Introduction
Diabetic nephropathy (DN) is a common end-stage
renal disease that is characterized by mesangial cell proliferation
and extracellular matrix (ECM) accumulation with mesangial
expansion, glomerular hypertrophy, tubulointerstitial fibrosis and
glomerular sclerosis in later stages (1–3).
Mesangial cell proliferation is one of the major pathological
characteristics in the early stage of DN (4–6). The
expression of a number of cyclin-dependent kinase (CDK) inhibitors
may also downregulate progressive glomerular hypertrophy (7).
Mesangial cells are hypothesized to serve an
important role in the metabolism of type IV collagen. Regulation of
type IV collagen may result in ECM expansion, leading to mesangial
lesion hypercellularity (8).
High-glucose (HG) cell culture conditions induce mesangial cells to
overexpress ECM proteins (9,10).
Increased generation of reactive oxygen species (ROS) is a mark of
the diabetic environment, and studies have demonstrated that ROS
induce the production of profibrotic growth factors, including
transforming growth factor (TGF)-β and type IV collagen (11,12).
Connective tissue growth factor (CTGF) expression may lead to
diabetic renal disease by inducing ECM synthesis and inhibiting ECM
degradation. In renal mesangial cells, HG-induced ECM degradation
occurs through changes in the expression levels of the matrix
metalloproteinases (MMPs) and their specific inhibitors, the tissue
inhibitors of MMPs (TIMP-1 and TIMP-2) (13). A previous study reported that
TIMP-2 can bind to MMP-2 and membrane-bound type 1 (MT1)-MMP, and
it has been demonstrated to be upregulated in diabetic conditions
(14). In addition, CTGF has been
reported to mediate the effects of HG in the inhibition of
mesangial matrix expansion, via increasing the expression of TIMPs
(15).
TGF-β is the most potent and ubiquitous
profibrogenic cytokine, and serves an important role in the
mechanisms underlying mesangial cell hypertrophy and
fibrotic/sclerotic manifestations of DN (16,17).
The TGF-β/Smad signaling pathway is crucial for profibrogenic
cellular responses. Active TGF-β binds to a TGF-β type II receptor
on the cell membrane, which activates a type I receptor, leading to
the subsequent activation of receptor-regulated Smads (R-Smads),
such as Smad-2 and Smad-3. The phosphorylated (p)-R-Smads form a
complex with the common mediator-Smad (Smad-4), which translocates
into the nucleus to regulate gene transcription by binding to the
Smad binding element in the promoter of the target genes (18). Conversely, the inhibitory Smads
(I-Smads, Smad-6 and Smad-7) negatively regulate TGF-β signaling by
binding to a type I receptor or by activating Smad signaling.
I-Smads have been revealed to antagonize TGF-β-mediated signaling
through various mechanisms. Smad7 has been reported to form a
stable complex with TGF-β type I receptors, thus leading to the
inhibition of R-Smad phosphorylation and hetero-complex formation
between R-Smads and Smad-4 (19).
Nuclear factor (NF)-κB is a transcription factor
that regulates the initiation and termination of inflammation.
Activation of NF-κB by hyperglycemia has been implicated in the
pathogenesis of diabetes and the associated complications of DN
(20). NF-κB is maintained in an
inactive form in the cytoplasm by binding to the inhibitor of NF-κB
(IκB) protein (21). In diabetes,
activated NF-κB translocates into the nucleus and induces the
expression of proinflammatory factors, including intercellular
adhesion molecule (ICAM)-1, monocyte chemotactic protein (MCP)-1
and TGF-β1, which in turn induces persistent and enhanced
inflammation, fibronectin overproduction and ECM accumulation, and
finally leads to accelerated renal injury, including
glomerulosclerosis and renal fibrosis (22).
Samchuleum (SCE) was originally recorded in the
ancient Korean medical book, Donguibogam, and is a well-known
traditional blended herbal formula specifically used for dysuria
caused by a shifted bladder in pregnant woman. SCE is composed of
nine dried herbs: Rehmannia glutinosa, Paeonia lactiflora,
Cnidium officinale Makino, Angelica sinensis, Panax
japonicus, Atractylodes ovata, Pinellia ternata, Citrus
reticulata and Glycyrrhiza glabra. However, the
protective effects of SCE on renal dysfunction have not previously
been studied, to the best of our knowledge. Therefore, the present
study attempted to determine whether SCE is able to prevent
HG-induced mesangial cell fibrosis and glomerulosclerosis in
primary human mesangial cell cultures.
Materials and methods
Preparation of a water extract of
SCE
The formula for SCE consists of nine herbs,
including: Rehmanniae Radix Preparata [Rehmannia glutinosa
(Gaertn.) DC; rhizome, steamed and dried]; Paeoniae Radix
(Paeonia lactiflora Pall; root), Cnidii Rhizoma (Cnidium
officinale Makino; rhizome); Angelicae Gigantis Radix
[Angelica sinensis (Oliv.) Diels; root]; Ginseng Radix
(Panax japonicus C.A. Meyer; root); Atractylodis Rhizoma
Alba [Atractylodes ovata (Thunb.) DC; root]; Pinelliae
Rhizoma [Pinellia ternata (Thunb.) Breit; tuberous root];
Citri Pericarpium (Citrus reticulata Blanco; pericarp); and
Glycyrrhizae Radix (Glycyrrhiza glabra L.; root). These were
mixed in equal weights (30 g) and placed in a 5 l conical flask.
The mixed sample (270 g) was boiled with 2 l distilled water for 2
h at 100°C and then centrifuged at 990 × g for 20 min at 4°C. The
supernatant was filtered with Whatman no. 3 filter papers (Whatman;
GE Healthcare Life Sciences, Chalfont, UK), and then concentrated
using a rotary evaporator. The concentrated supernatant was
lyophilized to produce a powder (44.92 g), which was then stored at
−70°C until use. A herbarium voucher specimen (Samchuleum; cat. no.
HBG192-01) was deposited in Hanbang Body-fluid Research Center,
Wonkwang University (Jeonbuk, Korea).
Mesangial cell cultures
Human renal mesangial cells (HRMC; cat. no. 4200)
were purchased from ScienCell Research Laboratories, Inc.
(Carlsbad, CA, USA). Mesangial cells were cultured in Mesenchymal
Stem Cell Medium (ScienCell Research Laboratories, Inc.), and
incubated in a humidified (50–70%) CO2 incubator at 37°C
under 95% air and 5% CO2. Cells between passages three
and seven were employed in the present study.
Measurement of cell proliferation
[3H]-thymidine incorporation was
performed to examine the effects of SCE on renal mesangial cell
proliferation. Mesangial cells were incubated in a 24-well plate
until ~70% confluent and subsequently treated with glucose (25 mM)
and SCE (10–50 µg/ml), followed by the addition of 1 µCi of
[3H]-thymidine [methyl-(3H) thymidine 50
Ci/mM; Nycomed; Takeda Pharmaceuticals International GmbH, Zurich,
Switzerland]. Following incubation at 37°C for 24 h, the plate was
washed once with ice-cold PBS (pH 7.4), treated 3 times with 10%
trichloroacetic acid (2 ml) for 5 min each time, and then lysed in
0.3 N NaOH (1 ml) and 1% SDS for at least 30 min at room
temperature. Following lysis, [3H]-thymidine activity
was measured using the Beckman LS 7500 Liquid Scintillation Counter
(Beckman Coulter, Inc., Brea, CA, USA) and the resulting data were
analyzed using the software provided by the manufacturer. Each
experiment was performed in triplicate or quadruplicate.
