Introduction
Galactomannans, being polysaccharides built up of a
β-(1–4)-D-mannan backbone with single D-galactose branches linked
α-(1–6), are present in the endosperm of numerous plants,
particularly the Leguminosae (1).
The ratios of mannose/galactose may differ according to the species
(2). Due to their different
physicochemical properties, galactomannans are frequently used in
the pharmaceutical, biomedical, cosmetics and food industries,
including as thickeners, gels, flocculating agents and stabilizing
agents (3,4). In addition, the bioactivities of
galactomannan have been demonstrated, including anticoagulation,
immune regulation, anti-ulcer, antiviral, blood sugar-lowering and
antioxidant activities (5–10).
For commercial purposes, galactomannans are
extracted primarily from Cyamopsis tetragonolobus L. (guar
beans), Ceratonia siliqua (locust beans) and Caesalpinea
spinosa (tara). However, the sources of these plants are
limited, and research has focused on the identification of
alternative sources (11).
Crotalaria mucronata Desv. is a common and productive herb
in the south of China. The seed endosperm of C. mucronata
has abundant galactomannan, which may be used as a potential
thickening agent (12). In the
present study, it was hypothesized that the viscous property of
Crotalaria galactomannan was associated with to spatial
structure. Therefore, it was necessary to elucidate the structure
of Crotalaria galactomannan.
Unlike the electron microscope, which provides a
two-dimensional projection of sample, the atomic force microscope
(AFM) is able to provide a three-dimensional surface profile.
Therefore, AFM is widely used in the spatial formation analysis of
different polymer chains, including DNA, proteins, polysaccharides
and other macromolecular chains (13–15).
In the present study, galactomannan isolated from C.
mucronata was analyzed by gas chromatography (GC) and AFM in
order to elucidate the association between the structure and
molecular properties of Crotalaria galactomannan.
Materials and methods
Materials
Mature seeds of C. mucronata were purchased
from the Traditional Chinese Medicine Market of Puning (Puning,
China). The authentication of plant materials was performed
morphologically by Dr Shou-Jun Guo (Hanshan Normal University,
Chaozhou, China). The samples were deposited in Department of
Biology, Hanshan Normal University.
Isolation and purification of
galactomannans
The seeds (20 g) were immersed in 100 ml boiling
water for 20 min, and subsequently incubated for 24 h at room
temperature (28°C). When the seeds had doubled in size, the
endosperm was manually collected following removal of the peel and
embryo. The endosperm was ground and homogenized for 24 h in 100 ml
water at 75°C. The viscous solution was centrifuged with 3,200 × g
for 30 min at 4°C and the supernatant was precipitated with 2X
(v/v) 95% ethanol. The precipitate was collected on a glass filter
(fine grade), and washed successively with 75% ethanol. Finally,
the polysaccharide precipitate was dried using a vacuum lyophilizer
and weighed.
Conditions for GC
GC experiments were performed on a Shimadzu GC-14A
gas chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped
with a DB-5MS column (30 m × 0.25 mm × 0.25 µm) and a flame
ionization detector, using a high purity of nitrogen as the carrier
gas at a flow rate of 8 cm3/min. The temperature was
heated from 180 to 240°C at 10°C/min, and kept at 240°C for 25 min.
The vaporizer temperature was 260°C and the detector temperature
was 240°C. The detector voltage was 350 V. An aliquot of 15 µl was
injected for each run. The quantitative analysis of D-mannose and
D-galactose by GC was optimized in a concentration range of
0.03–1.00 mg/ml. The calibration curve of D-mannose was
Y=0.3458X+0.1341 (r2=0.9921) and that of
D-galactose was Y=0.9541X-0.6234 (r2=0.9934).
Sample preparation for AFM
The purified Crotalaria galactomannan was
dissolved in deionized water at 90°C for 1 h using a magnetic
stirrer, and subsequently cooled to room temperature. The
concentrations of galactomannan solution were prepared at 1 and 2
µg/ml. A peeled-off mica slide was pretreated with 5 µl 10 mM NiNO3
for 1 min, followed by the addition of 1 or 2 µg/ml galactomannan
solution on the Ni+-treated mica surface, which was
air-dried at room temperature for 10 min. Finally, the mica slide
was rinsed with deionized water and dried, and was observed and
imaged using the AFM in tapping mode.
Conditions for AFM
The AFM instrument used was a Nanoscope III, a
multimode scanning probe microscope equipped with a type EV scanner
(Digital Instruments; Bruker Corporation, Billerica, MA, USA). The
tapping mode was employed by using etched silicon cantilever probes
of 224 µm nominal length, at a spring constant of 20–70 N/m and a
contact force of 3–4 nN. Images were obtained at ambient
temperature and humidity. For any given image, the height was
analyzed in at least three distinct regions of the structure using
Nanoscope software (version 5.12; Bruker Corporation). Section
analysis of AFM allows depth, height, width and angular
measurements to be taken.
