Autophagy is imperative in normal and pathological
conditions, including inflammation, adaptation to stress, aging,
immunity, metabolic and neurodegenerative disorders, and cancer
(1–7). Both in vivo animal models and
in vitro cell culture studies suggest that autophagy serves
a role in the maintenance of renal function under pathological
conditions including nephrotoxic drugs treatment or
ischemia/reperfusion (I/R) injury (8,9).
Acute kidney injury (AKI) is characterized by
tubular cell injury and death that leads to a rapid and progressive
loss of renal function, including declined glomerular filtration,
accumulation of nitrogenous wastes and imbalance of water,
electrolytes and acid-base reactions. AKI is a common disease and
constitutes a risk factor for chronic kidney disease (10). The incidence of AKI during an ICU
stay ranges from 22 to 67% (11).
Although there is certain progress in basic research and clinical
application, AKI with an increasing morbidity and mortality rate
and few prevention and treatment approaches, remains a common and
serious clinical condition in hospitalized patients (11).
Autophagy is classified into three subtypes:
macroautophagy, chaperone-mediated autophagy, and microautophagy
(12). Macroautophagy, generally
referred to as autophagy, is well-studied and is the focus of this
review. Autophagy is an ‘auto-eating’ process in a cell where
intracellular organelles, proteins, and other macromolecules are
sequestered into autophagic vesicles (known as autophagosomes) and
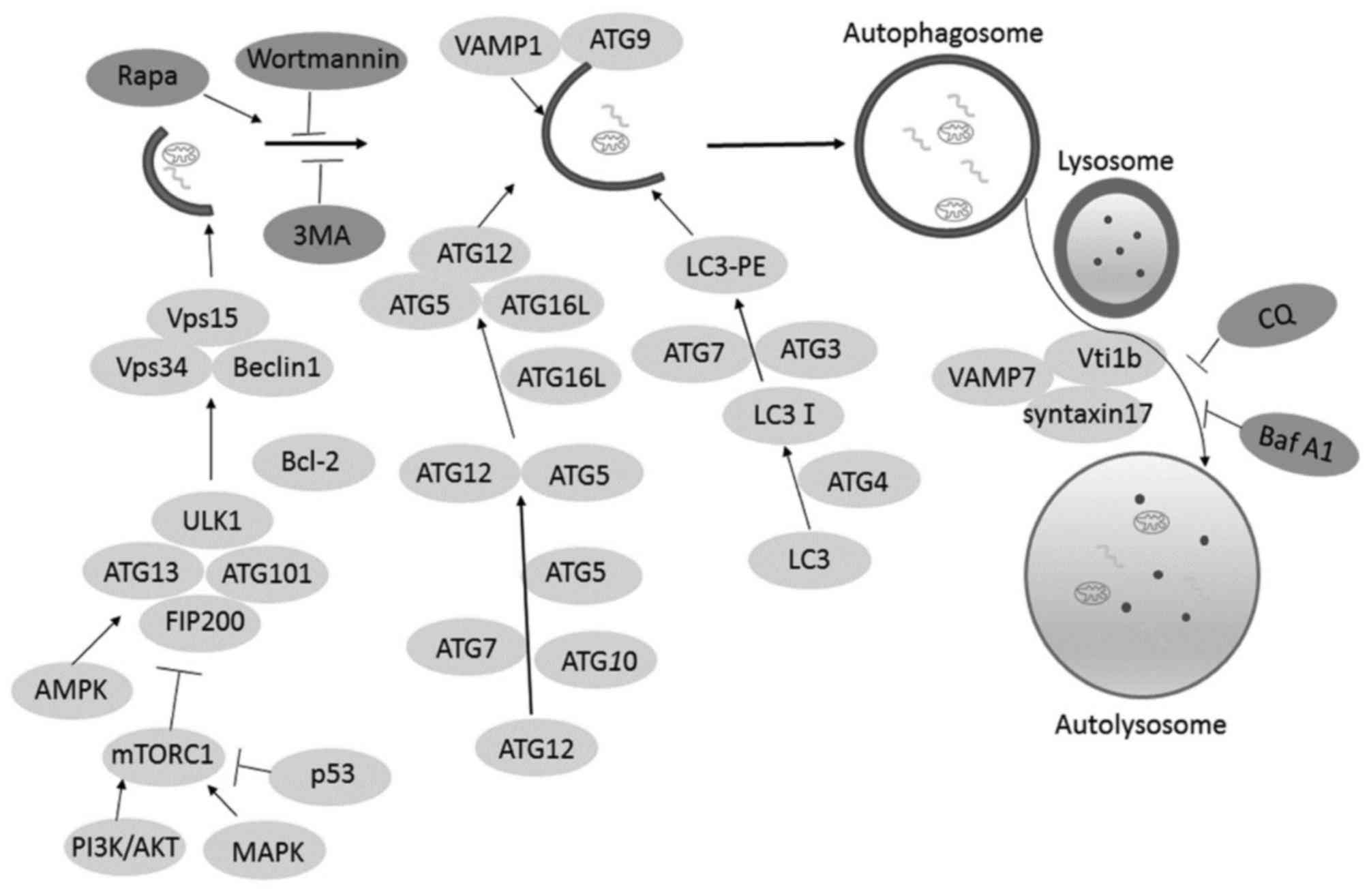
then degraded by the hydrolases of lysosomes (13). The formation of autophagosomes
involves the following steps: Induction, nucleation and elongation
that form a complete isolation membrane. Subsequently,
double-membrane autophagosomes with sequestered materials inside
fuses with the lysosome and form autolysosomes (Fig. 1). Cytoplasmic constituents are
degraded by the lysosomal hydrolases for cyclic utilization
(14). The initiation step of
autophagosome formation involved the ULK1/2-Atg13-FIP200 complex,
which is required for the phagophore membranes. The phagophore
membrane PI3K/Vps34 complex containing Vps34, Vps15, Beclin-1 and
Atg14 L is recruited for nucleation. Autophagy-related gene 9 (Atg
9) and vacuole membrane protein 1 (VMP1) are necessary for membrane
expansion. Atg1-Atg13-Atg17 complex promote Atg9 cycling between
the phagophore assembly site (PAS) and non-PAS compartments and
aided in localization of Atg9 to the PAS (15,16).