Western blot analysis
Human mesangial cells were were pretreated with SCE
for 30 min and then stimulated with HG for 24 h. Subsequently,
cells were lysed using ice-cold lysis buffer with freshly added
protease inhibitor cocktail (Amresco, LLC, Solon, OH, USA) at 4°C
for 30 min, centrifuged at 14,000 × g at 4°C for 10 min and the
supernatants were collected. Protein concentration was measured
using a Bradford assay with bovine serum albumin (BSA) as the
standard. Equal amounts of protein samples (40 µg) were separated
by 10% SDS-PAGE and transferred onto nitrocellulose membranes.
Membranes were blocked with 5% skimmed milk powder in TBS
containing 0.05% Tween-20 [10 mM Tris-HCl (pH 7.6), 150 mM NaCl,
0.05% Tween-20] and incubated overnight at 4°C with the appropriate
primary antibodies (Table I). The
primary antibodies were detected with the corresponding horseradish
peroxidase-conjugated secondary antibodies (1:5,000; Table I). Protein bands were visualized
using Enhanced Chemiluminescence Detection Reagent (Amersham; GE
Healthcare Life Sciences) with the Chemi-doc image analyzer
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). β-actin was used
as the loading control. Lamin B was used as the loading control for
nuclear proteins. Blots were semi-quantified by densitometric
analysis using the ImageJ software version 1.49v (National
Institutes of Health, Bethesda, MD, USA).
 | Table I.Primary and secondary antibodies used
in the present study. |
Table I.
Primary and secondary antibodies used
in the present study.
| Antibody | Supplier | Catalogue
number | Dilution |
|---|
| Primary |
|
|
|
| CDK2
(H-298) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-748 | 1:1,000 |
| CDK4
(H-22) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-601 | 1:1,000 |
| Cyclin
D1 (A-12) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-8396 | 1:1,000 |
| Cyclin
E (HE12) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-247 | 1:1,000 |
| TGF-β1
(V) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-146 | 1:1,000 |
|
p-Smad-2 (Ser465/467) | EMD Millipore
(Billerica, MA, USA) | AB3849-I | 1:1,000 |
| Smad-2
(S-20) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-6200 | 1:1,000 |
| Smad-4
(B-8) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-7966 | 1:1,000 |
| Smad-7
(H-79) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-11392 | 1:1,000 |
| MT1-MMP
(V-16) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-12366 | 1:1,000 |
| TIMP-2
(3A4) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-21735 | 1:1,000 |
|
Collagen IV (H-57) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-135231 | 1:1,000 |
| CTGF
(B-6) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-373936 | 1:1,000 |
| ICAM-1
(H-108) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-7891 | 1:1,000 |
| MCP-1
(R-17) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-1785 | 1:1,000 |
| NF-κB
p65 (F-6) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-8008 | 1:1,000 |
| I-κB-α
(C-15) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-203 | 1:1,000 |
| β-actin
(4E8H3) | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-130065 | 1:1,000 |
| Secondary |
|
|
|
| Goat
anti-mouse IgG | Enzo Life Sciences,
Inc. (Farmingdale, NY, USA) | ADI-SAB-100 | 1:5,000 |
| Goat
anti-rabbit IgG | Enzo Life Sciences,
Inc. (Farmingdale, NY, USA) | ADI-SAB-300 | 1:5,000 |
| Mouse
anti-sheep/goat IgG | Enzo Life Sciences,
Inc. (Farmingdale, NY, USA) | ADI-SAB-400 | 1:5,000 |
Preparation of cytoplasmic and nuclear
extracts
Renal mesangial cells (8.8×106 cells)
were rapidly harvested in cold PBS on ice by sedimentation and
centrifuged at 10,000 × g for 10 min at 4°C. Cytoplasmic and
nuclear extracts were prepared using the Nuclear Extract kit
(Active Motif, Inc., Carlsbad, CA, USA), according to the
manufacturer's protocol. Briefly, cells were scraped, washed with
PBS, resuspended in hypotonic buffer (10 mM HEPES, 1.5 mM
MgCl2, 10 mM KCl, 0.2 mM phenylmethylsulfonyl fluoride
and 0.5 mM dithiothreitol), incubated on ice for 15 min, and then
lysed by adding 1% detergent, followed by vigorous vortexing for 10
sec and centrifugation at 4°C for 30 sec at 14,000 × g. The nuclear
pellet was resuspended in 30 µl of complete lysis buffer (1 mm DTT,
1% protease inhibitor cocktail, lysis buffer AM1). Nuclear proteins
were extracted by gentle agitation on ice for 30 min and
centrifugation at 4°C for 10 min at 14,000 × g. Subsequently, the
extracts were immediately transferred to clean screw-cap tubes and
stored at −80°C until use.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA isolation from cultured mesangial cells
(6×106) was performed using an RNeasy Plus Mini kit
(Qiagen GmbH, Hilden, Germany). RNA (1 µg) quality was measured at
the optical density ratio 260/280 nm by using a
UV-spectrophotometer. cDNA was synthesized using an HiPi RT-PCR kit
(ELPIS-Biotech. Inc., Daejeon, Korea). RT-qPCR analysis was
performed using the StepOnePlus Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
optimized with DyNAmo SYBR-Green 2-step RT-qPCR kit protocol
(Finnzymes; Thermo Fisher Scientific, Inc.). qPCR was initiated by
activating the AmpliTaq DNA polymerase by hot start at 95°C for 15
min followed by 40 cycles of denaturation at 94°C for 20 sec,
annealing at 60°C for 30 sec, extension at 72°C for 60 sec, and
plate reading at 60°C for 10 sec. The temperature of PCR products
was increased from 65 to 95°C at a rate of 0.2°C/sec and the
resulting data were analyzed by using the StepOne™
software version 2.3 provided by the manufacturer. The PCR products
were resolved by 1% agarose gel electrophoresis and visualized
using ethidium bromide (EMD Millipore, Billerica, MA, USA) to a
final concentration of ~0.5 µg/ml. The primers used in the present
study are presented in Table II.
Experiments were performed in triplicate and mRNA expression was
normalized to GAPDH. Gene expression was quantified using the
2−∆∆Cq method, as previously described (23).
 | Table II.Primers (forward and reverse) used
for reverse transcription-quantitative polymerase chain
reaction. |
Table II.
