Introduction
Inflammation serves a key role in the complex
biological response to harmful stimuli in atherosclerosis and
coronary heart disease. Lysophosphatidylcholine (LPC), the primary
constituent of oxidized low density lipoprotein (LDL), effectively
induces oxidative stress in vascular endothelial cells and serves a
key etiological role in atherosclerosis (1). LPC is upregulated under inflammatory
conditions. In addition, inflammation has a causal association with
innate immunity (2), diabetes
(3) and cancer (4). It may be attributed to a wide variety
of inflammatory cytokines, including tumor necrosis factor-α
(TNF-α), interleukin (IL)-6 and IL-8 (5). Therefore, intervention with an
efficacious anti-inflammatory agent is highly desirable in diseases
such as atherosclerosis.
Resveratrol (RES) occurs naturally as a polyphenol
in various fruits and vegetables and is abundant in grapes
(6). Various studies suggest that
RES has anticancer, anti-mutation, cardiovascular, anti-thrombotic,
anti-microbial, anti-oxidant and immune strengthening activities.
Additionally, RES serves a key anti-inflammatory role in diabetes
(3), cancer (4), cardiovascular disease (7) and neurodegenerative disease (8).
A previous study demonstrated that the
pharmacological activity of RES may be associated with Toll-like
receptor (TLR)-4 (9). TLR-4
belongs to the IL-1 receptor (R)/TLR superfamily. It activates the
innate immune system (10). In
addition, activation of TLR-4 induces NF-κB expression. The
activation of NF-κB induces the expression of IL-6 and TNF-α
(11). However, it is not clear
whether RES reduces lactate dehydrogenase (LDH) activity and
inflammatory cytokine levels via the TLR-4/MyD88/NF-κB signaling
pathway in LPC-induced damage and inflammation in vitro.
Therefore, the present study investigated the effect of
TLR-4-mediated NF-кB signaling in the anti-inflammatory response of
RES to LPC-induced damage and inflammation in the HUVE-12 vascular
endothelial cell line.
Materials and methods
Reagents
RES was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany; catalog no. R5010). RPMI1640 culture medium
was purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The lactic acid dehydrogenase assay kit was supplied by
Beckman Coulter, Inc. (Brea, CA, USA) and LPC and anti-β-actin
antibodies (cat. no. A4700) were purchased from Sigma-Aldrich;
Merck KGaA.
Human TNF-α ELISA (cat. no. BMS223HS) and human IL-6
ELISA (cat. no. EHC007) kits were from NeoBioscience (Shenzhen,
China). Anti-TLR-4 (cat. no. AP1504a) and anti-MyD88 (cat. no.
2E9C2) antibodies were from Abgent, Inc. (San Diego, CA, USA).
Anti-NF-κB p65 antibodies (cat. no. sc-8008) were provided by Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). OriGene Technologies,
Inc. (Rockville, MD, USA) supplied the lentiviral particles
packaging of pGFP-V-RS-TLR-4-short hairpin
(sh)RNAorpCMV6-AC-GFP-TLR-4-cDNAplasmids.
Cell culture and drug treatment
The human umbilical vein endothelial HUVE-12 cell
line was purchased from the Institute of Biochemistry and Cell
Biology (Shanghai, China). Cells were cultured at 37°C and 5%
CO2 in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% heat-inactivated fetal
calf serum (FCS; Invitrogen; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin, and 100 µg/ml streptomycin. HUVE-12 cells
stimulated by LPC (final concentrations: 1.0, 10.0 and 100.0
µmol/l) for 24 h and DMSO (final concentrations: 0.1%) served as
the control. In RES treatment experiments, HUVE-12 cells were
pretreated with 1, 3 and 10 µmol/l RES for 2 h prior to treatment
with 10 µmol/l LPC for 24 h at room temperature.
LDH activity
LDH activity was measured and results analyzed using
an automated biochemistry analyzer (Beckman Coulter Chemistry
analyzer AU-5800 with an Anjue Medical reagent pack, cat no. 1480,
Beckman Coulter, Inc., Brea, CA, USA).
ELISA analysis of IL-6 and TNF-α
Levels of TNF-α or IL-6 were quantified in cell
supernatant prepared by centrifugation for 5 min at 2,800 × g using
ELISA kits (cat. nos. EHC007 and BMS223HS), according to the
manufacturer's protocol. A total of 100 µl serially-diluted
standard samples or supernatant samples were added to the
microplate and incubated at 37°C for 1 h. Subsequently, 100 µl 1X
antibody solution against TNF-α or IL-6 was added to each well and
incubated for 37°C for 1 h. A total of 100 µl horseradish
peroxidase-conjugated secondary antibody was added to each well for
30 min at 37°C. The plate was washed four times with 100 µl PBS
containing 0.1% Tween-20 (PBST) and then the plate was incubated
with 100 µl/well substrate in the dark for 15 min. The optical
density was measured at a wavelength of 450 nm using a
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cell infection
HUVE-12 cells were seeded onto 24-well plates to a
confluence of 40–50% and incubated overnight at 37°C. The cells
were infected with lentiviral particles packaging of
pGFP-V-RS-TLR-4-shRNA
(CCGGCCGCTGGTGTATCTTTGAATACTCGAGTATTCAAAGATACACCAGCGGTTTTTG) or
pCMV6-AC-GFP-TLR-4-cDNA (NM_138557) plasmids supplied by OriGene
Technologies (Beijing, China) in Opti-MEM (cat. no. 11058–021;
Invitrogen; Thermo Fisher Scientific, Inc.) and enhanced infection
solution containing 6 µg/ml polybrene (cat. no. REVG0002; Genechem,
Inc., Daejeon, Korea). After 4 h, the medium was replaced with
RPMI1640 medium containing 10% FCS. Infected cells were cultured
for 48 h for the assessment of gene expression by western
blotting.
Western blotting
Cells were washed with PBS three times and were
lysed on ice with radioimmunoprecipitation assay buffer containing
1% phenylmethylsulfonyl fluoride (Santa Cruz Biotechnology, Inc.).
SDS-PAGE (10 or 12%) was used to separate the proteins in the
lysates (40 µg protein), followed by electroblotting of the
proteins onto a polyvinylidene difluoride membrane (EMD Millipore,
Billerica, MA, USA). Membranes were blocked in 5% non-fat milk in
PBST for 1 h at room temperature, and probed with the following
mouse anti-human primary monoclonal antibodies: Anti-TLR-4
(1:1,000), anti-MyD88 (1:1,000), anti-NF-κBp65 (1:1,000) and
anti-β-actin (1:2,000), under slight vibration at 4°C overnight.
Membranes were subsequently incubated with a goat polyclonal
secondary antibody (cat. no. 31430; 1:500; Invitrogen; Thermo
Fisher Scientific, Inc.) to mouse immunoglobulin Gse immunoglobulin
G for 1 h at room temperature, followed by an Enhanced
Chemiluminescence substrate solution (GE Healthcare; Chicago, IL,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS software version 15.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis and a one-way analysis of variance
followed by Tukey's post-hoc test was used to analyze significant
differences in mean values. P<0.05 was considered to indicate a
statistically significant difference.
