Introduction
Gastric cancer accounts for 9.7% of the total number
of cancer-associated mortalities globally, and accounts for 7.8% of
new malignant tumor diagnoses annually (1). Gastroscopy is one of the early
diagnosis methods in gastric cancer, and the 5-year survival rate
of patients with gastric cancer has been improved due to
gastroscopy (2). The early
recurrence of gastric cancer resulting from lymphatic vessel
micrometastasis, as well as increased tolerance to radiotherapy and
chemotherapy in advanced gastric cancer, are predominant risk
factors to be addressed in order to improve gastric cancer
prognosis (3,4). The lymph node and hematogenous
metastasis pathways are the primary metastasis pathways associated
with gastric cancer and are closely associated with gastric cancer
prognosis. Therefore, it is important to investigate potential
therapeutic targets associated with the occurrence and metastasis
of gastric cancer, and to explore molecular targeted therapy and
prognosis of gastric cancer.
The chicken ovalbumin upstream transcription factors
(COUP-TFs), belonging to the steroid/thyroid hormone receptor
family, were discovered in 1986 (5–7). The
COUP-TFs family members include two highly homologous proteins,
termed COUP-TFI (8,9) and COUP-TFII (10,11),
and their encoding genes are located on chromosome 5 and 15,
respectively. COUP-TFII is generally considered to be an orphan
nuclear receptor, as its ligand(s) have not yet been determined.
COUP-TFII is also known as nuclear receptor subfamily 2, group F,
member 2. According to previous studies, COUP-TFII is implicated in
the regulation of gene expression and organism development, cell
differentiation and homeostasis, and is also involved in
tumorigenesis and metastasis processes (12–14).
COUP-TFII expression levels are associated with tumorigenesis,
invasion and metastasis in numerous cancer types, such as breast,
prostate (12), pancreatic
(15), ovarian (16) and colorectal cancer (17). However, the association between
COUP-TFII and gastric cancer has not yet been fully determined.
This study aimed to investigate the potential use of COUP-TFII as a
gastric cancer biomarker, and its use as a molecular target in
targeted therapy. Further study of the COUP-TFII protein may
provide novel alternatives to the current therapeutic treatment
options available to patients with gastric cancer.
Materials and methods
Clinical tissue samples and cell
lines
Gastric cancer tissues and their matched normal
adjacent tissues were obtained from the Department of
Gastrointestinal Surgery, The First Affiliated Hospital of Xiamen
University (Xiamen, China), between September 2013 to February
2016. The patients' age ranged from 39 to 76-years-old with a
distribution of 57.9% of males among the patients. All samples were
collected with patients' informed consent, and all tissues were
pathologically confirmed. This study was approved by the Ethics
Committee of the First Affiliated Hospital of Xiamen University
(Xiamen, China). In the present study, tumor staging criteria were
based on the 2010 American Joint Committee on Cancer's TNM staging
criteria for gastric cancer (18).
BGC-823, MGC-803 and SGC-7901 gastric cancer cells, GES-1 normal
gastric mucosal epithelial cells and 293T cells, were obtained from
the Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai, China). All gastric cancer cells and GES-1
cells were maintained in RPMI1640 media (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) (19), whereas
the 293T cells were cultured in Dulbecco's modified Eagle's medium
(Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal bovine
serum (FBS, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). All cells were maintained at 37°C in a humidified incubator
with 5% CO2.
Plasmid construction and generation of
stable cell lines
COUP-TFII was amplified using cDNA and then inserted
into the pCDH-CMV-MCS-EF1-Puro plasmid (System
Biosciences, Palo Alto, CA, USA). The primers used to generate
these constructs were as follows: COUP-TFII forward,
5′-GCTCTAGAATGGCAATGGTAGTCAGCACGT-3′ and reverse,
5′-ATAAGAATGCGGCCGCTTATTGAATTGCCATATACGGCCAGT-3′; GAPDH (control)
forward, 5′-GTGGACCTGACCTGCCGTCT-3′, and reverse,
5′-GGAGGAGTGGGTGTCGCTGT-3′. For the generation of COUP-TFII stable
cell lines, a lentivirus-mediated packaging system containing four
plasmids, COUP-TFII or control plasmid (System Biosciences), pMDL,
REV and VSVG (Invitrogen; Thermo Fisher Scientific, Inc.) were used
as described (20). For the stable
knockdown COUP-TFII in GES-1 cells, pLL3.7-puro containing
COUP-TFII small interfering RNA (siRNA) or negative control was
co-transfected with pMDL, REV and VSVG as described (20). The siRNA sequences were as follows:
siRNA#1 forward, 5′-GCGAGCUGUUUGUGUUGAATT-3′ and reverse,
5′-UUCAACACAAACAGCUCGCTT-3′; siRNA#2 forward,
5′-GGAUCUUCCAAGAGCAAGUTT-3′ and reverse,
5′-ACUUGCUCUUGGAAGAUCCTT-3′; siRNA#3 forward,
5′-GGCCGUAUAUGGCAAUUCATT-3′ and reverse,
5′-UGAAUUGCCAUAUACGGCCTT-3′. Transfected cells were cultured for 72
h prior to subsequent experimentation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples and cell
lines using the TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.,) according to the manufacturer's instructions.
For mRNA reverse transcription, cDNA was synthesized using the
ReverTra AceH qPCR RT kit (Toyobo, Osaka, Japan) with 1 mg total
RNA. RT-qPCR was performed via SYBR Green assay (Invitrogen; Thermo
Fisher Scientific, Inc.) using the SYBRH Select Master Mix for CFX
(Invitrogen; Thermo Fisher Scientific, Inc.) as described (21). GAPDH was used as the control. The
reverse transcription primers and qPCR primers of COUP-TFII and
GAPDH were purchased from Thermo Fisher Scientific, Inc. COUP-TFII
relative expression was evaluated by using the 2−ΔΔCq
method (22). All PCR assays were
performed in triplicate.
Cell proliferation assay
In order to determine cell viability, a MTT assay
was performed according to a recent study (21). Firstly, MGC-803 and GES-1 cells
were seeded and transfected in a 96-well plate. At 24, 48, 72, 96
and 120 h time intervals post-transfection, MTT (0.5 mg/ml,
Sigma-Aldrich; Merck KGaA) was added to the cells, and the
absorbance at 490 nm was then measured 3 h later. For colony
formation assays, cells were seeded in 6-well plates and maintained
in RPMI 1640 medium containing for 10–14 days as described
(23). Colonies were fixed with 4%
paraformaldehyde and incubated for 15 min at room temperature,
stained with 0.1% crystal violet for 15 min at room temperature,
and then photographed using a digital camera (23). Experiments were repeated at least
three times.