Results
Morphology and structure of Crotalaria
galactomannan molecular chains
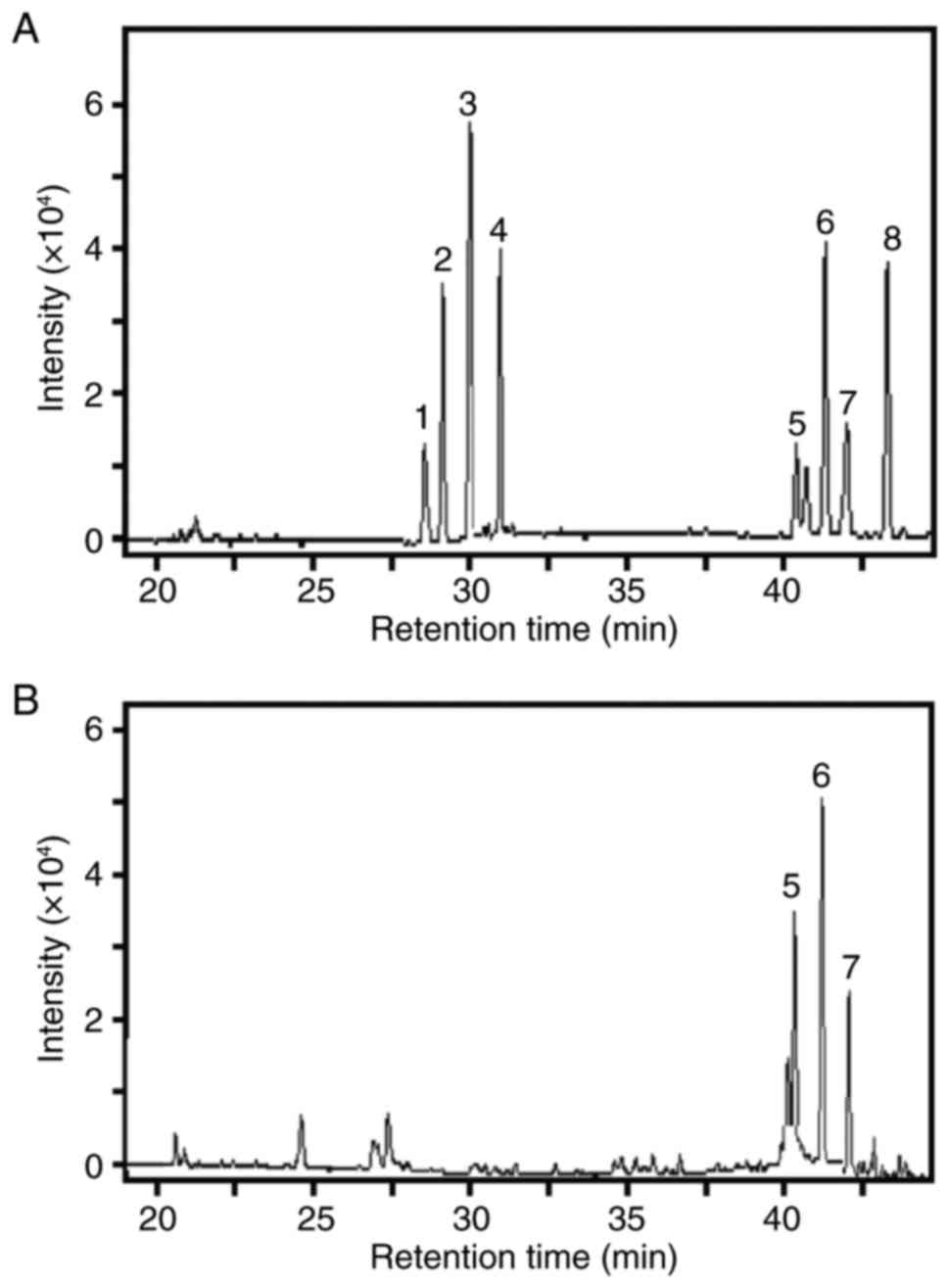
The GC profile of standard oligosaccharides was
revealed, including rhamnose, ribose, arabinose, xylose, inositol,
D-mannose, D-galactose and glucose (Fig. 1A).
Retention times indicated that galactomannan was
composed of D-mannose and D-galactose, and inositol served as an
internal control. The chirality of the sugars was confirmed from
these standard oligosaccharides (including D-mannose and
D-galactose) (Fig. 1B).
