Introduction
Osteoarthritis represents the most common form of
chronic joint disorder worldwide, the risk of which is
significantly increased with age (1). Currently, commonly prescribed
osteoarthritis medications include nonsteroidal anti-inflammatory
drugs, analgesic drugs, and joint injection with glucocorticoids
and visco-supplementation. However, these medications cannot halt
the progression of the disease or reverse any damage caused by
osteoarthritis. Surgical intervention is recommended for those
patients with symptoms that persist after the appropriate use of
nonsurgical treatment (2).
Osteoarthritis is characterized by progressive
degradation of the extracellular cartilage matrix (ECM) and loss of
chondrocytes (1,3). During the progression of
osteoarthritis, the production of reactive oxygen species (ROS)
(4) and the apoptosis of
chondrocytes (5,6) is gradually increased. Previous
evidence has suggested that the proinflammatory cytokine
interleukin (IL)-1β serves an important role in the development of
osteoarthritis. IL-1β levels are elevated in the synovial fluid,
synovial membrane, subchondral bone and cartilage of patients with
osteoarthritis (7). IL-1β induces
the expression of matrix metalloproteinases (MMPs) in cultured
chondrocytes, which leads to ECM degradation, abnormal bone
metabolism and inflammatory disease (8–10).
In addition, it has previously been reported that IL-1β can induce
in vitro release of nitric oxide (NO), DNA damage (11), ROS production and mitochondrial
damage in chondrocytes (12), thus
leading to chondrocyte apoptosis. These processes may require the
activation of nuclear factor (NF)-κB and mitogen-activated protein
kinase pathways (13–16).
Psoralidin is one of the active ingredients isolated
from the seeds of Psoralea corylifolia, which is extensively
used in Asian and African traditional medicines and exerts
therapeutic effects on cardiovascular and inflammatory diseases
(17,18). In addition, psoralidin possesses
osteoblast proliferation-stimulating (19), antibacterial (20) and antitumor activities (21–23).
Nevertheless, no data is currently available regarding whether
psoralidin can affect chondrocyte apoptosis. The present study
aimed to investigate the effects of psoralidin on IL-1β-induced
apoptosis of cultured rat chondrocytes and explored the possible
mechanisms.
Materials and methods
Collection, isolation, and culture of
rat chondrocytes
The present study was approved by the ethics
committee of Tongji Hospital (Shanghai, China). Sprague-Dawley (SD)
rats (n=6; age, 6 weeks; weight, 150–180 g) were purchased from
Shanghai Experimental Animal Center (Shanghai, China). The rats
were maintained at a controlled temperature (24±1°C) and controlled
relative humidity (20–30%), under a 12-h light/dark cycle with free
access to food and water. Following two days of acclimatization,
the rats were sacrificed and the articular cartilage was collected
and minced into small pieces before being digested with 0.4% type
II collagenase solution (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at 37°C for 5 h. Subsequently, the digested cartilage was
passed through a 70-µm cell strainer to remove undigested tissues,
and the chondrocytes were collected by centrifugation at 50 × g for
5 min. The cells were then cultured in Dulbecco's modified Eagle's
medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in an atmosphere
containing 5% CO2.
Experimental grouping
To establish the appropriate dose of IL-1β, the
cells were divided into five groups and treated with the following
concentrations of IL-1β (Sigma-Aldrich; Merck KGaA): 0, 1, 10, 50
or 100 ng/ml. Subsequently, a Cell Counting Kit (CCK)-8 assay was
performed after 0, 24 and 48 h to detect cell proliferation.
To investigate the effects of psoralidin on
chondrocyte proliferation, cells were incubated with various doses
of psoralidin (1, 5, 10, 15 or 20 µM; Shanghai YuanYe Biotechnology
Co., Ltd., Shanghai, China) or vehicle [dimethyl sulfoxide (DMSO)].
Subsequently, cell proliferation was detected after 0, 24 and 48 h.
To investigate the protective effects of psoralidin on
IL-1β-induced cytotoxicity, cells were incubated with 10 ng/m IL-1β
and various doses of psoralidin (1, 5, 10, 15 or 20 µM) or DMSO.
Cells in the control group were cultured with vehicle only (DMSO).
Cell proliferation was detected after 0, 24 and 48 h of
incubation.
For all other experiments, the cells were divided
into the following seven groups: Group 1 (control group), in which
cells were cultured with vehicle (DMSO); group 2, in which cells
were cultured with 10 ng/ml IL-1β and DMSO for 24 h; group 3, in
which cells were cultured with 10 ng/ml IL-1β and 5 µM psoralidin
for 24 h; group 4, in which cells were cultured with 10 ng/ml IL-1β
and 10 µM psoralidin for 24 h; group 5, in which cells were
cultured with 10 ng/ml IL-1β and 15 µM psoralidin for 24 h; group
6, in which cells were cultured with 30 µM
pyrriolidine-dithiocarbamate (PDTC, NF-κB inhibitor; Sigma-Aldrich;
Merck KGaA) and 10 ng/ml IL-1β for 24 h; and group 7, in which
cells were cultured with 10 ng/ml IL-1β and 500 µM N-acetylcysteine
(NAC; Sigma-Aldrich; Merck KGaA) for 24 h.
