Introduction
Parkinson's disease (PD) is a slow-progressing
neurological disease that results in degeneration of nerve cells
(1). As a result, the majority of
patients exhibit signs of dementia in the earlier stages (2), and when the disease progresses they
start to exhibit clinical symptoms including tremor, bradykinesia,
positional imbalance and rigidity (3). Ageing is a key factor in the
development of PD, as it results in morphological and neurochemical
alterations in dopamine-producing neurons (4–6).
Treating PD in earlier stages has a neuroprotective role in
preventing or prolonging the development of disease complications
(7). Treatment at later stages of
PD currently has no effect, and in most cases it results in
treatment resistance (1).
Earlier detection of PD is essential in the clinical
management of PD. Physiological symptoms occur following neural
degeneration, and thus, suitable biomarkers that detect earlier
stages of PD need to be identified (8). Currently available biomarkers fail to
specifically identify PD; further complications include variation
within individual results and a lack of experimental
reproducibility (8–10). To identify an effective biomarker
it is helpful to understand the pathogenesis of PD development;
however, the underlying mechanism underlying the neural
degeneration of PD and its associated neural dysfunction remain to
be clearly elucidated (1). An
improvement in the basic knowledge of this disease is required to
improve understanding of its pathogenesis, and to aid in the
identification and validation of biomarkers associated with
different pathological stages.
C-Jun N-terminal kinase 3 (JNK3) is markedly
expressed in brain tissue (11)
and has demonstrated roles in inflammation, apoptosis, cellular
proliferation and neural degeneration (12,13).
The present study used PD mouse models to investigate the role of
apoptosis and JNK3 in PD, and how they associate with different two
pathological stages of this disease.
Materials and methods
Mouse model with PD
Initially, 30 BALB/c male mice (age, 6 weeks;
weight, 30–40 mg) were purchased from The Jackson Laboratory (Bar
Harbor, ME, USA) were used in the present study. Mice were treated
twice daily with MPTP and maintained under a 12 h light/dark cycle
at 25±1°C with 45±5% relative humidity and food and water provided
freely. The PD mouse model was developed using
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which was
directly administered into the nasal cavity of the mice at a dosage
of 1.5 mg/nostril (total dose, 60 mg/kg) (14). Following treatment with MPTP, the
mice displayed early symptoms of PD by the 2nd week,
including hind limb weakness and restricted movements. On the
4th week, mice exhibited advanced symptoms of PD with
two hind limb paralyses. Limb weakness was used to assess the
development of Parkinson's disease (15). The animals, chemical used and the
experimental methods for developing the PD model were approved by
the animal care board of The First People's Hospital of Shangqiu
(Shangqiu, China).
Treatment with rasagiline
The mice were divided into the following
experimental groups (n=6/group): Normal (untreated); PD early; PD
advanced; PD early + rasagline; PD advanced + rasagline. Mice with
early and advanced PD were treated with rasagiline by dissolving
the drug in water and administering orally at a dose of 1 mg/day
(30 mg/kg), for one week. Water was used as a vehicle control.
Following treatment, the clinical symptoms including limb movement
and brain histological variation of the mice were analysed. The
mice were subsequently sacrificed and whole brain samples were
removed for further investigation.
