Introduction
Cardiovascular disease is a generic term,
incorporating cardiovascular and cerebrovascular diseases caused by
hyperlipidemia, sticky blood, atherosclerosis, and hypertension
(1,2). Cardiovascular disease presents
systemic vascular lesions affecting the performance of the heart
and brain, and is the most frequent cause of mortality in the adult
population of economically developed countries (3,4).
Coronary heart disease is a type of disease that includes coronary
atherosclerosis heart disease, coronary arterial atherosclerosis
and vascular cavity stenosis or occlusion, which cause myocardial
ischemia, hypoxia or necrosis of the heart (5,6).
Anoxia-reoxygenation injury of the heart is a serious threat to
human health and coronary heart disease, caused by myocardial
ischemia-reperfusion injury, is the primary reason for mortality
and disability during cardiovascular disease, which emerges during
myocardial infarction, cardiopulmonary bypass surgery, heart
attack, and heart transplantation, and during other types of
cardiovascular disease (7,8). Anoxia-reoxygenation injury of heart
frequently and ultimately results in irreversible fatal injury and
even mortality (9). Therefore,
elucidating the molecular mechanism of anoxia-reoxygenation injury
is considered to be essential for the prevention and treatment of
cardiovascular disease.
Inflammation is one of the most common
characteristics of anoxia-reoxygenation injury of the heart
(10). A previous study indicated
that preventing inflammation efficiently protects against
myocardial ischemia-reperfusion injury (11). Vinten-Johansen et al
(12) demonstrated that
inflammation and pro-inflammatory mediators are involved in
myocardial ischemia-reperfusion injury, post-ischemic injury and
gradually restore blood flow. In addition, a previous study
indicated that inhibition of apoptosis and inflammation contributes
to rehabilitation of myocardial ischemia/reperfusion injury
(13). Furthermore, a previous
study demonstrated the beneficial role of prazosin on
cardiovascular manifestations and pulmonary edema (14). However, to the best of our
knowledge, the efficacy of prazosin on inflammation has not been
investigated. In the present study, changes in serum inflammatory
factors were analyzed in mice with anoxia-reoxygenation injury
following treatment with prazosin.
Oxidative stress is another inducer of
anoxia-reoxygenation injury of the heart and may lead to
dyslipidemia, impairment of energy metabolism and chronic kidney
disease (15). Matsuda et
al (16) indicated that
anti-oxidative agents improve insulin resistance and restore
adiponectin production in obesity-associated metabolic and
cardiovascular diseases (16). In
addition, oxidative stress in cardiovascular disease has
demonstrated important implications in physiological and
pathophysiological cardiovascular processes (17). Furthermore, oxidative stress and
antioxidant strategies in cardiovascular disease and cardiovascular
disease-associated parameters have been investigated in a model for
metabolic syndrome (18,19). Furthermore, the long-term effects
of prazosin administration on blood pressure, heart and structure
of coronary artery have been evaluated in young spontaneously
hypertensive rats (20). In the
present study, the efficacy of prazosin on oxidative stress was
analyzed in a mouse model of anoxia-reoxygenation injury. Notably,
the molecular mechanism of prazosin on anoxia-reoxygenation injury
was investigated in experimental mice.
The aim of the present study was to investigate the
protective effects of prazosin on myocardial cells against
anoxia-reoxygenation injury in a mouse model. The regulatory
effects of prazosin on inflammation responses, oxidative stress,
blood lipid levels, blood pressure and apoptosis were analyzed in
experimental mice. The potential mechanism of ERK-mediated nuclear
factor of activated T cells (NF-AT), AP-1, NF-κB signaling pathways
was also investigated in myocardial cells in mice with
anoxia-reoxygenation injury following prazosin treatment. These
investigations indicate that the long-term effect of prazosin is
beneficial for improvement of anoxia-reoxygenation injury.
Materials and methods
Ethical approval
The current study was performed according to the
recommendations of the Guide for the Care and Use of Laboratory
Animals of the General Hospital of Chinese People's Armed Police
Force (Beijing, China). All surgery and experiments were performed
under anesthetic to minimize pain.
Cell cultures
Myocardial cells from experimental mice were
cultured in Minimum Essential Medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and incubated overnight at
37°C in a humidified atmosphere of 5% CO2. Myocardial
cells were treated with 10 mg/ml prazosin (P7791; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 24 h to analyze the therapeutic
effects of prazosin in vitro.
ELISA
In the protein detection assay, mouse IL-6 (D6050),
TNF-α, (MTA00B) IL-10 (DY417), IL-1 (MLB00C), interferon (IFN)-γ
(DY485) and IL-2 (M2000; all R&D Systems, Inc., Minneapolis,
MN, USA). ELISA kits were used to determine the serum levels of
inflammatory factors. The procedures were conducted according to
the manufacturer's instructions. The final results were recorded at
a wavelength of 450 nm using an ELISA plate reader.
Western blot analysis
Myocardial cells were homogenized in lysate buffer
containing protease-inhibitor (P8340; Sigma-Aldrich; Merck KGaA)
and were centrifuged at 8,000 × g at 4°C for 10 min. The
supernatant was used for analysis of protein expression. For
detection of purpose protein, transmembrane proteins were extracted
using a Transmembrane Protein Extraction kit (Qiagen Sciences,
Inc., Gaithersburg, MD, USA) according to the manufacturer's
instructions. Protein concentration was measured using a
bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.).
Protein samples (20 µg) were separated by 12% SDS-PAGE and
transferred onto PVDF membranes (EMD Millipore, Billerica, MA, USA)
as previously described (21). For
western blotting, primary antibodies against the following were
used: Reactive oxygen species (ROS; 1:1,000; ab151922), glutathione
(GSH; 1:1,000; ab94733), heme oxygenase (HO)-1 (1:1,000; ab68477),
P38 mitogen activated protein kinase (MAPK; 1:1,000; ab197348),
catalase (CAT; 1:1,000; ab52477), superoxide dismutase (SOD;
1:1,000; ab80946), Caspase-3 (1:1,000; ab13847), Caspase-9
(1:1,000; ab202068), B-cell lymphoma (Bcl)-2 (1:1,000; ab692), P53
(1:1,000; ab1431), apoptotic protease activating factor 1 (Apaf-1;
1:1,000; ab2001), Fas (1:1,000; ab82419), ERK (1:1,000; ab54230),
pERK (1:1,000; ab65142), NF-AT (1:1,000; ab25916), AP-1 (1:1,000;
ab21981), NF-κB (1:1,000; ab32360) and β-actin (1:1,000; ab5262;
all Abcam, Cambridge, UK) for 12 h at 4°C following blocking with
5% skimmed milk for 1 h at 37°C. Membranes were subsequently
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG mAb (1:2,000; PV-6001; OriGene Technologies, Inc., Beijing,
China) for 24 h at 4°C. A Ventana Benchmark automated staining
system was used to analyze protein expression (Olympus BX51;
Olympus Corp., Tokyo, Japan).