Migration and invasion assays
The Transwell migration assay was performed as
described (21). A total of
5×104 MGC-803 or GES-1 cells were plated on the top
chambers of 8 µm pore size Transwell plates (Corning Incorporated,
Corning, NY, USA). In order to perform the Matrigel-coated
Transwell invasion assay as described (21), Matrigel and 8×104
MGC-803 or GES-1 cells were plated on the top chambers of 8 µm pore
size Transwell plates (Corning Incorporated, Corning, NY, USA). The
migration and invasion assays were conducted for 48 h; all
experiments were performed at least three times in triplicate.
Western blot analysis
Western blotting was performed as described
(21). Cell lysates (10 mg) were
subjected to 10% SDS-PAGE analysis and immunoblotting analysis
using antibodies against COUP-TFII (1:5,000; ab64849, Abcam,
Cambridge, UK), and either β-actin (1:5,000; ab8227, Abcam,
Cambridge, UK) was used as a protein loading control. Proteins were
visualized with an ECL kit and film (Kodak, Rochester, NY, USA) in
a dark room; the film was scanned using a HP ScanJet Pro 2000
(Hewlett-Packard, Palo Alto, CA, USA) and analyzed with ImageJ
software (v 1.46r; National Institutes of Health, Bethesda, MD,
USA).
Immunohistochemistry
Sections were then cut and stained using
immunohistochemistry as described (20). Briefly, the sections were
deparaffinized, rehydrated by using a descending alcohol series,
subjected to microwave antigen retrieval buffer (Sigma-Aldrich;
Merck KGaA) and then incubated for 10 min with 3% hydrogen peroxide
at room temperature. Non-specific binding was blocked using 5%
bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 30 min at
37°C. The sections were then incubated with rabbit polyclonal
anti-COUP-TFII antibody (1:200; ab64849, Abcam) overnight at 4°C,
and then incubated with goat anti-mouse immunoglobulin G H&L
biotinylated secondary antibody (1:200; ab6788, Abcam) bound to a
streptavidin-horseradish peroxidase complex for 30 min in the dark
at room temperature. The bound antibody was detected using
3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocols, and the sections were then counterstained
with hematoxylin at room temperature for 1 min, dehydrated and
mounted. Two pathologists scored all sections independently. The
staining index was calculated as the product of the staining
intensity (−, no staining; +, weak, light yellow; ++, moderate,
yellow brown; +++, strong, brown). Staining intensity was
