Introduction
Periodontitis is a kind of chronic inflammatory
diseases caused by bacteria, which can impact soft and hard tissue
around tooth (1). The ideal
results of periodontal treatment is to obtain tissue regeneration
(2). Periodontal regeneration is a
complex process that requires the coordination of proliferation and
differentiation of functional cells. Firstly, residual periodontal
stem cells (PDLSCs), alveolar perivascular or systemic mesenchymal
stem cells (MSCs) begin to proliferate and migrate to the defect.
Then these cells differentiate multidirectionally, forming new
cementoblast, periodontal fibroblasts and osteoblasts (3). Apparently, the number and quality of
regenerative cells in defect area is the key to periodontal
regeneration. However, due to the chronic inflammation, the number
of regenerative cells in the periodontal defect area is inadequate
and the function is compromised. Tissue engineering technique
centered on stem cell therapy is one of the main strategies for the
current study of periodontal regeneration. In brief, tissue
engineering is an emerging discipline that combines seed cells,
scaffold materials and cytokines. After a period of time of
cultivation in vitro, the compounds were implanted in
vivo to form new tissues and organs (4). The development of tissue engineering
leads to new prospects for tissue or organ repair, but there are
still some disadvantages: exogenous stem cells may cause immune
rejection; autologous stem cells probably cause a secondary injury
to the patient; although PDLSCs and dental pulp stem cells can be
derived from extracted teeth under special circumstances, the
process of collection, cultivation and re-transplantation for seed
cells takes long time and high cost (5). Therefore, the clinical transformation
of tissue engineering techniques in periodontal regeneration faces
challenges. In order to overcome the shortcomings of traditional
tissue engineering techniques, the researchers tried to strengthen
the endogenous wound healing process by stimulating body's own
repair ability. This strategy of tissue regeneration without the
need for exogenous cell transplants is named in situ tissue
engineering technique (6). It has
been proved in medical disciplines that, through the endogenous
stem cell migration to the damaged area, tissue regeneration can be
achieved without exogenous cell transplantation (7–9).
Recruitment of enough endogenous functional cells to the defect
regions and promotion of their committed differentiation at
appropriate times to re-establish the destroyed periodontium
becomes a new strategy for periodontal regeneration (10).
The key elements of in situ tissue
engineering are the application of chemokine and biomaterials with
chemotaxis. The recruitment for MSCs can be accomplished through
different bioactive factors such as stromal cell-derived factor-1
(SDF-1), bone morphogenetic protein (BMP), fibroblast growth factor
(FGF) and platelet derived growth factor (PDGF) (11). However, the optimal choice of
factors has not been determined. SDF-1, now named as CXCL12, is a
kind of classical chemotactic agent, which is constitutively
expressed by human gingival fibroblasts (HGFs) and by human
periodontal ligament (PDL) fibroblasts (HPDLFs) (12). SDF-1 and its receptor, C-X-C motif
receptor 4 (CXCR4) play a vital role in the development of
embryonic organs (13),
maintaining tissue homeostasis after birth (14) and bone remodeling (15). CXCR4 expression is found on the
cell surface in human and rat MSCs (16) and human PDLSCs (12). A series of studies have shown that
the local expression of SDF-1 increases after injury of tissues
like heart, brain, liver and bone, and MSCs can be recruited and
repair damaged tissues (17–20).
Moreover, SDF-1 can promote the migration and proliferation of stem
cells and then enhance periodontal bone regeneration (10,21).
Besides, SDF-1 has the ability to promote angiogenesis (22) and reduces inflammation, which could
prevent the host from strong immune response to the implant
(23). bFGF also has extensive
biological activities, which is present in basement membranes, in
the subendothelial extracellular matrix of blood vessels in normal
tissue and in periodontal ligament (24). The study showed that bFGF can
regulate cell proliferation and differentiation (25,26)
and is able to promote angiogenesis (27) and nerve regeneration (28,29).
In an in vitro experiment, bFGF was found to sustain
self-renewal ability, while maintaining differentiation potency
(30,31). Furthermore, documents and our
previous studies show that bFGF significantly promote migration of
MSCs (25,32) and chemotactic activity of bFGF for
MSCs is even stronger than SDF-1 (33) or BMP-2 (32).