Flow cytometric analysis of myocardial
cell apoptosis
Apoptosis rates of myocardial cells were evaluated
using Annexin V-fluorescein isothiocyanate (FITC) and propidium
iodide (PI) apoptosis detection kit (556547; BD Biosciences, San
Jose, CA, USA). Myocardial cells were collected and suspended with
Annexin V-FITC and PI according to the manufacturer's instructions.
Fluorescence was measured via FACS scan flow cytometry (BD
CellQuest Software Version 3.3; BD Biosciences).
Animal experiments
A total of 120 male C57BL/6 mice were purchased from
Charles River Laboratories (Shanghai, China) and housed under
pathogen-free conditions. Mice were maintained under a 12 h
light/dark cycle with free access to food and water. The myocardial
injury mouse model was established according to a previous study
(22). Following establishment of
the anoxia-reoxygenation injury mouse model, the mice were
randomized into two groups (n=60 per group). The animals received
prazosin (10 mg/kg) treatment or the same volume of
phosphate-buffered saline (PBS) once per day and the treatment
continued for 30 days. Cardiac function was further analyzed to
evaluate the efficacy of prazosin.
Immunohistochemistry
The myocardial tissues were flash frozen at −20°C
and cut into sections (4 µm), deparaffinized in xylene and
rehydrated through a graded ethanol series. Sections were prepared
and epitope retrieval was performed using a Lab Vision™
Tris-HCl Buffer for Heat-Induced Epitope Retrieval kit (AP9005;
Invitrogen; Thermo Fisher Scientific, Inc.) for further analysis.
The paraffin sections were subjected to hydrogen peroxide (3%) for
10–15 min and subsequently blocked using 5% skim milk for 10–15 min
at 37°C. Finally, the sections were stained with hematoxylin and
eosin at 4°C for 12 h. All sections were washed three times with
PBS. The area of myocardial injury, circumference fragmentation and
segmentation of myocardial cells were determined by observation of
six randomly selected views under a fluorescence microscope.
Statistical analysis
All data are presented as means and standard error
of the mean. Unpaired data was analyzed using Student's t-test.
Comparison of data between multiple groups was performed by one-way
analysis of variance and P<0.05 was considered to indicate a
statistically significant difference.
Results
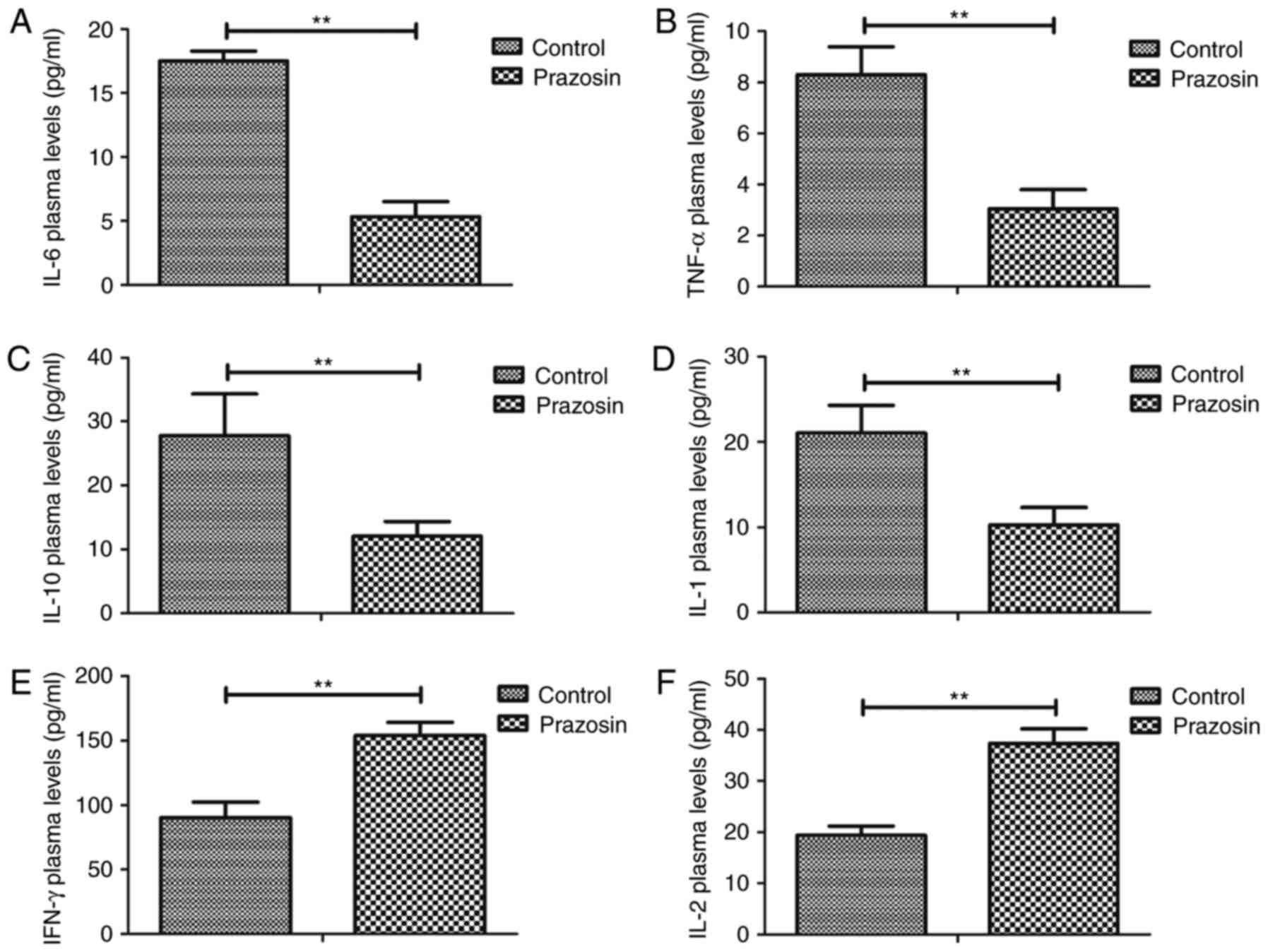
Prazosin decreases serum levels of
inflammatory factors in the mouse model of anoxia-reoxygenation
injury
Expression levels of inflammatory factors were
analyzed in the anoxia-reoxygenation injury mouse model prior to
and post treatment with prazosin, as determined by ELISA. As shown
in Fig. 1A-D, inflammatory factor
expression levels of IL-6, TNF-α, IL-10 and IL-1 were decreased by
prazosin treatment in the serum of mice with hypoxia/reoxygenation
injury. However, plasma concentration levels of IFN-γ and IL-2 were
significantly upregulated by prazosin in mice with
hypoxia/reoxygenation injury (Fig. 1E,
F). These findings indicate that prazosin decreased the levels
of inflammatory factors in the serum and promoted secretion of
anti-inflammatory factors in the mouse model of
anoxia-reoxygenation injury.