determined using Image Pro Plus software (version 6.0; Media
Cybernetics, Inc., Rockville, MD, USA); the average integrated
optical density (IOD) was obtained by analyzing five fields per
slide. The average IOD of tumor tissue was divided by the average
IOD of paired normal tissue to attain the relative average IOD.
Tumorigenicity in vivo
The Animal Care and Use Committee of Xiamen
University approved this study and all its experimental protocols.
For tail vein metastasis, either 5×105 MGC-803/COUP-TFII
cells or 5×105 MGC-803 control cells were injected into
6-week-old male BALB/C mice (n=4 per group; weight=26.3±2.4 g;
provided by the Experimental Animal Center of Xiamen University,
Xiamen, China). Hepatic metastasis on day 14 was monitored as
described (20) once a week for 4
weeks, using the live animal Lumina II system (Xenogen IVIS system;
PerkinElmer, Inc., Waltham, MA, USA).
Database analysis
A dataset with clinical pathological parameters in
80 gastric patients was obtained from the Oncomine database Cui
Gastric (3610110; https://www.oncomine.org/); the relevance between
COUP-TFII expression level and patients' clinical stage and overall
survival was investigated using the analysis of ‘Gene summary view’
and ‘Dataset view’ (24,25). Data of COUP TFII expression levels
of gastric cancer tissue and relative gastric tissue from a dataset
were standardized and compared; if the value obtained was greater
within gastric cancerous tissue than the corresponding healthy
tissue, expression would be regarded as ‘overexpressed’. However,
low expression would be observed if the values were lower within
gastric cancer tissue compared with in the healthy tissue.
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. All images were made using GraphPad
Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA). Data
are presented as mean ± standard deviation. The two-tailed
Student's t-test and one-way analysis of variance, followed by a
Chi-square post-hoc test were used in order to determine P-values,
and P<0.05 was considered to indicate a statistically
significant difference.
Results
COUP-TFII is downregulated in gastric
cancer tissues and gastric cancer cell lines
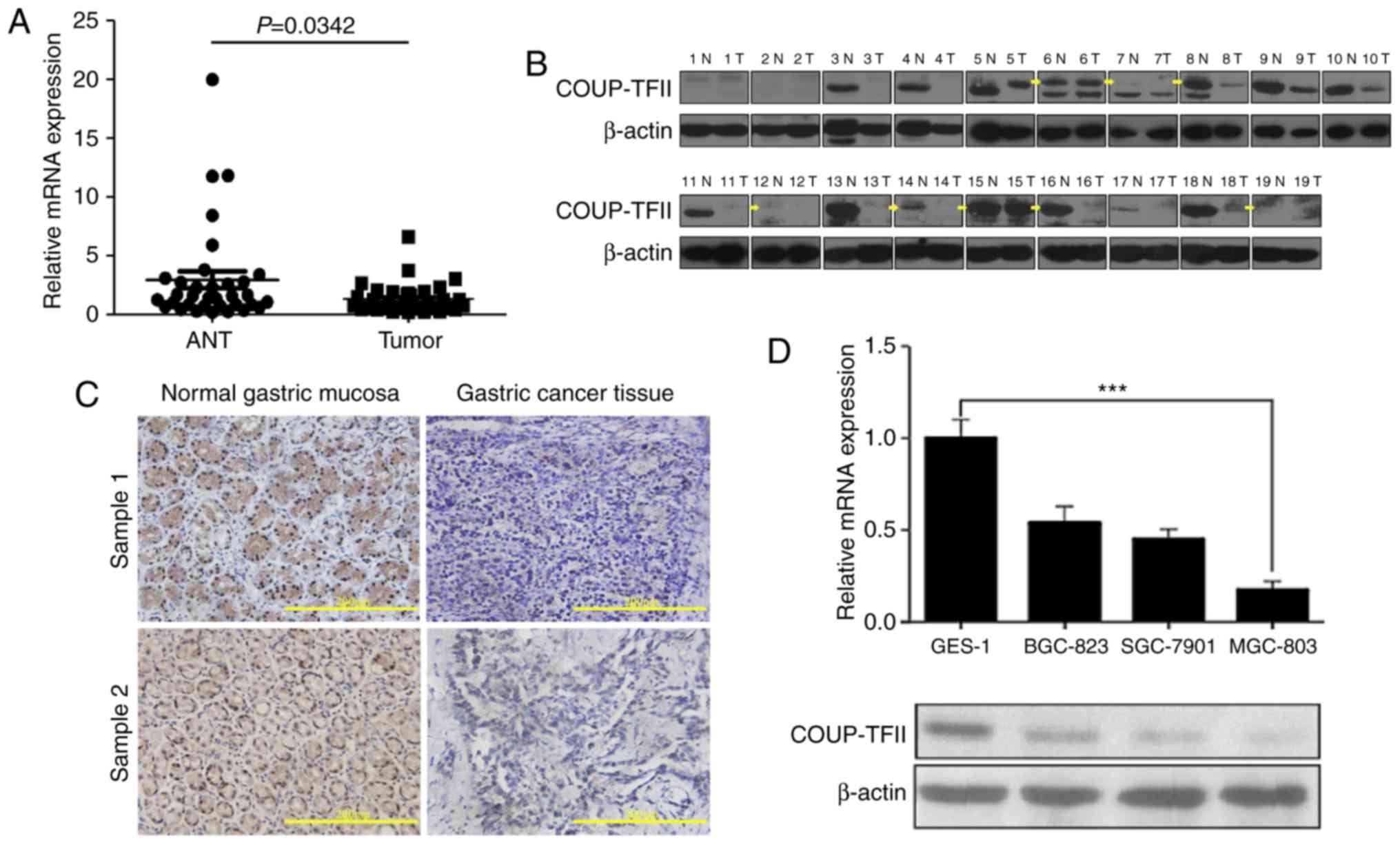
In order to investigate the difference in COUP-TFII
expression between gastric cancer tissues and their matched
adjacent non-neoplastic tissues (ANT), the COUP-TFII expression
levels were investigated using RT-qPCR and western blot analysis on