Indeed, Atg12-Atg5-Atg16 complex and Atg8/light chain 3-II are two
ubiquitin-like conjugation systems that are involved in the
elongation and expansion steps during autophagosome formation
(17). Mi et al (18) demonstrated that actin served a role
in autophagosome formation. They demonstrated that polymerized
actin puncta were co-localized with DFCP1 [a PtdIns(3)P-binding ER
protein that marks omegasomes] and LC3-positive puncta. PtdIns(3)P
promoted actin network formation inside membranes, thereby branched
actin networks are essential for autophagosomal membrane
shaping.
Recent studies have documented the key role of
autophagy in normal physiological conditions as well as in
pathogenesis conditions. In physiological processes, a low level of
basal autophagy occurs to maintain cellular homeostasis. In
pathological conditions, cellular stress including hypoxia, cell
starvation, oxidant injury, genotoxic agents, and other damaging
factors contribute to the induction of autophagy (19,20).
Autophagy has both pro-survival and pro-death
functions in gastric cancer cells (21). Autophagy is upregulated in cancer
cells to provide nutritive material for cell survival (22). Pla et al (23) demonstrated that autophagy decreased
ethanol toxicity in mouse neurons. Autophagy and autophagic flux
decreased in aging hearts, stimulation of autophagy alleviated
aging-associated pathology in the heart (24). Furthermore, autophagy is considered
to play a renoprotective role in kidney injury (25).
AKI is mainly caused by I/R injury and nephrotoxic
drugs which impair renal function Adequate hydration control,
maintenance of arterial pressure, pre-emptive use of antioxidants
may prevent AKI; few treatments could change the development of AKI
(26). Previous studies have
demonstrated autophagy serves a role in AKI (27–33).
I/R injury is a leading cause of AKI, which is
frequently associated with many clinical conditions but lacks
effective therapies (27). Recent
studies have reported that autophagy is activated in renal I/R
injury in vitro and in vivo models irrespective of
the genetic or pharmacological impairment of autophagy (27–35).
Pharmacological inhibitors or inducers of autophagy
have non-specific effects and therefore previous studies
demonstrated the effect of autophagy during renal I/R by using
conditional kidney proximal tubule-specific Atg5- or Atg7-knockout
mice (31,33). Kimura et al (33) demonstrated that I/R injury
increases proximal tubule cell apoptosis in kidney proximal
tubule-specific Atg5 mice compared with the wild-type mice. It is
also demonstrated that autophagy serves a critical role in
maintaining tubular cell integrity during stress conditions
(34).
On the other hand, excessive stimulation of
autophagy has been described to exacerbate I/R injury in the kidney
(37). Isaka et al
(38) used LC3-GFP transgenic mice
and demonstrated that I/R injury promoted the formation of LC3-GFP
dots. By contrast, increased B-cell lymphoma (Bcl)-2 protein
protected tubular epithelial cells from I/R injury by suppressing
autophagy and inhibiting apoptosis (38). Furthermore, overexpression of
Bcl-xL and Bcl-2 eliminate both apoptosis and autophagy (39). Suzuki et al (40) used HK-2 cells subjected to hypoxia
or activation of reactive oxygen species and demonstrated that
compared to normoxic conditions LC3-labeled autophagic vacuoles
slightly increased and lysosome-associated membrane
protein2-labeled lysosomes markedly increased following 48 h of
hypoxia. Using lysosomal protease inhibitors autophagosomes
increased significantly under hypoxia, suggesting that hypoxia
highly induces autophagic generation and degradation. Moreover, HK2
cells with Atg7 deletion significantly inhibit
H2O2-induced cell death (40). These results indicate that
autophagy may contribute to cell death during renal
ischemia-reperfusion injury. In another study it was demonstrated
that kidneys of GFP-LC3 transgenic mice that were subjected to 48 h
of cold ischemia in the presence of lysosomal inhibitor bafilomycin
A1, the number of apoptotic cells were significantly reduced
(41) suggesting that autophagy
may serve a role in I/R injury.
Non-coding RNAs, including lncRNA and microRNAs,
exhibit an effect on regulating autophagy during I/R injury
(42–44). MicroRNAs serve a significant effect
in autophagy during kidney I/R injury both in vivo and in
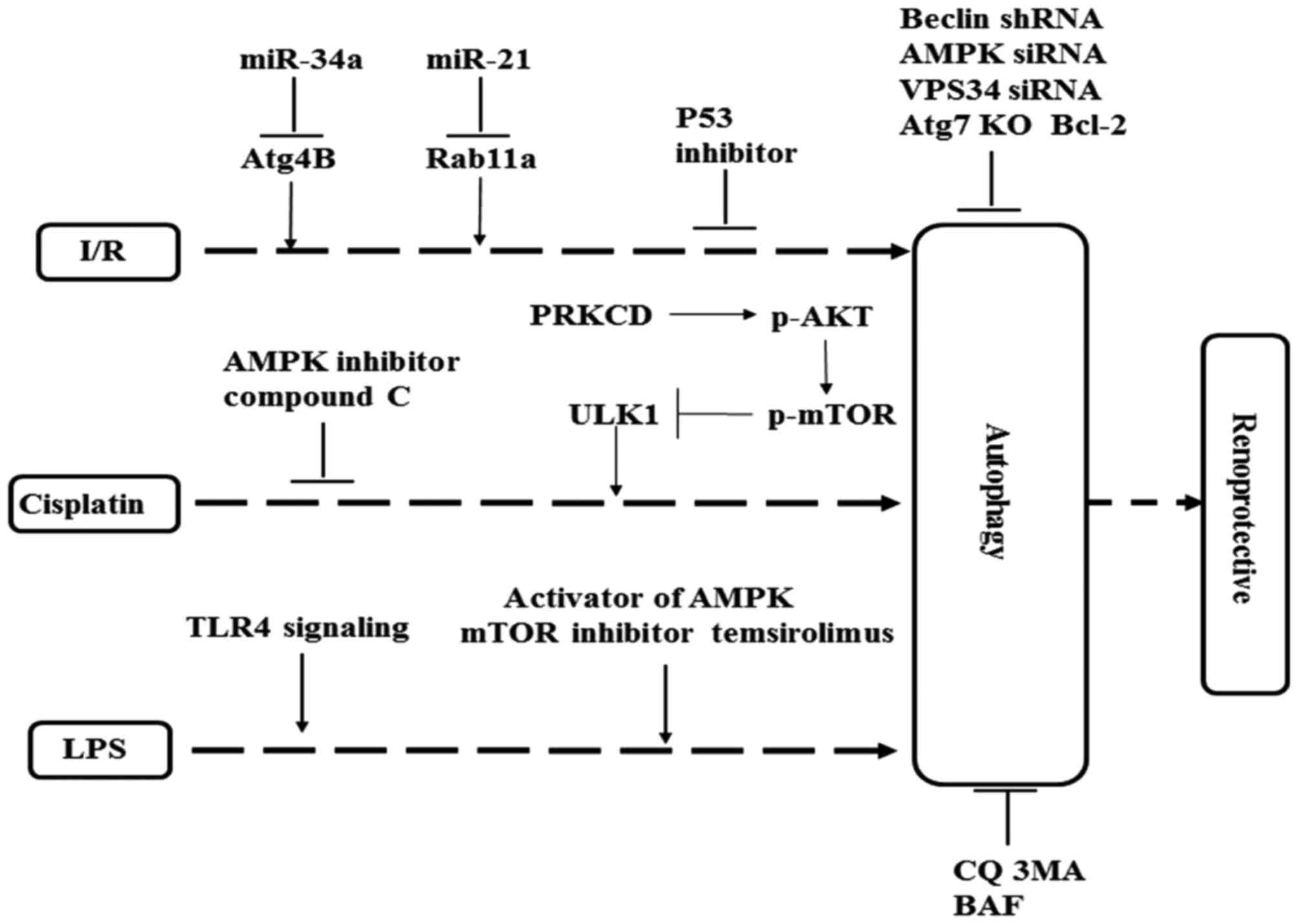