Results and Discussion
Effects of LPC on LDH activity, IL-6
and TNF-αsecretion, and TLR-4, MyD88 and NF-κB expression in
HUVE-12 cells
Treatment of HUVE-12 cells with 10 and 100 µmol/l
LPC significantly enhanced LDH activity and the levels of IL-6 and
TNF-α compared with cells treated with 0.1% DMSO (0 µmol/l LPS;
P<0.01; Fig. 1A-C), which
suggested that LPC induced damage and inflammation. In addition,
treatment with 1, 10 and 100 µmol/l LPC elevated the expression
levels of TLR-4, MyD88 and NF-κB p65 in HUVE-12 cells in a
concentration-dependent manner compared with cells treated with 0
µmol/l LPC (Fig. 1D-F). These
results suggested that TLR-4 signaling mediates HUVE-12 cell injury
induced by LPC. LDH is a marker of injury and diseases, including
heart failure (12). Oxidation and
enzymatic modification of low density lipoprotein (LDL) leads to
lysophosphatidylcholine (LPC) synthesis in atherosclerosis
(12). LPC induces inflammation in
coronary artery smooth muscle cells (13). Qin et al (14) demonstrated that LPC maintains
macrophage polarization towards a classically activated phenotype
in inflammation. Li et al (15) reported that LPC induces the
secretion of inflammatory factors in human umbilical vein
endothelial cells. The results of the present study suggested that
LPC increased LDH activity and expression of the inflammatory
cytokines, IL-6 and TNF-α, suggesting that LPC induced injury and
inflammation in HUVE-12 cells.
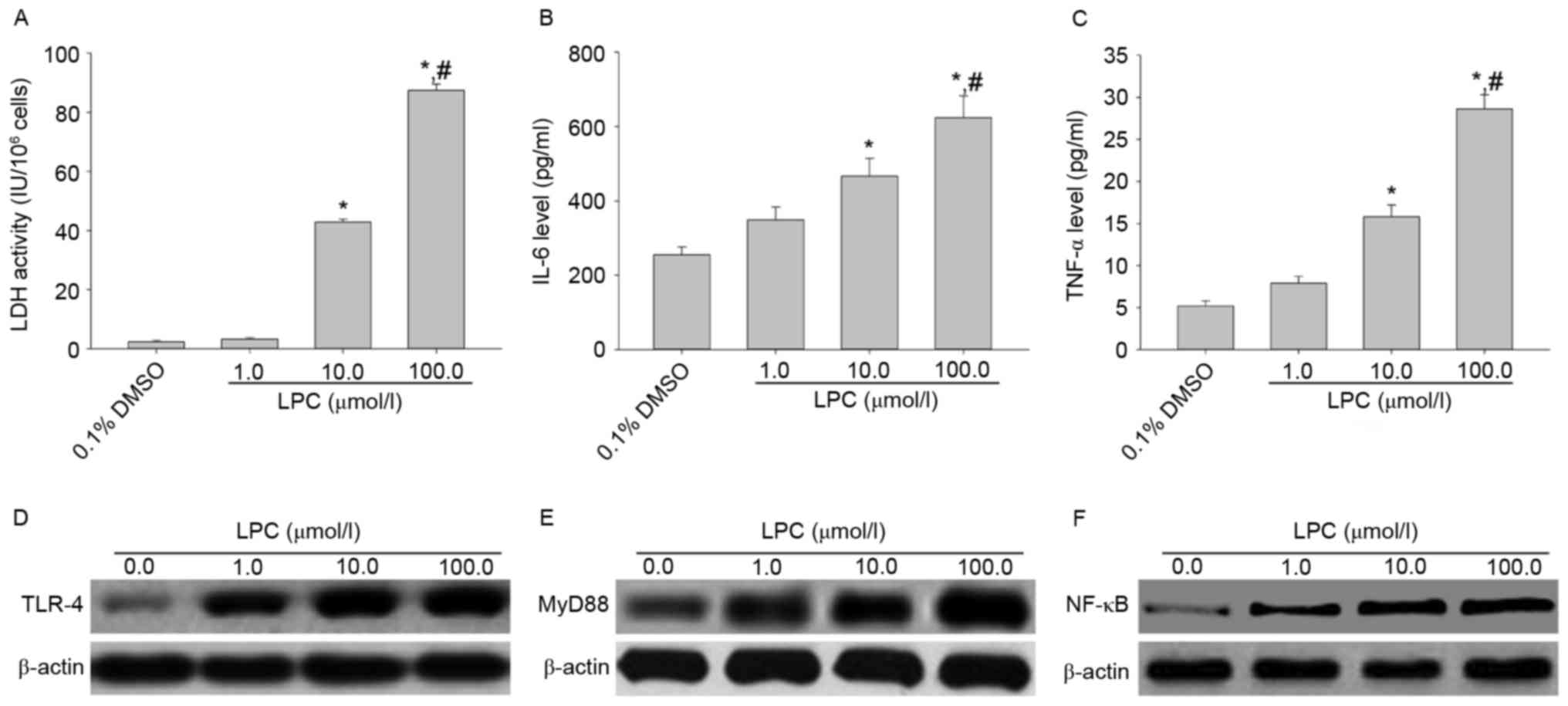 | Figure 1.LPC induces inflammation and activates
the TLR-4/MyD88/NF-κB signaling pathway in HUVE-12 cells. (A)
Activity of LDH and secretion of (B) IL-6 and (C) TNF-α were
elevated after treatment with 10.0 and 100.0 µM LPC. Data are
expressed as the mean ± standard deviation. *P<0.05 vs. 0 µmol/l
(0.1% DMSO) and #P<0.05 vs. 1.0 µmol/l LPC. In
addition, 1.0, 10.0 and 100.0 µM LPC treatment resulted in
increased levels of (D) TLR-4, (E) MyD88 and (F) NF-κB. LPC,
lysophosphatidylcholine; TLR-4, Toll-like receptor-4; MyD88,
myeloid differentiation primary response gene 88; NF-κB, nuclear
factor-κB; LDH, lactate dehydrogenase; IL-6, interleukin-6; TNF-α,
tumor necrosis factor-α. |
Pathogens, cytokines and environmental stimuli alter
TLR-4 expression in vascular injury and the inflammatory response.
TLRs mediate zinc/nickel-induced inflammation in endothelial cells
(16). Wang et al (17) reported that TLR-4 stimulates
proliferation and an inflammatory response in LPS-induced Hep G2
cells. Bomfim et al (18)
suggested that TLR-4 mediates hypertension and vascular
inflammation via NF-κB signaling. The interaction between TLR-4 and
proteinase-activated receptor 2 [PAR (2)] contributes to vascular homeostasis
(19). The present study
demonstrated that TLR-4 signaling may be involved in LPC-induced
injury and inflammation in HUVE-12 cells.
Effect of RES on LDH activity, IL-6
and TNF-αsecretion, and TLR-4, MyD88 and NF-κB p65 expression
To examine whether RES protects against LPC-induced
injury and inflammation, HUVE-12 cells were pretreated with 1, 3
and 10 µmol/l RES prior to treatment with 10 µmol/l LPC. RES
inhibited the effects of LPC on LDH activity and cytokine
expression compared with cells treated with 0 µmol/l RES and LPC
(P<0.01; Fig. 2A-C). In
addition, RES suppressed the expression levels of TLR-4, MyD88 and
NF-κB compared with cells treated with 0 µmol/l RES (Fig. 2D-F), which were upregulated by LPC
treatment alone. RES has been reported to exhibit anti-atherogenic
effects (20). Various studies
suggested that RES protects cardiomyocytes against injury via the
TLR-4/NF-κB signaling pathway (21,22).