samples derived from patients with gastric cancer. Subsequently, it
was revealed that COUP-TFII mRNA and protein expression levels in
gastric carcinoma samples were significantly reduced compared with
normal gastric mucosa tissues in 34 cases and 19 cases,
respectively (Fig. 1A and B).
Furthermore, immunohistochemical analysis revealed that COUP-TFII
expression in gastric cancer tissues (11/19) was reduced compared
with COUP-TFII expression in ANT (Fig.
1C, scale bar, 200 µm; Table
I). The expression levels of COUP-TFII mRNA and protein in
three gastric cancer cell lines (SGC-7901, SGC-823 and MGC-803),
and a normal gastric mucosa cell line (GES-1), were then determined
via RT-qPCR and western blotting (Fig.
1D). Compared with GES-1, the expression level of COUP-TFII was
reduced in all gastric cell lines, particularly in the MGC-803 cell
line (P<0.001). These results therefore suggest that COUP-TFII
expression has an association with gastric cancer prognosis.
 | Table I.COUP-TFII expression in clinical
gastric cancer samples and ANT tissues revealed by
immunohistochemistry. |
Table I.
COUP-TFII expression in clinical
gastric cancer samples and ANT tissues revealed by
immunohistochemistry.
|
|
| Staining index of
COUP-TFII expression |
|---|
|
|
|
|
|---|
| Sample | Number | (−) | (+) | (++) | (+++) |
|---|
| ANT | 19 | 5 | 1 | 2 | 11 |
| Gastric cancer | 19 | 11 | 1 | 3 | 4 |
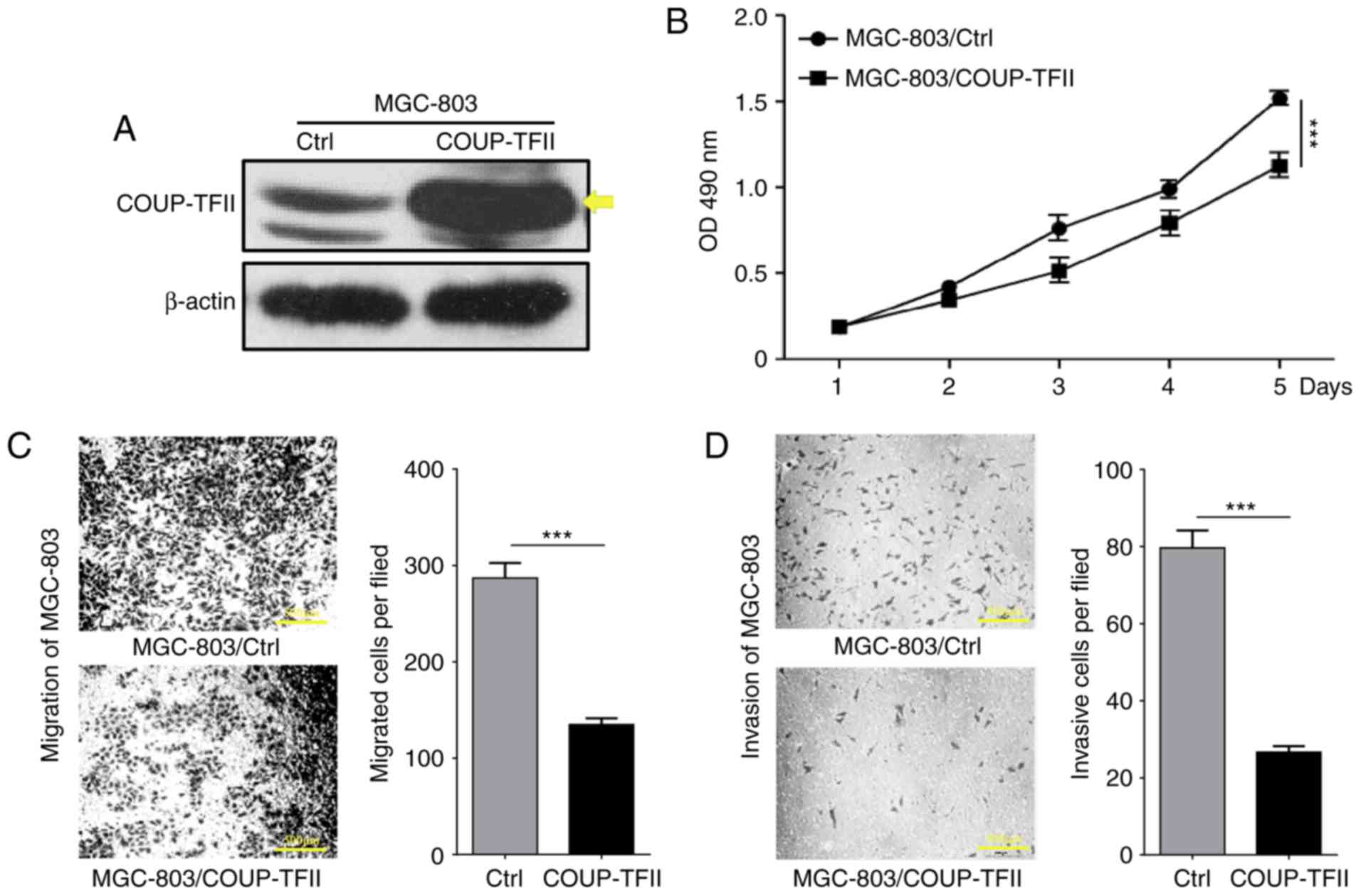
COUP-TFII inhibits proliferation,
migration and invasion of gastric cancer cell in vitro
To determine the effect of COUP-TFII on gastric
cancer cell proliferation, a COUP-TFII expression plasmid was
transfected into the MGC-803 cell line (Fig. 2A), and cell proliferation was
determined via MTT assay. As revealed by Fig. 2B, the level of cellular
proliferation in the MGC-803/COUP-TFII group was significantly
reduced compared with the MGC-803 and MGC-803 control groups
(P<0.001). In order to determine whether COUP-TFII could
modulate the metastasis of gastric cancer cells, Transwell assays
were performed to evaluate the migration and invasion capacities of
the cells in vitro. This revealed a significant reduction in
the migratory and the invasive abilities of the MGC-803/COUP-TFII
group (P<0.001; Fig. 2C and D;
scale bar, 500 µm). In conclusion, these results demonstrate that
COUP-TFII expression inhibits proliferation, migration and invasion
of gastric cancer cells in vitro.
