcification in vascular smooth muscle. Am J Physiol Heart
Circ Physiol. 301:H61–H68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Q, Kingman J, Sundberg JP, Levine MA
and Uitto J: Dual effects of bisphosphonates on ectopic skin and
vascular soft tissue mineralization versus bone microarchitecture
in a mouse model of generalized arterial calcification of infancy.
J Invest Dermatol. 136:275–283. 2016. View Article : Google Scholar : PubMed/NCBI
|