Histology and
immunohistochemistry
Brain samples obtained from early and advanced
stages of PD and samples from untreated mice were sectioned into
smaller pieces, fixed with 10% formaldehyde and embedded in
paraffin. Formalin-fixed paraffin embedded tissue samples were
subjected to histological and immunohistochemical analysis
(16). Using a microtome, tissue
samples were evenly sliced into 5 µm sections and placed on a slide
warming table. Following deparrafinization and rehydration, the
slides were stained with haematoxylin and eosin for
histopathological analysis. For immunohistochemistry, the
endogenous peroxidise activity was blocked using 10%
H2O2 and nonspecific binding was blocked in
4% bovine serum albumin (BSA; Sigma-Aldrich; Merck KgaA, Darmstadt,
Germany) solution for 1 h. The tissue sections were incubated with
an anti-JNK3 antibody (cat. no. ab126591; Abcam, Cambridge, UK;
1:500) or with an anti-caspase-3 antibody that specifically detects
cells with early apoptosis (cat. no. ab13847, Abcam; 1:500)
overnight at 4°C. Following washing with PBS twice to reduce
non-specific binding of the primary antibody, the tissues sections
were incubated with a polyclonal goat anti-rabbit IgG horseradish
peroxidase-conjugated secondary antibody (ab6721, Abcam; 1:5,000)
for 1 h at room temperature. The slides were then washed and
developed using diaminobenzidine solution and counterstained with
haematoxylin and eosin. The experiments were repeated three times
and the signals obtained were examined and documented under a light
microscope equipped with NIS-Elements Viewer 4.3 (Nikon,
Corporation, Tokyo, Japan).
Western blot analysis
To analyse the differential expression of JNK3 in
samples obtained from control, and PD treated and untreated mice,
western blot analysis was performed. The samples were dissected out
and crushed on ice along with 2X sample buffer [500 mM Tris (pH
6.8), 20% glycerol, 4% SDS, 5% β-mercaptoethanol, 0.1% bromophenol
blue], then the tube are heated in a boiling water bath for 5 min
to obtain the protein lysate. The extracted protein samples were
measured using the Lowry method and 70 µg/well was separated by 10%
SDS-PAGE according to a previously described protocol (17). Separated proteins were transferred
to polyvinyl difluoridene membranes, and the membranes were blocked
with 4% BSA solution to limit non-specific binding of the antibody.