vitro (43,44). Liu et al (43) demonstrated that miR-34a reduced the
autophagic activity and caused injury in I/R tubular epithelial
cells via a direct binding to the Atg4B 3′-untranslated region. In
addition, miR-21 mimics directly targeted Rab11a 3′-UTR reducing
beclin-1, LC3-II expression and increased p62 expression in the I/R
model. Overexpression of Rab11a weakened the effect of miR-21
mimics and I/R on cell apoptosis (44). In a rat I/R model, pre-treatment
with miR-21 inhibitor injection ameliorated the renal injury, and
increased the expression of LC3-II and beclin-1 (44). The role of autophagy in I/R damage
depends on the injury situation such as the duration of hypoxia,
ischemia and reperfusion, and the effect of autophagy in different
studies is shown in Table I.
Cisplatin is a widely used chemotherapeutic drug,
with major side effects in kidneys, leading to cell death and
tissue damage (45,46). Several studies demonstrated that
cisplatin is capable of activating autophagy both in vitro
and in vivo AKI models (47–51).
Depending on the experimental conditions, autophagy
could act as a mechanism of cytoprotection (47,48).
Cisplatin induced an increase of LC3-II expression that is further
enhanced by emodin treatment in NRK-52E cells (49). Autophagy activity is increased
after 6 h of cisplatin treatment and began a gradual decrease from
12 h in proximal tubular cells (RPTCs), which was transiently
transfected with GFP-LC3, indicating that autophagy is induced
before apoptosis in RPTCs during cisplatin treatment (50). Consistently, using
mRFP-LC3-transfected NRK-52E cells it was demonstrated that emodin
increased the formation of mRFP-LC3 dots and decreased apoptosis
during cisplatin treatment (51).
Inhibition of autophagy with either 3-MA or bafilomycin A1 elevated
renal proximal tubular cell apoptosis and abolished the protective
effects of emodin during cisplatin treatment (50,51),
suggesting a renoprotective role of autophagy in cisplatin-induced
AKI. HEK cells with Beclin-1 knockdown prevent the formation of
GFP-LC3 dots during cisplatin treatment and sensitize cells to
cisplatin-induced apoptosis (50).
By contrast, overexpressed beclin-1 and Atg5 in LLC-PK1 cells
inhibit caspase activation and protecte tubular cells from
cisplatin toxicity (51). This
further supports that autophagy serves a protective role against
cisplatin injury to proximal tubular epithelial cells.
The activity of autophagy is crucial to protect
kidney against chemotherapeutics injury (49). The underlying mechanism of how
autophagy protects the kidney from the damage induced by cisplatin
is not clearly understood (31).
P53 is reported to participate in cisplatin-induced renal cell
apoptosis in in vitro and in vivo models (52–58).
Feng et al (59) suggested
that p53 regulates autophagy. Periyasamy-Thandavan et al
(50) demonstrated that
pifithrin-α, a pharmacological inhibitor of p53, inhibited
autophagosome formation during cisplatin treatment in RPTCs.
Increased p53 and JNK activation aggravate cisplatin-induced
proximal tubular cells apoptosis in PT-Atg7-KO mice (31).
mTORC1 is a critical serine-threonine kinase in the
autophagy regulation pathway that negatively regulates autophagy
activity (49). However, AMPK
inhibited the activity of mTORC1, a AMPK inhibitor compound C
significantly suppressed emodin-induced AMPK phosphorylation and
LC3 conversion, consequently inhibited the autophagic activity and
increased the cisplatin-induced proximal tubular cells apoptosis
(49). Cells transfected with AMPK
small interference (si)RNA were sensitive to cisplatin-induced AKI
(60). NAD(P)H: quinone
oxidoreductase 1 (NQO1) knockout enhances autophagy in ACHN cells
and mice during cisplatin treatment by the AMPK/mTOR signaling
pathway (61).
Caspase inhibitor zVAD-fmk prevented the degradation
of Atg5, Atg12, and beclin-1 thereby increasing GFP-LC3-II dots in
LLC-PK1 during cisplatin treatment. However, autophagosome
formation and p62 expression were not significantly increased in
the presence of zVAD-fmk and chloroquine, demonstrating that
zVAD-fmk impaired autophagic flux by blocking the autophagosome
clearance (62). zVAD-fmk
suppressed lysosomal function and impaired autophagic flux by
inhibiting lysosomal cathepsins (63) and calpains (64,65).
A similar result is obtained zVAD-fmk prevented beclin-1 cleavage
to impair autophagic flux and increase cisplatin-induced cellular
injury in a mouse model (62).