Primers (forward and reverse) used
for reverse transcription-quantitative polymerase chain
reaction.
| Gene | Primer |
|---|
| TGF-β1 | F:
5′-GCACGTGGAGCTGTACCA-3′ |
|
| R:
3′-CAGCCGGTTGCTGAGGTA-5′ |
| Collagen IV | F:
5′-TGTCAGCAATTAGGCAGGTC-3′ |
|
| R:
3′-CACCATGTTTCGGAATGGTT-5′ |
| CTGF | F:
5′-CTGCAGGCTAGAGAAGCAGAG-3′ |
|
| R:
3′-GATGCACTTTTTGCCCTTCT-5′ |
| ICAM-1 | F:
5′-GGCCGGCCAGCTTATACAC-3′ |
|
| R:
3′-TAGACACTTGAGCTCGGGCA-5′ |
| MCP-1 | F:
5′-ACTGAAGCTCGTACTCTC-3′ |
|
| R:
3′-CTTGGGTTGTGGAGTGAG-5′ |
| GAPDH | F:
5′-CAAGGCTGAGAATGGGAAGC-3′ |
|
| R:
3′-AGCATGTGGGAACTCAGATC-5′ |
Immunofluorescence microscopy
assay
Renal mesangial cells (3×106) were fixed
with 4% paraformaldehyde at room temperature for 30 min in the
culture dishes and permeabilized with 0.4% Triton X-100 in PBS at
room temperature for 5 min. Samples were blocked with 1% BSA (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) for 30 min at room
temperature and incubated with anti-Smad2 and anti-NF-κB subunit
p65 primary antibodies (Table I)
at 4°C overnight. Samples were then incubated with secondary
antibodies labeled with Alexa Fluor 488 (cat. no. A-11001; 1:200;
Molecular Probes; Thermo Fisher Scientific, Inc.) for 60 min at
room temperature. Nuclei were counterstained with 1 µg/ml DAPI at
room temperature for 5 min. The images were captured using an
Eclipse Ti fluorescence microscope (Nikon Corporation, Tokyo,
Japan) and analyzed using ImagePro software version 5.0 (Media
Cybernetics, Inc., Rockville, MD, US).
Luciferase promoter assay
Cells at 60–70% confluence were transiently
co-transfected with the plasmids according to the Lipofectamine LTX
kit (Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA) and
the manufacturer's plasmid transfection protocol. Plasmids linked
to a luciferase reporter (MMP-2 promoter) were kindly provided from
Dr Lee ST (Yonsei University, Seoul, Republic of Korea). The
plasmid mixture containing 5 µg of the MMP-2-promoter-luciferase
reporter or the Renilla-luciferase reporter and 5 µl of
Opti-MEM™ Media (Thermo Fisher Scientific, Inc.) was
blended with the Lipofectamine LTX reagent. Following incubation
for 48 h at 37°C, the cells were pretreated with SCE for 30 min and
25 mM D-glucose (HG) was added for 24 h. Cells in the control group
were cultured with 5.4 mmol/l glucose at 37°C. Cells were then
lysed with 100 µl of reporter lysis buffer and the extracts (30 µl)
were used to assess luciferase activity using the
Pierce™ Renilla-Firefly Luciferase Dual Assay kit
(Thermo Fisher Scientific, Inc.), as previously described (24). Luciferase activity was normalized
to Renilla activity and expressed as a percentage of the
control.
Intracellular ROS production
analysis
A fluorescent dye
(5,6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate;
CM-H2DCFDA) was used to examine the intracellular
generation of ROS. Renal mesangial cells (~70% confluent) in
96-well plates were pretreated with SCE (10–50 µg/ml) or
N-acetyl-L-cysteine (NAC; 1 mM) for 30 min and then stimulated with
HG (25 mM) for 24 h at 37°C. Following incubation, 2 µM
CM-H2DCFDA were added for 30 min at 37°C. The
fluorescence intensity was measured with an Infinite F200 PRO
Spectrofluorometer (Tecan Group Ltd., Männedorf, Switzerland) and
cells were observed under an Eclipse Ti fluorescence microscope
(Nikon Corporation). Recording and analysis of fluorescence signals
was performed using the NIS-Elements Basic Research Microscope
Imaging software version 4.30.00 (Nikon Corporation).
Statistical analysis
All experiments were repeated at least three times.
The results are presented as the mean ± standard error of the mean,
and the data were analyzed using one-way analysis of variance
followed by a Dunnett's test or Student's t-test to decide any
significant differences. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of SCE on HG-induced mesangial
cell proliferation
To investigate the effects of SCE on HG-induced
renal mesangial cell proliferation, [3H]-thymidine
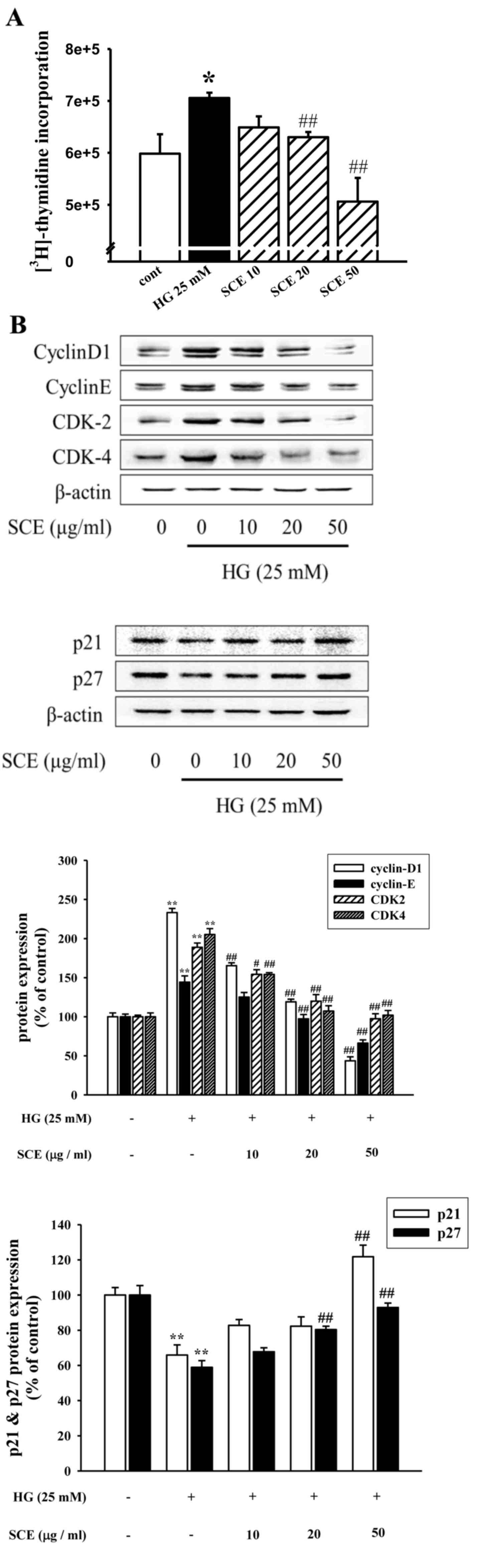
incorporation was measured. As demonstrated in Fig. 1A, the [3H]-thymidine
incorporation assay revealed that the HG-induced increase in cell
proliferation was significantly inhibited by pretreatment with
20–50 µg/ml SCE (P<0.01). HG treatment also resulted in the
increased expression of CDK-2 or CDK-4, cyclin D1 and cyclin E
proteins and the decreased expression levels of CDK inhibitory
proteins p21waf1/cip1 and p27kip1 (Fig. 1B). By contrast, exposure to SCE
reduced the expression levels of cell-cycle regulated proteins
(Fig. 1B). Therefore, it was
demonstrated that SCE treatment reduced HG-induced mesangial cell
proliferation through the downregulation of the expression of cell
cycle regulatory factors.