Membranes were subsequently incubated with an anti-JNK3 primary
antibody (cat. no. ab126591, Abcam; 1:500) or an anti-caspase-3
antibody (cat. no. ab13847, Abcam; 1:500) overnight at 4°C. Actin
(cat. no. ab3280, Abcam; 1:500) served as a loading control.
Following incubation, the membrane was washed three times with
TBS-Tween 20 (TBST) buffer and incubated with a goat anti-rabbit
IgG alkaline phosphatase-conjugated secondary antibody (Abcam,
ab97048; 1:5,000) for 1 h at room temperature. The membrane was
subsequently washed in TBST and developed with
5-Bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium
(Sigma-Aldrich; Merck KGaA) to obtain the signal.
Results
Histological differences between
healthy, PD and rasagiline-treated brain tissue
Histological examination of brain tissue was
performed to assess the pathological stages of PD mice. The
analysis of histopathological features is useful in understanding
the etiology of disease progression, which other techniques, such
as magnetic resonance imaging, cannot provide. Analysis of the
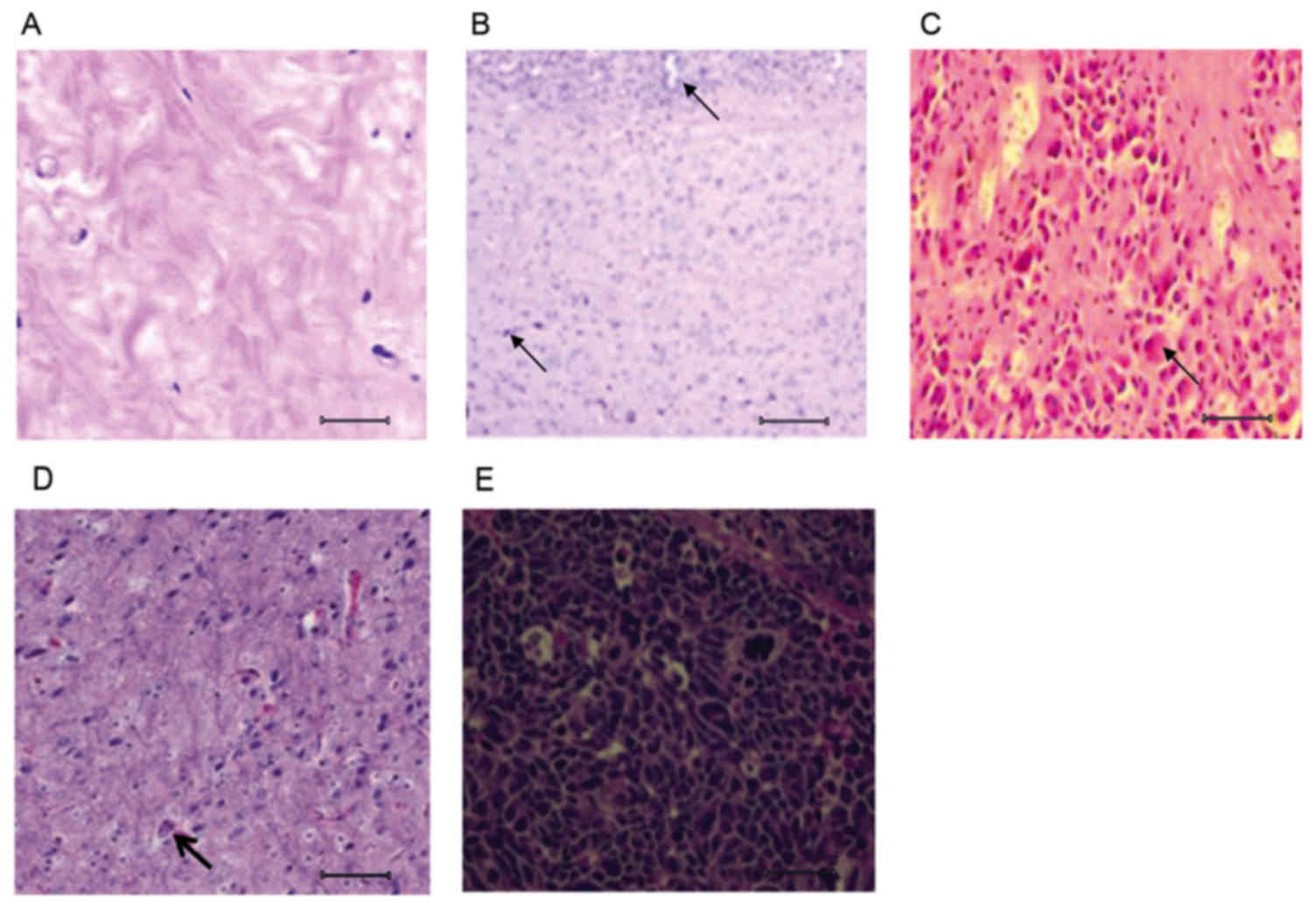
control brain tissue revealed cell bodies and axons that were
scattered uniformly throughout the tissue (Fig. 1A). The mice treated with MPTP for 2
weeks developed early stages of PD, with the development of lesions
throughout the brain tissue (Fig.
1B); the affected mice demonstrated hind limb weakness.
Similarly, the mice treated with MPTP for 4 weeks exhibited poor
movement, with paralyses of both hind limbs, representing an
advanced stage of PD. Histological analysis of brain tissue from
the advanced-stage PD mice revealed extensive lesions that severely
affected the white matter, which is important for motor activity
(Fig. 1C).
Following treatment with rasagiline for one week,
the early-stage PD mice exhibited improvement in their hind limb
weakness. These mice demonstrated active movement and histological
examination revealed that the brain lesions were reduced in number,
compared with the untreated early-stage PD mice (Fig. 1D). Mice with advanced-stage PD
presented no clinical or histological improvements. Furthermore,
the two hind-limb paralyses deteriorated clinically with
progressive restricted in limb movement. The brain tissue also
exhibited more degenerative tissue, potentially preventing the mice
from recovering (Fig. 1E).
