Introduction
Aseptic loosening induced by wear particles has
become one of the most critical contributors to arthroplasty
failure (1). Wear particles are
debris from joint replacement implants that are able to induce
inflammation and bone resorption at the interface between the
prosthesis and its adjoining bone (2,3).
Various measures have been used for the prevention and treatment of
aseptic loosening. Strontium ranelate (SR) is an anti-osteoporotic
drug, and has the potential to reduce the risk of spinal and hip
fractures in postmenopausal women (4). SR is able to promote the
proliferation of pre-osteoblasts, suppress the production and
activity of osteoclasts, and increase osteoclast apoptosis
(5,6). Therefore, SR may be considered to be
a potential treatment for aseptic loosening.
Receptor activator of nuclear factor-κB ligand
(RANKL) is secreted by osteoblasts and other cell types, including
endothelial and active T cells (7,8), and
various inflammatory factors may stimulate its secretion (9–11).
Upon binding to its membrane receptor (RANK), RANKL activates the
nuclear factor (NF)-κB signaling pathway and induces osteoclast
differentiation, inhibits osteoclast apoptosis, and promotes
osteoclast adhesion to the bone surface (12–14).
Osteoprotegerin (OPG), a soluble competitive decoy receptor for
RANK, is able to inhibit the NF-κB signaling pathway by interfering
with the RANKL-RANK interaction (11,15).
OPG is secreted by a number of types of cells, including
osteoblasts and mesenchymal stem cells (16). The interaction between OPG, RANKL
and RANK, therefore, may serve an essential role in the regulation
of bone metabolism (17–19).
The present study aimed to investigate whether
treatment with SR may inhibit aseptic loosening in an experimental
mouse model that simulates artificial joint replacement, and
reflects the interaction between wear particles and periprosthetic
tissues (20), and to examine the
potential biochemical mechanisms of action of SR.
Materials and methods
Preparation of wear particles
Unmixed titanium (Ti) particles (Zimmer Biomet,
Warsaw, IN, USA) with an average size of 5 µm were used. Prior to
injection, the particles were rinsed in 70% ethanol for 48 h at
room temperature, washed twice in PBS, and autoclaved at 180°C for
6 h to remove endotoxins. A commercial detection kit (E-Toxate;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to test
whether the treated wear debris contained endotoxins or not
(21).
Animal experiment
A total of 45 10-week-old female C57BL/6J mice, each
weighing 20±2 g, were used in the present study. All mice were
maintained with pressure-controlled ventilation at a constant
temperature of 25°C and a relative humidity of 40–70% in a 12/12-h
light/dark cycle, and were given lab chow and water ad
libitum. The study protocol was approved by the Animal Ethics
Committee of Ningxia Medical University (Yinchuan, China).
Animal experiments were performed as previously
described (20). In all mice, an
intraperitoneal injection of Nembutal (0.6% pentobarbital sodium)
was given to induce general anesthesia, and the murine joint
prosthesis model was established in the right lower extremities.
Under sterile conditions, the tibial plateau was exposed through
the medial parapatellar approach and one Ti pin was gently
implanted into the proximal tibia, with the pin head being
maintained in the same plane as the tibial plateau surface. The
skin incision was washed with normal saline containing 100 U/ml
penicillin and 100 mg/ml streptomycin, and each layer was
separately closed with absorbable sutures (20). Prior to surgically inserting the Ti
pin, the mouse tibial canal was injected with 10 µl Ti suspension
(4×104 particles of Ti in normal saline). Subsequently,
every 2 weeks following surgery, 20 µl Ti particles were injected
into the joint capsule at week 2, 4, 6, 8, 10 and 12. Mice were
randomly divided into three groups for treatment with SR (S12911-2;
PROTELOS®; Servier, Stoke Poges, UK): Control group
(joint prosthesis only), SR625 group (joint prosthesis and SR at a
dose of 625 mg/kg/day), and SR1800 group (joint prosthesis and SR
at a dose of 1,800 mg/kg/day). A total of 7 days post-surgery, mice
were given SR via intragastric gavage. Animals were treated
consecutively for 12 weeks and were sacrificed for histological
analysis, immunohistochemical (IHC) analysis, Ti prosthesis
steadiness examination and micro-computed tomography (µCT)
analysis.
Pullout test to assess Ti prosthesis
steadiness
Following sacrifice, the tibia containing the Ti pin
was removed (20). To expose the
Ti pin head, all muscles and tissues around the bone were carefully
removed. Each bone was fixed to a special clamp using dental
cement, which was designed to align the long axis of the implant
with the long axis of the HP-100 Control Electronic Universal
Testing Machine (Yueqing Zhejiang Instrument Scientific Co., Ltd).
With the position of the mouse limb and the custom fixture
controlled, the pin was pulled out of the tibial canal at a rate of
2.0 mm/min. Load data were recorded using automatic software
(Edburg version 1.0; Yueqing Instrument Co., Ltd., Yueqing,
China).
µCT scans
Following removal of all soft tissues, tibias from
four mice per group were fixed in 4% paraformaldehyde, at 4°C for 4
weeks. The fixed shin bones were scanned by µCT (SkyScan 1176;
Bruker microCT, Kontich, Belgium) at a resolution of 9 µm. The µCT
scans were acquired at a 900-ms exposure time, 45-kW voltage and
550-mA current. Automatic data analysis software (NRecon version
1.1.11; Bruker microCT) was used to reconstruct and acquire images
based on the µCT analyses, and to determine the bone volume
fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number
(Tb.N), bone volume (BV), and specific bone surface (BS/BV) of the
shin bone surrounding the Ti pin. All horizontal cutting images
were captured at two-fifths of the titanium nail, which was 2 mm
from the lower edge of the top hat.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocol. The 260/280 absorbance
ratio was measured to verify RNA purity (NanoDrop; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). First strand cDNA was
synthesized with 1 µg total RNA using the RevertAid First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). A total of 2
µl cDNA was used for each PCR mixture, containing SYBR®
Premix Ex Taq™ II (Tli RNaseH Plus; Takara Biotechnology Co., Ltd.,
Dalian, China). The reaction was subjected to a 40-cycle
amplification of 95°C for 30 sec, 95°C for 5 sec, and 60°C for 30
sec. The relative mRNA expression of selected genes was normalized
to GAPDH and quantified using the 2−ΔΔCq method
(22).