The results of the present study revealed that RES may protect from
the LPC-induced damage and inflammation by inhibiting the
TLR-4/MyD88/NF-κB signaling pathway.
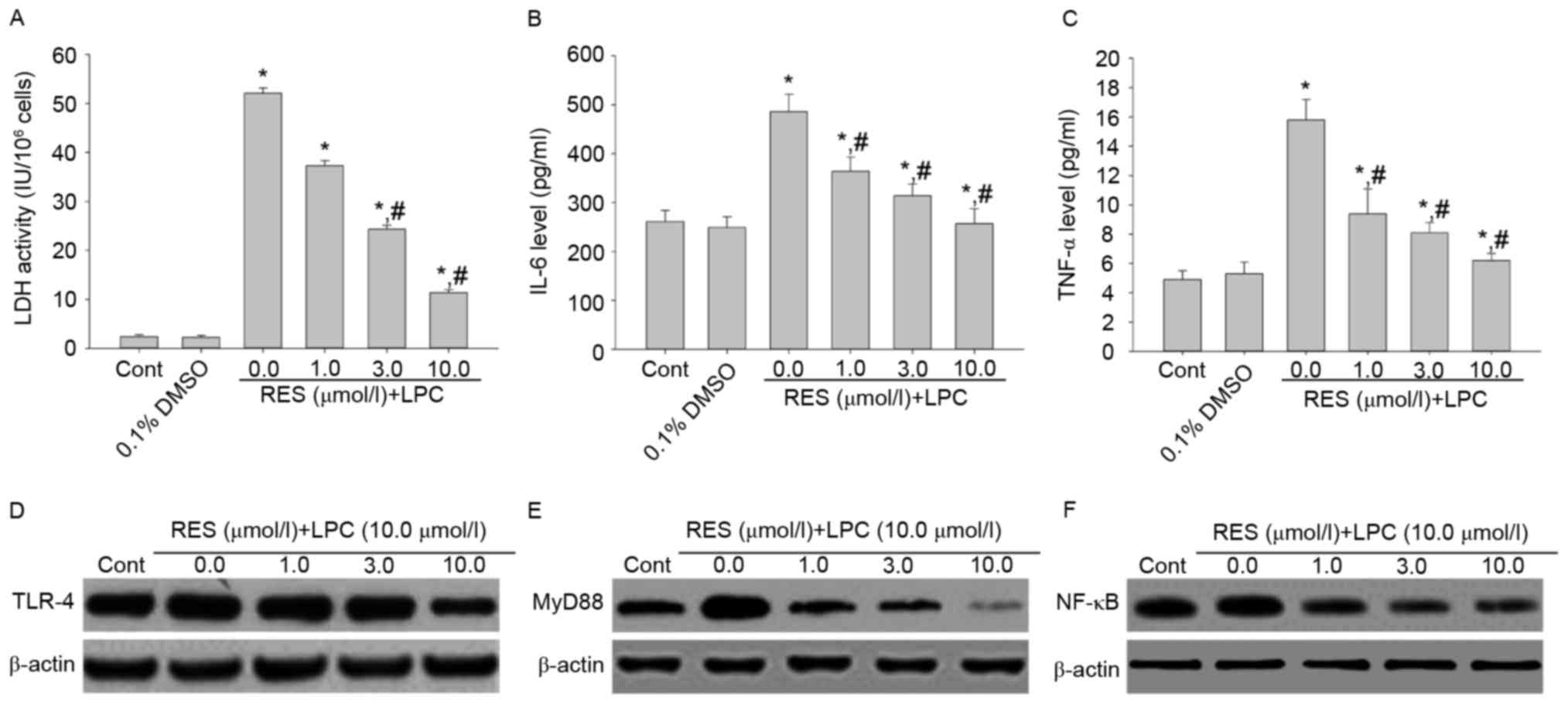 | Figure 2.Pretreatment with RES suppresses the
inflammation induced by the subcytotoxic concentration of 10.0
µmol/l LPC and decreases activation of the TLR-4/MyD88/NF-κB
signaling pathway in HUVE-12 cells. The effect of LPC on (A) LDH
activity, (B) IL-6 and (C) TNF-α was suppressed by 1.0, 3.0 and
10.0 µM RES in HUVE-12 cells. Furthermore, LPC-induced upregulation
of (D) TLR-4 (E) MyD88 and (F) NF-κB was suppressed by RES
treatment. Data are expressed as the mean ± standard deviation.
*P<0.05 vs. 0 µmol/l (0.1% DMSO); #P<0.05 vs. 0.0
µmol/l RES. Cont, untreated HUVE-12 cells; RES, resveratrol; LPC,
lysophosphatidylcholine; TLR-4, Toll-like receptor-4; MyD88,
myeloid differentiation primary response gene 88; NF-κB, nuclear
factor-κB; LDH, lactate dehydrogenase; IL-6, interleukin-6; TNF-α,
tumor necrosis factor-α. |
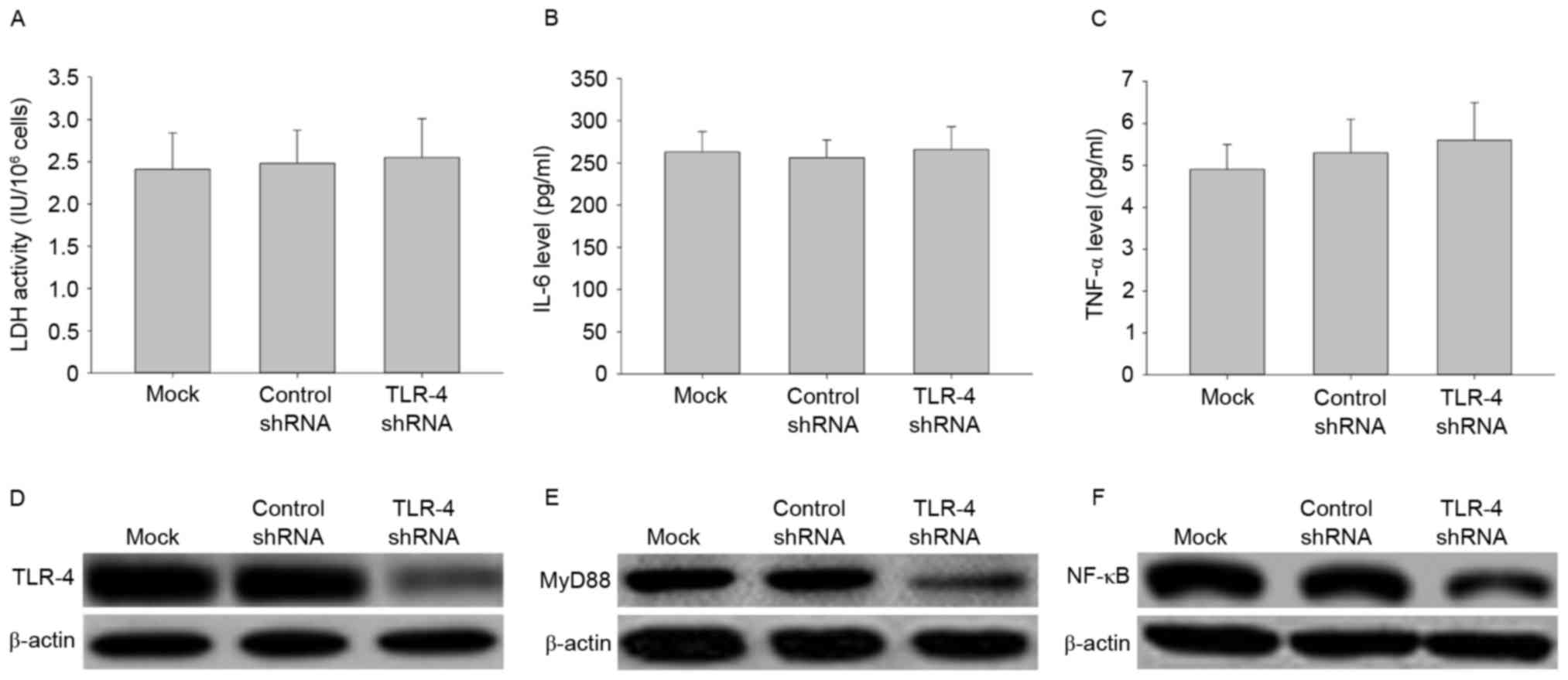
Effects of TLR-4 shRNA transfection on
LDH activity, IL-6 and TNF-αsecretion, and signal transduction
To further investigate the role of TLR-4 in
LPC-induced damage and inflammation in HUVE-12 cells, TLR-4 shRNA
transduction was performed to silence the TLR-4 gene.