Effects of SCE on HG-induced
TGF-β1/Smad signaling pathway
The present study investigated whether SCE treatment
was able to reverse HG-induced renal mesangial cell fibrosis
through the regulation of TGF-β/Smad signaling. Western blot
analysis revealed that HG stimulation enhanced the protein
expression of TGF-β1, p-Smad-2 and Smad-4, whereas SCE
co-administration appeared to prevent this effect (Fig. 2A). In addition, HG stimulation
enhanced the nuclear expression of p-Smad-2 protein, whereas SCE
(20–50 µg/ml) reduced p-Smad-2 protein expression, particularly in
the nuclear extracts. Conversely, cytoplasmic Smad-7 protein
expression was increased by SCE co-treatment. PCR and RT-qPCR
analyses revealed that the HG-induced TGF-β1, Smad-2 and Smad-4
mRNA levels were decreased following co-treatment with SCE
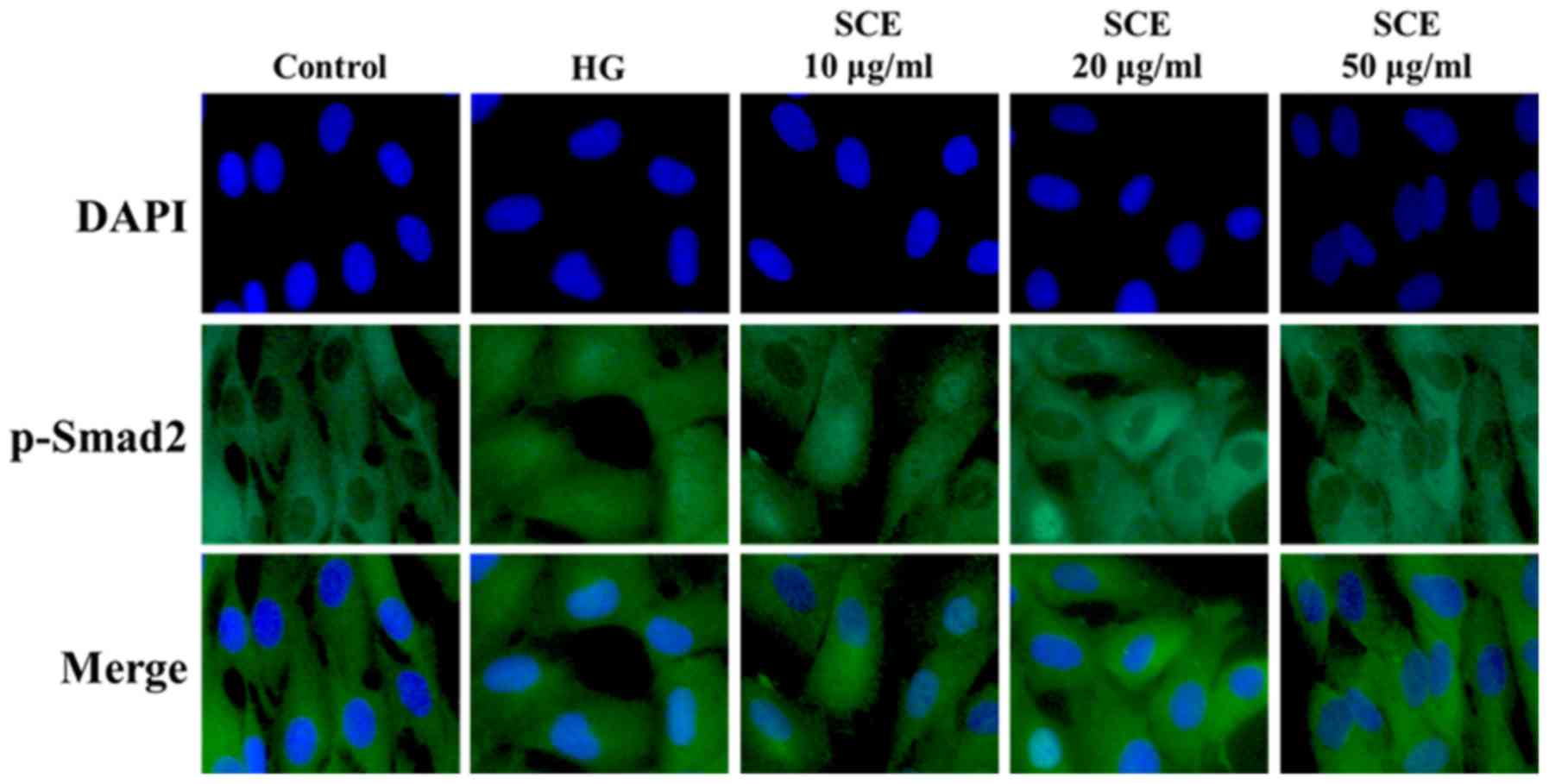
(Fig. 2B). As demonstrated in
Fig. 3, HG-stimulated mesangial
cells exhibited increased staining intensities of p-Smad-2 in the
nucleus. Nuclear p-Smad-2 expression levels were decreased
following treatment with SCE in dose-dependent manner. As TGF-β
signaling acts through Smad-2 and Smad-4, these results
demonstrated that SCE may block TGF-β-mediated fibrosis by
interrupting downstream Smad signaling.