In the GC analysis, the calibration curves of
D-mannose and D-galactose were linear over a concentration range of
0.03–1.00 mg/ml. The GC method was precise, accurate and sensitive
enough for simultaneous quantitative evaluation of
mannose/galactose detection. Peak areas (n=3) of mannose/galactose
were used to calculate their contents, and the results indicated
that the ratio of D-mannose to D-galactose was ~2.375:1 in
galactomannan (Fig. 1B).
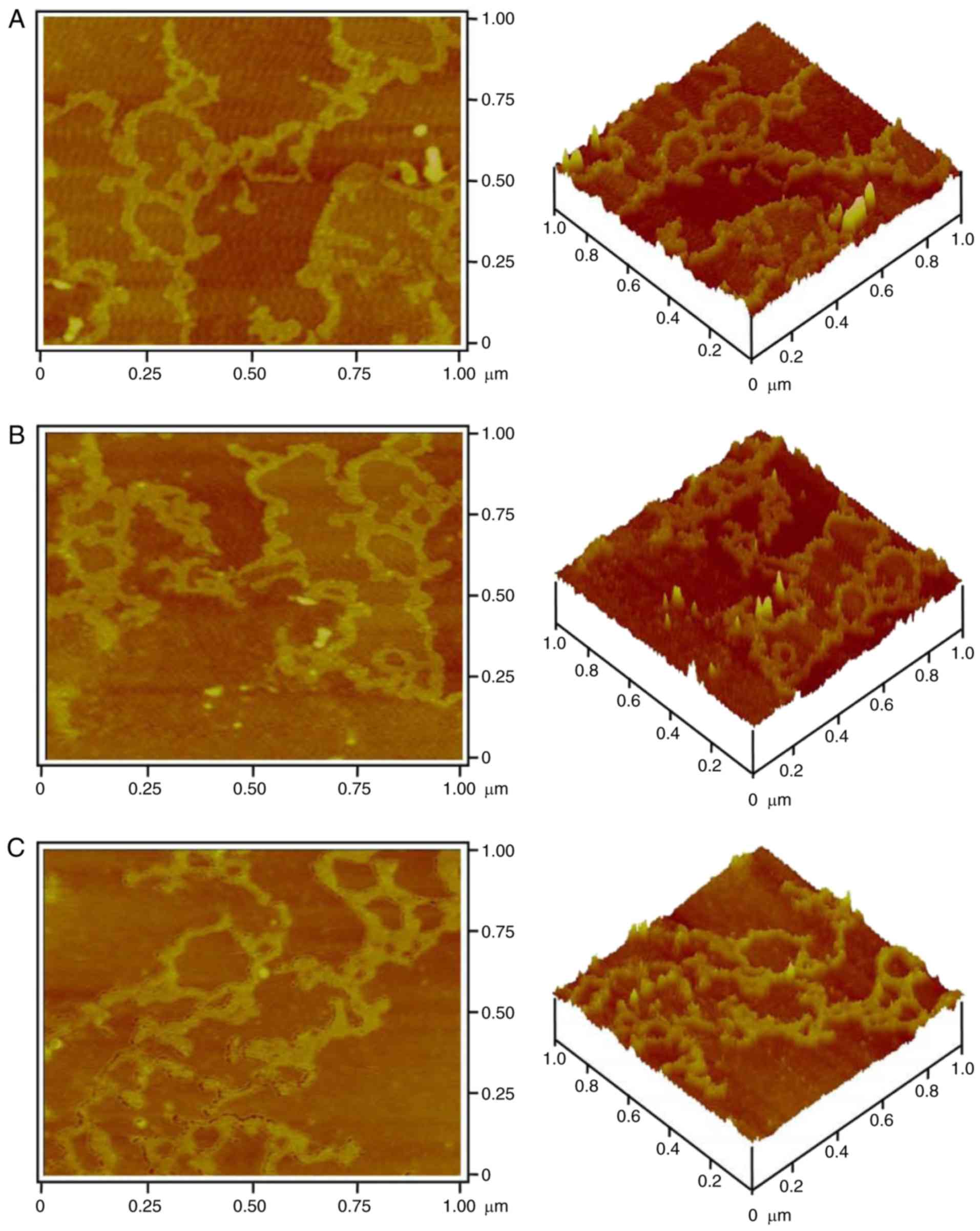
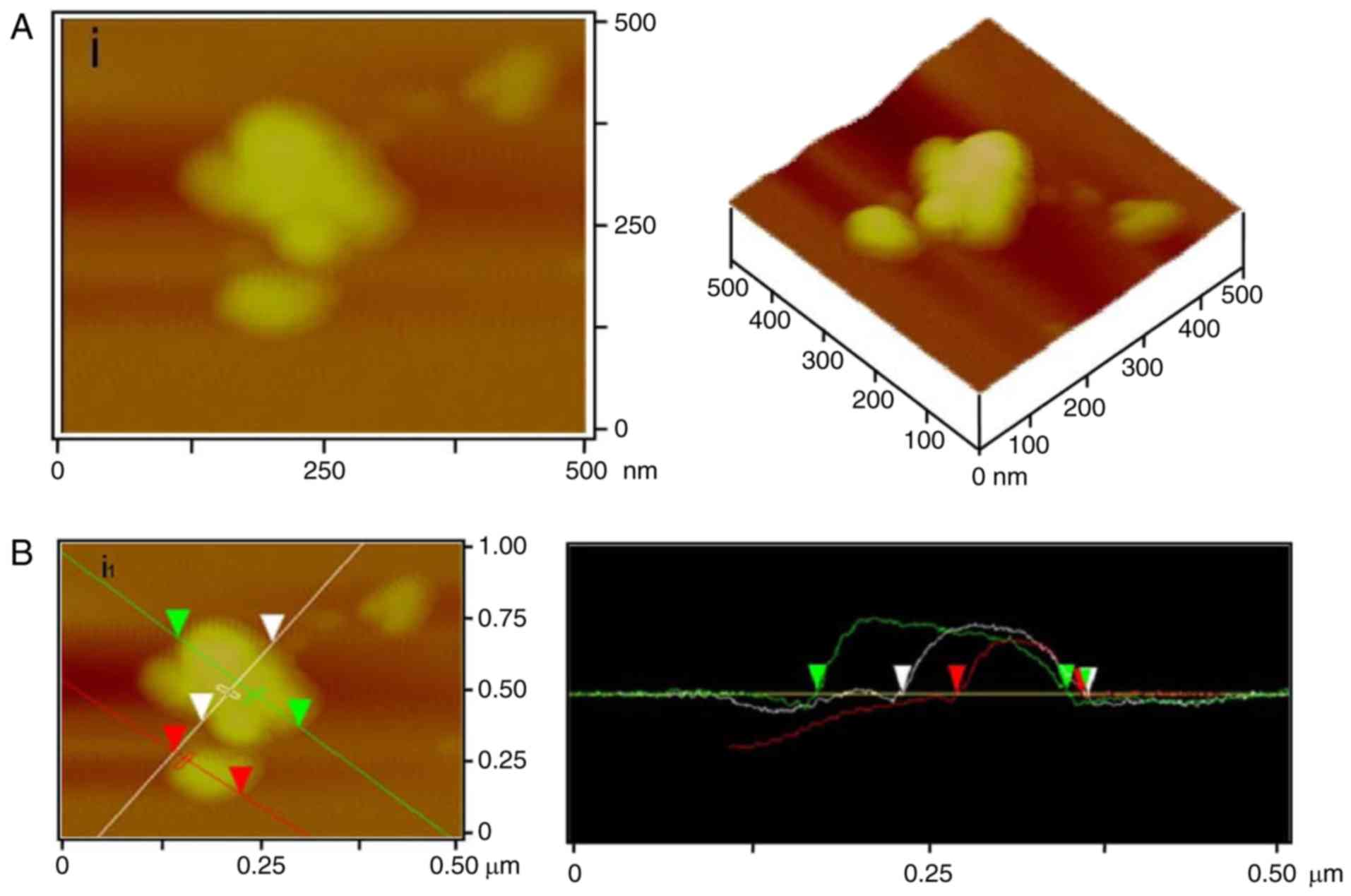
In order to reveal its molecular morphology, 1 µg/ml
galactomannan was analyzed by AFM. The two-dimensional and
three-dimensional images (Fig. 2)
demonstrated that the large molecular chains of polysaccharides
exhibited different structures containing numerous rings and
branches. The density of the molecular chains depended on the
initial concentration of polysaccharide and its deposition amount
on the mica chip. The contrast of the image depended on the force
on the probe tip of the AFM. Therefore, the optimal force for
obtaining a clear and stable three-dimensional image of the
polysaccharide was ~3–4 nN.
The single chains and multi-branches were observed
(Fig. 2A and B). These single
polysaccharide chains were able to intertwine to form polymerized
molecules. In these polymers, there were numerous different rings
that were created at different connections (Fig. 2A and B). In addition, coral-like
branches were observed (Fig. 2C).