There is a connection between apoptosis and autophagy, where a
signaling pathway that regulates autophagy can also regulate
apoptosis (17). Therfore, 10 µM
cisplatin induced autophagy to maintain cell homeostasis, whereas
50 µM cisplatin induced cell apoptosis in NRK-52E cells. However,
Rovetta et al (66)
demonstrated that ER signaling pathway regulated the balance
between autophagy and apoptosis induced by cisplatin. Autophagy
mostly served a renoprotective action in cisplatin-induced AKI
(Table II).
Cyclosporine A (CsA) is an immune-suppressor used in
renal allograft transplantation (67). Previous studies demonstrated that
CsA induced the accumulation of autophagosomes and inhibited
autophagic clearance (68). Yadav
et al (69) demonstrated
that expression of LC3-II was increased and SQSTM1 accumulation was
decreased following CsA treatment in transmembrane BAX inhibitor
motif containing 6 (TMBIM6)-expressing HK-2 cells compared to NC
cells, suggesting that the autophagic flux was standard in TMBIM6
cells. A similar result was obtained in vivo, in CsA-treated
TMBIM6−/− mice where autophagosome formation was increased and the
formation and activity of lysosome were decreased (69). The mechanism of TMBIM6-induced
autophagy was that it activated PRKAA and suppressed mTORC1 in
CsA-treated HK-2 cells. This further demonstrated that TMBIM6
reversed the impaired autophagic flux by stimulating lysosome
biogenesis through TFEB activation.
Sepsis-related AKI is an important clinical issue
occurring during severe infection including cecal ligation and
puncture (CLP) and LPS (70,71).
The effect of autophagy in septic AKI remains unclear, compared
with the genetic evidence in I/R and cisplatin models (17). Lipopolysaccharide (LPS)-induced
accumulation of LC3-II in HPT1 cells derived from human proximal
tubular epithelial cells (PTEC) (72). Preincubation of rapamycin decreased
tumor necrosis factor (TNF) and induces NRK-52E cell death, whereas
the knockdown of Atg7 exaggerated TNF-induced DNA fragmentation
(73). This demonstrated that
autophagy serves a cytoprotective role in the sepsis-induced AKI.
Similar results were found in vivo, LPS induces the
accumulation of LC3-positive puncta in GFP-LC3 transgenic mice
(74). CLP-induced autophagy was
time-dependent in rat, LC3-II was elevated transiently at 3 h but
declined at 9 h until 18 h after CLP, renal dysfunction occurred at
9 and 18 h after CLP (73),
indicating that autophagy occurred prior to apoptosis and the
decline of autophagy aggravated TNF-α-induced cell death.
Chloroquine, an inhibitor of lysosomes also aggravates LPS-induced
AKI in C57BL/6 mice (74). Tubular
injury was more severe in ATG7KO mice than in controls and the
expression of IL-6 was significantly increased in ATG7KO kidneys
compared with controls following LPS treatment (73), indicating that autophagy protected
kidneys from CLP and LPS-induced sepsis injury. Leventhal et
al (72) reported that
LPS-induced autophagy in RTEC was TLR4-dependent, there were no
differences in LC3-II accumulation in C57Bl\10ScN mice, which was
no functional TLR4 compared with saline-injected mice. Autophagy
was decreased in adult mice during LPS-induced AKI, administration
of the mTOR inhibitor temsirolimus increased autophagy and improved
renal function in adult mice during LPS-induced AKI (75). In young mice (8 weeks of age), the
inhibitor of VPS34 damaged renal function after LPS treatment
(75). These results suggest that
autophagy serves a renoprotective role irrespective of age (young
or aged mice) during LPS treatment (Table III).
Mesenchymal stem cells (MSCs) are multipotent stem
cells isolated from different tissues including bone marrow,
umbilical cord, adipose tissue or muscle (76). These cells have self-renewal and
multiple differentiation potential including adipocytes,
chondrocytes and osteocytes (77).
A large number of studies have documented the potential therapeutic
effects of MSC, including cardiopathy (78), hepatic diseases (79), and renal injury (80). The therapeutic potential of MSCs
includes the anti-inflammatory, antioxidant, anti-fibrotic,
anti-apoptotic, pro-angiogenic, stimulation of endogenous
progenitor cells, and promotion of cellular reprogramming (78–80).
Exosomes derived from MSC have beneficial effects in distinct
models of injury. Our previous studies suggested that exosomes
derived from human umbilical cord MSCs alleviated liver fibrosis
(81), cutaneous wound healing
(82), and acute myocardial
ischemic injury (83).
MSCs are recruited to injured tissues and release
certain cytokines and growth factors, such as insulin-like,
hepatocyte and vascular endothelial growth factors, which could
activate endogenous cellular repair programs contributing to the
growth and survival of endothelial and epithelial tubular cells
thus promoting renal angiogenesis and regeneration (84,85).
Bruno et al (86)
demonstrated that MSC-derived microvesicles express MSC markers and
transferr cellular materials to neighboring cells, including RNA
and proteins, consequently promoting cell proliferation and
inhibited apoptosis.
Human MSCs repair HK2 cell after ischemia injury by
stimulating normal reactive oxygen species handling, anti-apoptotic
activity, energy production, protein synthesis, cytoskeleton
organization and cell proliferation (87). In I/R rats, MSCs repair kidney by
anti-inflammatory, anti-apoptotic and by enhancing the repair of
peritubular capillaries and tubular epithelial cells (88). Furthermore, extracellular vesicles
derived from MSCs alleviate kidney injury through anti-oxidation by
strengthening Nrf2/ARE activation in I/R rats (89). BM-MSCs ameliorate cisplatin-induced
AKI by increasing Foxp3+ T-regulatory cell infiltration
in a monkey model (90). Human
adipose-derived mesenchymal stem cells protect against
cisplatin-induced AKI via anti-apoptotic pathways (91). In a previous study, it demonstrated
that human umbilical cord mesenchymal stem cells-derived exosomes
decrease cisplatin-induced renal oxidative stress and apoptosis in
NRK-52E cells and in rats (92),
indicating that MSCs and microvesicles released by MSCs may improve
renal injury in AKI.
Autophagy plays a critical role in MSCs-based
therapy of tissues injury. Hypoxia and heat shock pretreatment
enhance survival in BM-MSCs and increase the therapeutic potential
of the stem cell by increasing autophagy, providing a novel
strategy to improve MSC-based therapies (93,94).