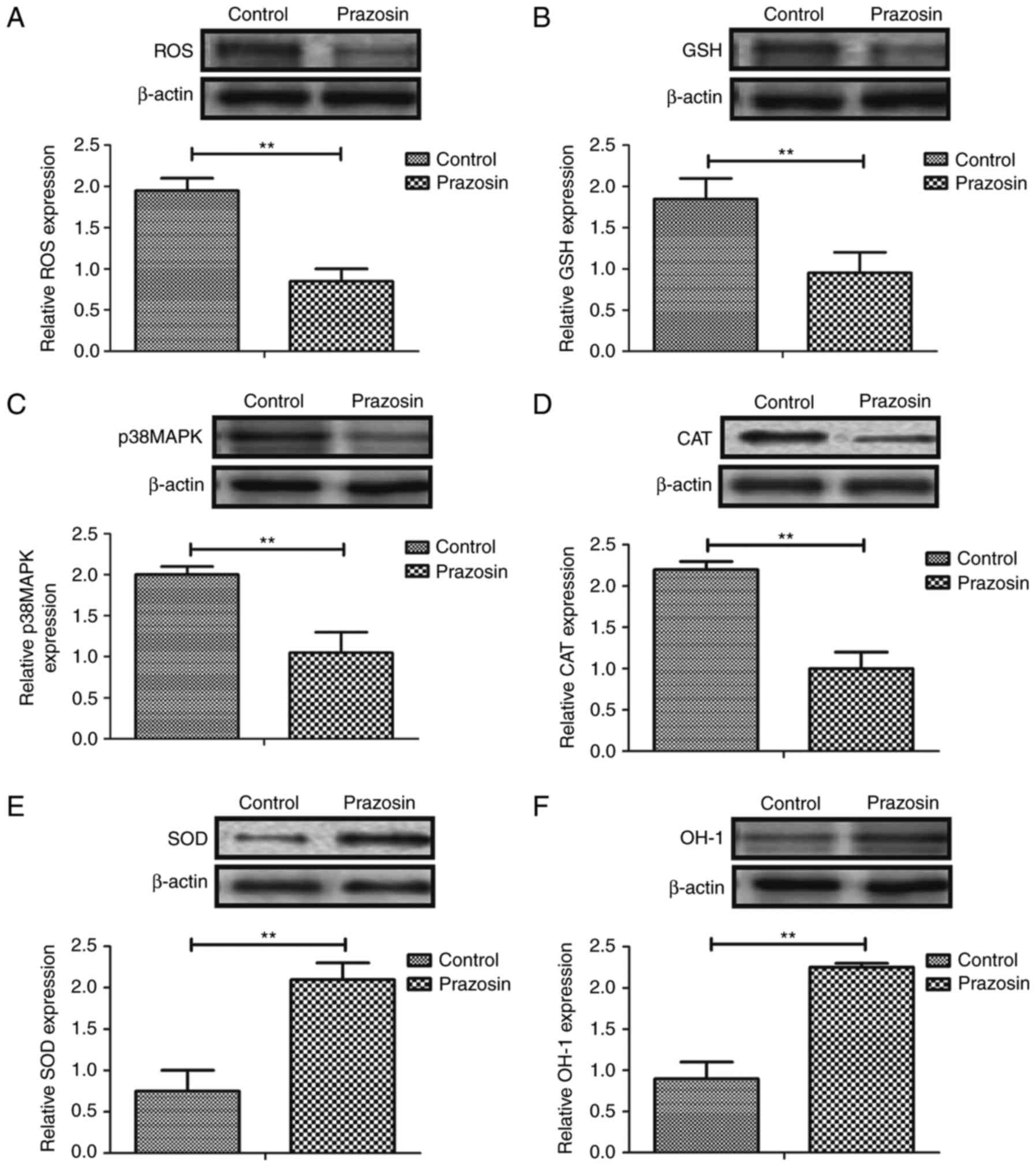
Prazosin downregulates oxidative
stress in the mouse model of anoxia-reoxygenation injury
To investigate the biological effects of prazosin on
myocardial cells, oxidative stress was analyzed in the myocardial
cells of mice with anoxia-reoxygenation injury. As shown in
Fig. 2A-D, ROS, GSH, p38MAPK and
CAT were markedly downregulated in myocardial cells in a
prazosin-treated mouse model of anoxia-reoxygenation injury.
However, the expression levels of SOD and HO-1 were upregulated in
the myocardial cells of prazosin-treated mice with
anoxia-reoxygenation injury (Fig. 2E,
F). These results indicate that prazosin downregulates
oxidative stress and upregulates SOD and HO-1 activation in mice
exhibiting anoxia-reoxygenation injury.