Downregulated COUP-TFII enhances the
proliferative, migratory and invasive abilities of GES-1
As a result of siRNA transfection, three COUP-TFII
knockdown cell lines were established (group si#1, si#2, si#3).
Western blotting analysis demonstrated that COUP-TFII expression
was reduced by the siRNA compared with the control group
(P<0.01; Fig. 3A). Furthermore,
the MTT assay and the colony formation experiment revealed that
knockdown of COUP-TFII in GES-1 cells enhanced their proliferative
ability to different extents (P<0.05; Fig. 3B and C; scale bar, 6 mm). In
addition, the results of the Transwell assays revealed that the
migratory (P<0.01) and invasive (P<0.001) abilities of GES-1
cells were also significantly increased by COUP-TFII knockdown
compared with the negative control (Fig. 3D; scale bar, 500 µm).
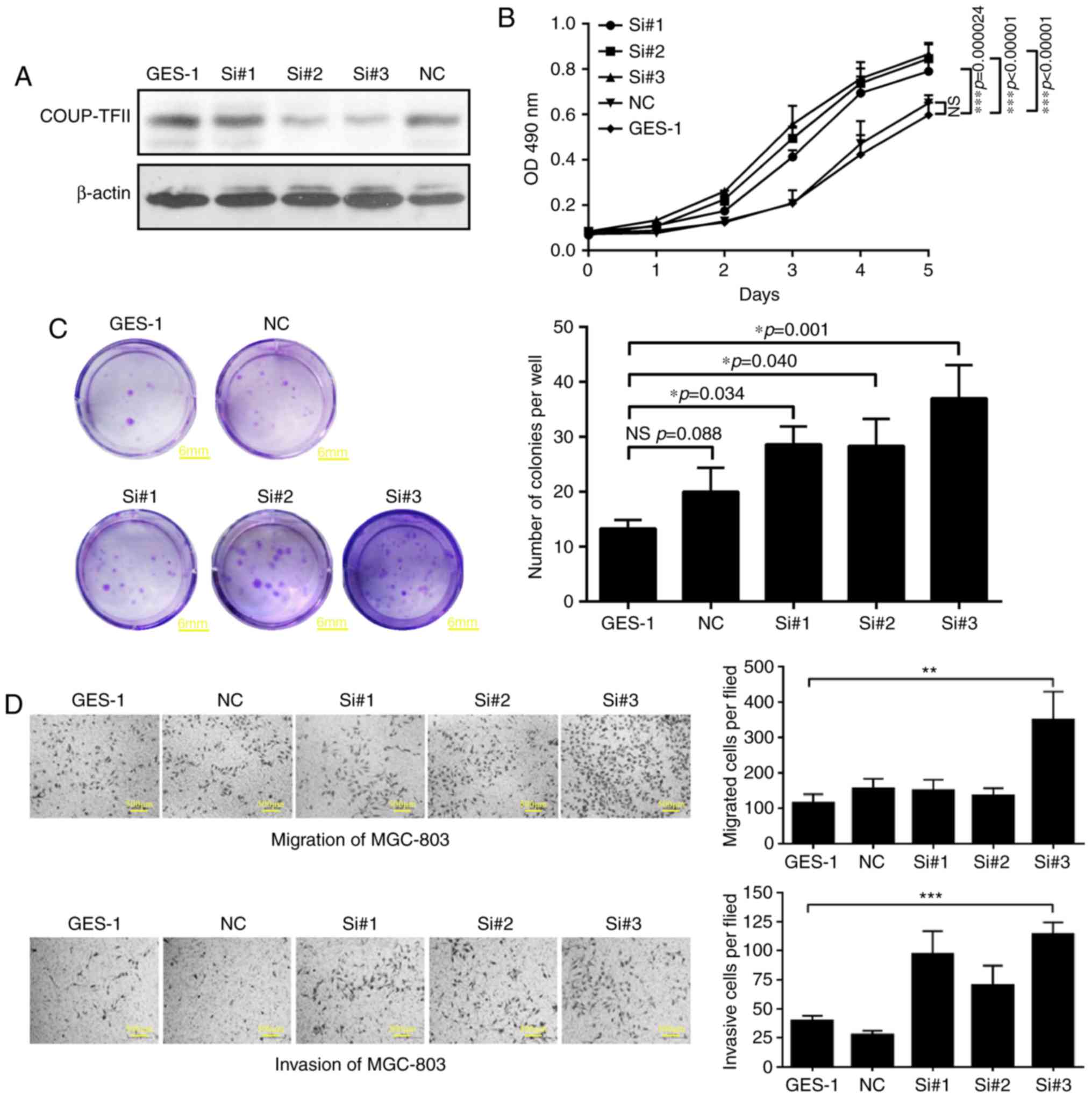 | Figure 3.Downregulation of COUP-TFII
expression enhances the proliferation, migration and invasion of
GES-1 cells. (A) Relative COUP-TFII expression revealed by western
blotting analysis in three COUP-TFII knockdown cell lines (groups
si#1, si#2, and si#3). (B) MTT assay and the (C) colony formation
experiments demonstrate that following downregulation of COUP-TFII
gene expression in GES-1 cells, cellular proliferative ability is
differentially affected. Scale bar, 6 mm. (D) Transwell migration
assays and invasion assays with Matrigel-coated membranes of three
COUP-TFII knockdown cell lines. Scale bar, 500 µm. *P<0.05,
**P<0.01, ***P<0.001. COUP-TFII, chicken ovalbumin upstream
promoter transcription factor 2; NS, no significance; NC, negative
control; Si, siRNA. |
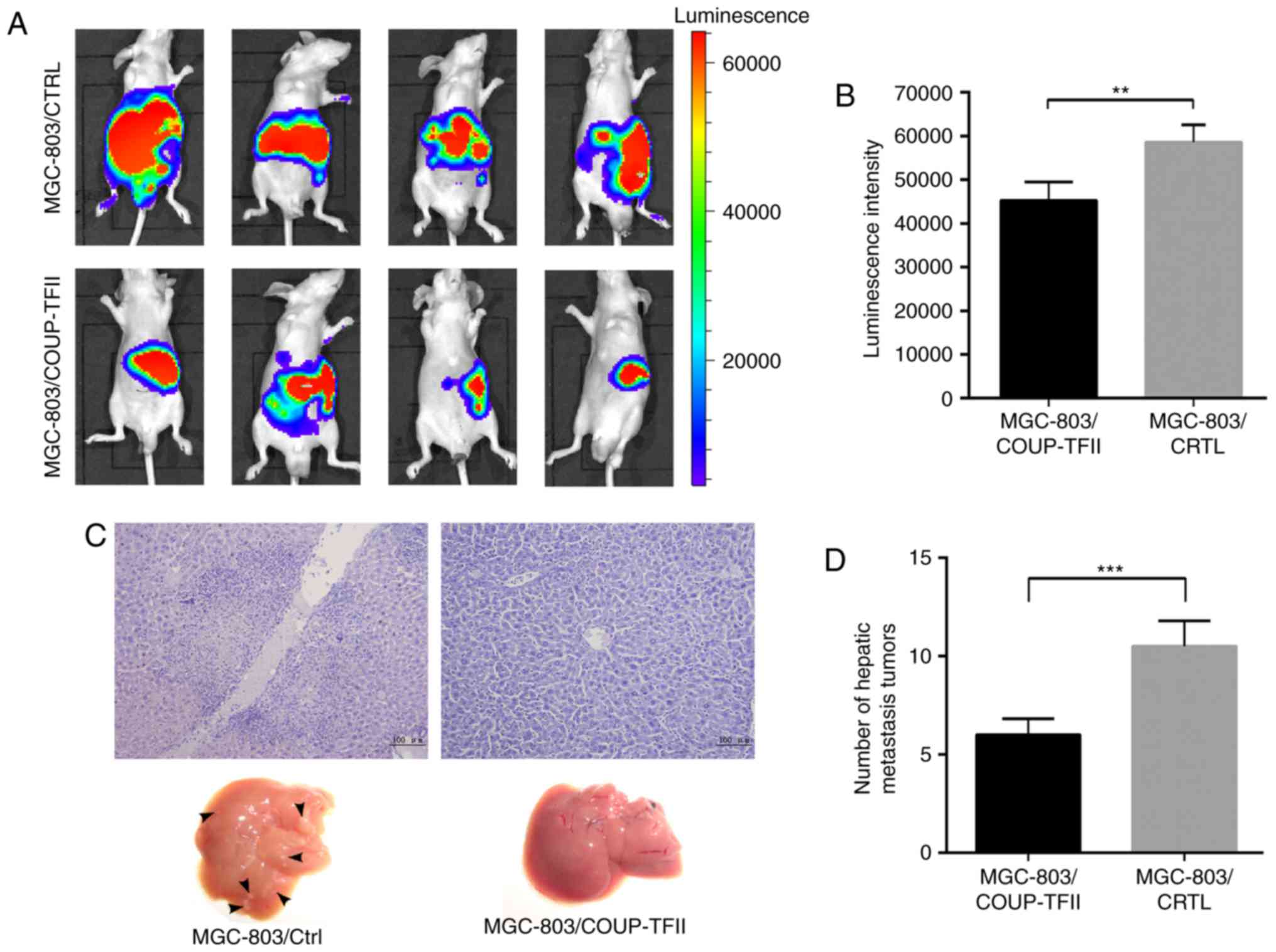
COUP-TFII inhibits tumor growth and
hepatic metastasis of gastric cancer in vivo
In order to determine whether COUP-TFII expression
could inhibit tumor growth and metastasis of gastric cancer cells
in vivo, MGC-803/COUP-TFII cells and MGC-803 control cells
were labeled with luciferase, injected into nude mice, and then
analyzed 2 weeks later. Bioluminescence imaging results revealed
that MGC-803/COUP-TFII cells produced significantly smaller tumors
compared with the mice injected with MGC-803 control cells
(Fig. 4A). Associated statistical
analysis of luminescence intensity is presented in Fig. 4B (P<0.01). Furthermore, the
number of hepatic metastases in the MGC-803/COUP-TFII group was
less than in the MGC-803 control group (P<0.001; Fig. 4C and D; scale bar, 100
µm).