Bone marrow (BM)-MSCs alleviate chronic high
glucose-induced injury in INS-1 cells (95) and reduce the severity of lung I/R
injury in human pulmonary microvascular endothelial cells (HPMVECs)
(96) by enhanced autophagic
activity. Similar results are presented in vivo, where
BM-MSCs promote the recovery of pancreatic damage in T2D rats
(95) and reduce the severity of
lung I/R injury in C57BL/6J mice (96). Shin et al (97) demonstrated that MSCs significantly
enhance autophagy and the clearance of amyloid-β in Alzheimer
disease models to increase neuronal survival against Aβ toxicity.
In a Parkinsonian model, BM-MSCs increase the cell viability and
reduce α-synuclein by upregulating autophagolysosome formation
(98). Tonsil-derived MSCs
ameliorate liver fibrosis via the downregulation of TGF-β and
autophagy activation (99). These
findings support the protective effect of autophagy in MSCs,
thereby repairing the pancreas, lung, nervous diseases. In our
previous studies, it was demonstrated that human umbilical cord
mesenchymal stem cell-derived exosomes prevent cisplatin-induced
AKI by autophagy (100) which
providing a novel strategy of MSC-based therapy for AKI.
In conclusion, autophagy is induced in kidneys in
response to AKI, autophagy in AKI is multifaceted and complex, and
it can protect against kidney injury or promote cell death
depending on experimental conditions (17). The activity of autophagy is
time-dependent. In the early stages of renal injury, autophagy
contributes to cell survival, whereas in later stages, autophagy
may activate apoptosis signaling pathways sequentially promoting
cell death and renal function injury, but the mechanism remains
unknown. Autophagy and apoptosis share similar regulators and are
mutually inhibitory (101). Many
factors that activate autophagy also activate cell apoptosis, which
usually precedes cell apoptosis (17). Several critical signaling pathways
positively regulate autophagy and apoptosis, such as the tumor
suppressor p53, bcl-2 family, death-associated protein kinases and
c-Jun N terminal kinases (101).
In the model of cisplatin-induced nephrotoxicity, PRKCD suppressed
autophagy and promoted renal cell death by AKT/mTOR signal pathway
(102). The possible protective
mechanism of autophagy in AKI is summarized in Fig. 2. Autophagic flux is frequently
defined as a measure of autophagic degradation activity. Continuous
observation autophagy is imperative, and to assess the efficiency
of the autophagic flux is necessarily in measuring autophagic
degradation activity. However, the molecules participating in the
autophagosome formation and the regulating mechanism are not known.
The signaling pathways, which have been reported in AKI models,
such as AMPK and mTOR, are critical in inducing and regulating
autophagy but these are also poorly understood.
Pharmacological inhibitors (3-MA or bafilomycin A or
wortmannin) or inducers (Rapa) of autophagy are non-specific for
autophagy (17). It is necessary
to find autophagy inducers in proximal tubules and examine their
effects in models of AKI. A comprehensive understanding of
autophagy may improve renal function and prevent AKI
progression.
MSC-based therapies have been extensively studied as
a potential treatment for several diseases (78–80).
However, our understanding of the regulatory mechanisms of MSCs or
MSC-derived vesicles in kidney disease, remain to be fully
elucidated. Autophagy serves a key role in MSCs-based therapy, but
effects of autophagy in AKI, which are repaired by MSCs are not
very clear. Therefore, elucidating its role may provide a novel
approach towards the therapy of AKI.
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272481), the Major
Research Plan of Jiangsu Higher Education (grant no. 15KJA320001)
and a project funded by the Priority Academic Program Development
of Jiangsu Higher Education Institutions.
|
1
|
Qian M, Fang X and Wang X: Autophagy and
inflammation. Clin Transl Med. 6:242017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomiyama R, Takakura K, Takatou S, Le TM,
Nishiuchi T, Nakamura Y, Konishi T, Matsugo S and Hori O:
3,4-dihydroxybenzalacetone and caffeic acid phenethyl ester induce
preconditioning ER stress and autophagy in SH-SY5Y cells. J Cell
Physiol. 233:1671–1684. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antikainen H, Driscoll M, Haspel G and
Dobrowolski R: TOR-mediated regulation of metabolism in aging.
Aging Cell. 16:1219–1233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Germic N, Stojkov D, Oberson K, Yousefi S
and Simon HU: Neither eosinophils nor neutrophils require
ATG5-dependent autophagy for extracellular DNA trap formation.
Immunology. 152:517–525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Long M, Li X, Li L, Dodson M, Zhang DD and
Zheng H: Multifunctional p62 effects underlie diverse metabolic
diseases. Trends Endocrinol Metab. 28:818–830. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu ZY, Chen B, Zhang JP and Ma YY:
Up-regulation of autophagy-related gene 5 (ATG5) protects
dopaminergic neurons in a zebrafish model of Parkinson's disease. J
Biol Chem. 292:18062–18074. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi HS, Jeong EH, Lee TG, Kim SY, Kim HR
and Kim CH: Autophagy inhibition with monensin enhances cell cycle
arrest and apoptosis induced by mTOR or epidermal growth factor
receptor inhibitors in lung cancer cells. Tuberc Respir Dis
(Seoul). 75:9–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z and Choi ME: Autophagy in kidney
health and disease. Antioxid Redox Signal. 20:519–537. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huber TB, Edelstein CL, Hartleben B, Inoki
K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, et
al: Emerging role of autophagy in kidney function, diseases and
aging. Autophagy. 8:1009–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He L, Wei Q, Liu J, Yi M, Liu Y, Liu H,
Sun L, Peng Y, Liu F, Venkatachalam MA and Dong Z: AKI on CKD:
Heightened injury, suppressed repair and the underlying mechanisms.
Kidney Int. 92:1071–1083. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trongtrakul K, Sawawiboon C, Wang AY,
Chitsomkasem A, Limphunudom P, Kurathong S, Prommoon S,
Trakarnvanich T and Srisawat N: Acute kidney injury in critically
Ill surgical patients: Epidemiology, risk factors and outcomes.