These results indicated that Crotalaria galactomannan may be
a single chain containing ring and branch structures. The structure
of the single-chain molecule may be affected by interactive forces
and the environment; therefore, the appearance of the
polysaccharide molecule chain may be changeable. Additionally,
polysaccharides may entangle with each other to form the coil
structure, finally forming a ring and a short rod-like branch.
Single-molecule structure of
Crotalaria galactomannan
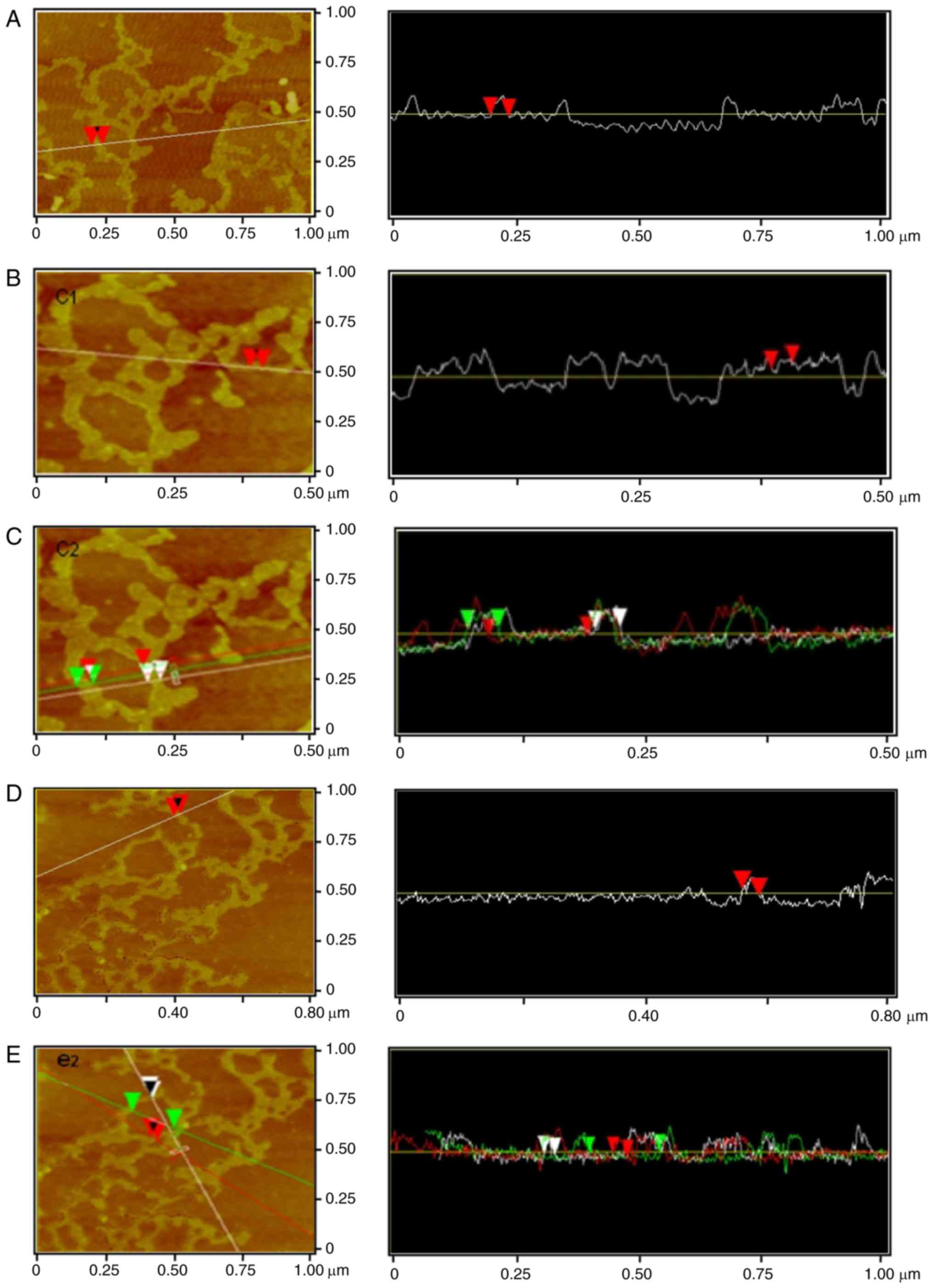
Via software analysis, Crotalaria
galactomannan was observed to form a single chain ring (Fig. 3A) of 37.34-nm surface width,
37.14-nm horizontal width and 0.10-nm thickness.
Galactomannan was able to form a short single-chain
with rod-like branches (Fig. 3B);
the surface width of the rod-like branch was 21.62 nm, the
horizontal width of the branch was 21.48 nm and the thickness of
the branch was 0.27 nm.