JNK3 expression at different
pathological stages of PD
Inflammatory responses and various stress stimuli
can activate JNK3, which in turn has the ability to regulate
important cellular activities including cell differentiation,
survival and death (18). The
present study analyzed JNK3 expression at two different
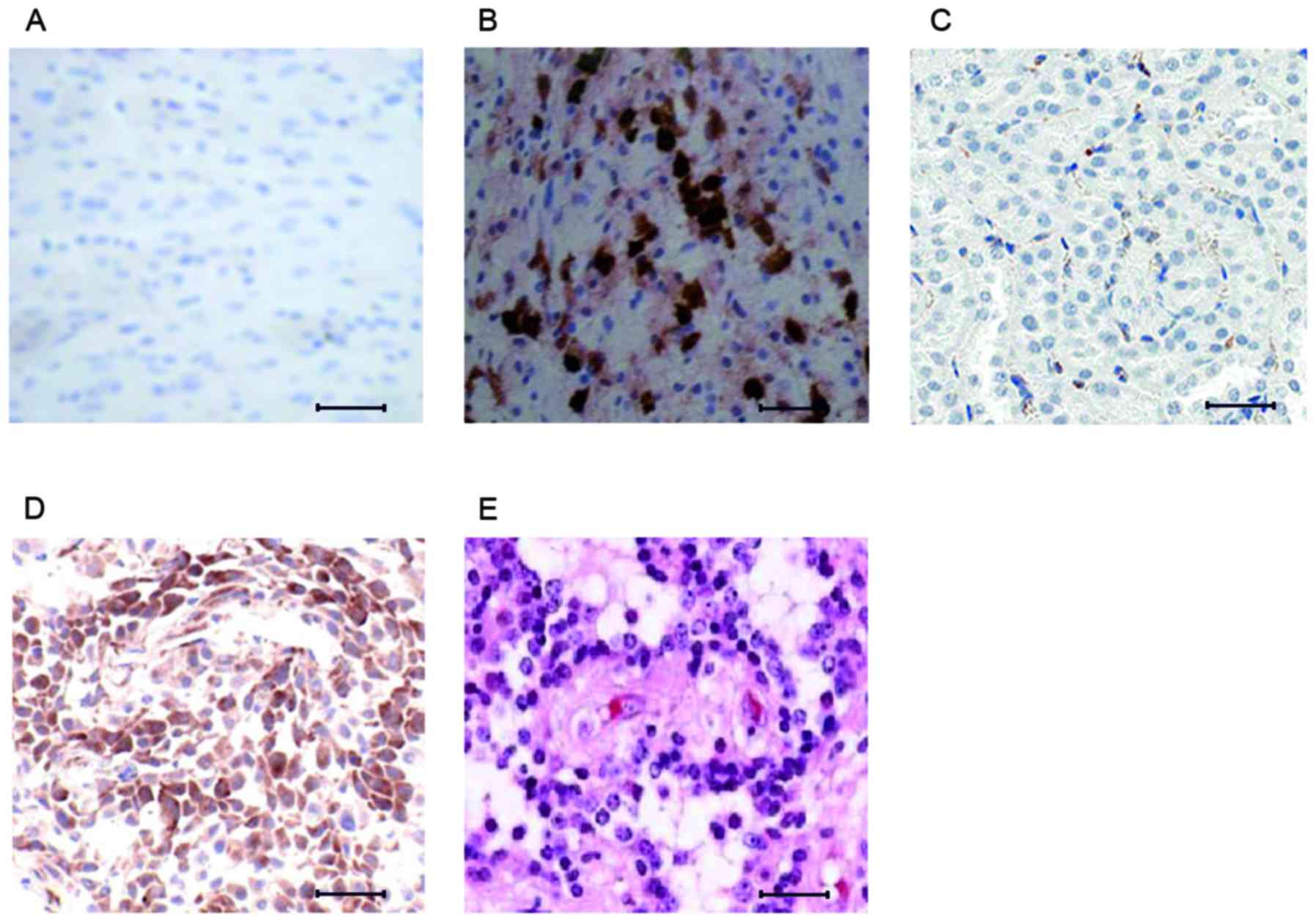
pathological stages of PD using immunohistochemistry. Analysis of
brain tissue from control mice revealed very mild expression of
JNK3 (Fig. 2A), due to the
regulation of JNK3 expression. Following treatment with MPTP for 2
weeks, the JNK3 signal demonstrated upregulation (Fig. 2B), which may have been due to
MPTP-induced neurological stress. Following treatment with MPTP for
4 weeks, the advanced-stage PD brain tissue revealed elevated JNK3
expression when compared with the early PD brain tissue (Fig. 2C).
 | Figure 2.Immunohistological differences
associated with JNK3 expression and response to rasagiline.