Cell proliferation assay
The chondrocytes were plated in 96-well plates
(2×103 cells/well) and were cultured at 37°C for 12 h in
serum-free DMEM. Following the appropriate treatment, cells were
incubated with CCK-8 (Beyotime Institute of Biotechnology,
Shanghai, China) at 37°C for 1 h. Optical density of each well was
measured at a wavelength of 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell apoptosis assay
Apoptotic analysis was performed using an Annexin
V-FITC flow cytometry apoptosis detection kit according to the
manufacturer's protocol (Beyotime Institute of Biotechnology).
After treatment, 1×106 cells were harvested and
resuspended in binding buffer, after which the cells underwent
Annexin V labeling for 15 min and propidium iodide (PI) labeling
for 5 min in the dark at 4°C. The samples were analyzed using a
flow cytometer (BD Biosciences, San Jose, CA, USA) with FlowJo
analysis software version 7.6.5 (FlowJo LLC, Ashland, OR, USA) The
percentage of Annexin V+/PI− cells was used
to quantify the proportion of early apoptotic cells.
Protein extraction and western
blotting
Total protein was extracted from the treated cells
using radioimmunoprecipitation assay buffer with freshly added
0.01% protease inhibitor cocktail (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China). Nuclear and cytosolic
proteins were extracted using NE-PER™ Nuclear and Cytoplasmic
Extraction Reagents (Thermo Fisher Scientific, Inc.). A total of 30
µg of proteins were separated by 10 or 15% SDS-PAGE and were then
transferred onto nitrocellulose membranes (EMD Millipore, Bedford,
MA, USA). Protein expression levels were analyzed by western
blotting. The membranes were blocked with 5% skimmed milk at room
temperature for 1 h, incubated with the appropriate primary
antibodies overnight at 4°C. Antibodies against B-cell lymphoma 2
(Bcl-2; sc-492; 1:200) and Bcl-2-associated X protein (Bax; sc-493;
1:200) were purchased from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). The MMP-13 antibody (ab39012; 1:3,000) was obtained from
Abcam (Cambridge, UK), and the MMP-1 antibody (10371–2-AP; 1:1,000)
was purchased from ProteinTech Group, Inc. (Chicago, IL, USA).
Antibodies against GAPDH (5174; 1:2,000), NF-κB p65 (8242;
1:1,000), β-actin (4970; 1:1,000) and histone H3 (4499S; 1:2,000)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). The membranes were then incubated with secondary antibodies
(A0208 and A0216; 1:1,000; Beyotime Institute of Biotechnology) at
room temperature for 1 h. Densitometric analysis was performed with
ImageJ software version 1.41 (National Institutes of Health,
Bethesda, MD, USA).
Caspase-3 and −9 activity assay
Caspase-3 and −9 activity was measured using the
Caspase colorimetric assay kit (Nanjing Keygen Biotech Co., Ltd.,
Nanjing, China) according to the manufacturer's protocol. Briefly,
cells were collected and lysed using the lysis buffer provided.
Absorbance values at 400 nm were determined using a microplate
reader (Bio-Rad Laboratories, Inc.). The control group values were
set at 100%, and results are expressed as a percentage of the
control.
Measurement of intracellular ROS
levels
Following treatment, the levels of intracellular ROS
were measured by flow cytometry following incubation with
dichlorofluorescein diacetate (Beyotime Institute of Biotechnology)
for 20 min in the dark at 37°C. Results were expressed as a
percentage of the control group fluorescence intensity.
NO production
NO concentration in the medium was measured
according to the Griess reaction method. Briefly, 50 µl Griess
reagent (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) was mixed with 50 µl cultured medium from treated cells and
was incubated in the dark for 10 min. The absorbance was measured
at 550 nm using a microplate reader (Bio-Rad Laboratories, Inc.).
The control group values were set at 100%, and results are
presented as a percentage of the control.
Statistical analysis
Data were analyzed using GraphPad Prism statistical
software version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Data are presented as the mean ± standard deviation of three
independent experiments. One-way analysis of variance followed by
Dunnett's multiple comparisons test was used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of psoralidin on IL-1β-induced
damage
To establish the appropriate dose of IL-1β, isolated
primary chondrocytes were incubated with 0, 1, 10, 50 or 100 ng/ml
IL-1β. A total of 24 and 48 h after treatment, cell proliferation
was significantly decreased by IL-1β (Fig. 1A). The results indicated that 10
ng/ml IL-1β had comparable effects to 50 and 100 ng/ml IL-1β;
therefore, 10 ng/ml IL-1β was used in the subsequent
experiments.