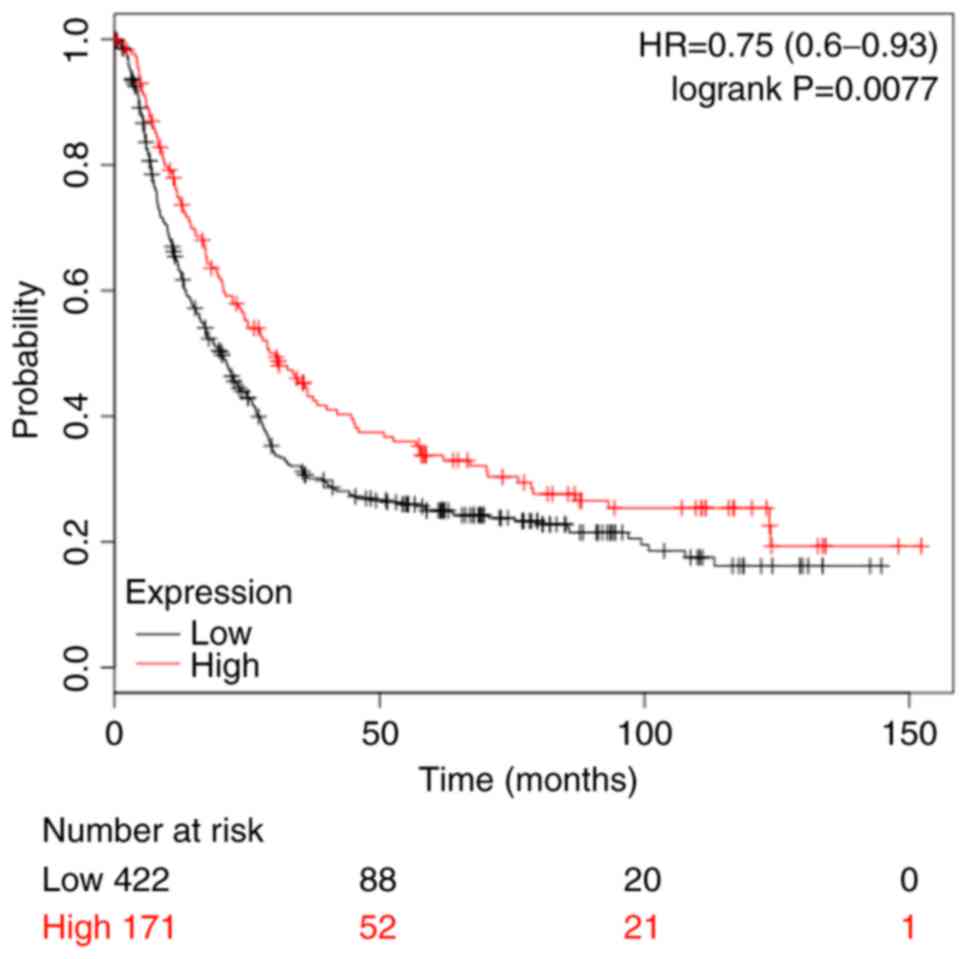
Database analysis suggests that high
expression level of COUP-TFII is significantly associated with
patients' clinical stage and overall survival
As presented in Table
II, analysis of the clinical pathological parameters in 80
gastric patients obtained from the Oncomine database (https://www.oncomine.org/) suggested that the
expression level of COUP-TFII in gastric cancer has no association
with the patient's sex, age and grade (P>0.05), however, it does
have an association with the patient's clinical stage (P<0.01;
Table II). The Kaplan-Meier plot
revealed that high expression levels of COUP-TFII improved overall
survival in patients with gastric cancer (Fig. 5).
 | Table II.Statistical analysis of gastric
cancer clinical sample data from Oncomine database. |
Table II.
Statistical analysis of gastric
cancer clinical sample data from Oncomine database.
| Clinical data | Samples (n) | High expression of
COUP-TFII (%) | Low expression of
COUP-TFII (%) | χ2 | P-value |
|---|
| Total samples | 80 | 32 (40) | 48 (60) |
| 0.006 |
| Age |
|
|
| 0.02 | >0.05 |
|
>50 | 67 | 28 (41.8) | 39 (58.2) |
|
|
|
<50 | 10 | 5 (50) | 5 (50) |
|
|
| Gender |
|
|
| 1.52 | >0.05 |
|
Male | 52 | 25 (48) | 27 (52) |
|
|
|
Female | 25 | 8 (32) | 17 (68) |
|
|
| Stage |
|
|
| 0.55 | >0.05 |
|
1–2 | 15 | 6 (40) | 9 (60) |
|
|
|
3–4 | 37 | 19 (51.4) | 18 (48.6) |
|
|
| Clinical stage |
|
|
| 22.4 | <0.01 |
|
I–II | 11 | 3
(27.3) | 8
(72.7) |
|
|
|
III–IV | 61 | 29 (47.5) | 32 (52.5) |
|
|
Discussion
Gastric cancer is one of the most prevalent
gastrointestinal malignant tumors, and poses a significant threat
to health and life expectancy. Well-established treatments, such as
surgical procedures, do not always meet the requirements of
patients. As a consequence of accumulating research in recent
years, molecular targeted therapy is fast becoming an increasingly
prominent field within oncology research. Nuclear receptors are
significantly implicated in the development of tumors. Nuclear
receptors themselves, or their downstream target molecules, are
often abnormally expressed in breast cancer, prostate cancer and
other cases of tumorigenesis (26). COUP-TFs are important members of
the nuclear receptor family, and COUP-TFII, when isolated from the
nuclear extracts of HeLa cells, can bind directly to the upstream
promoter region of chicken ovalbumin in order to regulate its
transcription (7). COUP-TFII has
an important function in physiological development and
tumorigenesis. In recent years, the involvement of COUP-TFII in
malignant tumor progression has fast become a major focus of cancer
research.
Previous studies have revealed that numerous
malignant tumor tissues abnormally express COUP-TFII, which
regulates the invasion and migration of tumor cells (27). Qin et al (12) demonstrated that COUP-TFII inhibits
the transforming growth factor β (TGF-β) signaling pathway and
promotes the growth and migration of prostate cancer cells.
Furthermore, Qin et al (13) revealed that ~60% of prostate cancer
samples had moderate to high expression levels of COUP-TFII;
whereas only 5% of normal prostate tissues demonstrated abnormal
expression of COUP-TFII. In addition, Bao et al (17) detected the expression of COUP-TFII
in colon cancer tissue samples and revealed that the expression
levels of COUP-TFII were elevated in cancerous tissues compared
with normal mucosa tissues, and demonstrated that COUP-TFII could
promote the invasion and migration of colorectal cancer cells via
inhibition of the expression of adhesion molecules, such as tight
junction protein 1, epithelial cadherin and β-catenin. Polvani
et al (15) demonstrated
that COUP-TFII was highly expressed in pancreatic cancer tissues.