Crotalaria galactomannan may constitute a
ring structure with a 101.98-nm diameter (Fig. 3C). The single chains exhibited
different properties: On the left side, the surface width was 31.50
nm, the horizontal width was 31.25 nm and the thickness was 0.10
nm; on the other side, the surface width was 25.80 nm, the
horizontal width was 25.39 nm and the thickness was 0.11 nm.
The software analysis indicated that
Crotalaria galactomannan single-chain rod-like branches had
a surface width of 27.85 nm, a horizontal width of 27.34 nm and a
thickness of 0.54 nm (Fig. 3D).
Galactomannan was additionally able to form a single chain ring
with a diameter of 168.76 nm (Fig.
3E), and a single chain ring with a surface width of 33.61 nm,
a horizontal width of 33.20 nm and a thickness of 0.26 nm; in
addition, there was another single chain with a surface width of
27.29 nm, a horizontal width of 25.39 nm and a thickness of 0.09
nm.
Spiral-coil structure of Crotalaria
galactomannan
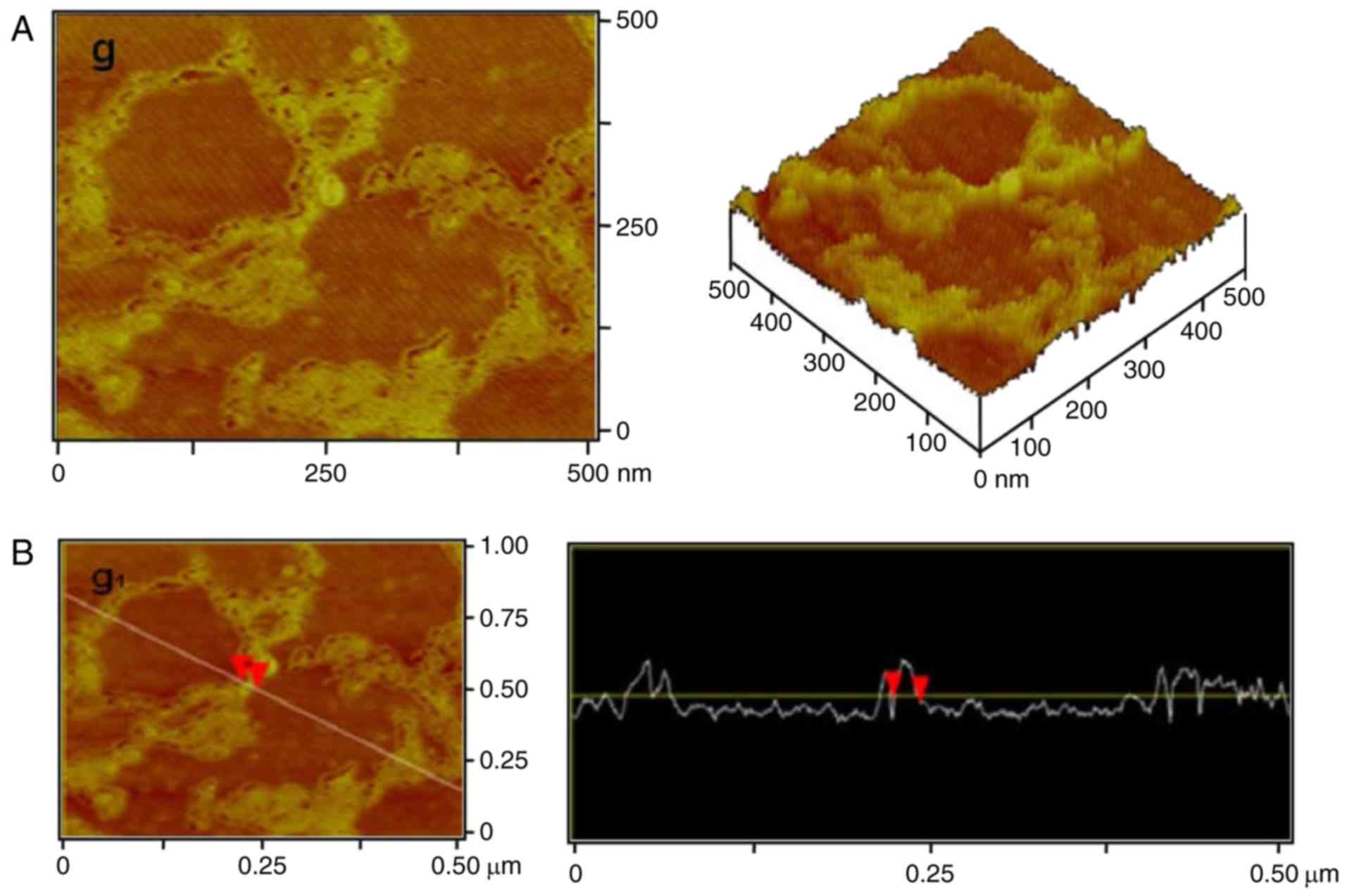
A high-resolution image of Crotalaria
galactomannan is presented in Fig.