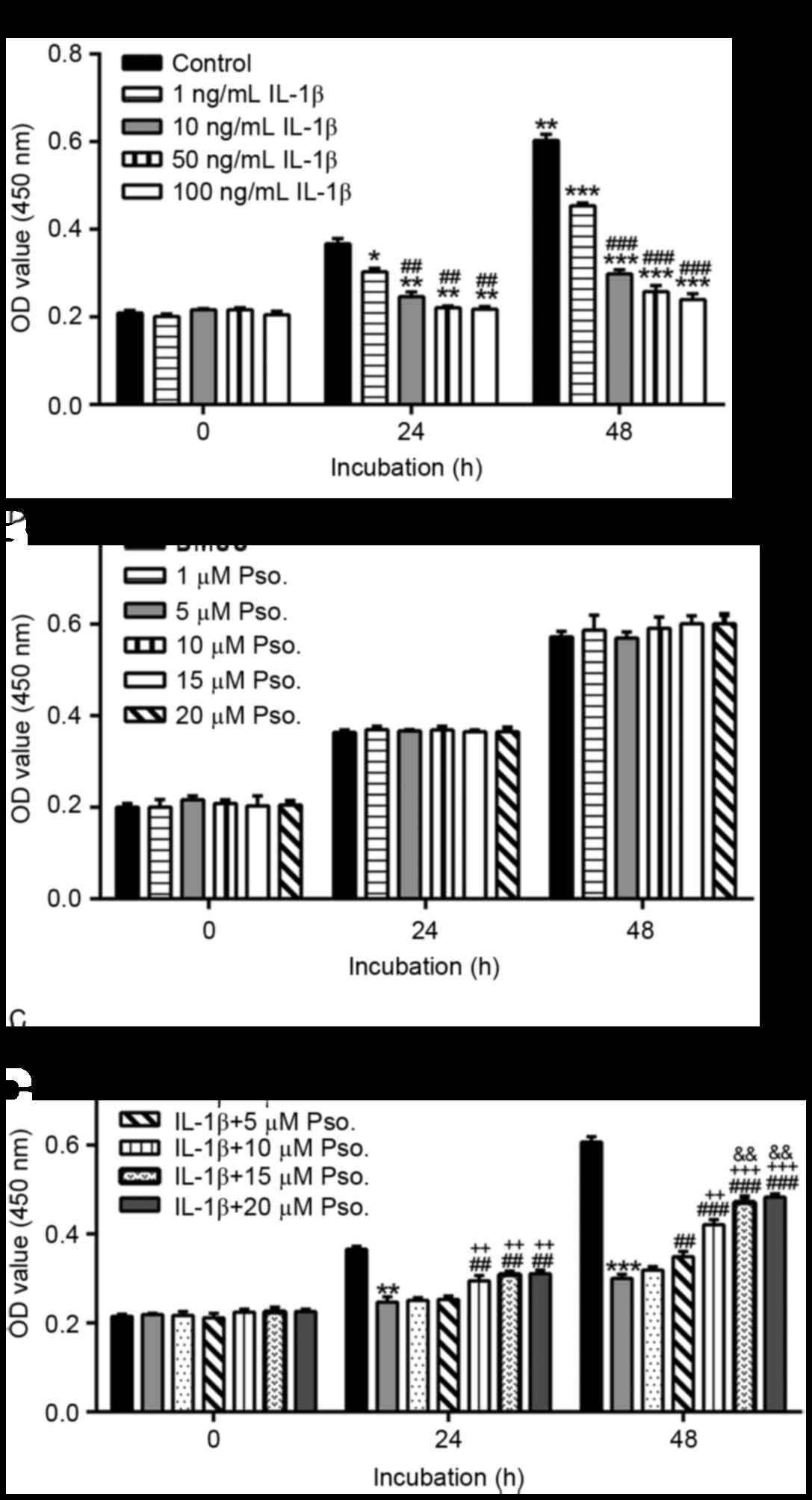 | Figure 1.Effects of Pso. on IL-1β-induced
damage. Proliferation of chondrocytes in various treatment groups
was assessed using Cell Counting kit-8 assay. (A) Chondrocytes were
treated with 1, 10, 50 or 100 ng/ml IL-1β for 0, 24 or 48 h. Cells
in the control group were cultured in media alone. *P<0.05,
**P<0.01 and ***P<0.001 vs. the control group;
##P<0.01 and ###P<0.001 vs. the 1 ng/ml
IL-1β group. (B) Chondrocytes were treated with Pso. (1, 5, 10, 15
or 20 µM) or vehicle (DMSO). (C) Chondrocytes were treated with
Pso. (1, 5, 10, 15 or 20 µM) or vehicle (DMSO) alongside 10 ng/ml
IL-1β. **P<0.01 and ***P<0.001 vs. the control (DMSO) group;
##P<0.01 and ###P<0.001 vs. the IL-1β +
DMSO; ++P<0.01 and +++P<0.001 vs. the
IL-1β + 1 µM Pso. group; &&P<0.01 vs. the
IL-1β + 5 µM Pso. group. Data are presented as the mean ± standard
deviation obtained from three separate experiments performed in
triplicate. DMSO, dimethyl sulfoxide; IL-1β, interleukin-1β; OD,
optical density; Pso., psoralidin. |
The present study also investigated the effects of
psoralidin on chondrocyte proliferation (Fig. 1B). Cell proliferation was similar
in cells treated with psoralidin (1, 5, 10, 15 or 20 µM) and cells
treated with vehicle (DMSO), thus suggesting that psoralidin
exerted no cytotoxic effects on chondrocytes. In addition, the
protective effects of psoralidin on IL-1β-induced cytotoxicity were
analyzed. Cells were treated with 10 ng/ml IL-1β and various
concentrations of psoralidin (1, 5, 10, 15 or 20 µM) or vehicle
(DMSO). Cell proliferation was significantly decreased by IL-1β
(Fig. 1C) at 24 and 48 h
post-treatment. A total of 24 h after treatment, IL-1β-induced cell
damage was significantly reduced by the addition of psoralidin at
doses of 10, 15 and 20 µM (P<0.01). Treatment with 5, 10 or 15
µM psoralidin for 48 h exerted protective effects in a
dose-dependent manner; however, there was no significant difference
between the 15 and 20 µM psoralidin groups. Therefore, 5, 10 and 15
µM psoralidin were used in subsequent experiments.