Nephrology (Carlton). 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng X, Xie Y and Zhang A: Advance of
autophagy in chronic kidney diseases. Ren Fail. 39:306–313. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sekito T, Kawamata T, Ichikawa R, Suzuki K
and Ohsumi Y: Atg17 recruits Atg9 to organize the
pre-autophagosomal structure. Genes Cells. 14:525–538. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livingston MJ and Dong Z: Autophagy in
acute kidney injury. Semin Nephrol. 34:17–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mi N, Chen Y, Wang S, Chen M, Zhao M, Yang
G, Ma M, Su Q, Luo S, Shi J, et al: CapZ regulates autophagosomal
membrane shaping by promoting actin assembly inside the isolation
membrane. Nat Cell Biol. 17:1112–1123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sureshbabu A, Ryter SW and Choi ME:
Oxidative stress and autophagy: Crucial modulators of kidney
injury. Redox Biol. 4:208–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ravanan P, Srikumar IF and Talwar P:
Autophagy: The spotlight for cellular stress responses. Life Sci.
188:53–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang JC, Feng YL, Liang X and Cai XJ:
Autophagy in 5-fluorouracil therapy in gastrointestinal cancer:
Trends and challenges. Chin Med J (Engl). 129:456–463. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Degenhardt K, Mathew R, Beaudoin B, Bray
K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pla A, Pascual M and Guerri C: Autophagy
constitutes a protective mechanism against ethanol toxicity in
mouse astrocytes and neurons. PLoS One. 11:e01530972016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shirakabe A, Ikeda Y, Sciarretta S,
Zablocki DK and Sadoshima J: Aging and autophagy in the heart. Circ
Res. 118:1563–1576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang XY, Yang H, Wang MG, Yang DB, Wang ZY
and Wang L: Trehalose protects against cadmium-induced cytotoxicity
in primary rat proximal tubular cells via inhibiting apoptosis and
restoring autophagic flux. Cell Death Dis. 8:e30992017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Almeida DC, Donizetti-Oliveira C,
Barbosa-Costa P, Origassa CS and Câmara NO: In search of mechanisms
associated with mesenchymal stem cell-based therapies for acute
kidney injury. Clin Biochem Rev. 34:131–144. 2013.PubMed/NCBI
|
|
27
|
Zhang YL, Zhang J, Cui LY and Yang S:
Autophagy activation attenuates renal ischemia-reperfusion injury
in rats. Exp Biol Med (Maywood). 240:1590–1598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guan X, Qian Y, Shen Y, Zhang L, Du Y, Dai
H, Qian J and Yan Y: Autophagy protects renal tubular cells against
ischemia/reperfusion injury in a time-dependent manner. Cell
Physiol Biochem. 36:285–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ling H, Chen H, Wei M, Meng X, Yu Y and
Xie K: The effect of autophagy on inflammation cytokines in renal
ischemia/reperfusion injury. Inflammation. 39:347–356. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang M, Liu K, Luo J and Dong Z:
Autophagy is a renoprotective mechanism during in vitro hypoxia and
in vivo ischemia-reperfusion injury. Am J Pathol. 176:1181–1192.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang M, Wei Q, Dong G, Komatsu M, Su Y
and Dong Z: Autophagy in proximal tubules protects against acute
kidney injury. Kidney Int. 82:1271–1283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chandrika BB, Yang C, Ou Y, Feng X, Muhoza
D, Holmes AF, Theus S, Deshmukh S, Haun RS and Kaushal GP:
Endoplasmic reticulum stress-induced autophagy provides
cytoprotection from chemical hypoxia and oxidant injury and
ameliorates renal ischemia-reperfusion injury. PLoS One.
10:e01400252015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kimura T, Takabatake Y, Takahashi A,
Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T,
Soga T, et al: Autophagy protects the proximal tubule from
degeneration and acute ischemic injury. J Am Soc Nephrol.
22:902–913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu S, Hartleben B, Kretz O, Wiech T,
Igarashi P, Mizushima N, Walz G and Huber TB: Autophagy plays a
critical role in kidney tubule maintenance, aging and
ischemia-reperfusion injury. Autophagy. 8:826–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsumoto T, Urushido M, Ide H, Ishihara
M, Hamada-Ode K, Shimamura Y, Ogata K, Inoue K, Taniguchi Y,
Taguchi T, et al: Small heat shock protein beta-1 (HSPB1) is
upregulated and regulates autophagy and apoptosis of renal tubular
cells in acute kidney injury. PLoS One. 10:e01262292015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang LT, Chen BL, Wu CT, Huang KH, Chiang
CK and Hwa Liu S: Protective role of AMP-activated protein
kinase-evoked autophagy on an in vitro model of
ischemia/reperfusion-induced renal tubular cell injury. PLoS One.
8:e798142013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Decuypere JP, Ceulemans LJ, Agostinis P,
Monbaliu D, Naesens M, Pirenne J and Jochmans I: Autophagy and the
kidney: Implications for ischemia-reperfusion injury and therapy.
Am J Kidney Dis. 66:699–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Isaka Y, Suzuki C, Abe T, Okumi M,
Ichimaru N, Imamura R, Kakuta Y, Matsui I, Takabatake Y, Rakugi H,
et al: Bcl-2 protects tubular epithelial cells from
ischemia/reperfusion injury by dual mechanisms. Transplant Proc.
41:pp. 52–54. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chien CT, Shyue SK and Lai MK: Bcl-xL
augmentation potentially reduces ischemia/reperfusion induced
proximal and distal tubular apoptosis and autophagy.
Transplantation. 84:1183–1190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suzuki C, Isaka Y, Takabatake Y, Tanaka H,
Koike M, Shibata M, Uchiyama Y, Takahara S and Imai E:
Participation of autophagy in renal ischemia/reperfusion injury.
Biochem Biophys Res Commun. 368:100–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Turkmen K, Martin J, Akcay A, Nguyen Q,
Ravichandran K, Faubel S, Pacic A, Ljubanović D, Edelstein CL and
Jani A: Apoptosis and autophagy in cold preservation ischemia.
Transplantation. 91:1192–1197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu SY, Dong B, Zhou SH and Tang L: LncRNA
MALAT1: A potential regulator of autophagy in myocardial
ischemia-reperfusion injury. Int J Cardiol. 247:252017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu XJ, Hong Q, Wang Z, Yu YY, Zou X and
Xu LH: MicroRNA-34a suppresses autophagy in tubular epithelial
cells in acute kidney injury. Am J Nephrol. 42:168–175. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu X, Hong Q, Wang Z, Yu Y, Zou X and Xu
L: MiR-21 inhibits autophagy by targeting Rab11a in renal
ischemia/reperfusion. Exp Cell Res. 338:64–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arany I and Safirstein RL: Cisplatin
nephrotoxicity. Semin Nephrol. 23:460–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: Mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Levine B and Yuan J: Autophagy in cell
death: An innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu H, Gu LB, Tu Y, Hu H, Huang YR and Sun
W: Emodin ameliorates cisplatin-induced apoptosis of rat renal
tubular cells in vitro by activating autophagy. Acta Pharmacol Sin.