4A. The molecular chain winding and spiral-coil structure was
clearly observed. The surface width of the coil was 22.86 nm, the
horizontal width was 22.46 nm and the thickness was 0.16 nm
(Fig. 4B).
The high-resolution image (Fig. 4B) demonstrated that the
single-stranded molecule of the polysaccharide formed an entangled
ring and the intramolecular ring of the spiral-coil structure.
Through the high resolution and software analysis, polysaccharide
single-chains were observed form a ring structure of molecules with
a winding diameter of ~50–170 nm and a single-chain length of
1.2–1.5 µm (Fig. 4B).
Macromolecular-aggregation structure
of Crotalaria galactomannan
In order to reveal the molecular aggregation of
Crotalaria galactomannan, 2 µg/ml galactomannan was analyzed
by AFM, producing two-dimensional and three-dimensional images
(Fig. 5). The results demonstrated
that the different-sized polysaccharide macromolecules were able to
wind and form spherical aggregates. There were two spherical
aggregations which were observed in the images in Fig. 5A. The diameter of the small
aggregation was 110.45 nm and its thickness was 0.086 nm, while the
length of large aggregation was 216.10 nm, its width was 161.79 nm
and the thickness was 0.12 nm. The average thickness of the
spherical aggregations was 0.10 nm (Fig. 5B).
This aggregation unit of polysaccharide may be
maintained by hydrogen bonds and van der Waals forces, which were
the principal components of the spherical structure. In this case,
two single-molecule chains may interact with each other to form the
double helix of polysaccharide. Additionally, the larger spherical
aggregations, higher-level structures, may be composed of four or
more globular units of polysaccharide with hydrogen bonds and van
der Waals forces.
Discussion
The three-dimensional structures of polysaccharide
are considered to be one of the determinants of their
bioactivities, and it has been hypothesized that the high-level
structure of a polysaccharide may be more important than the
primary structure (16,17). Therefore, it is of importance to
study the spatial association between single-chain polysaccharide
fragments and their conjugates. The conformational aspects of a
polysaccharide include the conformation of each monosaccharide, the
orientation of the monosaccharides with respect to each other and
the flexibility of the spatial structure, which is defined by
dihedral angles, torsion angles around glycosidic bonds,
inter-atomic distances and dynamic parameters (17). For Crotalaria galactomannan,
the secondary- and higher-level structures in solution are not
readily defined, due to their inherent flexibility. The
characterization of polysaccharide dynamics by experimental and
theoretical means remains an area of active research. With the
development of high resolution instrumental processes, including
various light scattering techniques (light scattering, X-ray and
neutron scattering), X-ray diffraction analysis, small-angle
neutron scattering, AFM and high-resolution nuclear magnetic
resonance spectroscopy, it may be possible to study the
conformation and three-dimensional structure of a polysaccharide at
the molecular level (17). The
present study used AFM to simulate and visualize the
three-dimensional structure of Crotalaria galactomannan.
In AFM observations of Crotalaria
galactomannan, the measured values were increased compared with the
estimated values for the polysaccharide molecules, which was due to
the broadening effect that occurred from scanning different parts
of the polysaccharide and the molecular chain. AFM observations of
galactomannan may illustrate the structure of a single molecular
chain and its various side-chain branches. In addition, the
molecular chains may entangle with each other via different
interactions, leading to numerous sizes of ring structure,
intramolecular spiral structure and spiral-coil structure. The
results of the present study thereby demonstrated that the
polysaccharide Crotalaria galactomannan exhibited numerous
branches.
Multi-strand branches of polysaccharides are tightly
arranged and mutually cross-linked, and this type of structure is
maintained by intermolecular van der Waals forces and hydrogen
bonds (17). Micelle formation
results from the effects of the intramolecular force and the
presence of micelles adjacent to the polysaccharide chain are
conducive to the formation of polysaccharides via hydrogen bonding.
A large amount of rod-like polysaccharide scattering was observed
on the mica surface in the present study. This rod-like
polysaccharide exhibited different lengths, and interacted to form
colloidal network structures. The spherical aggregates of
polysaccharide may be linked by hydrogen bonding and van der Waals
forces. Hydrogen bonds and van der Waals forces could drive two
single-molecule chains to associate with a double-helical coil and
to form an aggregate. A total of four aggregates with a
double-stranded helix globular structure may in turn form a large
spherical aggregate of polysaccharides.