Effects of psoralidin on IL-1β-induced
chondrocyte apoptosis
To determine the potential effects of psoralidin on
IL-1β-induced chondrocyte apoptosis, chondrocytes were incubated
with various concentrations of psoralidin (0, 5, 10 or 15 µM) and
IL-1β (10 ng/ml) for 24 h. NAC is known to prevent chondrocyte
apoptosis and cartilage destruction (24); therefore, additional treatment with
NAC (500 µM) was used as a positive control. The ratio of apoptotic
cells was assessed by Annexin V/PI labeling. As presented in
Fig. 2A, the average apoptotic
ratio of chondrocytes exposed to IL-1β was 20.6% compared with the
untreated control chondrocytes (1.5%; P<0.001), whereas the
addition of the NF-κB inhibitor PDTC or NAC markedly decreased the
apoptotic ratio (P<0.001). In addition, treatment with
psoralidin resulted in a dose-dependent reduction in the ratio of
apoptotic cells.
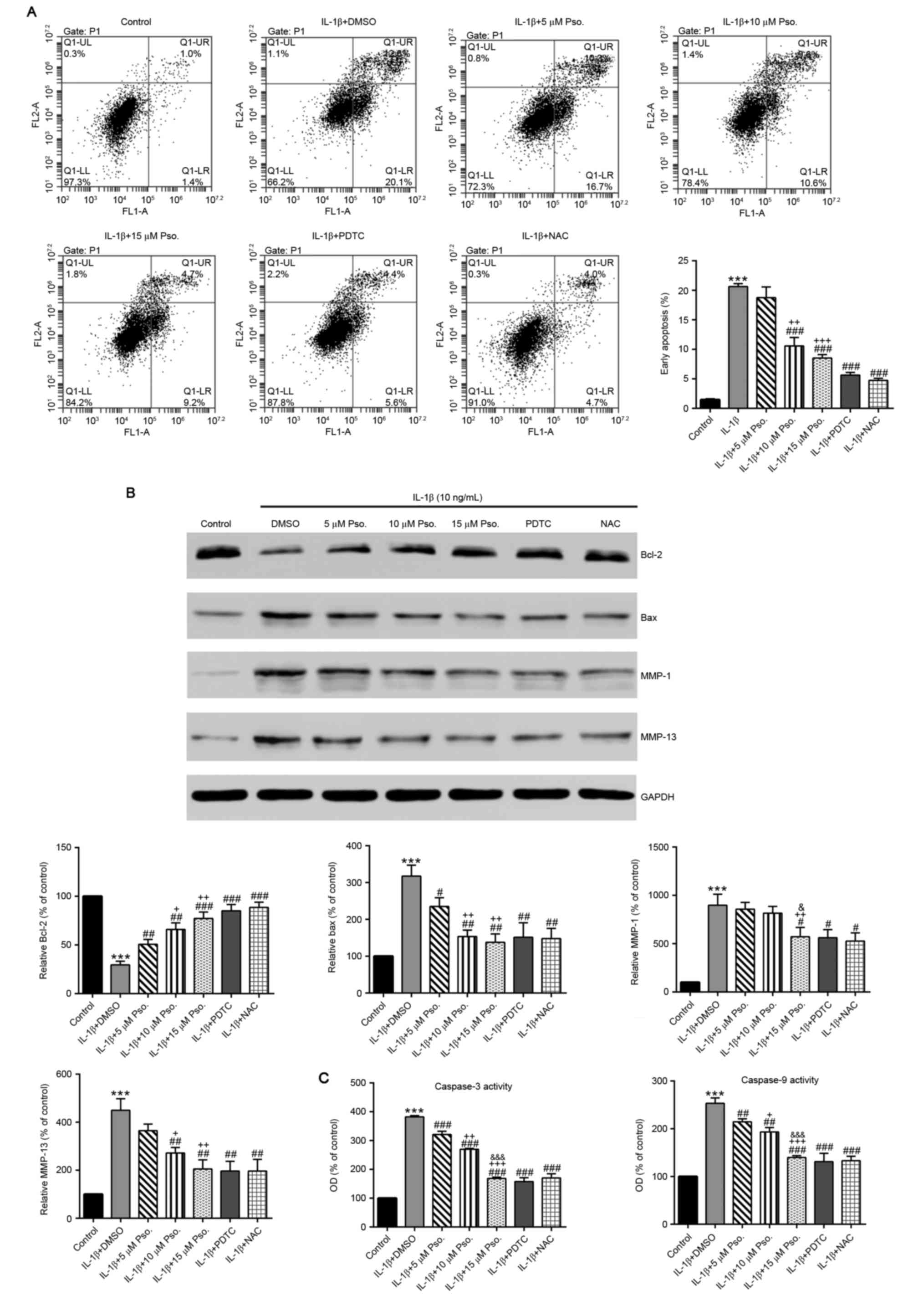 | Figure 2.Effects of Pso. on IL-1β-induced
chondrocyte apoptosis. Cells were treated with 10 ng/ml IL-1β, Pso.