PCR primers used in the present study were: RANKL
forward, 5′-TCCTGAGCCTCCATGAAAACG-3′ and reverse,
5′-CCCACACTGTGTTGCAGTTC-3′; OPG forward,
5′-TGAAGTACCGGAGCTGTCCCC-3′ and reverse,
5′-AGGCCATATGTGCTGCAGTTCG-3′; and GAPDH forward,
5′-TTGTCAAGCTCATTGGGCTCATTT-3′ and reverse,
5′-GCCATGTAGGTCCACCCATG-3′.
Histopathological and IHC
analysis
The tibia was fixed in 4% paraformaldehyde for 24 h
at 4°C, and immersed in EDTA solution for decalcification. The
samples were dehydrated in a graded series of ethanol followed by
xylene, prior to being embedded in paraffin at 60°C. Sections (5
µm) were cut perpendicular to the long axis of the tibia using an
RM2235 Rotary Microtome-Basic Instrument (Leica Microsystems, Inc.,
Buffalo Grove, IL, USA). Sections were stained with hematoxylin and
eosin (H&E) for histomorphometric analysis: 0.5% water-soluble
Eosin for 5 min at 23°C and Hematoxylin for 3 min at 23°C. IHC
staining for OPG and RANKL was implemented to assess the activity
of osteoclastogenesis. EDTA was preheated to 60°C in a pressure
cooker, then glass slides added to the autoclave for 2 min, then
allowed to cool for 20 min. Following washing with PBS, the slides
were incubated at 23°C for 10 min with 3% hydrogen peroxide, washed
again with PBS and incubated with primary antibodies overnight at
4°C. The primary antibodies used were: Rabbit polyclonal anti-OPG
(cat. no. ab183910; 1:300; Abcam, Cambridge, UK); and rabbit
polyclonal anti-RANKL (cat. no. ab9957; 1:300; Abcam). To exclude
the possibility of nonspecific staining, negative controls were
performed with PBS instead of primary antibodies. Then the slides
were incubated with secondary antibodies (Enzyme-labeled goat
anti-rabbit IgG polymer) part of the PV-9001 kit (Sino Biological,
Beijing, China) at 23°C for 40 min. Standardized IHC images were
obtained with a microscopic imaging system (DM2000 LED; Leica
Microsystems, Inc.), and positive expression was calculated using
Image-Pro Plus version 6.0 software (Media Cybernetics Inc.,
Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Results were analyzed by one-way analysis of variance, among the
three groups. The least significant difference post-hoc test was
performed for the distinction of means between different groups.
P<0.05 was considered to indicate a statistically significant
difference. SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
statistical analysis.
Results
Treatment with SR increases the
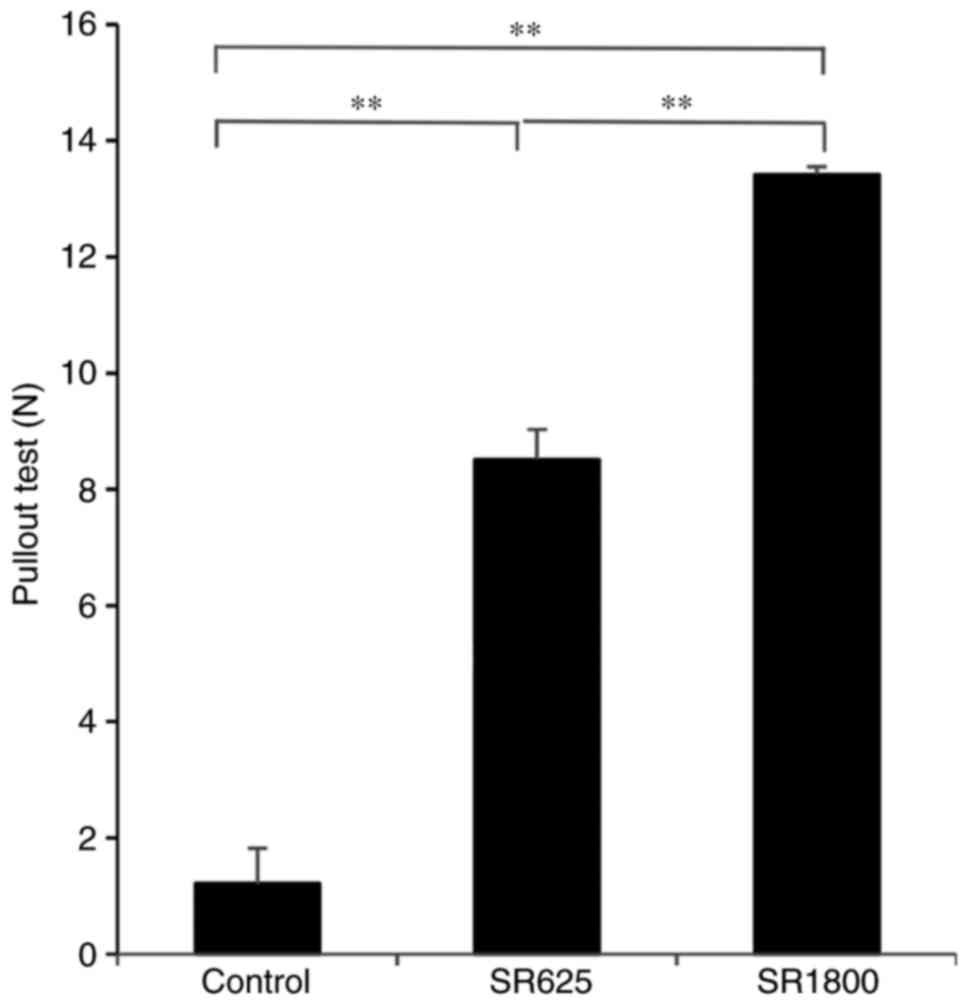
pulling force of the Ti pin
The average pulling load was 1.21±0.61 N for the
control group. Compared with the control group, significant
increases in pulling force were detected in the SR625 group
(8.51±0.52N, P<0.01) and SR1800 group (13.42±0.13N, P<0.01).
A significant difference in pulling load was additionally observed
between the SR625 and SR1800 groups (P<0.01; Fig. 1).