Immunohistological analysis of (A) control brain tissue, revealing
minimal expression of JNK3, (B) early-stage PD brain tissue, with
marginal expression of JNK3, (C) advanced-stage PD brain tissue,
with elevated expression of JNK3, (D) early-stage PD mice treated
with rasagiline, revealing downregulated expression of JNK3, and
(E) advanced-stage PD mice treated with rasagiline, revealing high
expression of JNK3. Scale bar=100 µm. JNK3, c-Jun N-terminal
kinase; PD, Parkinson's disease. |
JNK3 expression was investigated following treatment
with rasagiline, which has the ability to reversibly inhibit the
enzyme monoamine oxidase-B (19).
Rasagiline exhibited marked inhibitory effects on JNK3 expression
in early-stage PD (Fig. 2D).
However, rasagline treatment of mice with advanced-stage PD had no
effect, with the brain tissue exhibiting higher JNK3 expression
compared with the untreated advanced-stage mice (Fig. 2E).
Association between caspase-3 and JNK3
expression
In multicellular animals, apoptotic signals serve to
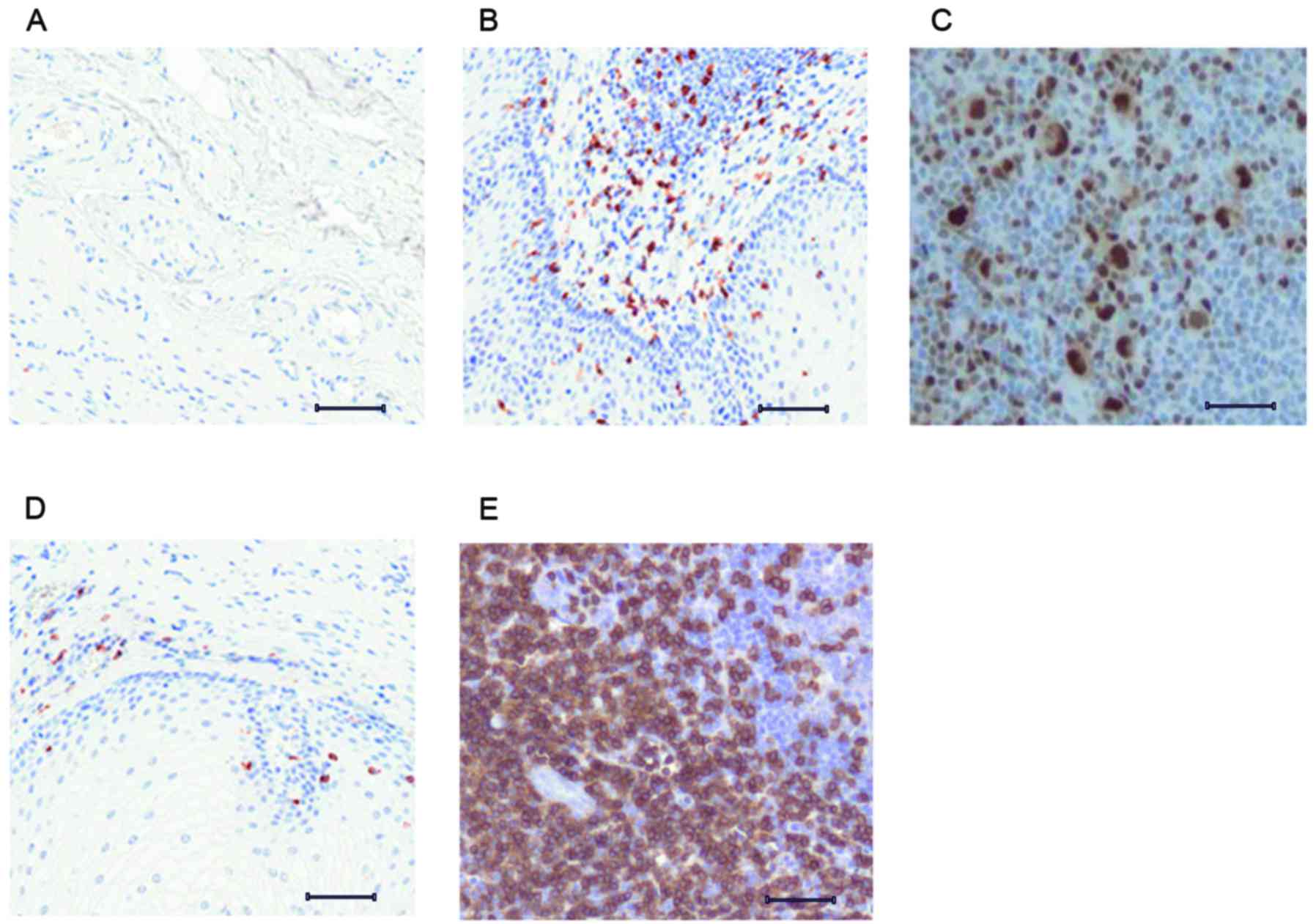
regulate the elimination of abnormal cells (20). Caspase-3 expression was assessed in
the brain tissue of early- and advanced-stage PD mice, to