(0, 5, 10 or 15 µM), 30 µM PDTC or 500 µM NAC for 24 h. Cells in
the control group were cultured in DMSO. (A) Apoptosis of
chondrocytes in the various treatment groups was determined by
Annexin V/propidium iodide staining and flow cytometry. (B) Protein
expression levels of Bcl-2, Bax, MMP-1 and MMP-13 were evaluated by
western blotting. (C) Caspase-3 and −9 activity were measured using
a Caspase colorimetric assay kit. All data are presented as the
mean ± standard deviation obtained from three separate experiments
performed in triplicate. The control group values were set at 100%,
and the results are expressed as a percentage of the control.
***P<0.001 vs. the control (DMSO) group; #P<0.05,
##P<0.01 and ###P<0.001 vs. the IL-1β +
DMSO group; +P<0.05, ++P<0.01 and
+++P<0.001 vs. the IL-1β + 5 µM Pso. group;
&P<0.05 and &&&P<0.001
vs. the IL-1β + 10 µM Pso. group. Bax, Bcl-2-associated X protein;
Bcl-2, B-cell lymphoma 2; DMSO, dimethyl sulfoxide; IL-1β,
interleukin-1β; MMP, matrix metalloproteinase; NAC,
N-acetylcysteine; OD, optical density; PDTC,
pyrriolidine-dithiocarbamate; Pso., psoralidin. |
Effects of psoralidin on the
expression of Bcl-2, Bax and MMP-1/13, and caspase-3/9 activation
of chondrocytes exposed to IL-1β
To investigate whether the mitochondrial apoptotic
pathway was affected, the protein expression levels of Bcl-2 and
Bax were evaluated by western blotting. As shown in Fig. 2B, compared with in the control
cells, IL-1β treatment led to a significant decrease in the
expression of the anti-apoptotic protein Bcl-2, and a significant
increase in the proapoptotic protein Bax (P<0.001). Conversely,
when psoralidin, PDTC or NAC were added, Bcl-2 expression was
significantly increased compared with in the IL-1β group, whereas
Bax expression was significantly reduced (P<0.01).
Alterations in caspase-3 and −9 activity were also
detected. As shown in Fig. 2C,
addition of psoralidin, PDTC or NAC significantly inhibited
IL-1β-mediated caspase-3 and −9 activation (P<0.01). Of the
three doses of psoralidin tested, 15 µM psoralidin had the most
marked effects.
IL-1β is able induce the expression of collagenases
(MMP-1 and −13), which degrade native collagen fibers and expedite
chondrocyte apoptosis (9).
Compared with in the control cells, IL-1β treatment induced a
significant increase in the expression levels of MMP-1 and −13.
(P<0.001), which was abrogated by the addition of 15 µM
psoralidin, PDTC or NAC (P<0.05).
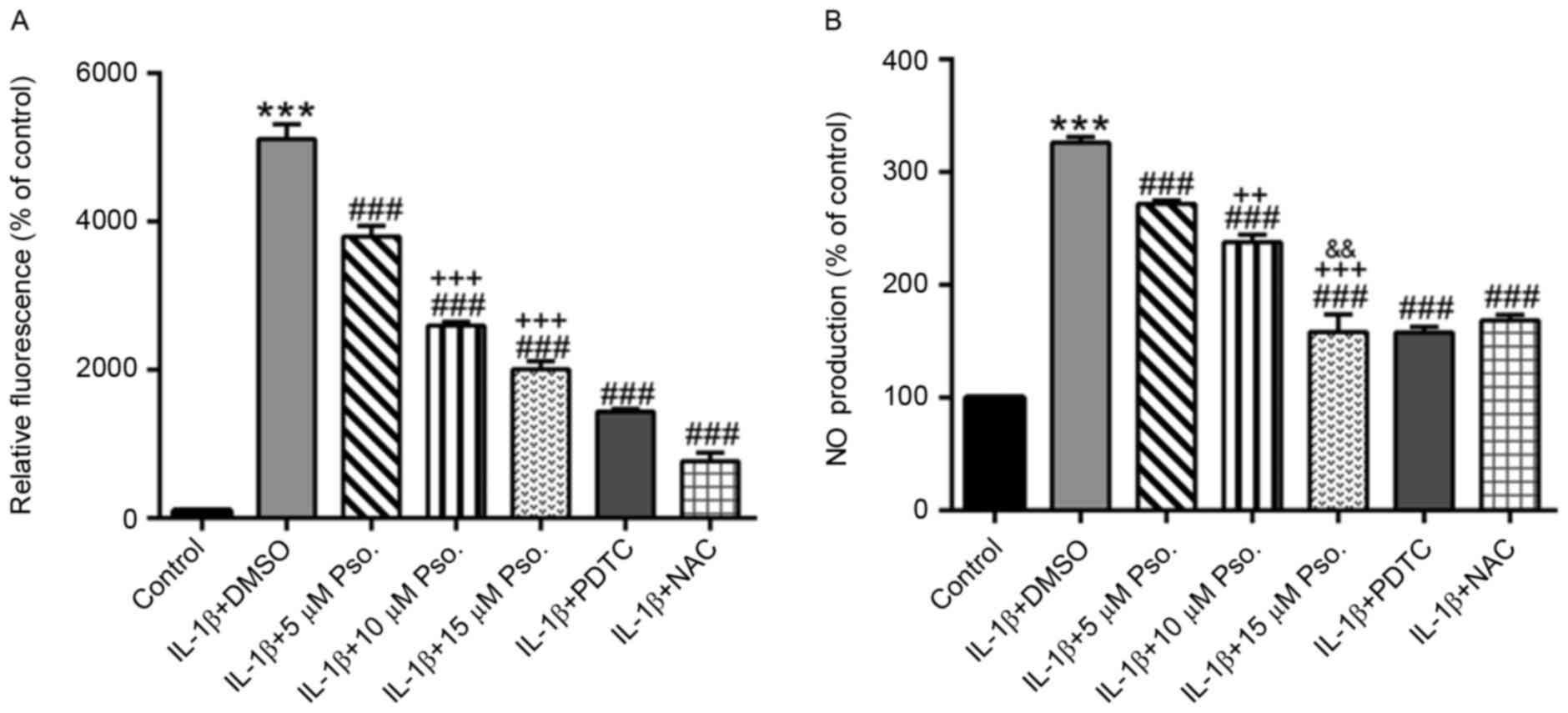
Effects of psoralidin on IL-1β-induced
ROS and NO production
The present study also analyzed intracellular ROS
and NO production (Fig. 3). IL-1β
(10 ng/ml) was able to significantly induce ROS and NO production
(P<0.001), which was markedly decreased by the addition of
psoralidin, PDTC or NAC (P<0.001). Psoralidin exerted its
effects in a dose-dependent manner.