In periodontal tissue regeneration, the
proliferation and differentiation of functional cells is a
continuous process. The first step is the migration and
proliferation of PDLSCs and MSCs, which make the periodontal defect
to be occupied by sufficient precursors. These precursors then
multi-directionally differentiate, forming the periodontal
ligament, alveolar bone and cementum. This implies that apart from
direct effect on migration and proliferation, the osteoblastic and
cementoblastic differentiation potency of MSCs treated by cytokines
will determine the final outcome of periodontal regeneration. To
our limited knowledge, comparing investigation of different
cytokines on this aspect has not been conducted, thus, osteogenic
differentiation capability of BMMSCs (ST2 cell line) pretreated
separately with bFGF and SDF-1 was compared here. It will provide
certain guidance for the selection of chemotactic agent in the
in situ periodontal tissue engineering.
Materials and methods
BMMSC culture
BMMSCs (ST2 cell line, donated by key laboratory of
oral tissue regeneration in Shandong province) were recovered from
cryopreservation. Then the cells were cultured in maintenance
medium (DMEM containing 10% fetal bovine serum, 100 U/ml penicillin
G and 100 µg/ml streptomycin, Hyclone, USA). BMMSCs were cultured
in an incubator at 37°C with an atmosphere comprising 95% air and
5% CO2 and 100% relative humidity. The medium was
changed every other day.
BMMSC pretreatment by bFGF and
SDF-1
BMMSCs were cultured till the 3rd generation,
non-adherent cells were discarded and adherent cells were washed
three times using phosphate buffered saline (PBS). All cells were
divided into three groups: the control group (cultured in
maintenance medium, 1% fetal bovine serum, 100 U/ml penicillin G
and 100 µg/ml streptomycin), bFGF-pretreated group (cultured in
maintenance medium with 20 ng/ml bFGF, 1% fetal bovine serum, 100
U/ml penicillin G and 100 µg/ml streptomycin), SDF-1-pretreted
group (cultured in maintenance medium with 200 ng/ml SDF-1, 1%
fetal bovine serum, 100 U/ml penicillin G and 100 µg/ml
streptomycin). Cells were incubated according to above grouping for
48 h.
BMMSC osteogenic differentiation
assay
After 48 h of culture, three groups of cells were
washed three times with PBS and cultured in an osteogenic medium
(10−8 M dexamethasone (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), 10 mM β-glycerophosphate (Sigma-Aldrich; Merck
KGaA) and 50 ng/ml ascorbic acid (Sigma-Aldrich; Merck KGaA) in
DMEM-F12 containing 10% FBS) for 3, 7, 14 and 28 days for following
experiments.
RT-PCR assay
After 3, 7, 14 days of osteogenic induction, cell
samples were collected. Total RNA was quantified with GeneQuant pro
spectrophotometer (Biochrom, Ltd., Cambridge, UK) and was reversely
transcribed into cDNA with Trizol kit (Triton X-100; Ameresco,
Inc., Framingham, MA, USA). Reverse transcription was conducted in
a 20 µl reaction volume with the following protocol: 37°C for 15
min and 85°C for 5 sec. RT-PCR was conducted by real-time
fluorescent quantitative PCR (Light Cycler II 480; Roche, Mannheim,
Germany). cDNA were kept at −20°C for the following measure.
Expression levels of osteoblastic genes including alkaline
phosphatase (ALP), runt related transcription factor 2 (Runx-2) and
bone sialoprotein (BSP). The primer sequences for ALP, BSP, Runx-2
were designed as Table I and GAPDH
used as a reference gene. Each reaction mixture had a total volume
of 10 µl and contained SYBR (SYBR® Premix Ex Taq™ II
kit; Takara Biotechnology Co., Ltd., Dalian, China) 3.6 µl, sterile
threefold-distilled water 5 µl, cDNA 1 µl, forward primer and
reverse peimer 0.2 µl respectively. RT-PCR was conducted with the
following protocols: 1 cycle of 95°C for 30 sec, followed by 45
cycles of 95°C for 5 sec and 60°C for 35 sec, and then 1 cycles of
95°C for 15 sec and 60°C for 60 sec, at last 1 cycle of 40°C for 30
sec.
 | Table I.Primer sequences for RT-PCR. |
Table I.