investigate a potential correlation in expression levels between
caspase-3 and JNK3, in early and advanced PD pathological stages.
The control mice revealed minimal expression of caspase-3 (Fig. 3A). Early-stage PD mice exhibited a
marked increase in caspase-3 expression (Fig. 3B); however, caspase-3 expression
was downregulated in the brain tissue of advanced-stage PD mice
(Fig. 3C). Rasagiline-treated
early-stage mice exhibited higher levels of caspase-3, compared
with the untreated early-stage mice (Fig. 3D), however, the advanced-stage PD
mice demonstrated minimal expression of caspase-3 (Fig. 3E).
Western blot analysis of JNK3 and
caspase-3 expression
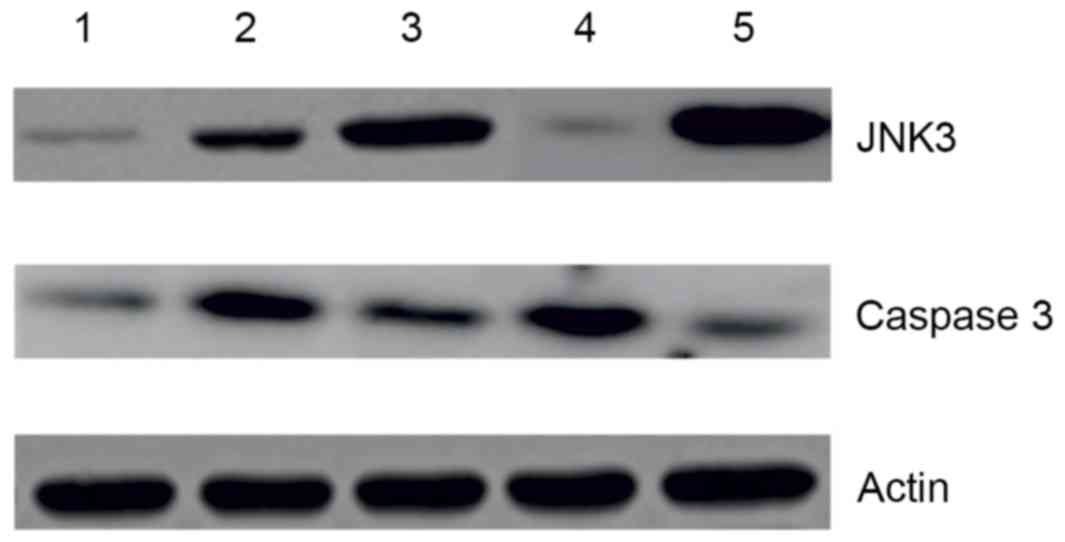
The immunohistochemistry results were further
investigated using western blot. Expression of JNK3 and caspase-3
was analysed, as presented in Fig. 4A
and B. The results supported the expression patterns observed
by immunohistochemical analysis. JNK3 demonstrated elevated
expression with progression of PD. Rasagline treatment was only
able to reduce the level of JNK3 in early-stage PD (Fig. 4A). In contrast, caspase-3 exhibited
elevated expression in early-stage PD (Fig. 4B), compared with advanced-stage PD
tissues.