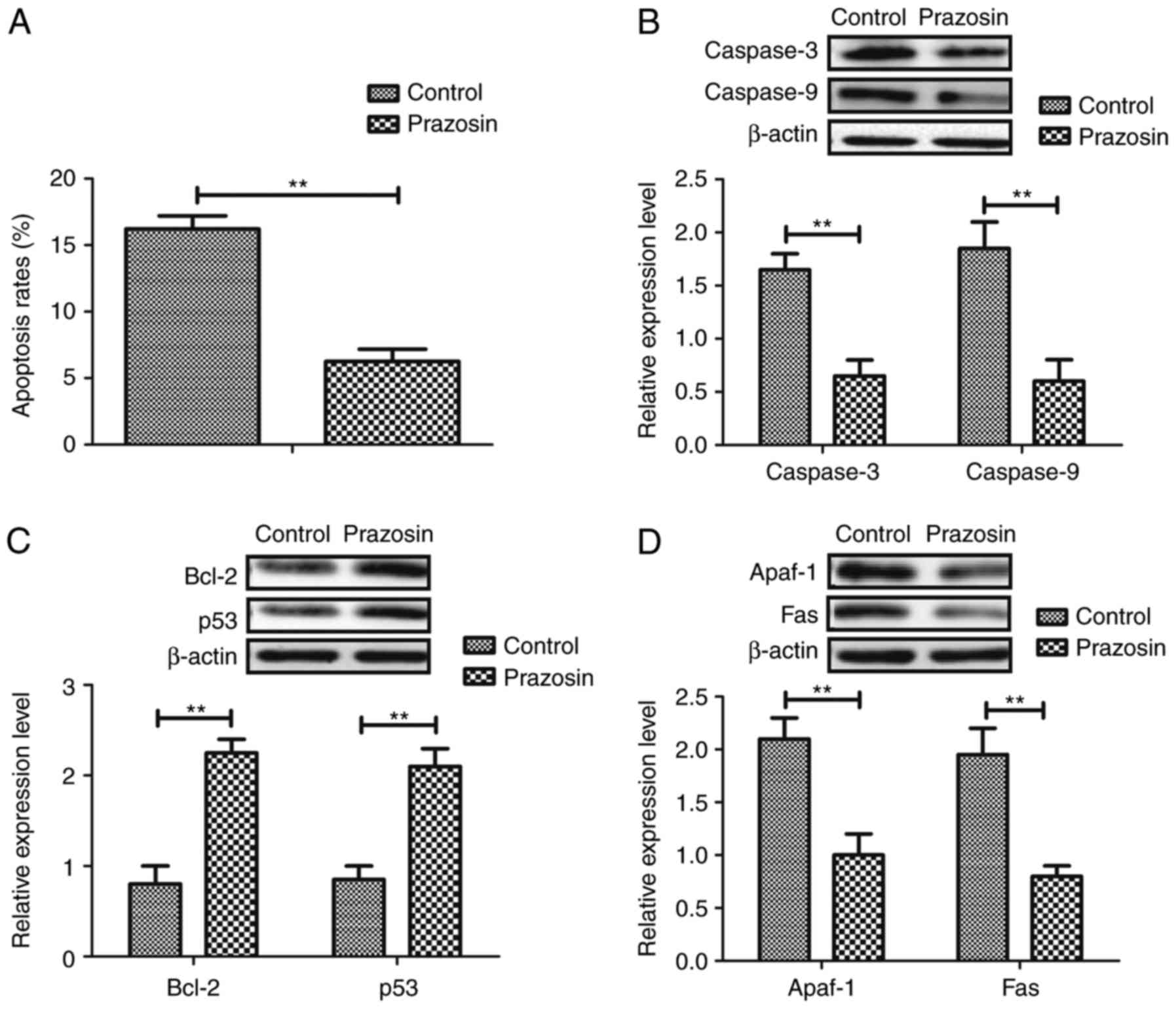
Prazosin suppresses apoptosis of
myocardial cells in a mouse model of anoxia-reoxygenation
injury
Subsequently, the improvement of apoptosis in
myocardial cells was analyzed in the mouse models of
anoxia-reoxygenation injury following treatment with prazosin. As
demonstrated in Fig. 3A, the
apoptosis rate was decreased in the experimental mice treated with
prazosin. In addition, pro-apoptotic gene expression levels of
caspase-3 and caspase-9 expression were observed to be
downregulated following prazosin (10 mg/ml) treatment (Fig. 3B). Furthermore, expression levels
of B-cell lymphoma 2 and p53 were upregulated in the myocardial
cells of the mouse models of anoxia-reoxygenation injury following
treatment with Prazosin (Fig. 3C).
Furthermore, following treatment with prazosin, the expression
levels of Apaf-1 and Fas were downregulated in the myocardial cells
of the mouse models of anoxia-reoxygenation injury (Fig. 3D). There results indicate that
prazosin contributes to the protection of prazosin against
apoptosis in myocardial cells.