Effects of psoralidin on IL-1β-induced
NF-κB nuclear translocation
IL-1β is known to induce NF-κB nuclear translocation
in chondrocytes (13). As
presented in Fig. 4, IL-1β was
able to significantly induce nuclear translocation of NF-κB
(P<0.01), which was partially abrogated by the addition of
psoralidin, PDTC or NAC (P<0.05).
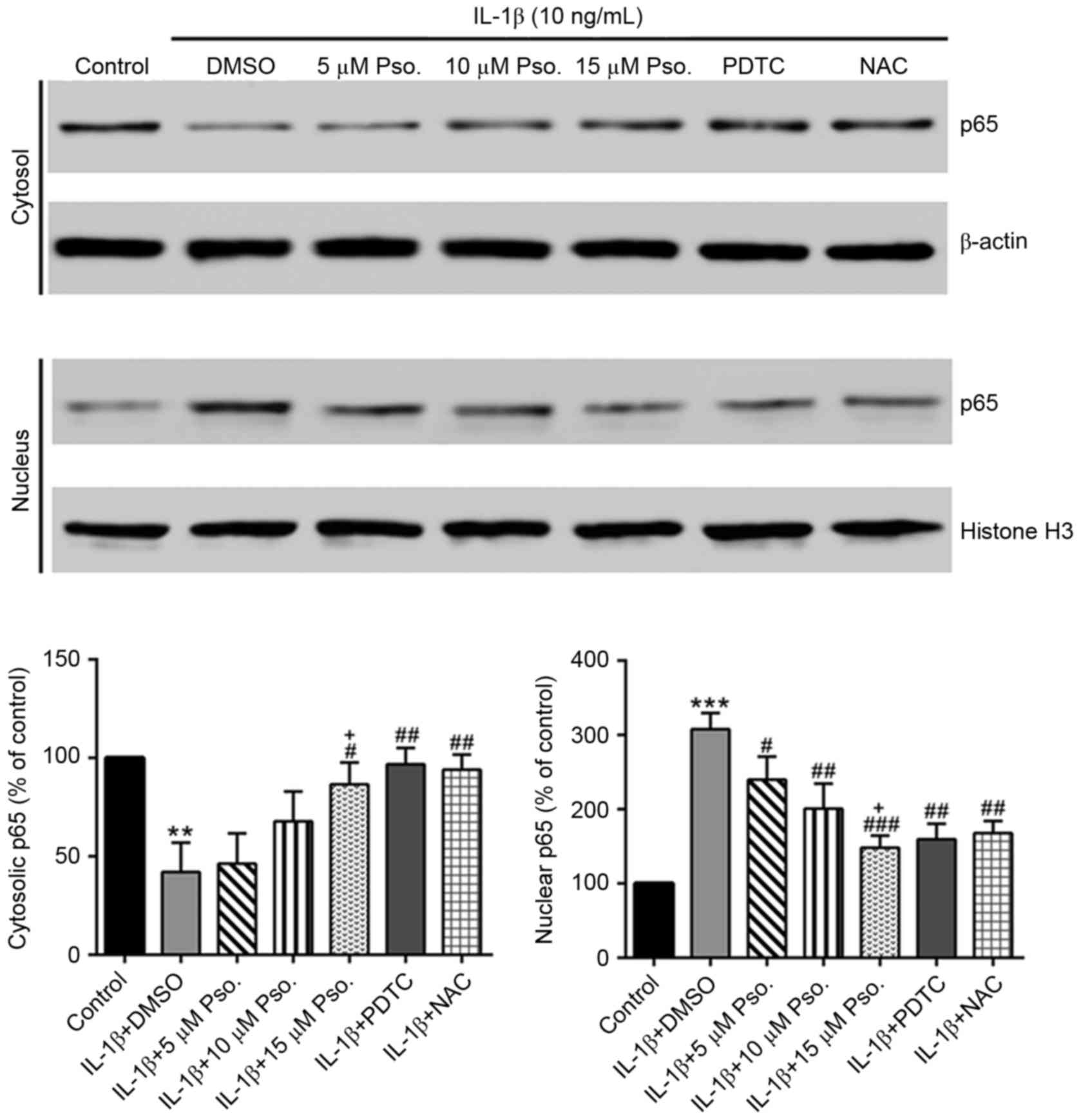 | Figure 4.Effects of Pso. on IL-1β-induced
nuclear factor-κB nuclear translocation. Nuclear and cytosolic
proteins were extracted with NE-PER™ Nuclear and Cytoplasmic
Extraction Reagents, and samples were analyzed by western blotting
with antibodies against p65, β-actin and histone H3. The
immunoreactive bands of cytosolic and nuclear p65 were normalized
to β-actin and histone H3, respectively. The control group values
were set at 100%, and the results are expressed as a percentage of
the control. **P<0.01 and ***P<0.001 vs. the control (DMSO)
group; #P<0.05, ##P<0.01 and
###P<0.001 vs. the IL-1β + DMSO group;
+P<0.05 vs. the IL-1β + 5 µM Pso. group. DMSO,
dimethyl sulfoxide; IL-1β, interleukin-1β; NAC, N-acetylcysteine;
PDTC, pyrriolidine-dithiocarbamate; Pso., psoralidin. |
Discussion
Psoralidin has been reported to possess osteoblast
proliferation-stimulating (19),
antibacterial (20) and antitumor
activities (21–23). However, the effects of psoralidin
on osteoarthritis have yet to be reported. The results of the
present study indicated that psoralidin protected rat chondrocytes
from IL-1β-induced apoptosis. In addition, it promoted Bcl-2
expression, reduced Bax expression, and decreased activation of
caspase-3 and −9. Psoralidin also inhibited the expression of MMP-1
and −13, the production of ROS, the release of NO, and NF-κB
nuclear translocation.