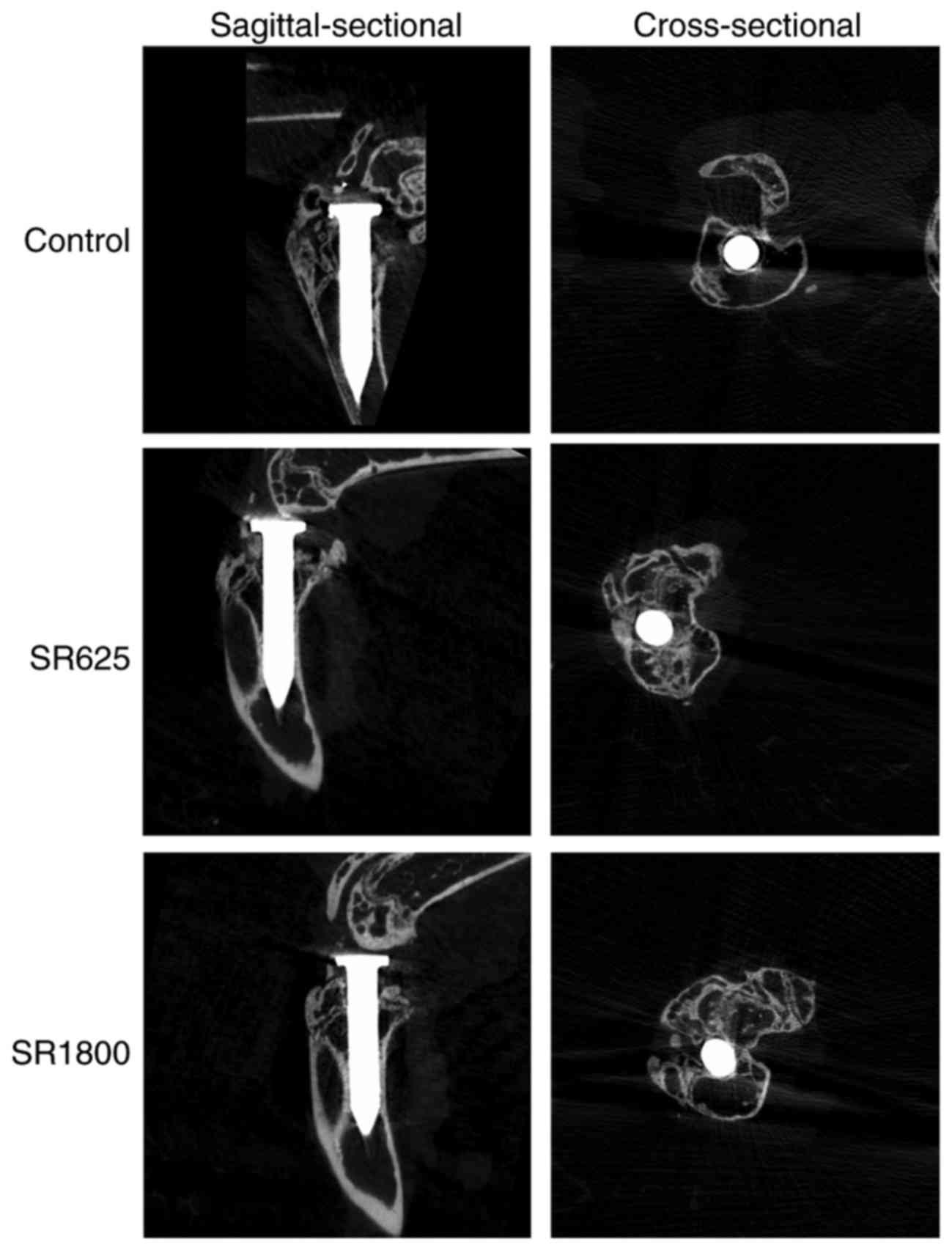
Treatment with SR improves bone
microstructure around the prosthesis
µCT scanning demonstrated differences in the bone
microstructure among the three groups. Osteolysis around the
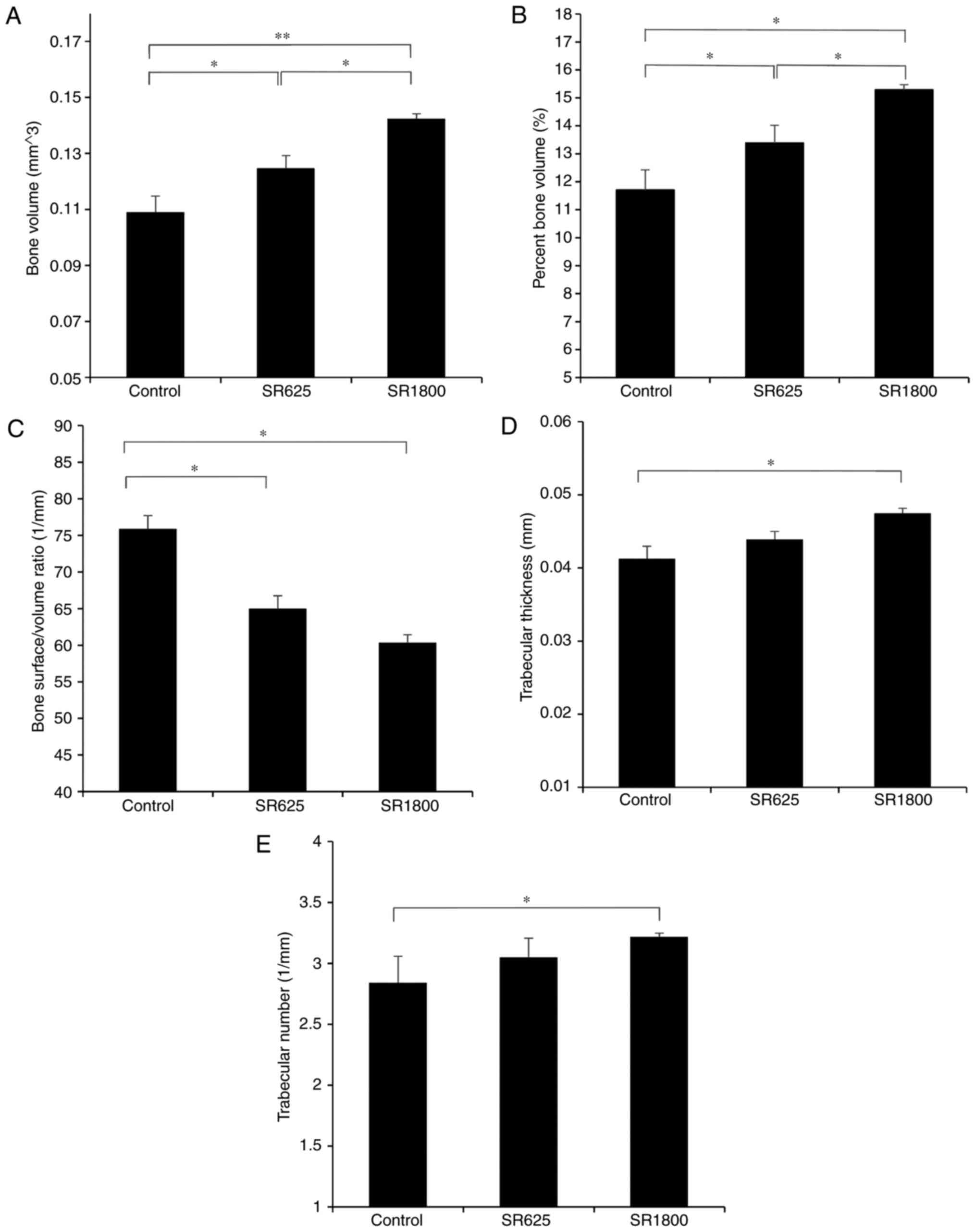
control group pin was most marked (Fig. 2). Tb.Th, Tb.N, BS/BV, BV and BV/TV
data were obtained from µCT analysis of the region of interest.