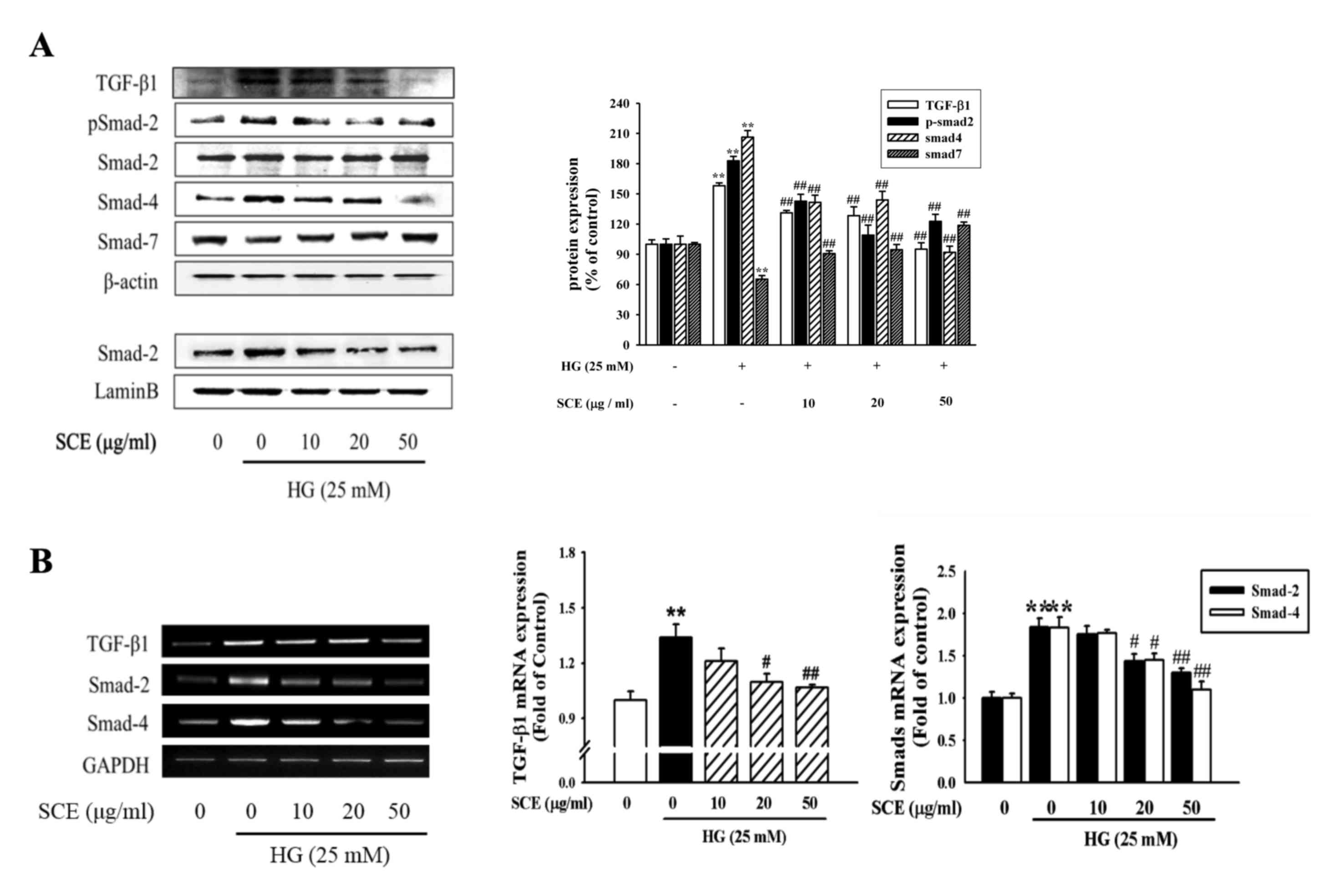 | Figure 2.Effects of SCE on the expression
levels of TGF-β1 and Smad proteins. (A) Western blot analysis with
specific antibodies against TGF-β1, p-Smad-2, Smad-2, Smad-4 and
Smad-7. β-actin and lamin B were used as the loadng controls for
cytoplasmic and nuclear protein expression, respectively. (B)
Levels of TGF-β1, Smad-2 and Smad-4 mRNA were analyzed by
electrophoresis using 1% agarose gel and visualized by ethidium
bromide staining. Each value represents the mean ± standard error
of the mean of three independent experiments. GAPDH was used as the
internal control. **P<0.01 vs. control; #P<0.05,
##P<0.01 vs. HG alone. SCE, samchuleum; TGF-β1,
transforming growth factor b1; HG, high glucose; p,
phosphorylated. |
Effects of SCE on MMP2 activation and
collagen IV expression
MMP-2 activity was measured by luciferase reporter
assay. As demonstrated in Fig. 4A,
pretreatment with SCE reversed the HG-induced decrease in MMP-2
promoter activity in human renal mesangial cells. MT1-MMP has been
revealed to activate MMP-2 in a process that requires TIMP-2,
thereby degrading ECM (25);
therefore, the effects of SCE treatment on the expression of
MT1-MMP and TIMP-2 in HG-treated mesangial cells were investigated.
Pretreatment with ≥20 µg/ml SCE upregulated the HG-inhibited
MT1-MMP expression, whereas the HG-induced expression of TIMP-2 was
downregulated following pretreatment with SCE (Fig. 4A).
 | Figure 4.Effects of SCE on MMP and ECM under
HG treatment. Cells were treated with HG (25 mM) with or without
pretreatment with SCE (10, 20 and 50 µg/ml). (A) Effect of SCE on
MMP-2 promoter activity and the expression of MMP-2 protein under
HG stimulation. Cells were transfected with an
MMP-2-promoter-luciferase reporter plasmid and reported activity
was normalized with the Renilla activity and expressed as a
percentage of the control. *P<0.05, **P<0.01 vs. control;
#P<0.05, ##P<0.01 vs. HG alone. (B)
Effects of SCE on collagen IV, CTGF, MMP and TIMP protein
expression levels. (C) Effects of SCE on collagen IV and CTGF mRNA
expression levels. SCE, samchuleum; MMP, matrix metalloproteinase;
ECM, extracellular matrix; HG, high glucose; MT1-MMP,
membrane-bound type 1 MMP; CTGF, connective tissue growth factor;
TIMP, tissue inhibitor of MMP. |
The inhibitory effects of SCE on HG-induced
mesangial matrix expansion were investigated by examining the
levels of collagen IV and CTGF protein expression levels by western
blotting (Fig. 4B). The expression
of collagen IV was elevated by HG stimulation, and this increase in
expression was reduced when cells were pretreated with ≥10 µg/ml
SCE. SCE inhibited the HG-induced expression CTGF protein in renal
mesangial cells. PCR analysis confirmed that SCE treatment affected
the HG-triggered expression levels of collagen IV and CTGF
(Fig. 4C). Collagen IV and CTGF
mRNA expression levels were markedly reduced by pretreatment with
≥20 µg/ml SCE in HG-exposed cells. The present results demonstrated
that treatment with SCE inhibited the mRNA and protein expression
of collagen IV, and decreased CTGF production. These results
demonstrated that SCE treatment has the potential to block
HG-induced glomerulosclerosis and kidney fibroblast.
Effects of SCE on HG-stimulated
inflammation
The present study suggested that SCE might alleviate
mesangial inflammation in the renal fibrogenetic process. ICAM-1
has been reported to promote the infiltration of inflammatory
cells, including mononuclear macrophages, into glomeruli and kidney
interstitial cells; inflammation-related factors, such as ICAM-1,
are known to accelerate glomerulosclerosis caused by diabetes
(26). SCE treatment inhibited the
HG-induced expression of ICAM-1 and MCP-1 proteins (Fig. 5A). In addition, ICAM-1 and MCP-1
mRNA levels were also reduced by 20–50 µg/ml SCE pretreatment in
HG-exposed mesangial cells, as evidenced by PCR and RT-qPCR
(Fig. 5A). The present study
hypothesized that SCE treatment may alleviate renal inflammatory
response related to the renal fibrotic process.