IL-1β can induce chondrocyte apoptosis; therefore,
it may be implicated in osteoarthritis pathophysiology (25). Consistent with the results of
previous studies (11,12), IL-1β treatment significantly
inhibited the proliferation and induced apoptosis of rat
chondrocytes, whereas the addition of psoralidin (10 or 15 µM)
markedly suppressed IL-1β-mediated apoptosis. Previous studies in
various cancer cell lines, including gastric, prostate, breast and
colon cancers, suggested that psoralidin exerts cytotoxic and
proapoptotic activities (23,26–29).
These inconsistent results may be due to the different cell types
and treatments used. Bcl-2 family proteins are able to alter
permeability of the mitochondrial membrane and can finally activate
caspase-9, −3 and −7 to initiate apoptosis (30). The present study demonstrated that
psoralidin alleviated the IL-1β-induced increase in the Bax to
Bcl-2 ratio, as well as the activity of caspase-3 and −9. The
results also suggested that psoralidin affected IL-1β-induced
apoptosis via the mitochondrial apoptosis pathway. In addition,
IL-1β can induce the release of NO (11) and the production of ROS (12), which eventually leads to
chondrocyte apoptosis. In the present study, psoralidin was able to
partially reverse the effects of IL-1β on NO release and ROS
production, thus affecting cell apoptosis.
In addition to chondrocyte apoptosis, cartilage
destruction is a main characteristic of osteoarthritis. In line
with previous observations that IL-1β can induce MMP expression
(8–10), the present study indicated that the
expression of MMP-1 and −13, which are two key regulators of
cartilage destruction, was induced by IL-1β. Psoralidin partially
abolished such effects, indicating the protective role of
psoralidin in cartilage destruction.
IL-1β can induce NF-κB activation in chondrocytes
(13). The present study also
analyzed the role of NF-κB in IL-1β-induced chondrocyte apoptosis.
IL-1β-induced NF-κB nuclear translocation was effectively inhibited
by PDTC. In addition, PDTC partially blocked IL-1β-induced
apoptosis, NO release, ROS production and MMP expression in
chondrocytes. These results suggested that IL-1β induced
proapoptotic effects via an NF-κB-dependent pathway. In addition, a
high dose of psoralidin (15 µM) had comparable effects to PDTC, and
therefore may be considered a drug candidate for the treatment of
osteoarthritis. Further in vivo animal experiments are
required to assess the therapeutic potential of psoralidin.
In conclusion, the findings of the present study
demonstrated that psoralidin may inhibit IL-1β-induced chondrocyte
apoptosis, NO production, ROS release, and MMP-1 and −13
expression, and may suppress IL-1β-activated NF-κB nuclear
translocation. Collectively, these data indicated the potential
therapeutic role of psoralidin in osteoarthritis treatment.
References
|
1
|
Loeser RF: Aging and osteoarthritis. Curr
Opin Rheumatol. 23:492–496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rönn K, Reischl N, Gautier E and Jacobi M:
Current surgical treatment of knee osteoarthritis. Arthritis.
2011:4548732011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thomas CM, Fuller CJ, Whittles CE and
Sharif M: Chondrocyte death by apoptosis is associated with
cartilage matrix degradation. Osteoarthritis Cartilage. 15:27–34.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li D, Xie G and Wang W: Reactive oxygen
species: The 2-edged sword of osteoarthritis. Am J Med Sci.
344:486–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharif M, Whitehouse A, Sharman P, Perry M
and Adams M: Increased apoptosis in human osteoarthritic cartilage
corresponds to reduced cell density and expression of caspase-3.