The invasion experiments demonstrated that the downregulation of
COUP-TFII expression levels in pancreatic cancer cells may inhibit
their invasiveness (15). However,
there is evidence that COUP-TFII demonstrated specific expression
patterns in certain tumor types (16,28),
such as in ovarian cancer, where low expression of COUP-TFII in
stromal tissue, and abnormally high expression in epithelial
tissues was detected, thus suggesting that COUP-TFII may have
different roles among different tumor types.
In the present study, the expression of COUP-TFII in
gastric cancer tissues was lower than that in the normal gastric
mucosa, thereby suggesting that the expression of the COUP-TFII
gene is reduced in gastric cancer tissues. This result is in
contrast to previous studies demonstrating an increase in the
expression of the COUP-TFII gene in prostate cancer (12), colon adenocarcinoma (17) and pancreatic cancer tissues
(15). In order to investigate the
effects of COUP-TFII on the proliferation and invasion of gastric
cancer cells, an MGC-803 gastric cancer cell line with
overexpressed COUP-TFII was established. The results of the MTT
assay revealed that after 3 days of culture, the 490 nm relative
absorbance of the MGC-803/COUP-TFII group was lower than that of
the MGC-803 control group (P<0.05), thus suggesting that
overexpression of COUP-TFII may significantly reduce the
proliferative ability of gastric cancer cells. Furthermore, the
results of the migration and invasion assays revealed that the
migratory abilities of the MGC-803/COUP-TFII cells was
significantly reduced compared with the MGC-803 control cells
(P<0.001), thereby suggesting that overexpression COUP-TFII can
decrease the mobility and invasive abilities of gastric cancer
cells. In the present study, the COUP-TFII gene was knocked down in
GES-1 cells. The MTT assay and the Transwell migration and invasion
assays demonstrated that knockdown of COUP-TFII expression in GES-1
cells significantly increased cellular proliferation and the
migratory and invasive abilities of the cells (P<0.01). These
results suggest that the expression level of COUP-TFII can inhibit
the proliferation, migration and invasion of gastric cancer cells,
as well as function as a tumor suppressor gene.
Direct spread, lymph node metastasis, hematogenous
metastasis and intraperitoneal implantation are the main transfer
pathways of gastric cancer, among which lymph node metastasis and
hematogenous metastasis are the predominant pathways responsible
for the development of distant metastasis of gastric cancer
(29). Previous studies have
revealed that COUP-TFII is involved in the regulation of vascular
and lymph angiogenesis via the regulation of vascular endothelial
growth factor as well as the expression level of its receptor, and
thus acts as a regulator of neovascularization and lymph
angiogenesis levels (14,30,31).
However, lymph node metastasis and hematogenous metastasis are
complex processes and include multiple regulatory factors. Lin
et al (32) demonstrated
that COUP-TFII can affect levels of lymphangiogenesis in a breast
cancer rat model via regulation of neuropilin 2 (NRP2) expression,
thus suggesting that COUP-TFII may enact its therapeutic effect via
regulation of NRP2 expression in order to affect the incidence of
lymph node metastasis in breast cancer. The present study revealed
that COUP-TFII expression in gastric carcinoma was reduced compared
with normal gastric tissue, and that overexpression of COUP-TFII
can downregulate the proliferation and migration of the MGC-803
cell line. Furthermore, the results of present study suggest that
COUP-TFII can inhibit the growth and invasion of gastric cancer and
may also be involved in the development of distant metastases.
Therefore, the present study use injection over expression of
COUP-TFII stable cell strain in order to nude mice spleen in
establishment of gastric cancer liver metastasis model by using
in vivo imaging system (33). The results revealed that the number
of liver metastases was lower in mice injected with COUP-TFII
overexpressing gastric cancer cells compared with the control group
(P<0.001), and hematoxylin and eosin analysis of liver
metastasis tissue sections confirmed that in cases of gastric
cancer, COUP-TFII can inhibit gastric cancer metastasis to the
liver.
Furthermore, the results of the present study
revealed that COUP-TFII can inhibit the growth and invasion of
gastric cancer, as well as limit the occurrence of liver
metastases. The grade, lymph node metastasis and distant metastasis
are all factors considered in gastric cancer clinical stage
classification. Database analysis of 80 patients with gastric
cancer revealed that early stage (I–II) expression level of
COUP-TFII was increased compared with expression levels of
COUP-TFII in late stages (III–IV; P<0.01), thus suggesting that
COUP-TFII may influence the occurrence and development of gastric
cancer. Clinical stages are closely associated with the prognosis
of patients with gastric cancer (34). Furthermore, database analysis
investigating survival rates demonstrated that increased expression
of COUP-TFII significantly correlated with increased survival rates
of patients with gastric cancer.
In conclusion, the results of the present study
revealed that COUP-TFII was reduced in cases of gastric cancer.
Furthermore, the results suggested that overexpression of COUP-TFII
can inhibit growth, invasion and migration of gastric cancer in
vitro and inhibit metastasis to the liver in vivo, and
thus, may improve the survival rate of patients with gastric
cancer. Therefore, the present study suggests that COUP-TFII may
serve as a potential tumor biomarker and a target for molecular
therapies. However, further studies aiming to uncover the
underlying regulatory mechanisms of COUP-TFII associated with
gastric cancer are required in order to determine the therapeutic
potential of COUP-TFII. Future studies should investigate the
association of COUP-TFII expression with regards to the TGF-β/SMAD
signaling pathway, the P53 signaling pathway, the
mitogen-associated protein kinase signaling pathway and the
epithelial-mesenchymal transition, in order to uncover the
molecular mechanism underpinning the link between COUP-TFII and
gastric cancer.
Acknowledgements
This study was supported by the Science and
Technology Project of Natural Science Foundation of Fujian Province
(grant nos. 2016J01639 and 2015J01564), the Medical Innovations
Topic in Fujian Province (grant nos. 2016-CXB-8 and 2012-CXB-29)
and Project of Xiamen Scientific and Technological Plan (grant no.