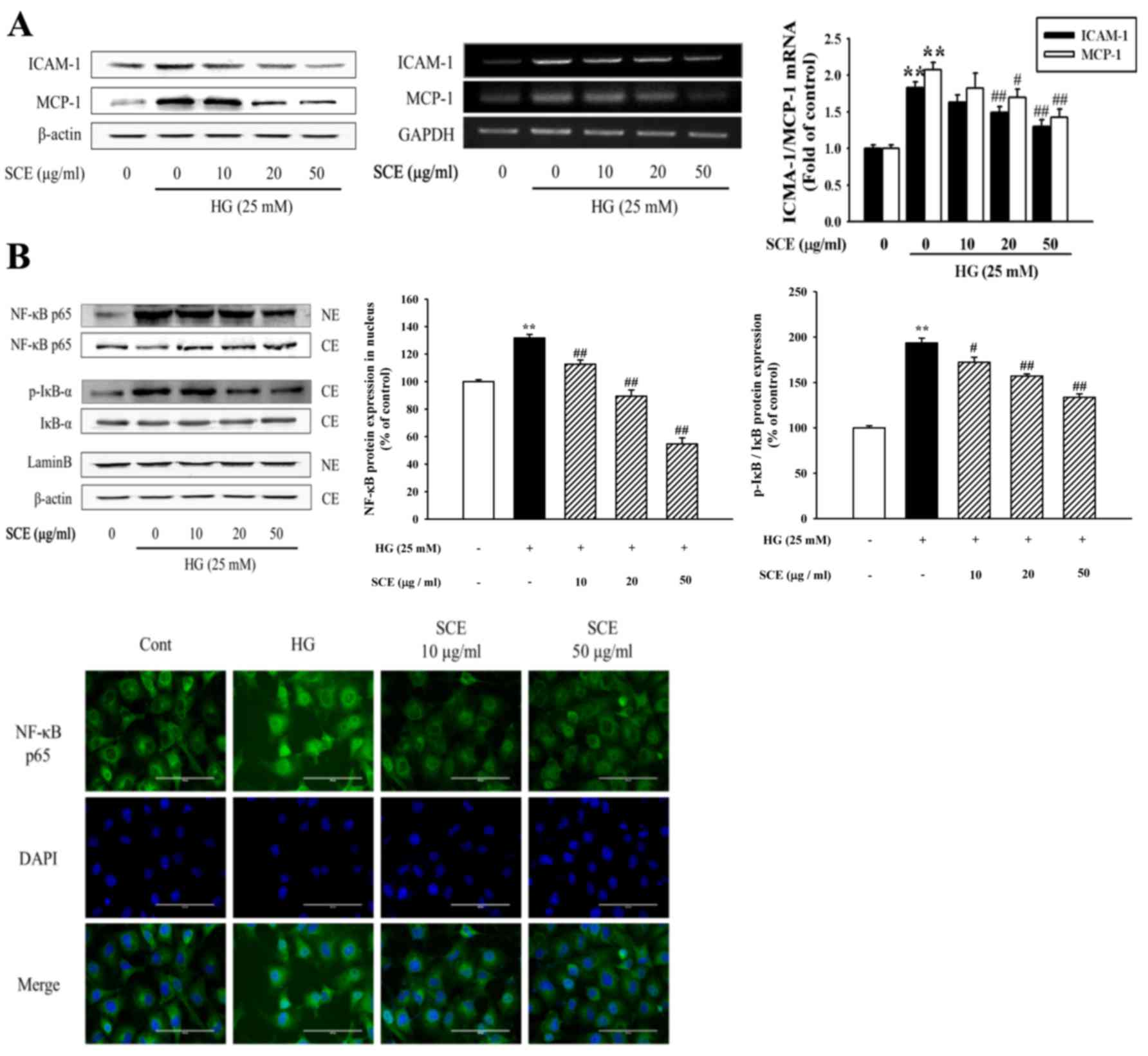 | Figure 5.Effects of SCE on HG-induced renal
inflammation. (A) Cells were treated with HG (25 mM) with or
without SCE pretreatment (10, 20 and 50 µg/ml). ICAM-1 and MCP-1
expression was analyzed by western blot, PCR and reverse
transcription-quantitative PCR. **P<0.01 vs. control;
#P<0.05, ##P<0.01 vs. HG alone. β-actin
and GAPDH were used as internal controls. (B) Effects of SCE on the
translocation of NF-κB subunit p65 into the nucleus. Cells were
incubated with 10–50 µg/ml SCE for 1 h prior to HG treatment.
Nuclear and cytoplasmic proteins were isolated and analyzed by
western blot assay using primary antibodies against NF-κB and
p-IκB-α. Localization of NF-κB was detected by immunofluorescence
assay (green, NF-κB; blue, nucleus; magnification, ×400).
Respective western blot data were obtained from three independent
experiments. SCE, samchuleum; HG, high glucose; ICAM, intercellular
adhesion molecule; MCP, monocyte chemotactic protein; PCR,
polymerase chain reaction; NF, nuclear factor; I, inhibitor; p,
phosphorylated; NE, nuclear extract; CE, cytoplasmic extract; cont,
control. |
Activated NF-κB translocates to the nucleus where it
binds to target gene promoters to trigger the transcription of
genes associated with the inflammatory response (27); this subsequently promotes mesangial
cell proliferation and mononuclear cell infiltration, and
eventually accelerates glomerulosclerosis in diabetes. Western blot
analysis revealed that HG exposure caused NF-κB subunit p65 nuclear
translocation, which was suppressed by pretreatment with SCE
(Fig. 5B). In addition, SCE
treatment reduced the HG-induced phosphorylation of IκB-α in the
cytoplasm. Immunofluorescence cell staining demonstrated that p65
was expressed at low levels in the cytoplasm of control cells,
whereas HG stimulation caused an increase in p65 staining level in
the nucleus. Pretreatment with SCE resulted in a decrease in
nuclear p65 expression levels (Fig.
5B).
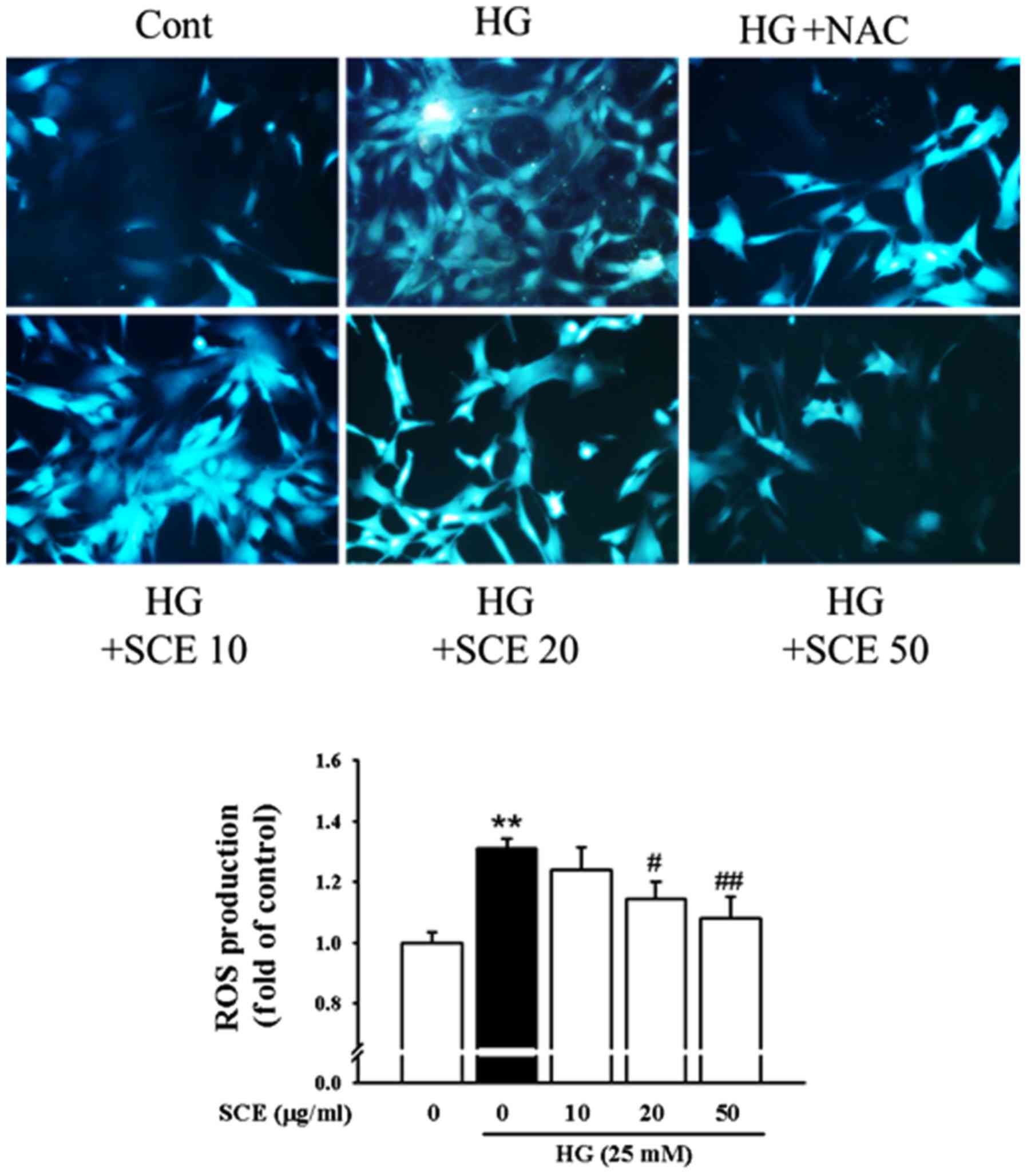
ROS are known to activate a number of transcription
factors as a common second messenger in various NF-κB activated
pathways (28). Therefore,
intracellular ROS production was measured to verify whether SCE
exposure causes a decrease in the levels of HG-induced oxidative
stress in renal mesangial cells (Fig.