Transfection with TLR-4-shRNA did not affect LDH activity or
expression of IL-6 and TNF-α in HUVE-12 cells (Fig. 3A-C), despite demonstrating that
transfection with TLR-4-shRNA significantly decreased the
expression levels of TLR-4 and its downstream targets, MyD88 and
NF-κB, compared with cells transfected with control shRNA (Fig. 3D-F). These results suggested that
knockdown of TLR-4 silenced the TLR-4 gene to inhibit the
TLR-4/MyD88/NF-κB signaling pathway; while it had little effect on
injury and inflammatory factor secretion of HUVE-12 cells.
TLR-4 shRNA transfection influences
LDH activity, IL-6 and TNF-αsecretion, and signal transduction
induced by LPC treatment
To evaluate the effect of TLR-4 shRNA on LPC-induced
damage and inflammation, the LDH activity and levels of IL-6 and
TNF-α were measured. As demonstrated in Fig. 4A-C, transfection with TLR-4 shRNA
significantly inhibited the effects of LPC on LDH activity and IL-6
and TNF-α cytokine secretion, compared with control shRNA
(P<0.01). Furthermore, TLR-4 shRNA suppressed LPC-induced
upregulation of signaling molecules compared with control shRNA
(Fig. 4D-F). This suggested that
TLR-4 gene silencing may protect from LPC-induced damage and
inflammation.
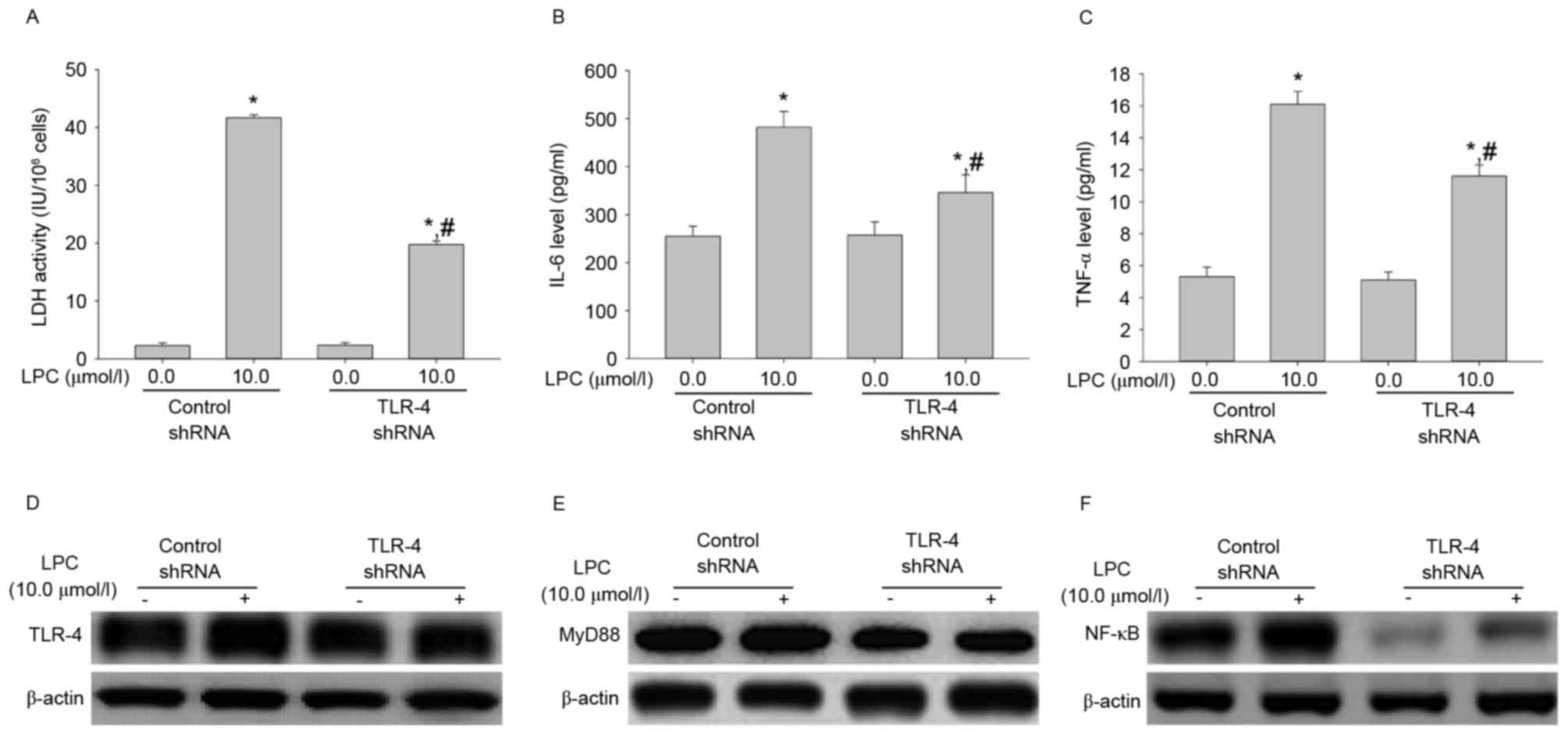 | Figure 4.TLR-4 shRNA transfection inhibits
LPC-induced damage and inflammation. (A) Compared with control
shRNA, transfection with TLR-4 shRNA suppressed LPC-induced
activity of LDH. (B) TLR-4 shRNA inhibited the effects of LPC on
secretion of IL-6 and (C) TNF-α. Furthermore, TLR-4shRNA
downregulated LPC-induced expression of (D) TLR-4, (E) MyD88 and
(F) NF-κB. Data are expressed as the mean ± standard deviation.