Compared with the control group (11.709±0.720%), BV/TV was
significantly increased in the SR625 group (13.390±0.628%, P=0.048)
and SR1800 group (15.288±0.184%, P=0.002) in a dose-dependent
manner (P=0.031). Similarly, compared with the control group
(0.109±0.006 mm3), BV was significantly increased in the
SR625 group (0.125±0.004 mm3, P=0.048) and SR1800 group
(0.142±0.002 mm3, P=0.002) in a dose-dependent manner
(P=0.031). Conversely, compared with the control group (75.89±1.82
1/mm), a significant decline in BS/BV was observed in the SR625
group (64.98±1.77 1/mm, P=0.005) and SR1800 group (60.36±1.06 1/mm,
P=0.001), although without a dose-dependent effect (P=0.123).
Additionally, compared with the control group
(0.041±0.001 mm), Tb.Th was increased in the SR625 group
(0.043±0.001 mm, P=0.175) and significantly increased in the SR1800
group (0.047±0.001 mm, P=0.011; Fig.
3D). Furthermore, compared with the control group (2.841±0.218
1/mm), Tb.N was increased in the SR625 group (3.050±0.157 1/mm,
P=0.154) and significantly increased in the SR1800 group
(3.219±0.027 1/mm, P=0.028; Fig.
3E).
Treatment with SR increases OPG
expression and decreases RANKL expression in the periprosthetic
tissue
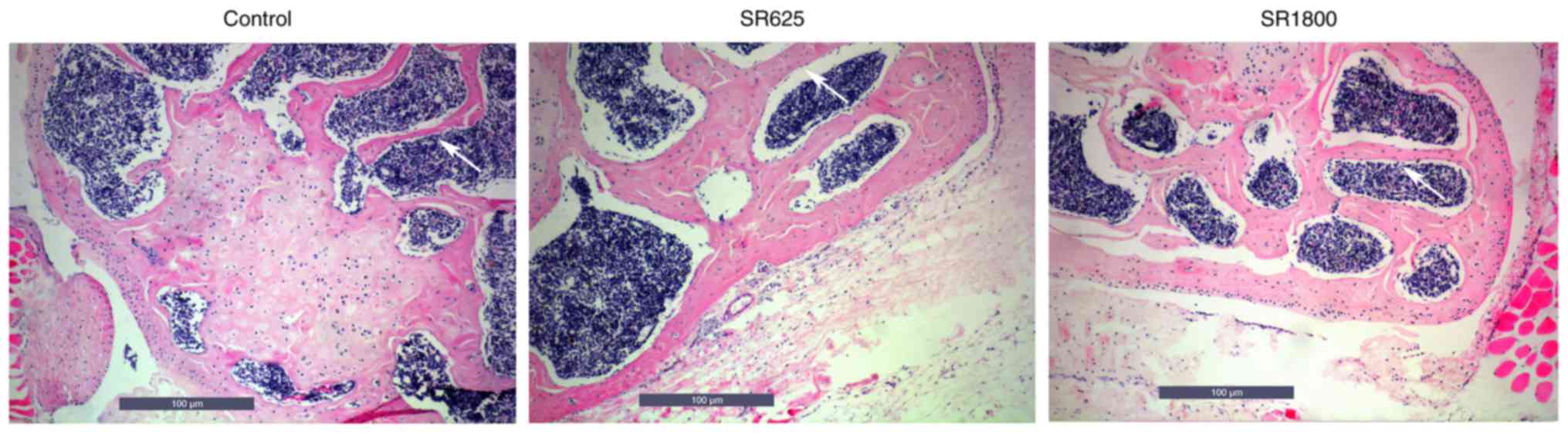
H&E staining indicated areas of bone resorption
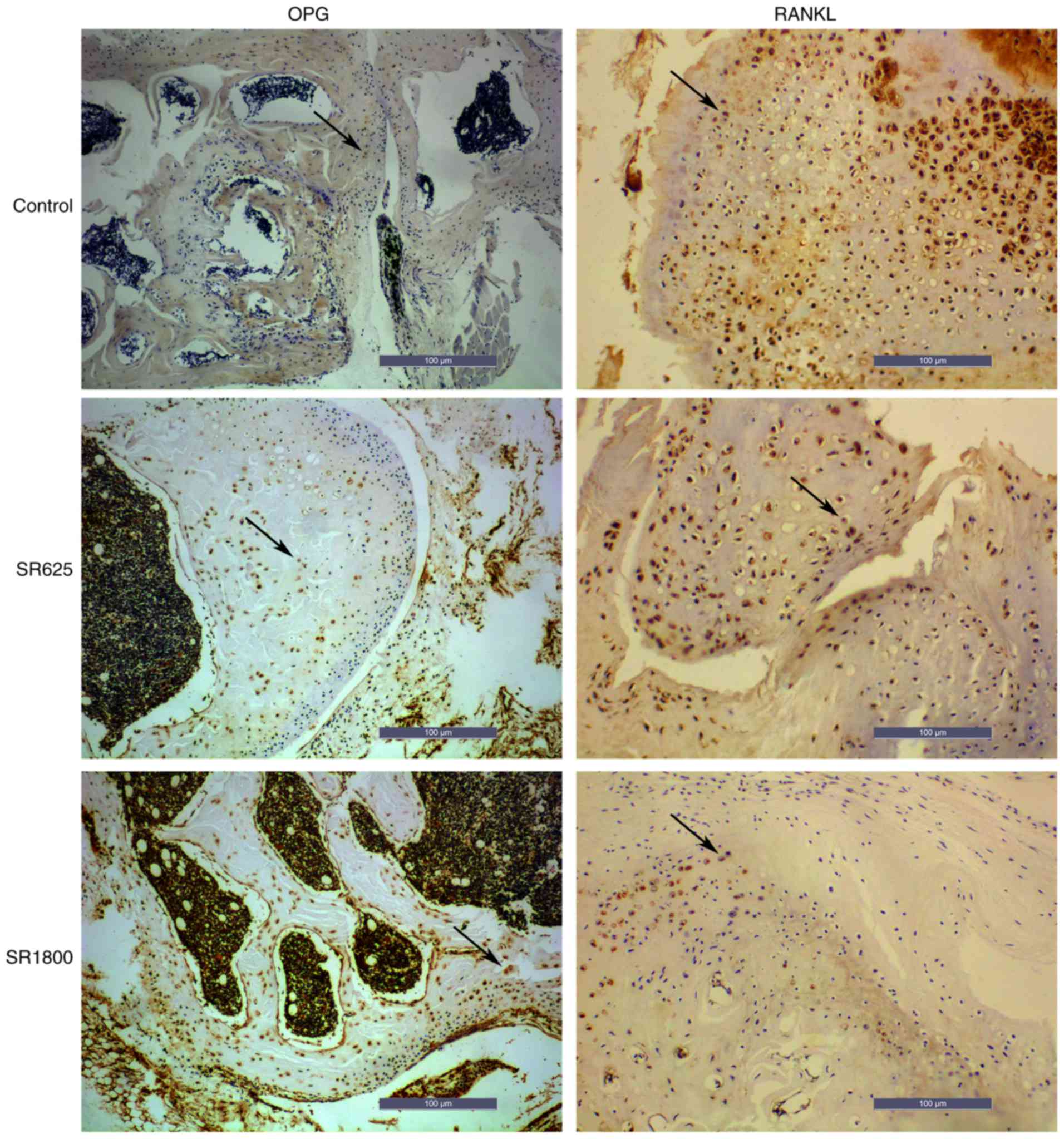
(Fig. 4). IHC was used to detect
the expression of OPG and RANKL in all groups. Fig. 5 illustrates the expression of OPG
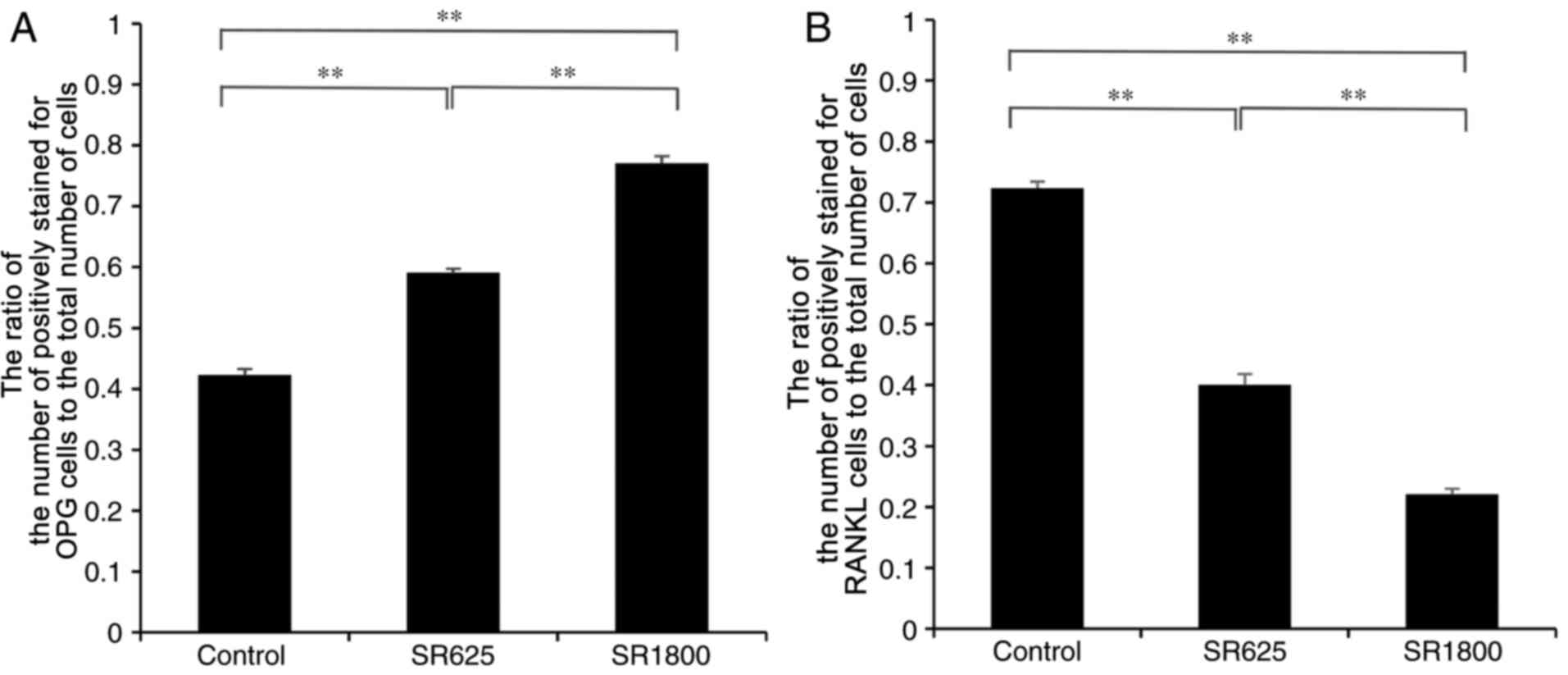
and RANKL in the bone around prosthesis. Compared with the control
group, the expression level of OPG was significantly increased in
the SR1800 group (0.422±0.010 vs. 0.770±0.012, respectively;
P<0.001; Fig. 6A) and the
expression levels of RANKL were significantly decreased
(0.723±0.011 vs. 0.221±0.009, respectively; P<0.01; Fig. 6B). Similarly, compared with the
control group, expression levels of OPG were significantly
increased in the SR625 group (0.422±0.010 vs. 0.590±0.007,
respectively; P<0.01; Fig. 6A)
and levels of RANKL were significantly decreased (0.723±0.011 vs.