6). HG increased ROS production (1.31 fold of control) compared
with the control group. However, pretreatment with 20 and 50 µg/ml
SCE decreased the HG-induced production of ROS to 1.14 and 1.08
fold, respectively (P<0.05 and P<0.01). Pretreatment with NAC
(1 mM), a potent antioxidant, reduced the HG-induced ROS
production.
NF-κB/ROS signaling may be involved in
diabetes-associated renal mesangial inflammation. The results of
the present study suggested that SCE treatment may suppress
HG-induced renal mesangial inflammatory response through the
disruption of the MCP-1, ICAM-1 and NF-κB/ROS signaling
pathways.
Discussion
In the present study, in vitro human renal
mesangial cell cultures were used to demonstrate that SCE
pretreatment inhibited HG-induced renal fibrosis and inflammation.
SCE exposure also suppressed renal hyperplasia and ECM accumulation
by inhibiting the expression of TIMP-2 in the matrix-degrading MMP
system. Development of DN is characterized by early-stage increases
in cell proliferation and ECM expansion (29). Cell proliferation is controlled by
cell cycle regulator proteins, which are necessary for progression
through the cell cycle. The present study performed
[3H]-thymidine incorporation experiments to demonstrate
that SCE pretreatment suppressed the HG-induced mesangial cell
proliferation. SCE treatment inhibited the expression of proteins
participating in CDK complexes (cyclin D1/CDK-4 and cyclin E/CDK-2)
and increased the expression of CDK inhibitors,
p21waf1/cip1 and p27kip1 under HG conditions.
The results of the present study demonstrated that SCE had an
inhibitory effect on mesangial proliferation by blocking the cell
cycle in the G0/G1 to S phase and inhibiting
DNA synthesis.
Mesangial ECM components, including fibronectin and
type IV collagen, serve an active role as a structural support for
the glomerular capillary tuft. They affect renal cell adhesion,
growth, migration and proliferation (30). The results of the present study
suggested that type IV collagen and CTGF, a profibrotic cytokine,
may be involved in the ECM-synthesizing process, and their mRNA and
protein expression levels were decreased following treatment with
SCE. In addition, SCE was demonstrated to improve the HG-triggered
dysfunction of the MMP system, via enhancing the expression of the
ECM-degrading MT1-MMP and inhibiting TIMP-2 expression. In
addition, the present results suggested that SCE may inhibit
HG-induced mesangial proliferation and fibrosis through the
regulation of MMP-2, which has been reported to induce the
enzymatic breakdown of ECM (31).
In mesangial cells, TGF-β/Smad signaling is hypothesized to serve a
significant role in the process of ECM accumulation and mesangial
expansion. HG-induced mesangial cell hypertrophy has been
associated with TGF-β signaling in DN (16,31,32).
The present study demonstrated that SCE pretreatment
decreased the mRNA and protein expression levels of TGF-β1,
p-Smad-2 and Smad-4, but increased the expression levels of Smad-7
in HG conditions. In addition, p-Smad-2 was mainly expressed in the
cytoplasm of normal mesangial cells; HG treatment promoted the
nuclear translocation of p-Smad-2, whereas SCE pretreatment
inhibited p-Smad-2 expression and its nuclear translocation. Thus,
these results suggested that SCE has the capacity to block
HG-induced renal fibrosis through the inhibition of factors
associated with ECM accumulation and TGF-β/Smad signaling in
DN.
Kidney inflammation is associated with the
progression of DN and understanding the inflammatory processes in
renal fibrosis is necessary to aid the development of new
therapeutics to halt the development of renal injury. In diabetes,
the activated NF-κB translocates into the nucleus and induces the
expression of activators and target genes of NF-κB, including
ICAM-1 and MCP-1, which in turn enhanced inflammation and finally
lead to the acceleration of the pathogenesis of glomerulosclerosis
and renal fibrosis through ECM accumulation (33,34).
The present study revealed that HG exposure enhanced ICAM-1 and
MCP-1 protein and mRNA expressions, which were inhibited by
pretreatment with SCE. In addition, SCE treatment not only reduced
HG-caused NF-κB subunit p65 translocation into the nucleus, but
also inhibited the phosphorylation of IκB-α in the cytoplasm.
Pretreatment with SCE also significantly suppressed HG-induced ROS
production and consequently inhibited mesangial cell proliferation
and inflammation. Results from the present study suggest that SCE
may control renal inflammation by regulating the expression of
inflammation-related genes and the NF-κB/ROS signaling pathway.
In conclusion, SCE pretreatment suppressed
HG-inflamed mesangial proliferation and ECM accumulation by
activating the matrix-degrading MMP system. SCE inhibited the
induction of CTGF and type IV collagen, through the regulation of
the TGF-β/Smad pathway. SCE exposure also inhibited the mesangial
inflammation possibly involved in renal fibrotic process. Thus, SCE
may be able to improve diabetic kidney disease by disturbing
TGF-β/Smad and NF-κB/ROS signaling pathways. These data provide the
initial evidence that SCE treatment may be a new approach for the
prevention and treatment of DN.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea (NRF) Grants funded by the Korean
government (grant no. NRF-2017R1A5A2015805) and the Ministry of
Education (grant no. NRF-2014R1A1A2008743).