*P<0.01 vs. control shRNA in absence of 10.0 µmol/l LPC;
#P<0.05 vs. control shRNA in presence of 10.0 µmol/l
LPC. TLR-4, Toll-like receptor-4; shRNA, short hairpin RNA; MyD88,
myeloid differentiation primary response gene 88; NF-κB, nuclear
factor-κB; LDH, lactate dehydrogenase; IL-6, interleukin-6; TNF-α,
tumor necrosis factor-α; LPC, lysophosphatidylcholine. |
Effects of RES therapy and TLR-4 shRNA
transfection on LDH activity, IL-6 and TNF-αsecretion, and TLR-4,
MyD88 and NF-κB expression
To investigate whether RES inhibits LPC-induced
damage and inflammation in human umbilical vein endothelial cells,
the effect of RES in combination with TLR-4 shRNA on LPC-induced
damage and inflammation in HUVE-12 cells was investigated. RES
treatment and TLR-4 shRNA transfection suppressed the effects of
LPC on LDH activity and IL-6 and TNF-α secretion (P<0.01;
Fig. 5A-C), the expression of
TLR-4 and MyD88 were weakly downregulated, and the expression of
NF-κB were markedly downregulated (Fig. 5D-F). These data suggested that RES
inhibited expression of NF-κB may have involved another
mechanism.
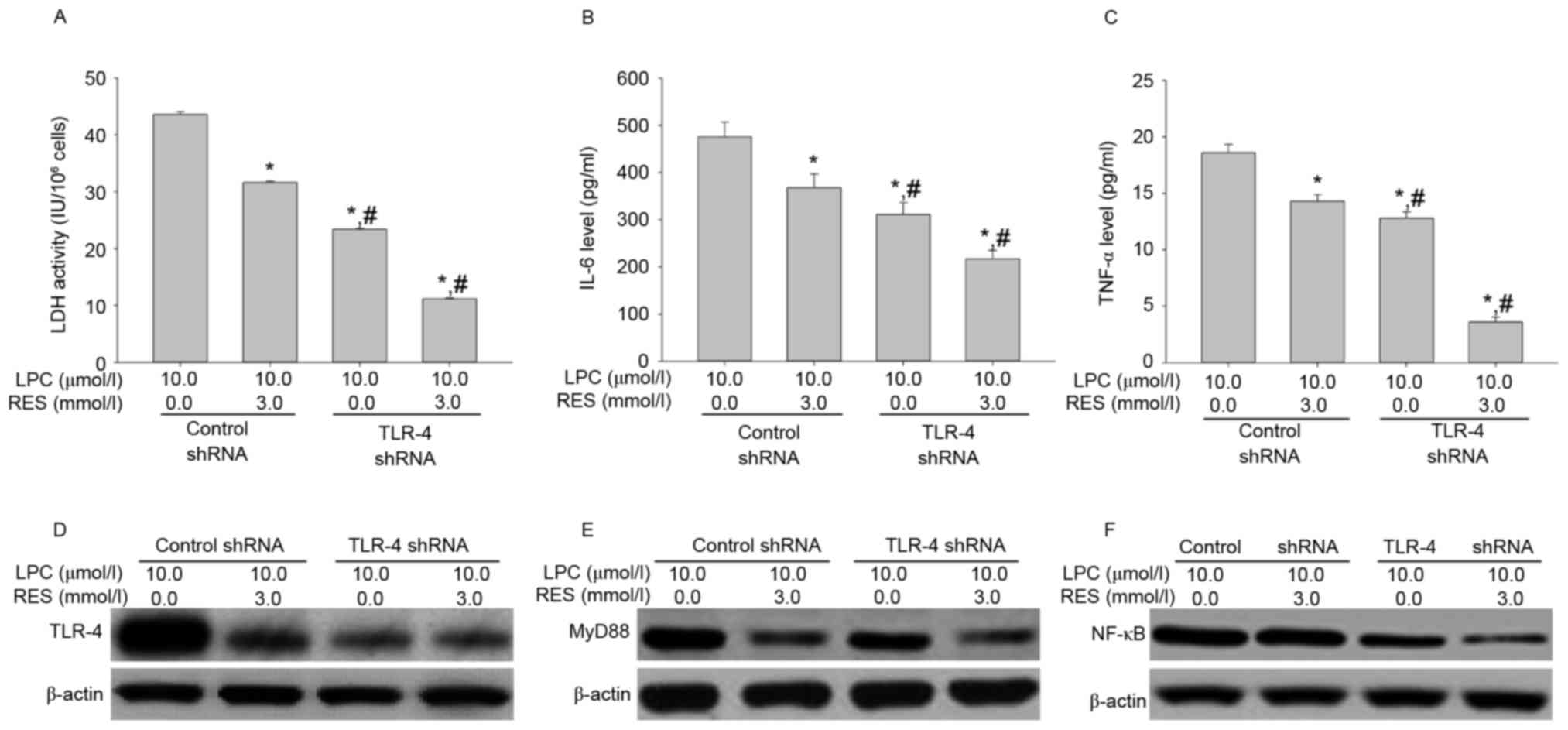 | Figure 5.RES and transfection with TLR-4 shRNA
cooperatively suppress LPC-induced inflammation by blocking the
TLR-4/MyD88/NF-κB signaling pathway in HUVE-12 cells. (A) RES and
TLR-4-shRNA transfection suppressed LPC-induced activity of LDH.
RES and TLR-4 shRNA transfection suppressed LPC-increased cytokine
expression of (B) IL-6 and (C) TNF-α. In addition, RES and
TLR-4-shRNA transduction suppressed LPC-mediated expression of (D)
TLR-4, (E) MyD88 and (F) NF-κB. Data are expressed as the mean ±
standard deviation. *P<0.01 vs. 0 µmol/l RES using control shRNA
transfection in presence of 10.0 µmol/l LPC; #P<0.05
vs. 3.0 µmol/l RES using control shRNA transfection in presence of
10.0 µmol/l LPC. RES, resveratrol; TLR-4, Toll-like receptor-4;
shRNA, short hairpin RNA; MyD88, myeloid differentiation primary
response gene 88; NF-κB, nuclear factor-κB; LDH, lactate
dehydrogenase; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α;
LPC, lysophosphatidylcholine. |
A previous study demonstrated that the
pharmacological activity of RES may be associated with TLR-4. The
interaction of TLR-4 with MyD88 activates TNF receptor-associated
factor, to activate the inflammatory cascade (23). TLR-4 may mediate MyD88-dependent
NF-κB activation, which increases the production of inflammatory
cytokines. The results of the present study suggested that RES
attenuates LPC-induced damage and inflammation in HUVE-12 cells by
inhibiting the TLR-4/MyD88/NF-κB signaling pathway.
Effect of TLR-4 overexpression on LDH
activity, IL-6 and TNF-αsecretion, and signal transduction
To further investigate the role of TLR-4 in
LPC-induced damage and inflammation in HUVE-12 cells, cells were
transfected with TLR-4 cDNA to overexpress the TLR-4 gene.
As demonstrated in Fig. 6A-C, no
significant differences were observed in LDH activity and cytokine
expression between cells transfected with the control cDNA and
TLR-4 cDNA (P>0.05). However, the expression levels of TLR-4 and
its downstream proteins including MyD88 and NF-κB, were elevated by
TLR-4 overexpression (Fig. 6D-F).
These results suggested that overexpression of TLR-4 may activate
the TLR-4/MyD88/NF-κB signaling pathway.