Discussion
Damage to the white matter of the brain is
particularly associated with PD; in advanced stages this results in
demyelination, which severely impacts upon the motor
activity-signalling capacity of the neuron (21). The damage that occurs in earlier
stages of PD may be postponed using novel drugs; however, advanced
stages of PD are difficult to control (22). The present study investigated the
expression pattern of JNK3 in two different pathological stages of
PD and compared them with the expression of caspase-3. Compared
with early-stage PD mice, greater clinical and histological
deterioration was observed in advanced-stage PD mice (Fig. 1A-C). The early-stage PD mice
treated with rasagiline demonstrated clinical and histological
improvements; however, there was no effect on advanced-stage PD
mice. This data indicated that rasagline may not be able to reverse
neural degeneration in advanced-stage PD.
Histopathological and immunohistochemical analysis
of different PD pathology stages, using a mouse model, may provide
enhanced understanding of PD pathology, due to the ability to
investigate pathological stages that are difficult to analyse with
other imaging techniques (23). In
the present study, a mouse model of PD was used to determine the
impact of rasagiline treatment in two different pathological stages
of PD.
JNK regulates cell growth, cell survival and
apoptosis, and has demonstrated a role in testicular (24) and breast (25) cancer; however, the role of JNK3 on
apoptosis has received limited research attention. Apoptosis is a
fundamental mechanism that regulates the survival of abnormal or
damaged cells (26). The results
of the present study indicated that the expression of JNK3 may be
associated with PD disease progression. Notably, following
treatment with rasagline, expression of JNK3 was only observed in
early-stage PD; however, no effect on advanced-stage PD was
observed. The expression of caspase-3 was elevated in early-stage
PD compared with control and advanced-stage PD. Following
rasagiline treatment, caspase-3 was highly upregulated in early-PD
samples, compared with advanced stage PD. Similar results were
obtained by western blot analysis.
In conclusion, as PD progresses, it results in the
accumulation of lesions with axonal and cell body damage. The
clinical manifestation and histological damage observed in
early-stage PD may be improved by treatment with with rasagiline.
Furthermore, rasagline may have the ability to inhibit the
expression of JNK3 and upregulate the expression of caspase-3 in
early-stage PD; however, it has no effect in advanced stages of PD.
The results of the present study may help to further understand the
complexities of this disease, and may aid the discovery of novel
therapeutic drugs to treat PD.
Acknowledgements
We sincerely thank our institutional support for the
successful completion of the project.
References
|
1
|
AlDakheel A, Kalia LV and Lang AE:
Pathogenesis-targeted, disease-modifying therapies in Parkinson
disease. Neurotherapeutics. 11:6–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buter TC, Van den Hout A, Matthews FE,
Larsen JP, Brayne C and Aarsland D: Dementia and survival in
Parkinson disease: A 12-year population study. Neurology.