37:235–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Periyasamy-Thandavan S, Jiang M, Wei Q,
Smith R, Yin XM and Dong Z: Autophagy is cytoprotective during
cisplatin injury of renal proximal tubular cells. Kidney Int.
74:631–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fang B and Xiao H: Rapamycin alleviates
cisplatin-induced ototoxicity in vivo. Biochem Biophys Res Commun.
448:443–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sprowl JA, Lancaster CS, Pabla N, Hermann
E, Kosloske AM, Gibson AA, Li L, Zeeh D, Schlatter E, Janke LJ, et
al: Cisplatin-induced renal injury is independently mediated by
OCT2 and p53. Clin Cancer Res. 20:4026–4035. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cummings BS and Schnellmann RG:
Cisplatin-induced renal cell apoptosis: Caspase 3-dependent and
-independent pathways. J Pharmacol Exp Ther. 302:8–17. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang
L and Dong Z: Regulation of PUMA-alpha by p53 in cisplatin-induced
renal cell apoptosis. Oncogene. 25:4056–4066. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang M, Yi X, Hsu S, Wang CY and Dong Z:
Role of p53 in cisplatin-induced tubular cell apoptosis: Dependence
on p53 transcriptional activity. Am J Physiol Renal Physiol.
287:F1140–F1147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Seth R, Yang C, Kaushal V, Shah SV and
Kaushal GP: p53-Dependent caspase-2 activation in mitochondrial
release of apoptosis-inducing factor and its role in renal tubular
epithelial cell injury. J Biol Chem. 280:31230–31239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wei Q, Dong G, Franklin J and Dong Z: The
pathological role of Bax in cisplatin nephrotoxicity. Kidney Int.
72:53–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wei Q, Dong G, Yang T, Megyesi J, Price PM
and Dong Z: Activation and involvement of p53 in cisplatin-induced
nephrotoxicity. Am J Physiol Renal Physiol. 293:F1282–F1291. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Feng Z, Zhang H, Levine AJ and Jin S: The
coordinate regulation of the p53 and mTOR pathways in cells. Proc
Natl Acad Sci USA. 102:pp. 8204–8209. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wei L, Chen W, Zou Y, Huang H, Pan B, Jin
S, Huang R, Nie S and Kong G: AMP-activated protein kinase
regulates autophagic protection against cisplatin-induced tissue
injury in the kidney. Genet Mol Res. 14:12006–12015. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim TW, Kim YJ, Kim HT, Park SR, Lee MY,
Park YD, Lee CH and Jung JY: NQO1 deficiency leads enhanced
autophagy in cisplatin-induced acute kidney injury through the
AMPK/TSC2/mTOR signaling pathway. Antioxid Redox Signal.
24:867–883. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Herzog C, Yang C, Holmes A and Kaushal GP:
zVAD-fmk prevents cisplatin-induced cleavage of autophagy proteins
but impairs autophagic flux and worsens renal function. Am J
Physiol Renal Physiol. 303:F1239–F1250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Scaringi L, Cornacchione P, Ayroldi E,
Corazzi L, Capodicasa E, Rossi R and Marconi P: Omeprazole induces
apoptosis in jurkat cells. Int J Immunopathol Pharmacol.
17:331–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bizat N, Galas MC, Jacquard C, Boyer F,
Hermel JM, Schiffmann SN, Hantraye P, Blum D and Brouillet E:
Neuroprotective effect of zVAD against the neurotoxin
3-nitropropionic acid involves inhibition of calpain.
Neuropharmacology. 49:695–702. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Madden DT, Egger L and Bredesen DE: A
calpain-like protease inhibits autophagic cell death. Autophagy.
3:519–522. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rovetta F, Stacchiotti A, Consiglio A,
Cadei M, Grigolato PG, Lavazza A, Rezzani R and Aleo MF: ER
signaling regulation drives the switch between autophagy and
apoptosis in NRK-52E cells exposed to cisplatin. Exp Cell Res.
318:238–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xu QX, Qiu XY, Jiao Z, Zhang M and Zhong
MK: FOXP3 rs3761549 polymorphism predicts long-term renal allograft
function in patients receiving cyclosporine-based immunosuppressive
regimen. Gene. 2017.(Epub ahead of print).
|
|
68
|
Lim SW, Hyoung BJ, Piao SG, Doh KC, Chung
BH and Yang CW: Chronic cyclosporine nephropathy is characterized
by excessive autophagosome formation and decreased autophagic
clearance. Transplantation. 94:218–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yadav RK, Lee GH, Lee HY, Li B, Jung HE,
Rashid HO, Choi MK, Yadav BK, Kim WH, Kim KW, et al: TMBIM6
(transmembrane BAX inhibitor motif containing 6) enhances autophagy
and reduces renal dysfunction in a Cyclosporine A-induced
nephrotoxicity model. Autophagy. 11:1760–1774. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yegenaga I, Tuglular S, Ari E, Etiler N,
Baykara N, Torlak S, Acar S, Akbas T, Toker K and Solak ZM:
Evaluation of sepsis/systemic inflammatory response syndrome, acute
kidney injury and RIFLE criteria in two tertiary hospital intensive
care units in Turkey. Nephron Clin Pract. 115:c276–c282. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schrier RW and Wang W: Acute renal failure
and sepsis. N Engl J Med. 351:159–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Leventhal JS, Ni J, Osmond M, Lee K,
Gusella GL, Salem F and Ross MJ: Autophagy limits endotoxemic acute
kidney injury and alters renal tubular epithelial cell cytokine
expression. PLoS One. 11:e01500012016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hsiao HW, Tsai KL, Wang LF, Chen YH,
Chiang PC, Chuang SM and Hsu C: The decline of autophagy
contributes to proximal tubular dysfunction during sepsis. Shock.
37:289–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mei S, Livingston M, Hao J, Li L, Mei C
and Dong Z: Autophagy is activated to protect against endotoxic
acute kidney injury. Sci Rep. 6:221712016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Howell GM, Gomez H, Collage RD, Loughran
P, Zhang X, Escobar DA, Billiar TR, Zuckerbraun BS and Rosengart
MR: Augmenting autophagy to treat acute kidney injury during
endotoxemia in mice. PLoS One. 8:e695202013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Golpanian S, Wolf A, Hatzistergos KE and
Hare JM: Rebuilding the damaged heart: Mesenchymal stem cells,
cell-based therapy and engineered heart tissue. Physiol Rev.