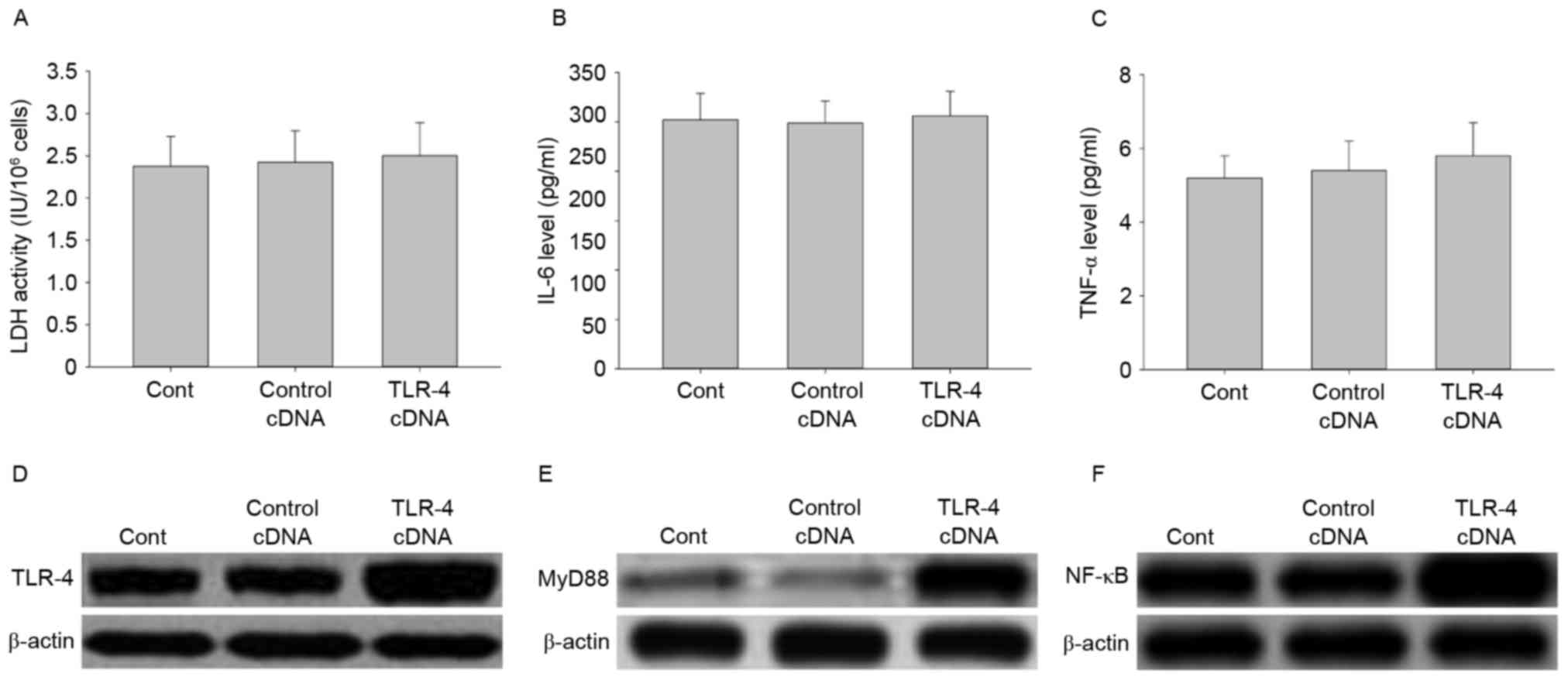 | Figure 6.Transfection with TLR-4 cDNA activates
the TLR-4/MyD88/NF-κB signaling pathway in HUVE-12 cells. (A)
Transfection with TLR-4 cDNA did not affect LDH activity or
expression of (B) IL-6 or (C) TNF-α. However, TLR-4 cDNA visibly
enhanced the expression of (D) TLR-4, (E) MyD88 and (F) NF-κB
compared with Cont or Control cDNA groups. Data are expressed as
the mean ± standard deviation. Cont, untreated HUVE-12 cells;
Contrl cDNA, GFP cDNA transduced HUVE-12 cells; TLR-4, Toll-like
receptor-4; MyD88, myeloid differentiation primary response gene
88; NF-κB, nuclear factor-κB; LDH, lactate dehydrogenase; IL-6,
interleukin-6; TNF-α, tumor necrosis factor-α. |
Effect of TLR-4 overexpression on LDH
activity, IL-6 and TNF-αsecretion, and signal transduction induced
by LPC
To determine the effect of TLR-4 overexpression on
LPC-induced damage and inflammation, the LDH activity and levels of
IL-6 and TNF-α were measured. Transfection with TLR-4 cDNA enhanced
LPC-induced LDH activity and IL-6 and TNF-α levels compared with
cells transfected with control cDNA in HUVE-12 cells (P<0.01;
Fig. 7A-C). The expression levels
of TLR-4, MyD88 and NF-κB in cells transfected with TLR cDNA was
higher than in the control cDNA group after LPC treatment (Fig. 7D-F), which suggested that TLR-4 may
be associated with NF-κB signaling during LPC-induced damage and
inflammation (18).
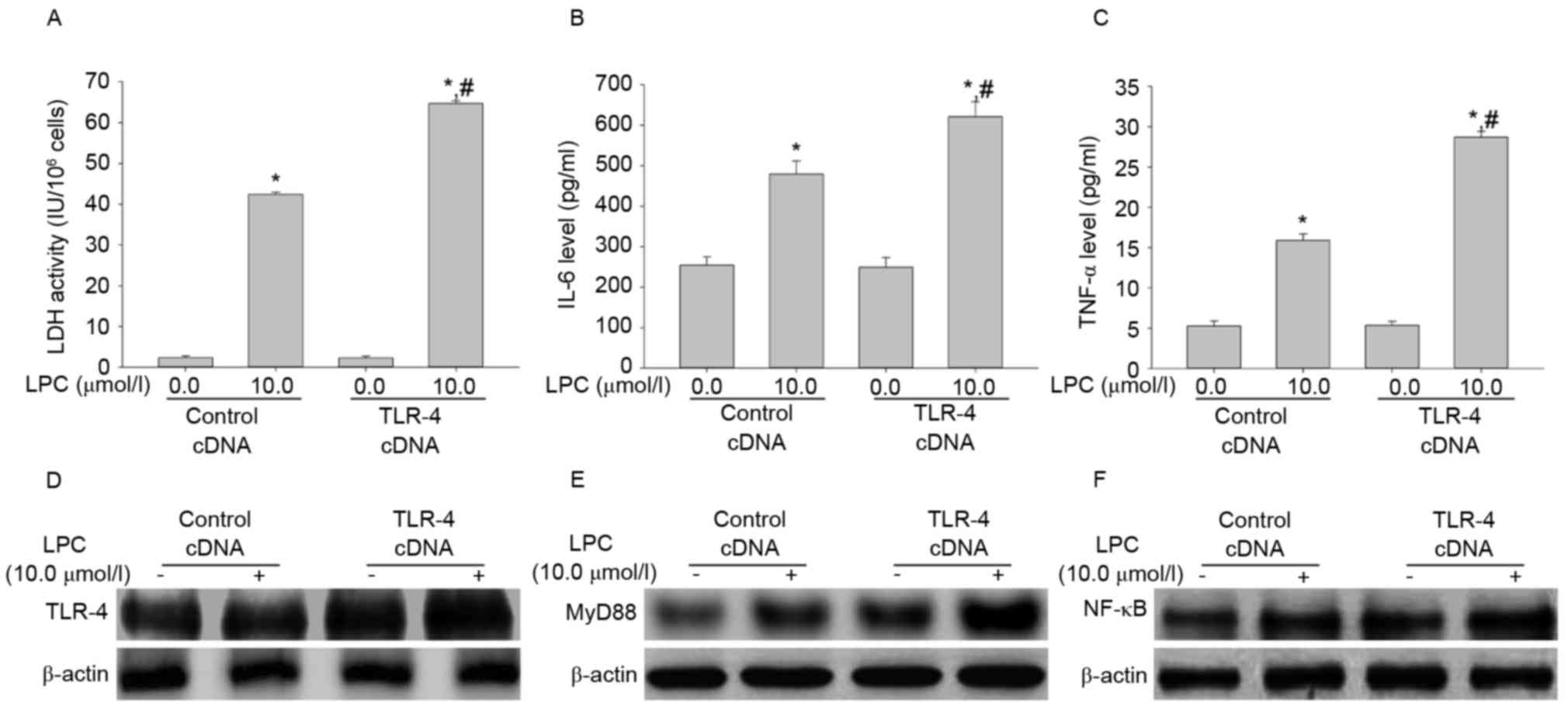 | Figure 7.TLR-4 cDNA transfection increases
LPC-induced damage and inflammation. (A) Compared with control
cDNA, the increased activity of LDH by LPC was elevated after
transfection with TLR-4 cDNA. TLR-4 cDNA transduction increased the
effect of LPC on (B) IL-6 and (C) TNF-α expression. Furthermore,
the levels of (D) TLR-4, (E) MyD88 and (F) NF-κB increased
following TLR-4 cDNA transduction compared with LPC-stimulated
HUVE-12 cells transduced with GFP cDNA. *P<0.01 vs. control cDNA
in the absence of 10.0 µmol/l LPC; #P<0.05 vs.