In conclusion, the results of the present study
demonstrated that Crotalaria galactomannan was composed of
one single D-mannose chain and a number of D-galactose branches,
and that the D-mannose/D-galactose ratio was 2.375:1. This chain
was able to intra-molecularly entangle itself into a helix
containing numerous ring structures of different sizes. A group of
galactomannan polysaccharides maybe linked together via hydrogen
bonding and van der Waals forces to form aggregations with small
rings or spiral windings.
Acknowledgements
The present study was supported by National Natural
Science Foundation of China (grant no. 81202907) and the National
Natural Science Foundation of Guangdong Province (grant nos.
2012B050300025 and 2014A020221058).
Glossary
Abbreviations
Abbreviations:
|
AFM
|
atomic force microscope
|
|
GC
|
gas chromatography
|
References
|
1
|
Stephen AM, Phillips GO and Williams PA:
Food Polysaccharides and Their Applications. Marcel Dekker, Inc.;
New York: pp. 155–186. 1995
|
|
2
|
Kök MS, Hill SE and Mitchell JR: Viscosity
of galactomannans during high temperature processing: Influence of
degradation and solubilisation. Food Hydrocolloid. 13:535–542.
1999. View Article : Google Scholar
|
|
3
|
Srivastava M and Kapoor VP: Seed
galactomannans: An overview. Chem Biodivers. 2:295–317. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vieira ÍGP, Mendes FNP, Gallão MI and de
Brito ES: NMR study of galactomannans from the seeds of mesquite
tree [Prosopis juliflora (Sw) DC]. Food Chem. 101:70–73. 2007.
View Article : Google Scholar
|
|
5
|
Hussein MM, Helmy WA and Salem HM:
Biological activities of some galactomannans and their sulfated
derivatives. Phytochemistry. 48:479–484. 1998. View Article : Google Scholar
|
|
6
|
Zia T, Hasnain SN and Hasan SK: Evaluation
of the oral hypoglycaemic effect of Trigonella foenum-graecum L.
(methi) in normal mice. J Ethnopharmacol. 75:191–195. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pandian RS, Anuradha CV and Viswanathan P:
Gastroprotective effect of fenugreek seeds (Trigonella foenum
graecum) on experimental gastric ulcer in rats. J Ethnopharmacol.
81:393–397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bin-Hafeez B, Haque R, Parvez S, Pandey S,
Sayeed I and Raisuddin S: Immunomodulatory effects of fenugreek
(Trigonella foenum graecum L.) extract in mice. Int
Immunopharmacol. 3:257–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woodgate DE and Conquer JA: Effects of a
stimulant-free dietary supplement on body weight and fat loss in
obese adults: A six-week exploratory study. Curr Ther Res.
64:248–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai F, Wen Q, Li L, Wu H and Li X:
Antioxidant activities of water-soluble polysaccharide extracted
from mung bean (Vigna radiata L.) hull with ultrasonic assisted
treatment. Carbohyd Polym. 81:323–329. 2010. View Article : Google Scholar
|
|
11
|
Joshi H and Kapoor VP: Cassia grandis
Linn. f. seed galactomannan: Structural and crystal lographical
studies. Carbohyd Res. 338:1907–1912. 2003. View Article : Google Scholar
|
|
12
|
Guo SJ, Yang YL, Zhang QM, She JB, Zhu YC
and Hong JJ: Optimization of polysaccharide extraction technology
from Crotalaria mucronata Desv. Seeds. Food Sci. 30:264–267.
2009.
|
|
13
|
Yi WS, Qin LH and Cao JB: Investigation of
morphological change of green tea polysaccharides by SEM and AFM.
Scanning. 33:450–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fuentes-Perez ME, Gwynn EJ, Dillingham MS
and Moreno-Herrero F: Using DNA as a fiducial marker to study SMC
complex interactions with the atomic force microscope. Biophys J.
102:839–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palacio ML, Schricker SR and Bhushan B:
Block copolymer arrangement and composition effects on protein
conformation using atomic force microscope-based antigen-antibody
adhesion. J Biomed Mater Res A. 100:978–988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pérez S, Mazeau K and Hervé du Penhoat C:
The three-dimensional structures of the pectic polysaccharides.
Plant Physiol Bioch. 38:37–55. 2000. View Article : Google Scholar
|
|
17
|
Zhang M, Cui SW, Cheung PCK and Wang Q:
Antitumor polysaccharides from mushrooms: A review on their
isolation process, structural characteristics and antitumor
activity. Trends Food Sci Tech. 18:4–19. 2007. View Article : Google Scholar
|