0.400±0.018, respectively; P<0.01; Fig. 6B). In addition, the expression
levels of OPG and RANKL were significantly increased and decreased,
respectively, to a greater extent in the SR1800 group compared with
the SR625 group (P<0.01).
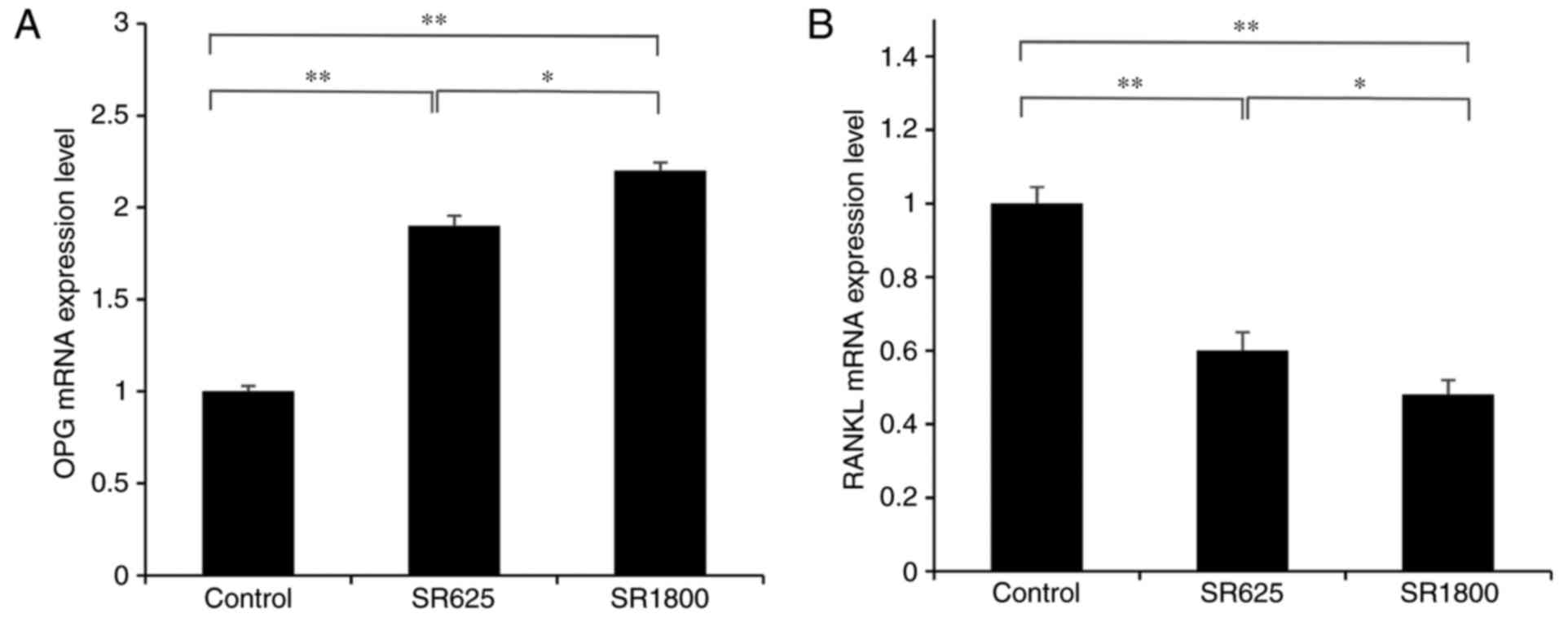
RT-qPCR analysis of OPG and RANKL in periprosthetic
tissues demonstrated that, compared with the control group, the
expression of OPG was significantly upregulated and the expression
of RANKL was significantly decreased in response to treatment with
SR (P<0.01). Additionally, this effect was significantly
enhanced in the SR1800 group compared with the SR625 group
(P<0.05; Fig. 7).
Discussion
Total knee arthroplasty is an effective and reliable
treatment for the terminal stage of knee arthritis. Following
surgery, symptoms may effectively be controlled and join function
restored (23–25). Aseptic loosening is one of the
long-term complications of total joint replacement and is an
important factor affecting the success rate of joint replacement.
The pathogenesis of aseptic loosening is not clear, although
previous studies indicated that an imbalance of osteogenesis and
osteolysis around the prosthesis is the root cause (5,6).
In the present study, the pulling force to remove
the Ti implant from the bone was enhanced following treatment with
SR in a dose-dependent manner. This finding supports the idea that
SR may be potentially effective against bone resorption. In
accordance with the above, Liu et al (26) demonstrated that BV and BV/TV were
significantly increased following treatment with SR. In another
study by Lu et al (27),
following treatment with SR, Tb.Th, bone density and BV/TV were
significantly enhanced compared with the control. However, no
significant differences in bone mineral density were noted between
the treatment groups and the control. In the present study, BV and
BV/TV around the periprosthetic tissue were significantly different
between the control group and treatment groups. In addition, BV/TV
was observed to be negatively associated with the dose of SR. SR
dose did not significantly affect BS/BV in the present study.
An aim of the present study was to determine the
effect of SR on Tb.Th and Tb.N in mice with periprosthetic
osteolysis. There were no statistically significant differences in
Tb.Th or Tb.N between the SR625 group and the control group.
However, significant differences in these parameters were observed
between the SR1800 group and the control group. These results
indicated that SR was able to increase BV, BS/BV (though not
significantly), BV/TV, Tb.N and Tb.Th following aseptic loosening
induced by wear particles, suggesting that SR may inhibit the
development of aseptic loosening. µCT and H&E staining
indicated that SR significantly reduced bone osteolysis compared
with the control group. In this experiment, the bone formation rate
was not measured, which is a limitation of the present study and
requires investigation in the future.
In agreement with previous studies (3,28),
it was demonstrated that SR significantly decreased the level of
RANKL and increased the secretion of OPG. The ratio of OPG to RANKL
serves an important role in the balance of bone mass and bone
metabolism (29–32). The homeostasis between bone
formation and resorption is essential for the regulation of bone
mass (33–36). Osteoclasts are responsible for
dynamic bone resorption, and their differentiation and apoptosis
are regulated by the ratio of OPG to RANKL (37,38).
The binding of RANKL to RANK may be prevented by OPG, therefore the
concentration of OPG and RANKL has an important influence on bone
resorption (39,40). The present study demonstrated that
OPG and RANKL were significantly upregulated and downregulated,
respectively, in the SR groups compared with the control group, at
the mRNA and protein level. These findings support a key role of SR
in inhibiting the differentiation of osteoclasts by regulating the
ratio of RANKL/OPG in the aseptic loosening model.