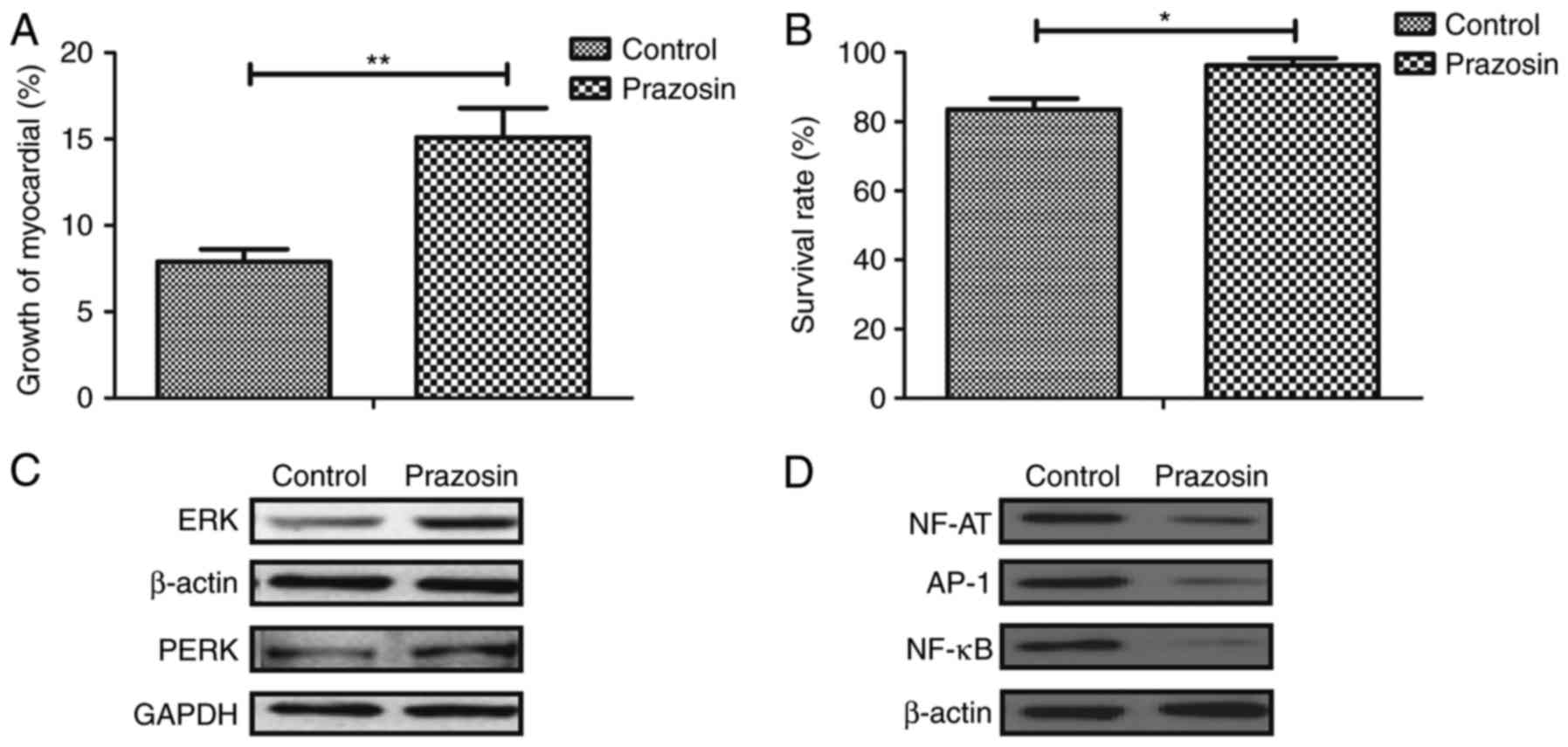
Prazosin improves survival of
myocardial cells in mice with anoxia-reoxygenation injury via the
ERK signaling pathway
The growth and molecular mechanism of
prazosin-mediated improvement of myocardial cells were further
analyzed in mice with anoxia-reoxygenation injury. As presented in
Fig. 4A, growth of myocardial
cells was promoted by prazosin in the experimental mice. The
survival rate of myocardial cells was improved by prazosin
treatment in mice with anoxia-reoxygenation injury (Fig. 4B). In addition, the expression and
phosphorylation levels of ERK were observed to be stimulated by
prazosin in experimental mice (Fig.
4C). Furthermore, the expression levels of NF-AT, AP-1, NF-κB
were downregulated in myocardial cells in mice treated with
prazosin (Fig. 4D). These results
indicate that prazosin improves survival in the myocardial cells of
mice with anoxia-reoxygenation injury via the ERK signaling
pathway.
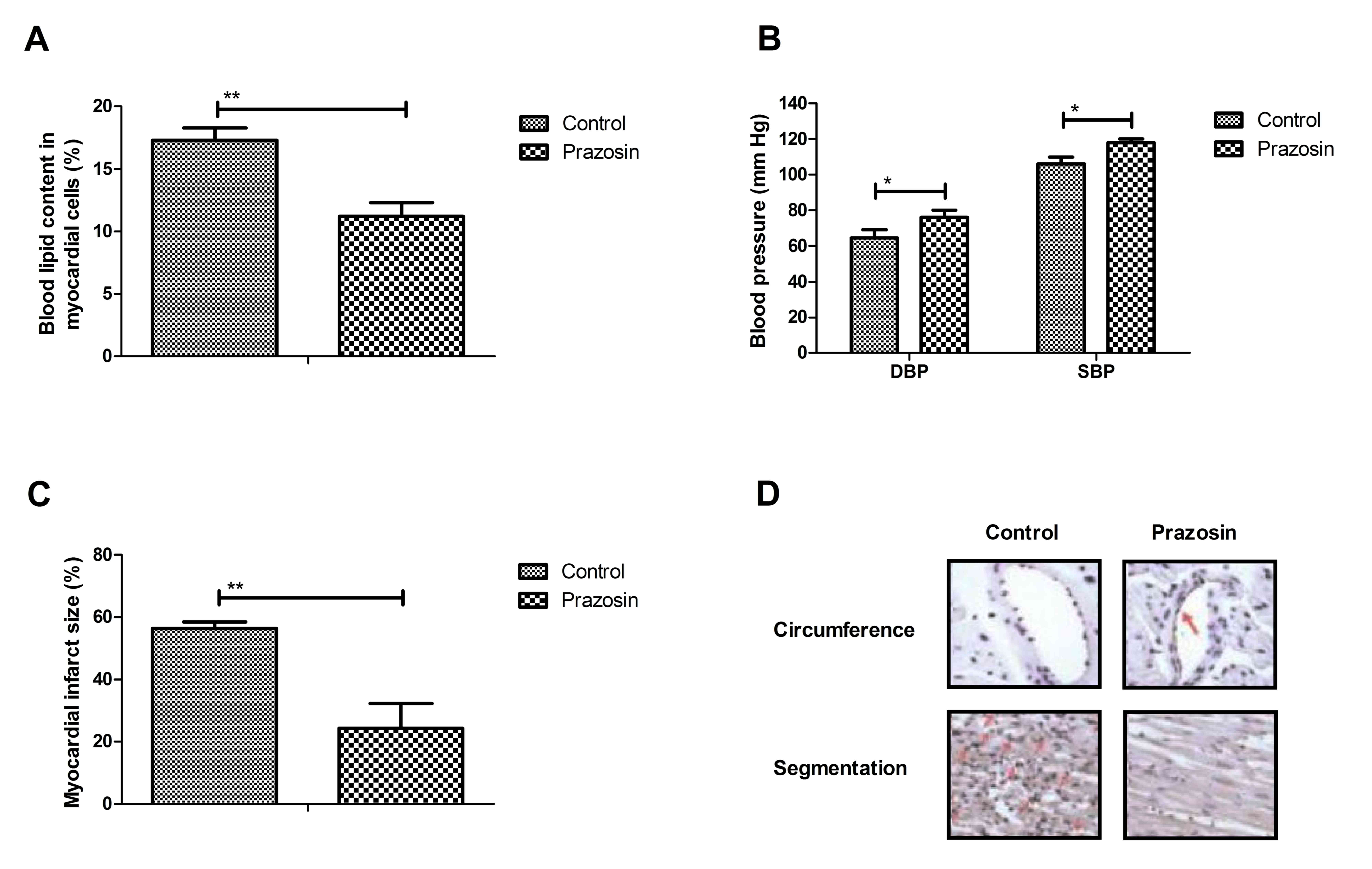
Prazosin improves blood lipid levels,
blood pressure and myocardial function in mice with
anoxia-reoxygenation injury
Myocardial function was analyzed in experimental
mice following treatment with prazosin. As presented in Fig. 5A, blood lipid levels were improved
in a prazosin-treated mouse model of anoxia-reoxygenation injury.