Arthritis Rheum. 50:507–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuo M, Nishida K, Yoshida A, Murakami T
and Inoue H: Expression of caspase-3 and −9 relevant to cartilage
destruction and chondrocyte apoptosis in human osteoarthritic
cartilage. Acta Med Okayama. 55:333–340. 2001.PubMed/NCBI
|
|
7
|
Koopman WJ and Moreland LW: Arthritis and
allied conditions: a textbook of rheumatology. Lippincott Williams
Wilkins; Philadelphia, PA: 2001
|
|
8
|
Koskinen A, Vuolteenaho K, Nieminen R,
Moilanen T and Moilanen E: Leptin enhances MMP-1, MMP-3 and MMP-13
production in human osteoarthritic cartilage and correlates with
MMP-1 and MMP-3 in synovial fluid from OA patients. Clin Exp
Rheumatol. 29:57–64. 2011.PubMed/NCBI
|
|
9
|
Aida Y, Maeno M, Suzuki N, Shiratsuchi H,
Motohashi M and Matsumura H: The effect of IL-1beta on the
expression of matrix metalloproteinases and tissue inhibitors of
matrix metalloproteinases in human chondrocytes. Life Sci.
77:3210–3221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elshaier AM, Hakimiyan AA, Rappoport L,
Rueger DC and Chubinskaya S: Effect of interleukin-1beta on
osteogenic protein 1-induced signaling in adult human articular
chondrocytes. Arthritis Rheum. 60:143–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou PH, Liu SQ and Peng H: The effect of
hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat
model of osteoarthritis. J Orthop Res. 26:1643–1648. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim J, Xu M, Xo R, Mates A, Wilson GL,
Pearsall AW IV and Grishko V: Mitochondrial DNA damage is involved
in apoptosis caused by pro-inflammatory cytokines in human OA
chondrocytes. Osteoarthritis Cartilage. 18:424–432. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HS, Lee CH, Tsai HC and Salter DM:
Inhibition of cyclooxygenase 2 expression by diallyl sulfide on
joint inflammation induced by urate crystal and IL-1beta.
Osteoarthritis Cartilage. 17:91–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wuertz K, Vo N, Kletsas D and Boos N:
Inflammatory and catabolic signalling in intervertebral discs: The
roles of NF-κB and MAP kinases. Eur Cell Mater. 23:103–120.
2012.PubMed/NCBI
|
|
15
|
Radons J, Bosserhoff AK, Grässel S, Falk W
and Schubert TE: p38MAPK mediates IL-1-induced down-regulation of
aggrecan gene expression in human chondrocytes. Int J Mol Med.
17:661–668. 2006.PubMed/NCBI
|
|
16
|
Sondergaard BC, Schultz N, Madsen SH,
Bay-Jensen AC and Karsdal MA: MAPKs are essential upstream
signaling pathways in proteolytic degradation-divergence in
pathways leading to aggrecanase and MMP mediated articular
cartilage degradation. Osteoarthritis Cartilage. 18:279–288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chopra B, Dhingra AK and Dhar KL: Psoralea
corylifolia L. (Buguchi)-Folklore to modern evidence: Review.
Fitoterapia. 90:44–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao L, Huang C, Shan Z, Xiang B and Mei
L: Fingerprint analysis of Psoralea corylifolia L. by HPLC and
LC-MS. J Chromatogr B Analyt Technol Biomed Life Sci. 821:67–74.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang D, Li F and Jiang Z: Osteoblastic
proliferation stimulating activity of Psoralea corylifolia extracts
and two of its flavonoids. Planta Med. 67:748–749. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khatune NA, Islam ME, Haque ME, Khondkar P
and Rahman MM: Antibacterial compounds from the seeds of Psoralea
corylifolia. Fitoterapia. 75:228–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pahari P and Rohr J: Total synthesis of
psoralidin, an anticancer natural product. J Org Chem.
74:2750–2754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu B, Wang AH, Zhou K, Chai LJ and Liu L:
The molecular pathway of psoralidin-induced apoptosis in HepG2 cell
line. Chin J Integr Med. Mar 29–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
23
|
Srinivasan S, Kumar R, Koduru S,
Chandramouli A and Damodaran C: Inhibiting TNF-mediated signaling:
A novel therapeutic paradigm for androgen independent prostate
cancer. Apoptosis. 15:153–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakagawa S, Arai Y, Mazda O, Kishida T,
Takahashi KA, Sakao K, Saito M, Honjo K, Imanishi J and Kubo T:
N-acetylcysteine prevents nitric oxide-induced chondrocyte
apoptosis and cartilage degeneration in an experimental model of
osteoarthritis. J Orthop Res. 28:156–163. 2010.PubMed/NCBI
|
|
25
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang YM, Hyun JW, Sung MS, Chung HS, Kim
BK, Paik WH, Kang SS and Park JG: The cytotoxicity of psoralidin
from Psoralea corylifolia. Planta Med. 62:353–354. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mar W, Je KH and Seo EK: Cytotoxic
constituents of Psoralea corylifolia. Arch Pharm Res. 24:211–213.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szliszka E, Czuba ZP, Sędek Ł, Paradysz A
and Król W: Enhanced TRAIL-mediated apoptosis in prostate cancer
cells by the bioactive compounds neobavaisoflavone and psoralidin
isolated from Psoralea corylifolia. Pharmacol Rep. 63:139–148.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bronikowska J, Szliszka E, Jaworska D,
Czuba ZP and Krol W: The coumarin psoralidin enhances anticancer
effect of tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL). Molecules. 17:6449–6464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gross A, Mcdonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|