3502Z20134011).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang Y and Ajani JA: Multidisciplinary
management of gastric cancer. Curr Opin Gastroenterol. 26:640–646.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi W, Wang J, Zhang M, Yu C, He W and Liu
P: The multivariate prognostic analysis of 1340 gastric cancer
patients who received radical resection. J Nanjing Med Univ (Nat
Sci Ed). 31:1310–1315. 2010.
|
|
4
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the human
development index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pastorcic M, Wang H, Elbrecht A, Tsai SY,
Tsai MJ and O'Malley BW: Control of transcription initiation in
vitro requires binding of a transcription factor to the distal
promoter of the ovalbumin gene. Mol Cell Biol. 6:2784–2791. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang LH, Tsai SY, Cook RG, Beattie WG,
Tsai MJ and O'Malley BW: COUP transcription factor is a member of
the steroid receptor superfamily. Nature. 340:163–166. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sagami I, Tsai SY, Wang H, Tsai MJ and
O'Malley BW: Identification of two factors required for
transcription of the ovalbumin gene. Mol Cell Biol. 6:4259–4267.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyajima N, Kadowaki Y, Fukushige S,
Shimizu S, Semba K, Yamanashi Y, Matsubara K, Toyoshima K and
Yamamoto T: Identification of two novel members of erbA superfamily
by molecular cloning: The gene products of the two are highly
related to each other. Nucleic Acids Res. 16:11057–11074. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang LH, Tsai SY, Sagami I, Tsai MJ and
O'Malley BW: Purification and characterization of chicken ovalbumin
upstream promoter transcription factor from HeLa cells. J Biol
Chem. 262:16080–16086. 1987.PubMed/NCBI
|
|
10
|
Wang LH, Ing NH, Tsai SY, O'Malley BW and
Tsai MJ: The COUP-TFs compose a family of functionally related
transcription factors. Gene Expr. 1:207–216. 1991.PubMed/NCBI
|
|
11
|
Ladias JA and Karathanasis SK: Regulation
of the apolipoprotein AI gene by ARP-1, a novel member of the
steroid receptor superfamily. Science. 251:561–565. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin J, Wu SP, Creighton CJ, Dai F, Xie X,
Cheng CM, Frolov A, Ayala G, Lin X, Feng XH, et al: COUP-TFII
inhibits TGF-β-induced growth barrier to promote prostate
tumorigenesis. Nature. 493:236–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin J, Chen X, Xie X, Tsai MJ and Tsai SY:
COUP-TFII regulates tumor growth and metastasis by modulating tumor
angiogenesis. Proc Natl Acad Sci USA. 107:pp. 3687–3692. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin J, Chen X, Yu-Lee LY, Tsai MJ and Tsai
SY: Nuclear receptor COUP-TFII controls pancreatic islet tumor
angiogenesis by regulating vascular endothelial growth
factor/vascular endothelial growth factor receptor-2 signaling.
Cancer Res. 70:8812–8821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Polvani S, Tarocchi M, Tempesti S, Mello
T, Ceni E, Buccoliero F, D'Amico M, Boddi V, Farsi M, Nesi S, et
al: COUP-TFII in pancreatic adenocarcinoma: Clinical implication
for patient survival and tumor progression. Int J Cancer.
134:1648–1658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hawkins SM, Loomans HA, Wan YW,
Ghosh-Choudhury T, Coffey D, Xiao W, Liu Z, Sangi-Haghpeykar H and
Anderson ML: Expression and functional pathway analysis of nuclear
receptor NR2F2 in ovarian cancer. J Clin Endocrinol Metab.
98:E1152–E1162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao Y, Gu D, Feng W, Sun X, Wang X, Zhang
X, Shi Q, Cui G, Yu H, Tang C and Deng A: COUP-TFII regulates
metastasis of colorectal adenocarcinoma cells by modulating Snail1.
Br J Cancer. 111:933–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu YM, Hu XP, Zhang L, Zhang SS and Xu JM:
Uptake characteristics of levofloxacin for the eradication of
Helicobacter pylori by GES-1 and MGC80-3 cells. Eur Rev Med
Pharmacol Sci. 20:486–490. 2016.PubMed/NCBI
|
|
20
|
Fan C, Lin Y, Mao Y, Huang Z, Liu AY, Ma
H, Yu D, Maitikabili A, Xiao H, Zhang C, et al: MicroRNA-543
suppresses colorectal cancer growth and metastasis by targeting
KRAS, MTA1 and HMGA2. Oncotarget. 7:21825–21839. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang C, Zhang Y, Ding W, Lin Y, Huang Z
and Luo Q: MiR-33a suppresses breast cancer cell proliferation and
metastasis by targeting ADAM9 and ROS1. Protein Cell. 6:881–889.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Xu G, Liu G, Ye Y, Zhang C, Fan
C, Wang H, Cai H, Xiao R, Huang Z and Luo Q: miR-411-5p inhibits
proliferation and metastasis of breast cancer cell via targeting
GRB2. Biochem Biophys Res Commun. 476:607–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McKenna NJ and O'Malley BW: Combinatorial
control of gene expression by nuclear receptors and coregulators.
Cell. 108:465–474. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Navab R, Gonzalez-Santos JM, Johnston MR,
Liu J, Brodt P, Tsao MS and Hu J: Expression of chicken ovalbumin
upstream promoter-transcription factor II enhances invasiveness of
human lung carcinoma cells. Cancer Res. 64:5097–5105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Safe S, Jin UH, Hedrick E, Reeder A and
Lee SO: Minireview: Role of orphan nuclear receptors in cancer and
potential as drug targets. Mol Endocrinol. 28:157–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu H and Xu Y: Recent advancement in
metastasis of gastric cancer. Chin J Prac. 31:666–669. 2011.
|
|
30
|
Nagasaki S, Suzuki T, Miki Y, Akahira J,
Shibata H, Ishida T, Ohuchi N and Sasano H: Chicken ovalbumin
upstream promoter transcription factor II in human breast
carcinoma: Possible regulator of lymphangiogenesis via vascular
endothelial growth factor-C expression. Cancer Sci. 100:639–645.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu M, Qin J, Tsai SY and Tsai MJ: The role
of the orphan nuclear receptor COUP-TFII in tumorigenesis. Acta
Pharmacol Sin. 36:32–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ
and Tsai SY: Direct transcriptional regulation of neuropilin-2 by
COUP-TFII modulates multiple steps in murine lymphatic vessel
development. J Clin Invest. 120:1694–1707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang S, Chen F, Zhang C, Gu X and Chen M:
Establishment of liver metastasis model in nude mice with human
gastric carcinoma. Beijing Lab Anim Sci. 9:131992.
|
|
34
|
Shan F, Li Z, Bu Z, Zhang L, Wu A, Wu X,
Wu Qi, Zong X, Li S, Ji X, Wu J and Ji J: The analysis of gastric
cancer staging AJCC 6th edition and 7th edition. Chin J Prac Surg.
31:675–680. 2011.
|