In addition, blood pressure was improved following prazosin
treatment in the experimental mice (Fig. 5B). In addition, the area of
myocardial injury was markedly decreased in the myocardial tissue
of mice treated with prazosin (Fig.
5C). Furthermore, circumference fragmentation and segmentation
of myocardial cells were markedly improved by prazosin treatment,
as determined by histological analysis (Fig. 5D). These results indicate that
prazosin improves blood lipid levels, blood pressure, and
myocardial function in mice with anoxia-reoxygenation injury.
Discussion
Coronary heart disease remains one of biggest causes
of death in the world and is closely associates with metabolic
disorders of endogenous substances (23). Anoxia-reoxygenation injury is a
serious type of coronary heart disease that significantly promotes
apoptosis of myocardial cells, and increases inflammation and
oxidative stress (24,25). In the current study, the efficacy
of prazosin against anoxia-reoxygenation injury in a mouse model
was investigated. The results demonstrated that prazosin decreases
the plasma concentration of inflammatory factors, and improves
oxidative stress in myocardial cells (26). Notably, it was observed that
prazosin treatment exerts significant anti-apoptotic effects in
myocardial cells of mice models of anoxia-reoxygenation injury.
These findings indicate that prazosin regulates survival of
myocardial cells via the ERK-mediated signaling pathway in a mouse
model of anoxia-reoxygenation injury.
Prazosin is a selective postsynaptic a1 receptor
blocker, which is used for treating mild and moderate hypertension
(27). Prazosin reduces the load
of the heart, which has been used for the treatment of cardiac
insufficiency (28). Zhao and Xu
(29) investigated the
ameliorative effect of prazosin on diabetic nephropathy patients
with positive a1-adrenergic receptor autoantibodies and refractory
hypertension. In addition, long-term effects of prazosin treatment
on blood pressure, carotid arteries, heart rate, and acetylcholine
have been reported in a previous study (30). Furthermore, Qazi et al
(31) indicated that prazosin
treatment exerts regulatory effects in posttraumatic stress and
alcohol use disorders. These reports indicate that prazosin may
present as an efficient therapeutic agent for the treatment of
anoxia-reoxygenation injury.
Inflammation is essential in the initiation and
development of coronary heart disease caused by
anoxia-reoxygenation injury of heart (32). Bobbert et al (33) have suggested that myocardial injury
induced by platelet activation and thrombus formation is associated
with myocardial inflammation in patients with cardiomyopathy. In
addition, the biological importance of inflammatory factor,
IL-6-mediated signaling pathways have been elucidated in patients
with acute myocardial infarction and dysglycemia (34). Additionally, previous studies
indicated that myocardial injury is aggravated by systemic
inflammation, which may further induce other types of heart disease
(35,36). Furthermore, TNF-α and IL-10 were
reported as markers of the inflammatory response in coronary artery
disease patients with acute myocardial infarction (37,38).
The current results indicate that prazosin is beneficial for
improvement of inflammatory factor expression in serum in mice with
anoxia-reoxygenation injury. Decreasing serum expression levels of
IL-6, TNF-α, IL-10 and IL-1 may contribute to upregulation of
anti-apoptotic genes expressed in myocardial cells.
Previous studies have indicated that apoptosis of
cardiomyocytes is crucial in cardiac dysfunction following acute
myocardial infarction in the development of coronary heart disease
(39,40). Numerous strategies aimed at
preventing or mitigating the extent of apoptosis have been
attempted to protect the heart against coronary heart
disease-induce apoptosis or death of cardiomyocytes (41–43).
In the current study, prazosin treatment significantly inhibited
apoptosis of myocardial cells by upregulation of Bcl-2 and p53, as
well as downregulation of caspase-3, caspase-9, Bcl-2 and p53 in
myocardial cells in a mouse model of anoxia-reoxygenation injury.
The results also indicated that the ERK signaling pathway is
involved in the protection effects of prazosin-mediated
anoxia-reoxygenation injury.
Currently, the molecular mechanisms of treatment for
coronary heart disease have been widely investigated (44–46).
Research has indicated that upregulation of the MAPK-ERK signaling
pathway regulates myocardial ischemia-reperfusion and exerts
protective roles in myocardial ischemia-reperfusion injury
(47). Furthermore, a previous
study demonstrated that activating p-ERK ameliorates acute
myocardial infarction in rats by decreasing the expression of
inflammatory-associated cytokines (48). In the present study, the activity
and expression levels of NF-AT, AP-1 and NF-κB were downregulated
in myocardial cells following prazosin treatment. The experiments
demonstrate that prazosin-induced upregulation of ERK contributes
to survival and growth of myocardial cells, which may attenuate
inflammation and oxidative stress in myocardial cells in mice with
anoxia-reoxygenation injury.
In conclusion, the current study indicates that
prazosin protects myocardial cells against inflammation, oxidative
stress and apoptosis by stimulating ERK expression and activity.
Thus, prazosin presents as an efficient drug for improving survival
of myocardial cells, blood lipid levels, blood pressure, and
myocardial function in mice with anoxia-reoxygenation injury. In
addition, the findings identify and provide evidence of the
cardioprotective advantage of prazosin in animal models of
anoxia-reoxygenation injury, and illustrate a potential mechanistic
pathway for the beneficial effects of prazosin in cardiovascular
disease.
References
|
1
|
Kaligis RW, Adiarto S, Erwinanto 2,
Nugroho J, Pradnyana BA, Lefi A and Rifqi S: Carotid intima-media
thickness in indonesian subjects with cardiovascular disease risk
factors who were not receiving lipid-lowering agents. Int J Angiol.