control cDNA in the presence of 10.0 µmol/l LPC. Data are expressed
as the mean ± standard deviation. TLR-4, Toll-like receptor-4;
MyD88, myeloid differentiation primary response gene 88; NF-κB,
nuclear factor-κB; LDH, lactate dehydrogenase; IL-6, interleukin-6;
TNF-α, tumor necrosis factor-α; LPC, lysophosphatidylcholine. |
Effects of RES treatment combined with
TLR-4 cDNA transduction on LDH activity, IL-6 and TNF-αsecretion,
and signal transduction
To confirm that the effects of RES inhibited
LPC-induced damage and inflammation in human umbilical vein
endothelial cells via regulation of the TLR-4/MyD88/NF-κB signaling
pathway, the effect of RES treatment and TLP4 overexpression on
LPC-induced damage and inflammation in HUVE-12 cells was
investigated. RES significantly suppressed the effects of TLR-4
overexpression on LPC-induced damage and inflammation (P<0.01;
Fig. 8A-F).
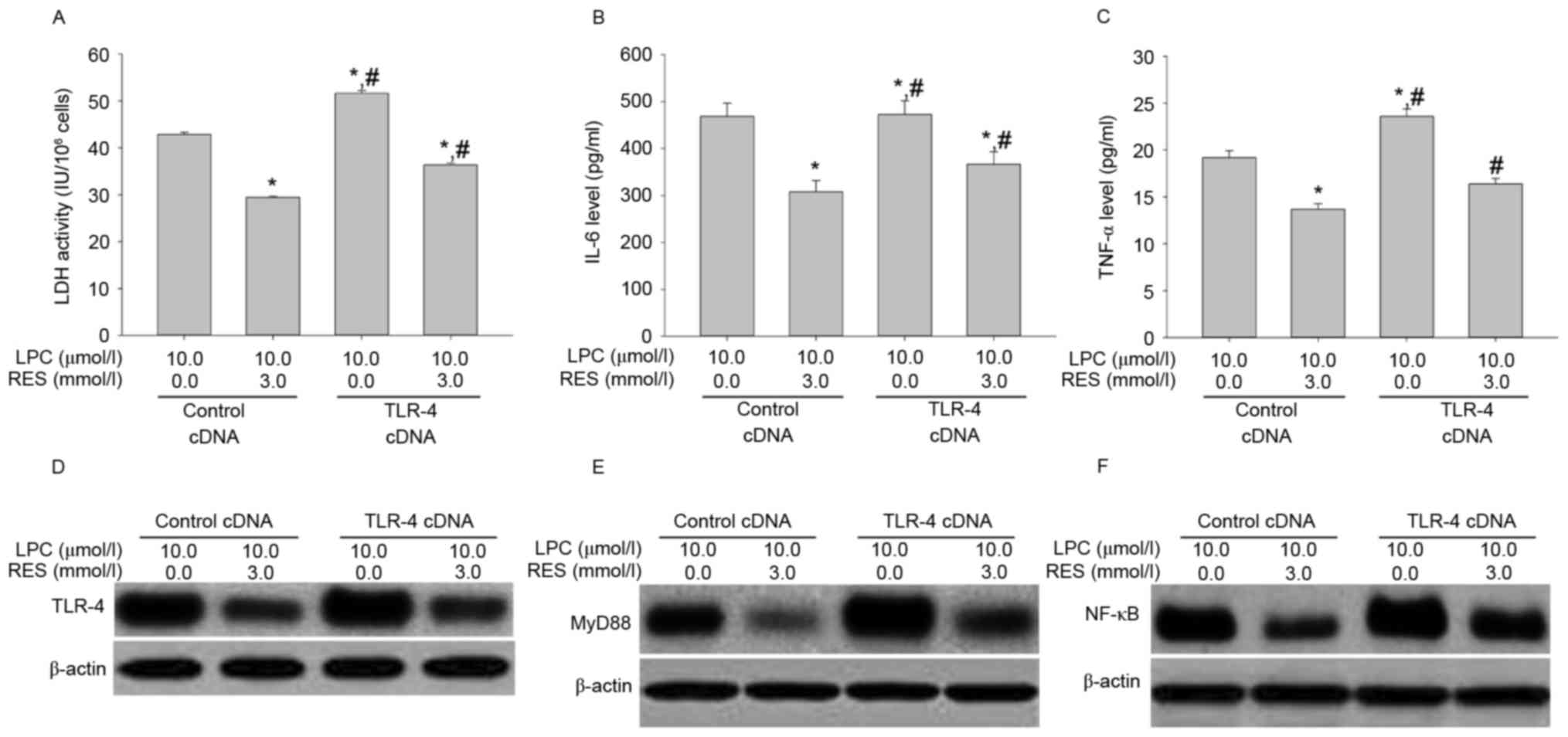 | Figure 8.Transfection with TLR-4 cDNA
antagonizes the inhibitory effects of RES on LPC-induced
inflammation by activating the NF-κB signaling pathway in HUVE-12
cells. (A) Inhibition of LPC-induced LDH activity by RES was
antagonized by TLR-4 cDNA transduction. The reduction in (B) IL-6
and (C) TNF-α by RES in the presence of LPC was attenuated by TLR-4
cDNA transfection. Furthermore, the downregulation inexpression of
(D) TLR-4, (E) MyD88 and (F) NF-κB by RES in the presence of LPC
was reversed following transfection with TLR-4 cDNA.*P<0.01 vs.