References
|
1
|
Kolset SO, Reinholt FP and Jenssen T:
Diabetic nephropathy and extracellular matrix. J Histochem
Cytochem. 60:976–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mason RM and Wahab NA: Extra-cellular
matrix metabolism in diabetic nephropathy. J Am Soc Nephrol.
14:1358–1373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abrass CK, Peterson CV and Raugi GJ:
Phenotypic expression of collagen types in mesangial matrix of
diabetic and non-diabetic rats. Diabetes. 37:1695–1702. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mauer SM, Steffes MW, Ellis EN, Sutherland
DE, Brown DM and Goetz FC: Structural-functional relationships in
diabetic nephropathy. J Clin Invest. 74:1143–1155. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ziyadeh FN, Sharma K, Ericksen M and Wolf
G: Stimulation of collagen gene expression and protein synthesis in
murine mesangial cells by high glucose is mediated by autocrine
activation of transforming growth factor-beta. J Clin Invest.
93:536–542. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Young BA, Johnson RJ, Alpers CE, Eng E,
Gordon K, Floege J, Couser WG and Seidel K: Cellular events in the
evolution of experimental diabetic nephropathy. Kidney Int.
47:935–944. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuan CJ, Al-Douahji M and Shankland SJ:
The cyclin kinase inhibitor p21WAF1, CIP1 is increased in
experimental diabetic nephropathy: Potential role in glomerular
hypertrophy. J Am Soc Nephrol. 9:986–993. 1998.PubMed/NCBI
|
|
8
|
Adler S: Structure-function relationships
associated with extracellular matrix alterations in diabetic
glomerulopathy. J Am Soc Nephrol. 5:1165–1172. 1994.PubMed/NCBI
|
|
9
|
Huang K, Liu W, Lan T, Xie X, Peng J,
Huang J, Wang S, Shen X, Liu P and Huang H: Berberine reduces
fibronectin expression by suppressing the S1P-S1P2 receptor pathway
in experimental diabetic nephropathy models. PLoS One.
7:e438742012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Köppel H, Riedl E, Braunagel M,
Sauerhoefer S, Ehnert S, Godoy P, Sternik P, Dooley S and Yard BA:
L-carnosine inhibits high-glucose-mediated matrix accumulation in
human mesangial cells by interfering with TGF-β production and
signalling. Nephrol Dial Transplant. 26:3852–3858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pozzi A, Voziyan PA, Hudson BG and Zent R:
Regulation of matrix synthesis, remodeling and accumulation in
glomerulosclerosis. Curr Pharm Des. 15:1318–1333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abboud HE: Mesangial cell biology. Exp
Cell Res. 318:979–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McLennan SV, Fisher EJ, Yue DK and Turtle
JR: High glucose concentration causes a decrease in mesangium
degradation. A factor in the pathogenesis of diabetic nephropathy.
Diabetes. 43:1041–1045. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bernardo MM and Fridman R: TIMP-2 (tissue
inhibitor of metalloproteinase-2) regulates MMP-2 (matrix
metalloproteinase-2) activity in the extracellular environment
after pro-MMP-2 activation by MT1 (membrane type 1)-MMP. Biochem J.
374:739–745. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McLennan SV, Wang XY, Moreno V, Yue DK and
Twigg SM: Connective tissue growth factor mediates high glucose
effects on matrix degradation through tissue inhibitor of matrix
metalloproteinase type 1: Implications for diabetic nephropathy.
Endocrinology. 145:5646–5655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma K and Ziyadeh FN: Hyperglycemia and
diabetic kidney disease: The case for transforming growth
factor-beta as a key mediator. Diabetes. 44:1139–1146. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ask K, Bonniaud P, Maass K, Eickelberg O,
Margetts PJ, Warburton D, Groffen J, Gauldie J and Kolb M:
Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1
but not TGF-beta3. Int J Biochem Cell Biol. 40:484–495. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signaling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fornoni A, Ijaz A, Tejada T and Lenz O:
Role of inflammation in diabetic nephropathy. Curr Diabetes Rev.
4:10–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clavreul N, Sansilvestri-Morel P, Magard
D, Verbeuren TJ and Rupin A: (Pro)renin promotes fibrosis gene
expression in HEK cells through a Nox4-dependent mechanism. Am J
Physiol Renal Physiol. 300:F1310–F1318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bondar IA, Klimontov VV and Nadeev AP:
Urinary excretion of proinflammatory cytokines and transforming
growth factor beta at early stages of diabetic nephropathy. Ter
Arkh. 80:52–56. 2008.(In Russian). PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
Relative Gene Expression Data Using Real-Time Quantitative PCR and
the 22DDCT Method. METHODS. 25:402–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Michelini E, Cevenini L, Mezzanotte L,
Ablamsky D, Southworth T, Branchini B and Roda A: Spectral-resolved
gene technology for multiplexed bioluminescence and high-content
screening. Anal Chem. 80:260–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang SW, Choi JS, Choi YJ, Bae JY and Li
J: Licorice isoliquiritigenin dampens angiogenic activity via
inhibition of MAPK-responsive signaling pathways leading to
induction of matrix metalloproteinases. J Nutr Nutr Nutr Biochem.
21:55–65. 2010. View Article : Google Scholar
|
|
26
|
Okada S, Shikata K, Matsuda M, Ogawa D,
Usui H, Kido Y, et al: Intercellular adhesion molecule-1-deficient
mice are resistant against renal injury after induction of
diabetes. Diabetes. 52:2586–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Poljakovic M, Nygren JM and Persson K:
Signalling pathways regulating inducible nitricoxide synthase
expression in human kidney epithelial cells. Eur J Pharmacol.
469:21–8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ha H and Lee HB: Oxidative stress in
diabetic nephropathy: Basic and clinical information. Curr Diab
Rep. 1:282–7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mahadevan P, Larkins RG, Fraser JR and
Dunlop ME: Effect of prostaglandin E2 and hyalurunan on mesangial
cell proliferation: A potential contribution to glomerular
hypercellularity in diabetes. Diabetes. 45:44–50. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pozzi A, Voziyan PA, Hudson BG and Zent R:
Regulation of matrix synthesis, remodeling and accumulation in
glomerulosclerosis. Curr Pharm Des. 15:1318–33. 2005. View Article : Google Scholar
|
|
31
|
Park IS, Kiyomoto H, Abboud SL and Abboud
HE: Expression of transforming growth factor-β and type IV collagen
in early streptozotocin-induced diabetes. Diabetes. 46:473–80.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huse M, Muir TW, Xu L, Chen YG, Kuriyan J
and Massague J: The TGFβ receptor activation process: Aan
inhibitor- to substrate-binding switch. Mol Cell. 8:671–82. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohga S, Shikata K, Yozai K, Okada S, Ogawa
D and Usui H: Thiazolidinedione ameliorates renal injury in
experimental diabetic rats through anti-inflammatory effects
mediated by inhibition of NF-κB activation. Am J J Physiol.
292:F1141–50. 2007.
|
|
34
|
Yin D, Yao W, Chen S, Hu R and Gao X:
Salidroside, the main active compound of Rhodiola plants, inhibits
high glucose-induced mesangial cell proliferation. Planta Med.
75:1191–5. 2009. View Article : Google Scholar : PubMed/NCBI
|