It may be noted that previous studies have reported
serious side effects with SR, such as Stevens-Johnson syndrome and
toxic epidermal necrolysis (41,42)
although these were not observed in the present study. Topical
application of SR is a promising method (43). Prostheses coated with SR may be
able to inhibit aseptic loosening (44,45).
In conclusion, SR inhibited wear particle-associated
osteolysis effectively, in a dose-dependent manner. SR additionally
downregulated the RANKL/OPG ratio, implying that SR may be a
potential therapy for aseptic loosening.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460333/H0606).
Glossary
Abbreviations
Abbreviations:
|
BV
|
bone volume
|
|
BV/TV
|
bone volume fraction
|
|
OPG
|
osteoprotegerin
|
|
RANKL
|
receptor activator of nuclear
factor-κB ligand
|
|
SR
|
strontium ranelate
|
|
Tb.N
|
trabecular number
|
|
Tb.Th
|
trabecular thickness
|
|
Ti
|
titanium
|
|
µCT
|
micro-computed tomography
|
References
|
1
|
Landgraeber S, Putz S, Schlattjan M,
Bechmann LP, Totsch M, Grabellus F, Hilken G, Jager M and Canbay A:
Adiponectin attenuates osteolysis in aseptic loosening of total hip
replacements. Acta Biomater. 10:384–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang H, Xu Y, Zhu M, Gu Y, Zhang W, Shao
H, Wang Y, Ping Z, Hu X, Wang L and Geng D: Inhibition of
titanium-particle-induced inflammatory osteolysis after local
administration of dopamine and suppression of osteoclastogenesis
via D2-like receptor signaling pathway. Biomaterials. 80:1–10.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu S, Virdi AS, Sena K and Sumner DR:
Sclerostin antibody prevents particle-induced implant loosening by
stimulating bone formation and inhibiting bone resorption in a rat
model. Arthritis Rheum. 64:4012–4020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reginster JY, Brandi ML, Cannata-Andia J,
Cooper C, Cortet B, Feron JM, Genant H, Palacios S, Ringe JD and
Rizzoli R: The position of strontium ranelate in today's management
of osteoporosis. Osteoporos Int. 26:1667–1671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karakan NC, Akpinar A, Göze F and Poyraz
Ö: Investigating the effects of systemically administered strontium
ranelate on alveolar bone loss histomorphometrically and
histopathologically on experimental periodontitis in rats. J
Periodontol. 88:e24–e31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rybchyn MS, Slater M, Conigrave AD and
Mason RS: An Akt-dependent increase in canonical Wnt signaling and
a decrease in sclerostin protein levels are involved in strontium
ranelate-induced osteogenic effects in human osteoblasts. J Biol
Chem. 286:23771–23779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferreira E, Bortolin RH, Freire-Neto FP,
Souza KSC, Bezerra JF, Ururahy MAG, Ramos AMO, Himelfarb ST, Abreu
BJ, Didone TVN, et al: Zinc supplementation reduces RANKL/OPG ratio
and prevents bone architecture alterations in ovariectomized and
type 1 diabetic rats. Nutr Res. 40:48–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiong J and O'Brien CA: Osteocyte RANKL:
New insights into the control of bone remodeling. J Bone Miner Res.
27:499–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong BR, Josien R, Lee SY, Vologodskaia M,
Steinman RM and Choi Y: The TRAF family of signal transducers
mediates NF-kappaB activation by the trance TRANCE receptor. J Biol
Chem. 273:28355–28359. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yasuda H, Shima N, Nakagawa N, Mochizuki
SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, et
al: Identity of osteoclastogenesis inhibitory factor (OCIF) and
osteoprotegerin (OPG): A mechanism by which OPG/OCIF inhibits
osteoclastogenesis in vitro. Endocrinology. 139:1329–1337. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Jia T, Zacharias N, Gong W, Du HX,
Wooley PH and Yang SY: Combination gene therapy targeting on
interleukin-1β and RANKL for wear debris-induced aseptic loosening.
Gene Ther. 20:128–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa N, Kinosaki M, Yamaguchi K, Shima
N, Yasuda H, Yano K, Morinaga T and Higashio K: RANK is the
essential signaling receptor for osteoclast differentiation factor
in osteoclastogenesis. Biochem Bioph Res Commun. 253:395–400. 1998.
View Article : Google Scholar
|
|
13
|
Hofbauer LC and Schoppet M: Clinical
implications of the osteoprotegerin/RANKL/RANK system for bone and
vascular diseases. JAMA. 292:490–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katsuyama H, Otsuki T, Tomita M, Fukunaga
M, Fukunaga T, Suzuki N, Saijoh K, Fushimi S and Sunami S:
Menaquinone-7 regulates the expressions of osteocalcin, OPG, RANKL
and RANK in osteoblastic MC3T3E1 cells. Int J Mol Med. 15:231–236.
2005.PubMed/NCBI
|
|
15
|
Mamolini E, Cervellati C, Greco P,
Carrieri A, Massari L, Crivellari I, Scapoli C and Bonaccorsi G:
VDR, RANKL and OPG polymorphisms as possible predisposing cofactors
of postmenopausal osteoporosis: Explorative study in Italian
population. Gynecol Endocrinol. 33:937–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chakravarti A, Marceau AA, Flamand L and
Poubelle PE: Normal human primary CD4+ T lymphocytes synthesize and
release functional osteoprotegerin in vitro. Lab Invest.
88:171–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuzaki K, Udagawa N, Takahashi N,
Yamaguchi K, Yasuda H, Shima N, Morinaga T, Toyama Y, Yabe Y,
Higashio K and Suda T: Osteoclast differentiation factor (ODF)
induces osteoclast-like cell formation in human peripheral blood
mononuclear cell cultures. Biochem Bioph Res Commun. 246:199–204.
1998. View Article : Google Scholar
|
|
18
|
Theoleyre S, Wittrant Y, Tat SK, Fortun Y,
Redini F and Heymann D: The molecular triad OPG/RANK/RANKL:
Involvement in the orchestration of pathophysiological bone
remodeling. Cytokine Growth Factor Rev. 15:457–475. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Ding L, Zhang S, Jiang T, Yang Y
and Li R: Effects of icariin on the regulation of the
OPG-RANKL-RANK system are mediated through the MAPK pathways in
IL-1β-stimulated human SW1353 chondrosarcoma cells. Int J Mol Med.