control cDNA in absence of 3.0 µmol/l RES; #P<0.05
vs. control cDNA in presence of 3.0 µmol/l RES. Data are expressed
as the mean ± standard deviation. TLR-4, Toll-like receptor-4;
MyD88, myeloid differentiation primary response gene 88; NF-κB,
nuclear factor-κB; LDH, lactate dehydrogenase; IL-6, interleukin-6;
TNF-α, tumor necrosis factor-α; LPC, lysophosphatidylcholine; RES,
resveratrol. |
RES was reported to exhibit anti-atherogenic effects
(20). Various studies suggested
that RES protects cardiomyocytes against injury via the TLR-4/NF-κB
signaling pathway (21,22). The results of the present study
supported the critical role served by TLR-4/MyD88/NF-κB signaling
in LPC-induced injury and pro-inflammatory responses. In addition,
the present study demonstrated that RES attenuated the inflammatory
reaction induced by LPC in HUVE-12 cells via downregulation of the
TLR-4/MyD88/NF-κB signaling pathway. The present study reported
that RES exercises protective actions in the first steps of the
atherogenic process. Reducing the expression of adhesion molecules
(intercellular adhesion molecule-1, and vascular cell adhesion
molecule-1) via inhibition of NF-κB pathway activation by RES was
demonstrated by Deng et al (24). The present study also provided
evidence that RES inhibited NF-κB activation through blocking
TLR-4/MyD88/NF-κB signal pathway. The results highlight the
anti-inflammatory properties and potential molecule mechanism of
RES. Bonnefont-Rousselot (25)
suggested that RES is a good candidate, owing to its protective
action of vascular walls towards oxidation, inflammation, platelet
oxidation and thrombus formation. RES may be beneficial in
preventing the development of atherosclerosis. However, further
studies with animal models are required to validate the findings of
the present study.
Glossary
Abbreviations
Abbreviations:
|
RES
|
resveratrol
|
|
LPC
|
lysophosphatidylcholine
|
|
LDH
|
lactate dehydrogenase
|
|
HUVE-12 cells
|
human umbilical vein endothelial-12
cells
|
|
LDL
|
low density lipoprotein
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-6
|
interleukin-6
|
|
OD
|
optical density
|
References
|
1
|
Domeij H, Hua X, Su J, Bäcklund A, Yan Z,
Frostegard AG, Haeggström JZ, Modéer T and Frostegard J: Annexin A5
inhibits atherogenic and pro-inflammatory effects of
lysophosphatidylcholine. Prostaglandins Other Lipid Mediat.
106:72–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sheldon IM, Cronin JG, Healey GD, Gabler
C, Heuwieser W, Streyl D, Bromfield JJ, Miyamoto A, Fergani C and
Dobson H: Innate immunity and inflammation of the bovine female
reproductive tract in health and disease. Reproduction.
148:R41–R51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao W, Zhou Y, Li Q, Zhou Q, Tan L, Song
Y, Zhao X, Yu M, Zheng S, Ye H, et al: Analysis of global gene
expression profiles suggests a role of acute inflammation in type
3C diabetes mellitus caused by pancreatic ductal adenocarcinoma.
Diabetologia. 58:835–844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Strowig T, Henao-Mejia J, Elinav E and
Flavell R: Inflammasomes in health and disease. Nature.
481:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bradamante S, Barenghi L and Villa A:
Cardiovascular protective effects of resveratrol. Cardiovasc Drug
Rev. 22:169–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim D, Nguyen MD, Dobbin MM, Fischer A,
Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et
al: SIRT1 deacetylase protects against neurodegeneration in models
for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J.
26:3169–3179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Youn HS, Lee JY, Fitzgerald KA, Young HA,
Akira S and Hwang DH: Specific inhibition of MyD88-independent
signaling pathways of TLR3 and TLR4 by resveratrol: Molecular
targets are TBK1 and RIP1 in TRIF complex. J Immunol.
175:3339–3346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang RL, Yuan Y, Zou GM, Liu G, Tu J and
Li Q: LPS-stimulated inflammatory environment inhibits
BMP-2-induced osteoblastic differentiation through crosstalk
between TLR4/MyD88/NF-kB and BMP/Smad signaling. Stem Cells Dev.
23:277–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Augoff K, Hryniewicz-Jankowska A and
Tabola R: Lactate dehydrogenase 5: An old friend and a new hope in
the war on cancer. Cancer Lett. 358:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aiyar N, Disa J, Ao Z, Ju H, Nerurkar S,
Willette RN, Macphee CH, Johns DG and Douglas SA:
Lysophosphatidylcholine induces inflammatory activation of human
coronary artery smooth muscle cells. Mol Cell Biochem. 295:113–120.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin X, Qiu C and Zhao L:
Lysophosphatidylcholine perpetuates macrophage polarization toward
classically activated phenotype in inflammation. Cell Immunol.
289:185–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li JZ, Wu JH, Yu SY, Shao QR and Dong XM:
Inhibitory effects of paeoniflorin on
lysophosphatidylcholine-induced inflammatory factor production in
human umbilical vein endothelial cells. Int J Mol Med. 31:493–497.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsou TC, Liou SH, Yeh SC, Tsai FY and Chao
HR: Crucial role of Toll-like receptors in the zinc/nickel-induced
inflammatory response in vascular endothelial cells. Toxicol Appl
Pharmacol. 273:492–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Tu Q, Yan W, Xiao D, Zeng Z,
Ouyang Y, Huang L, Cai J, Zeng X, Chen YJ and Liu A: CXC195
suppresses proliferation and inflammatory response in LPS-induced
human hepatocellular carcinoma cells via regulating
TLR4-MyD88-TAK1-mediated NF-kB and MAPK pathway. Biochem Biophys
Res Commun. 456:373–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bomfim GF, Echem C, Martins CB, Costa TJ,
Sartoretto SM, Dos Santos RA, Oliveira MA, Akamine EH, Fortes ZB,
Tostes RC, et al: Toll-like receptor 4 inhibition reduces vascular
inflammation in spontaneously hypertensive rats. Life Sci. 122:1–7.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bucci M, Vellecco V, Harrington L,
Brancaleone V, Roviezzo F, Mattace Raso G, Ianaro A, Lungarella G,
De Palma R, Meli R and Cirino G: Cross-talk between Toll-like
receptor 4 (TLR4) and proteinase-activated receptor 2 (PAR (2)) is
involved in vascular function. Br J Pharmacol. 168:411–420. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riccioni G, Gammone MA, Tettamanti G,
Bergante S, Pluchinotta FR and D'Orazio N: Resveratrol and
anti-atherogenic effects. Int J Food Sci Nutr. 66:603–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang C, Lin G, Wan W, Li X, Zeng B, Yang
B and Huang C: Resveratrol, a polyphenol phytoalexin, protects
cardiomyocytes against anoxia/reoxygenation injury via the
TLR4/NF-κB signaling pathway. Int J Mol Med. 29:557–563. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Xie C, Zhuang J, Li H, Yao Y, Shao C
and Wang H: Resveratrol attenuates inflammation in the rat heart
subjected to ischemia-reperfusion: Role of the TLR4/NF-κB signaling
pathway. Mol Med Rep. 11:1120–1126. 2015.PubMed/NCBI
|
|
23
|
Feng Y and Longmore GD: The LIM protein
Ajuba influences interleukin-1-induced NF-kappaB activation by
affecting the assembly and activity of the protein kinase
Czeta/p62/TRAF6 signaling complex. Mol Cell Biol. 25:4010–4022.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng YH, Alex D, Huang HQ, Wang N, Yu N,
Wang YT, Leung GP and Lee SM: Inhibition of TNF-α-mediated
endothelial cell-monocyte cell adhesion and adhesion molecules
expression by the resveratrol derivative,
trans-3,5,4′-trimethoxystilbene. Phytother Res. 25:451–457.
2011.PubMed/NCBI
|
|
25
|
Bonnefont-Rousselot D: Resveratrol and
cardiovascular diseases. Nutrients. 8:2502016. View Article : Google Scholar :
|