96:1127–1168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gazdic M, Arsenijevic A, Markovic BS,
Volarevic A, Dimova I, Djonov V, Arsenijevic N, Stojkovic M and
Volarevic V: Mesenchymal stem cell-dependent modulation of liver
diseases. Int J Biol Sci. 13:1109–1117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen C and Hou J: Mesenchymal stem
cell-based therapy in kidney transplantation. Stem Cell Res Ther.
7:162016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen
L, Wang M, Zhou Y, Zhu W, Li W and Xu W: Exosomes derived from
human umbilical cord mesenchymal stem cells alleviate liver
fibrosis. Stem Cells Dev. 22:845–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang B, Wang M, Gong A, Zhang X, Wu X,
Zhu Y, Shi H, Wu L, Zhu W, Qian H and Xu W: HucMSC-exosome
mediated-Wnt4 signaling is required for cutaneous wound healing.
Stem Cells. 33:2158–2168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H,
Zhu W and Xu W: Exosomes derived from human umbilical cord
mesenchymal stem cells relieve acute myocardial ischemic injury.
Stem Cells Int. 2015:7616432015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Meirelles Lda S, Fontes AM, Covas DT and
Caplan AI: Mechanisms involved in the therapeutic properties of
mesenchymal stem cells. Cytokine Growth Factor Rev. 20:419–427.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tögel F, Zhang P, Hu Z and Westenfelder C:
VEGF is a mediator of the renoprotective effects of multipotent
marrow stromal cells in acute kidney injury. J Cell Mol Med.
13:2109–2114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bruno S, Grange C, Collino F, Deregibus
MC, Cantaluppi V, Biancone L, Tetta C and Camussi G: Microvesicles
derived from mesenchymal stem cells enhance survival in a lethal
model of acute kidney injury. PLoS One. 7:e331152012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
da Costa MR, Pizzatti L, Lindoso RS,
Sant'Anna JF, DuRocher B, Abdelhay E and Vieyra A: Mechanisms of
kidney repair by human mesenchymal stromal cells after ischemia: A
comprehensive view using label-free MS(E). Proteomics.
14:1480–1493. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Xing L, Cui R, Peng L, Ma J, Chen X, Xie
RJ and Li B: Mesenchymal stem cells, not conditioned medium,
contribute to kidney repair after ischemia-reperfusion injury. Stem
Cell Res Ther. 5:1012014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang G, Zou X, Huang Y, Wang F, Miao S,
Liu G, Chen M and Zhu Y: Mesenchymal stromal cell-derived
extracellular vesicles protect against acute kidney injury through
anti-oxidation by enhancing Nrf2/ARE activation in rats. Kidney
Blood Press Res. 41:119–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Moghadasali R, Azarnia M, Hajinasrollah M,
Arghani H, Nassiri SM, Molazem M, Vosough A, Mohitmafi S, Najarasl
M, Ajdari Z, et al: Intra-renal arterial injection of autologous
bone marrow mesenchymal stromal cells ameliorates cisplatin-induced
acute kidney injury in a rhesus Macaque mulatta monkey model.
Cytotherapy. 16:734–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y,
Zhang B, Wang M, Mao F, Yan Y, et al: Exosomes released by human
umbilical cord mesenchymal stem cells protect against
cisplatin-induced renal oxidative stress and apoptosis in vivo and
in vitro. Stem Cell Res Ther. 4:342013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yao W, Hu Q, Ma Y, Xiong W, Wu T, Cao J
and Wu D: Human adipose-derived mesenchymal stem cells repair
cisplatin-induced acute kidney injury through antiapoptotic
pathways. Exp Ther Med. 10:468–476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang W, Liu L, Huo Y, Yang Y and Wang Y:
Hypoxia-pretreated human MSCs attenuate acute kidney injury through
enhanced angiogenic and antioxidative capacities. Biomed Res Int.
2014:4624722014.PubMed/NCBI
|
|
94
|
Qiao PF, Yao L, Zhang XC, Li GD and Wu DQ:
Heat shock pretreatment improves stem cell repair following
ischemia-reperfusion injury via autophagy. World J Gastroenterol.
21:12822–12834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhao K, Hao H, Liu J, Tong C, Cheng Y, Xie
Z, Zang L, Mu Y and Han W: Bone marrow-derived mesenchymal stem
cells ameliorate chronic high glucose-induced β-cell injury through
modulation of autophagy. Cell Death Dis. 6:e18852015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li J, Zhou J, Zhang D, Song Y, She J and
Bai C: Bone marrow-derived mesenchymal stem cells enhance autophagy
via PI3K/AKT signaling to reduce the severity of
ischaemia/reperfusion-induced lung injury. J Cell Mol Med.
19:2341–2351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shin JY, Park HJ, Kim HN, Oh SH, Bae JS,
Ha HJ and Lee PH: Mesenchymal stem cells enhance autophagy and
increase β-amyloid clearance in Alzheimer disease models.
Autophagy. 10:32–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Park HJ, Shin JY, Kim HN, Oh SH and Lee
PH: Neuroprotective effects of mesenchymal stem cells through
autophagy modulation in a parkinsonian model. Neurobiol Aging.
35:1920–1928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim
HS, Park YS, Jo I, Park JW, Jung SC, et al: Tonsil-derived
mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in
mice via autophagy activation. Sci Rep. 5:86162015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang B, Jia H, Zhang B, Wang J, Ji C, Zhu
X, Yan Y, Yin L, Yu J, Qian H and Xu W: Pre-incubated with
hucMSC-Exosomes prevent cisplatin-induced nephrotoxicity by
activating autophagy. Stem Cell Res Ther. 8:752017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fougeray S and Pallet N: Mechanisms and
biological functions of autophagy in diseased and ageing kidneys.
Nat Rev Nephrol. 11:34–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang D, Xu X and Dong Z: PRKCD/PKCσ
contributes to nephrotoxicity during cisplatin chemotherapy by
suppressing autophagy. Autophagy. 13:631–632. 2017. View Article : Google Scholar : PubMed/NCBI
|