25:174–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lines SW and Carter AM: Complement and
cardiovascular disease-the missing link in haemodialysis patients.
Nephron. 134:1042016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seaman CD, George KM, Ragni M and Folsom
AR: Association of von Willebrand factor deficiency with prevalent
cardiovascular disease and asymptomatic carotid atherosclerosis:
The atherosclerosis risk in communities study. Thromb Res.
144:236–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgia G, Russo GI, Tubaro A, Bortolus R,
Randone D, Gabriele P, Trippa F, Zattoni F, Porena M, Mirone V, et
al: Prevalence of cardiovascular disease and osteoporosis during
androgen deprivation therapy prescription discordant to EAU
guidelines: Results from a multi-center cross-sectional analysis
from the CHOsIng treatment for prostate canCEr (CHOICE) study.
Urology. 96:165–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heikkilä K, Koskinen OA, Agarwal A,
Tikkinen KA, Mäki M and Kaukinen K: Associations of coeliac disease
with coronary heart disease and cerebrovascular disease: A
systematic review and meta-analysis. Nutr Metab Cardiovasc Dis.
25:816–831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tully PJ, Turnbull DA, Beltrame J,
Horowitz J, Cosh S, Baumeister H and Wittert GA: Panic disorder and
incident coronary heart disease: A systematic review and
meta-regression in 1131612 persons and 58111 cardiac events.
Psychol Med. 45:2909–2920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weinreuter M, Kreusser MM, Beckendorf J,
Schreiter FC, Leuschner F, Lehmann LH, Hofmann KP, Rostosky JS,
Diemert N, Xu C, et al: CaM Kinase II mediates maladaptive
post-infarct remodeling and pro-inflammatory chemoattractant
signaling but not acute myocardial ischemia/reperfusion injury.
EMBO Mol Med. 6:1231–1245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren-an Q, Juan L, Chuyuan L, Wenjuan F,
Chunyan H, Xuemei Y, Lin H and Hong N: Study of the protective
mechanisms of Compound Danshen Tablet (Fufang Danshen Pian) against
myocardial ischemia/reperfusion injury via the Akt-eNOS signaling
pathway in rats. J Ethnopharmacol. 156:190–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yndestad A, Sandanger Ø, Jong WMC, Aukrust
P and Zuurbier CJ: Response to letter from Toldo et al. on ‘NLRP3
inflammasome activation during myocardial ischemia reperfusion is
cardioprotective’. Biochem Biophys Res Commun. 474:328–329. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang M, Chen J, Zhao J and Meng M:
Etanercept attenuates myocardial ischemia/reperfusion injury by
decreasing inflammation and oxidative stress. PLoS One.
9:e1080242014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chao J, Yin H, Yao YY, Shen B, Smith RS Jr
and Chao L: Novel role of kallistatin in protection against
myocardial ischemia-reperfusion injury by preventing apoptosis and
inflammation. Hum Gene Ther. 17:1201–1213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vinten-Johansen J, Jiang R, Reeves JG,
Mykytenko J, Deneve J and Jobe LJ: Inflammation, proinflammatory
mediators and myocardial ischemia-reperfusion Injury. Hematol Oncol
Clin North Am. 21:123–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loubele ST, Spek CA, Leenders P, van Oerle
R, Aberson HL, Hamulyák K, Ferrell G, Esmon CT, Spronk HM and ten
Cate H: Activated protein C protects against myocardial
ischemia/reperfusion injury via inhibition of apoptosis and
inflammation. Arterioscler Thromb Vasc Biol. 29:1087–1092. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Al-Asmari AK, Al-Seif AA, Hassen MA and
Abdulmaksood NA: Role of prazosin on cardiovascular manifestations
and pulmonary edema following severe scorpion stings in Saudi
Arabia. Saudi Med J. 29:299–302. 2008.PubMed/NCBI
|
|
15
|
Vaziri ND: Role of dyslipidemia in
impairment of energy metabolism, oxidative stress, inflammation and
cardiovascular disease in chronic kidney disease. Clin Exp Nephrol.
18:265–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuda M and Shimomura I: Roles of
adiponectin and oxidative stress in obesity-associated metabolic
and cardiovascular diseases. Rev Endocr Metab Disord. 15:1–10.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Csanyi G and Miller FJ Jr: Oxidative
stress in cardiovascular disease. Int J Mol Sci. 15:6002–6008.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belló-Klein A, Khaper N, Llesuy S,
Vassallo DV and Pantos C: Oxidative stress and antioxidant
strategies in cardiovascular disease. Oxid Med Cell Longev.
2014:6787412014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Molinar-Toribio E, Pérez-Jiménez J,
Ramos-Romero S, Lluís L, Sánchez-Martos V, Taltavull N, Romeu M,
Pazos M, Méndez L, Miranda A, et al: Cardiovascular disease-related
parameters and oxidative stress in SHROB rats, a model for
metabolic syndrome. PLoS One. 9:e1046372014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kristek F and Koprdova R: Long-term effect
of prazosin administration on blood pressure, heart and structure
of coronary artery of young spontaneously hypertensive rats. J
Physiol Pharmacol. 62:295–301. 2011.PubMed/NCBI
|
|
21
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-based quantitative assay for screening of
kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jong WM, Ten Cate H, Linnenbank AC, de
Boer OJ, Reitsma PH, de Winter RJ and Zuurbier CJ: Reduced acute
myocardial ischemia-reperfusion injury in IL-6-deficient mice
employing a closed-chest model. Inflamm Res. 65:489–499. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Zhang J, Ren T and Dong Z:
Targeted metabolomic profiling of cardioprotective effect of Ginkgo
biloba L. extract on myocardial ischemia in rats. Phytomedicine.
23:621–631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caraballo JC, Borcherding J, Rector M,
Hornick E, Stoltz D, Zabner J and Comellas AP: Role of PON in
anoxia-reoxygenation injury: A Drosophila melanogaster transgenic
model. PLoS One. 9:e844342014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duan JL, Wang JW, Guan Y, Yin Y, Wei G,
Cui J, Zhou D, Zhu YR, Quan W, Xi MM and Wen AD: Safflor yellow A
protects neonatal rat cardiomyocytes against anoxia/reoxygenation
injury in vitro. Acta Pharmacol Sin. 34:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khaidakov M, Mercanti F, Wang X, Ding Z,
Dai Y, Romeo F, Sawamura T and Mehta JL: Prevention of export of
anoxia/reoxygenation injury from ischemic to nonischemic
cardiomyocytes via inhibition of endocytosis. Am J Physiol Heart
Circ Physiol. 306:H1700–H1707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanon O, Giacomino A, Troy S, Bernaud C,
Girerd X and Weber S: Efficacy of and tolerance to prolonged
release prazosin in patients with hypertension and non-insulin
dependent diabetes. Ann Cardiol Angeiol (Paris). 49:390–396.