70:1017–1022. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crabtree D: Modulation of alpha-synuclein
metabolism and toxicity by cathepsin D. Uni Alabama Birmingham;
2012
|
|
4
|
Bennett DA, Beckett LA, Murray AM, Shannon
KM, Goetz CG, Pilgrim DM and Evans DA: Prevalence of parkinsonian
signs and associated mortality in a community population of older
people. N Engl J Med. 334:71–76. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kish SJ, Shannak K, Rajput A, Deck JH and
Hornykiewicz O: Aging produces a specific pattern of striatal
dopamine loss: Implications for the etiology of idiopathic
Parkinson's disease. J Neurochem. 58:642–648. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hornykiewicz O: Ageing and neurotoxins as
causative factors in idiopathic Parkinson's disease-a critical
analysis of the neurochemical evidence. Prog Neuropsychopharmacol
Biol Psychiatry. 13:319–328. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lang AE: Clinical trials of
disease-modifying therapies for neurodegenerative diseases: The
challenges and the future. Nat Med. 16:1223–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schapira AH: Recent developments in
biomarkers in Parkinson disease. Curr Opin Neurol. 26:395–400.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schapira AH and Schrag A: Parkinson
disease: Parkinson disease clinical subtypes and their
implications. Nat Rev Neurol. 7:247–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goetz CG, Tilley BC, Shaftman SR, Stebbins
GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel
R, et al: Movement disorder society-sponsored revision of the
unified parkinson's disease rating scale (MDS-UPDRS): Scale
presentation and clinimetric testing results. Mov Disord.
23:2129–2170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|
|
12
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bogoyevitch MA: The isoform-specific
functions of the c-Jun N-terminal Kinases (JNKs): Differences
revealed by gene targeting. Bioessays. 28:923–934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prediger RD, Aguiar AS Jr, Moreira EL,
Matheus FC, Castro AA, Walz R, De Bem AF, Latini A, Tasca CI,
Farina M and Raisman-Vozari R: The intranasal administration of
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): A new rodent
model to test palliative and neuroprotective agents for Parkinson's
disease. Curr Pharm Des. 17:489–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duty S and Jenner P: Animal models of
Parkinson's disease: A source of novel treatments and clues to the
cause of the disease. Br J Pharmacol. 164:1357–1391. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su JH, Anderson AJ, Cummings BJ and Cotman
CW: Immunohistochemical evidence for apoptosis in Alzheimer's
disease. Neuroreport. 5:2529–2533. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:pp. 4350–4354. 1979; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vlahopoulos S and Zoumpourlis VC: JNK: A
key modulator of intracellular signaling. Biochemistry (Mosc).
69:844–854. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rabey JM, Sagi I, Huberman M, Melamed E,
Korczyn A, Giladi N, Inzelberg R, Djaldetti R, Klein C and Berecz
G; Rasagiline Study Group, : Rasagiline mesylate, a new MAO-B
inhibitor for the treatment of Parkinson's disease: A double-blind
study as adjunctive therapy to levodopa. Clin Neuropharmacol.
23:324–330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis 1. Annu Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanyu H, Asano T, Sakurai H, Takasaki M,
Shindo H and Abe K: Magnetisation transfer measurements of the
subcortical grey and white matter in Parkinson's disease with and
without dementia and in progressive supranuclear palsy.
Neuroradiology. 43:542–546. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donovan S: Parkinson's disease treatment
US 6620415 B2. Filed July 11, 2001; issued September 16. 2003
|
|
23
|
Wegner C and Stadelmann C: Gray matter
pathology and multiple sclerosis. Curr Neurol Neurosci Rep.
9:399–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rathinam R, Berrier A and Alahari SK: Role
of Rho GTPases and their regulators in cancer progression. Front
Biosci (Landmark Ed). 16:2561–2571. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cellurale C, Girnius N, Jiang F,
Cavanagh-Kyros J, Lu S, Garlick DS, Mercurio AM and Davis RJ: Role
of JNK in mammary gland development and breast cancer. Cancer Res.
72:472–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bellamy CO, Malcolmson RD, Harrison DJ and
Wyllie AH: Cell death in health and disease: The biology and
regulation of apoptosis. Semin Cancer Biol. 6:3–16. 1995.
View Article : Google Scholar : PubMed/NCBI
|