34:1720–1726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang S, Yu H, Gong W, Wu B, Mayton L,
Costello R and Wooley PH: Murine model of prosthesis failure for
the long-term study of aseptic loosening. J Orthop Res. 25:603–611.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo H, Zhang J, Hao S and Jin Q:
Adenovirus-mediated small interfering RNA targeting tumor necrosis
factor-α inhibits titanium particle-induced osteoclastogenesis and
bone resorption. Int J Mol Med. 32:296–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bourne RB, Laskin RS and Guerin JS:
Ten-year results of the first 100 genesis II total knee replacement
procedures. Orthopedics. 30 8 Suppl:S83–S85. 2007.
|
|
24
|
Harato K, Bourne RB, Victor J, Snyder M,
Hart J and Ries MD: Midterm comparison of posterior
cruciate-retaining versus-substituting total knee arthroplasty
using the genesis II prosthesis. A multicenter prospective
randomized clinical trial. Knee. 15:217–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meding JB, Galley MR and Ritter MA: High
survival of uncemented proximally porous-coated titanium alloy
femoral stems in osteoporotic bone. Clin Orthop Relat Res.
468:441–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Zhu S, Cui J, Shao H, Zhang W, Yang
H, Xu Y, Geng D and Yu L: Strontium ranelate inhibits
titanium-particle-induced osteolysis by restraining inflammatory
osteoclastogenesis in vivo. Acta Biomater. 10:4912–4918. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu YC, Chang TK, Yeh ST, Fang HW, Lin CY,
Hsu LI and Huang CH and Huang CH: The potential role of strontium
ranelate in treating particle-induced osteolysis. Acta Biomater.
20:147–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang T, Yu H, Gong W, Zhang L, Jia T,
Wooley PH and Yang SY: The effect of osteoprotegerin gene
modification on wear debris-induced osteolysis in a murine model of
knee prosthesis failure. Biomaterials. 30:6102–6108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park K, Ju WC, Yeo JH, Kim JY, Seo HS,
Uchida Y and Cho Y: Increased OPG/RANKL ratio in the conditioned
medium of soybean-treated osteoblasts suppresses RANKL-induced
osteoclast differentiation. Int J Mol Med. 33:178–184. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song R, Gu J, Liu X, Zhu J, Wang Q, Gao Q,
Zhang J, Cheng L, Tong X, Qi X, et al: Inhibition of osteoclast
bone resorption activity through osteoprotegerin-induced damage of
the sealing zone. Int J Mol Med. 34:856–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A,
et al: Osteoclast differentiation factor is a ligand for
osteoprotegerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:pp.
3597–3602. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Atkins GJ, Kostakis P, Pan B, Farrugia A,
Gronthos S, Evdokiou A, Harrison K, Findlay DM and Zannettino AC:
RANKL expression is related to the differentiation state of human
osteoblasts. J Bone Miner Res. 18:1088–1098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grimaud E, Soubigou L, Couillaud S,
Coipeau P, Moreau A, Passuti N, Gouin F, Redini F and Heymann D:
Receptor activator of nuclear factor kappaB ligand
(RANKL)/osteoprotegerin (OPG) ratio is increased in severe
osteolysis. Am J Pathol. 163:2021–2031. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holding CA, Findlay DM, Stamenkov R, Neale
SD, Lucas H, Dharmapatni AS, Callary SA, Shrestha KR, Atkins GJ,
Howie DW and Haynes DR: The correlation of RANK, RANKL and TNFalpha
expression with bone loss volume and polyethylene wear debris
around hip implants. Biomaterials. 27:5212–5219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ndip A, Williams A, Jude EB,
Serracino-Inglott F, Richardson S, Smyth JV, Boulton AJ and
Alexander MY: The RANKL/RANK/OPG signaling pathway mediates medial
arterial calcification in diabetic charcot neuroarthropathy.
Diabetes. 60:2187–2196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peng X, Guo W, Ren T, Lou Z, Lu X, Zhang
S, Lu Q and Sun Y: Differential expression of the RANKL/RANK/OPG
system is associated with bone metastasis in human non-small cell
lung cancer. PLoS One. 8:e583612013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Balsa JA, Lafuente C, Gomez-Martin JM,
Galindo J, Peromingo R, Garcia-Moreno F, Rodriguez-Velasco G,
Martinez-Botas J, Gomez-Coronado D, Escobar-Morreale HF, et al: The
role of serum osteoprotegerin and receptor-activator of nuclear
factor-κB ligand in metabolic bone disease of women after obesity
surgery. J Bone Miner Metab. 34:655–661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stuss M, Sewerynek E, Król I, Stępień-Kłos
W and Jędrzejczyk S: Assessment of OPG, RANKL, bone turnover
markers serum levels and BMD after treatment with strontium
ranelate and ibandronate in patients with postmenopausal
osteoporosis. Endokrynol Pol. 67:174–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee HY, Shen MX, Lim YL, Tay YK, Chan MM,
Pang SM, Xiao ZW, Ang SB and Ren EC: Increased risk of strontium
ranelate-related SJS/TEN is associated with HLA. Osteoporos Int.
27:2577–2583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rossini M, Adami G, Adami S, Viapiana O
and Gatti D: Safety issues and adverse reactions with osteoporosis
management. Expert Opin Drug Saf. 15:321–332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo X, Wei S, Lu M, Shao Z, Lu J, Xia L,
Lin K and Zou D: Dose-dependent effects of strontium ranelate on
ovariectomy rat bone marrow mesenchymal stem cells and human
umbilical vein endothelial cells. Int J Biol Sci. 12:1511–1522.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gu Z, Huang B, Li Y, Tian M, Li L and Yu
X: Strontium-doped calcium polyphosphate/ultrahigh molecular weight
polyethylene composites: A new class of artificial joint components
with enhanced biological efficacy to aseptic loosening. Mater Sci
Eng C Mater Biol Appl. 61:526–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tian A, Zhai JJ, Peng Y, Zhang L, Teng MH,
Liao J, Sun X and Liang X: Osteoblast response to titanium surfaces
coated with strontium ranelate-loaded chitosan film. Int J Oral
Maxillofac Implants. 29:1446–1453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Newman SD, Lotfibakhshaiesh N, O'Donnell
M, Walboomers XF, Horwood N, Jansen JA, Amis AA, Cobb JP and
Stevens MM: Enhanced osseous implant fixation with
strontium-substituted bioactive glass coating. Tissue Eng Part A.
20:1850–1857. 2014. View Article : Google Scholar : PubMed/NCBI
|