2000.(In French). PubMed/NCBI
|
|
28
|
Rudner S and Browne P: Case report:
Management of secondary hypertension in a feline with the use of
transdermal prazosin. Int J Pharm Compd. 14:488–491.
2010.PubMed/NCBI
|
|
29
|
Zhao LS and Xu CY: Effect of prazosin on
diabetic nephropathy patients with positive α1-adrenergic receptor
autoantibodies and refractory hypertension. Exp Ther Med.
9:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kristek F, Malekova M and Cacanyiova S:
Long-term effect of prazosin and losartan administration on blood
pressure, heart, carotid artery, and acetylcholine induced dilation
of cardiovascular system of young Wistar rats and SHR. Gen Physiol
Biophys. 32:235–243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qazi H, Wijegunaratne H, Savajiyani R and
Koola MM: Naltrexone and prazosin combination for posttraumatic
stress disorder and alcohol use disorder. Prim Care Companion CNS
Disord. 16:2014.PubMed/NCBI
|
|
32
|
Carmona F, Manso PH, Silveira VS, Cunha
FQ, de Castro M and Carlotti AP: Inflammation, myocardial
dysfunction, and mortality in children with septic shock: An
observational study. Pediatr Cardiol. 35:463–470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bobbert P, Weikert U, Schmidt-Lucke C,
Skurk C, Meyer A, Steffens D, Schultheiss HP and Rauch U: Platelet
activation and thrombus formation relates to the presence of
myocardial inflammation in patients with cardiomyopathy. J Cardiol.
63:379–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ritschel VN, Seljeflot I, Arnesen H,
Halvorsen S, Weiss T, Eritsland J and Andersen GØ: IL-6 signalling
in patients with acute ST-elevation myocardial infarction. Results
Immunol. 4:8–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Langhorn R, Persson F, Ablad B, Goddard A,
Schoeman JP, Willesen JL, Tarnow I and Kjelgaard-Hansen M:
Myocardial injury in dogs with snake envenomation and its relation
to systemic inflammation. J Vet Emerg Crit Care (San Antonio).
24:174–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Gaetano M, Quacquaruccio G, Di
Castelnuovo A, Nowak N, Dorn J, Donati MB, Freudenheim JL, Trevisan
M and Iacoviello L: Haplotypes and haplotype-pairs of IL-1 beta and
IL-6 genes and risk of non fatal myocardial infarction in the
Western New York Acute MI Study. Thromb Haemost. 106:1231–1233.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Luo R, Chen R, Song L, Zhang S, Hua
W and Chen H: Cleavage of IκBα by calpain induces myocardial NF-κB
activation, TNF-α expression and cardiac dysfunction in septic
mice. Am J Physiol Heart Circ Physiol. 306:H833–H843. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goswami B, Rajappa M, Mallika V, Shukla DK
and Kumar S: TNF-alpha/IL-10 ratio and C-reactive protein as
markers of the inflammatory response in CAD-prone North Indian
patients with acute myocardial infarction. Clin Chim Acta.
408:14–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hashemian M, Poustchi H,
Mohammadi-Nasrabadi F and Hekmatdoost A: Systematic review of zinc
biochemical indicators and risk of coronary heart disease. ARYA
Atheroscler. 11:357–365. 2015.PubMed/NCBI
|
|
40
|
Farvid MS, Ding M, Pan A, Sun Q, Chiuve
SE, Steffen LM, Willett WC and Hu FB: Response to letters regarding
article, ‘dietary linoleic acid and risk of coronary heart disease:
A systematic review and meta-analysis of prospective cohort
studies’. Circulation. 132:e23–e24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu LL, Lin LR, Lu CX, Fu JG, Chao PL, Jin
HW, Zhang ZY and Yang TC: Expression of inflammatory and apoptosis
factors following coronary stent implantation in coronary heart
disease patients. Int Immunopharmacol. 11:1850–1854. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Salmina AB, Shul'man VA, Nikulina SY,
Trufanova LV, Fursov AA, But'yanov PA, Kuskaev AP, Bol'shakova EV
and Kotlovskii MY: Apoptosis of leukocytes as a marker of
neutrophil-endotheliocyte interaction in coronary heart disease.
Bull Exp Biol Med. 144:39–41. 2007.(In English, Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Geng YJ: Molecular mechanisms for
cardiovascular stem cell apoptosis and growth in the hearts with
atherosclerotic coronary disease and ischemic heart failure. Ann N
Y Acad Sci. 1010:687–697. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ren Y, Yang H, Browning C, Thomas S and
Liu M: Performance of screening tools in detecting major depressive
disorder among patients with coronary heart disease: A systematic
review. Med Sci Monit. 21:646–653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim C, Cushman M, Kleindorfer D, Lisabeth
L, Redberg RF and Safford MM: A review of the relationships between
endogenous sex steroids and incident ischemic stroke and coronary
heart disease events. Curr Cardiol Rev. 11:252–260. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ray IB, Menezes AR, Malur P, Hiltbold AE,
Reilly JP and Lavie CJ: Meditation and coronary heart disease: A
review of the current clinical evidence. Ochsner J. 14:696–703.
2014.PubMed/NCBI
|
|
47
|
Jiang M, Wang L and Jiang HH: Role of
spinal MAPK-ERK signal pathway in myocardial ischemia-reperfusion
injury. Zhongguo Dang Dai Er Ke Za Zhi. 15:387–391. 2013.(In
Chinese). PubMed/NCBI
|
|
48
|
Duan J, Yang Y, Liu H, Dou PC and Tan SY:
Osthole ameliorates acute myocardial infarction in rats by
decreasing the expression of inflammatory-related cytokines,
diminishing MMP-2 expression and activating p-ERK. Int J Mol Med.
37:207–216. 2016. View Article : Google Scholar : PubMed/NCBI
|