Primer sequences for RT-PCR.
| Gene | Forward primer | Reverse primer |
|---|
| GAPDH |
5′-AGGTCGGTGTGAACGGATTTG-3′ |
5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
| ALP |
5′-CTTGCTGGTGGAAGGAGGCAGG-3′ |
5′-GGAGCACAGGAAGTTGGGAC-3′ |
| BSP |
5′-CAGGGAGGCAGTGACTCTTC-3′ |
5′-AGTGTGGAAAGTGTGGCGTT-3′ |
| Runx-2 |
5′-CCCAGCCACCTTTACCTACA-3′ |
5′-TATGGAGTGCTGCTGGTCTG-3′ |
Western blot assay
ALP, BSP and Runx-2 were also measured by western
blot analysis at 3, 7 and14 days of osteogenic induction. Proteins
were extracted from the cells by ice-cold RIPA lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) containing 1% phenylmethylsulfonyl fluoride (PMSF; Beijing
Solarbio Science & Technology Co., Ltd.) for 30 min. Lysate
were collected and centrifugated at 12,000 × g at 4°C for 15 min
and preserved at −80 °C for later examination. Bovine serum albumin
(BSA) was used for establishing standard curve of protein
concentration according to the manufacturer's instructions of BSA
(Beijing Solarbio Science & Technology Co., Ltd.).
Samples and BSA maker were subjected to 10% SDS-PAGE
and transferred to polyvinylidene difluoride membranes (PVDF; GE
Amersham, Fairfield, CT, USA) by electroblotting. Filters were then
blocked in 5% non-fat milk-Tris buffered saline (TBS)-0.05%
Tween-20 for 1 h and incubated with the primary antibodies
overnight at 4°C as followed: rabbit monoclonal anti-Runx-2
antibody (1:2,000 dilution, ab23981; Abcam, Cambridge, UK), rabbit
monoclonal anti-ALP antibody (1:500 dilution, ab108337; Abcam), and
rabbit monoclonal anti-BSP antibody (1:1,000 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). PVDF membranes were washed
by TBST three times for 10 min. Then, Filters were blocked in 5%
non-fat milk-Tris buffered saline (TBS)-0.05% Tween-20 with the
secondary antibodies (1:20,000 dilution) for 1 h. The bands
corresponding to Runx-2, ALP and BSP were detected using a
chemiluminescence reagent (Merck Millipore, Darmstadt, Germany).
Images were collected with image J (National Institutes of Health,
Bethesda, MD, USA).
Mineral nodules formation assay
After 28 days of culture, mineralized nodules were
stained. Cells were washed three times with PBS and incubated with
0.5 ml of alizarin red S solution (1%) for 30 min until the
mineralized nodules had stained red. Excessive reagents were washed
by distilled water and dried. Digital images were captured using a
light microscope (IX71; Olympus Corporation, Tokyo, Japan). Numbers
of calcium nodules were observed in the field of vision under
original magnification ×100.
Statistical analysis
The experimental measurement data were given as the
mean ± standard deviation for each experiment and analyzed using
SPSS (version 17.0; SPSS, Inc., Chicago, IL, USA). A one-way
analysis of variance (ANOVA) was used for comparison between the
three groups, and LSD used for pairwise comparison. For each test,
P<0.05 was considered to indicate a statistically significant
difference.
Results
Morphology observation for BMMSCs
Adherent growth of ST2 cells was visible after
recovery or passage. Cells were polygonal or long fibrous, with
elongated and intersected connections between cells (Fig. 1A). All cells were in good condition
and proliferate fast, with a 90% confluency at the 2 to 3 days
after inoculation. After pretreated by bFGF and SDF-1 for 24 h, no
significant change in cell morphology can be observed. However, the
number of BMMSCs treated by bFGF and SDF-1 increased and
bFGF-pretreated group increased more apparently than SDF-1
(Fig. 1B-D).
RT-qPCR analysis
RT-qPCT showed that at day 3, BMMSCs pretreated by
bFGF showed significantly lower ALP and Runx-2 mRNA expression and
BMMSCs pretreated by SDF-1 showed significantly lower ALP mRNA
expression compared with negative group (Fig. 2A). However, at day 7, bFGF
pretreatment significantly promoted Runx-2, BSP and ALP mRNA
expressions compared with negative group. Moreover, bFGF-pretreated
group exhibited significantly higher expression of BSP and Runx-2
mRNA at day 7 than SDF-1-pretreated group (Fig. 2B). At day 14, both bFGF- and
SDF-1-pretreated group had no significantly different effect on
osteogenic differentiation compared with negative group (Fig. 2C).
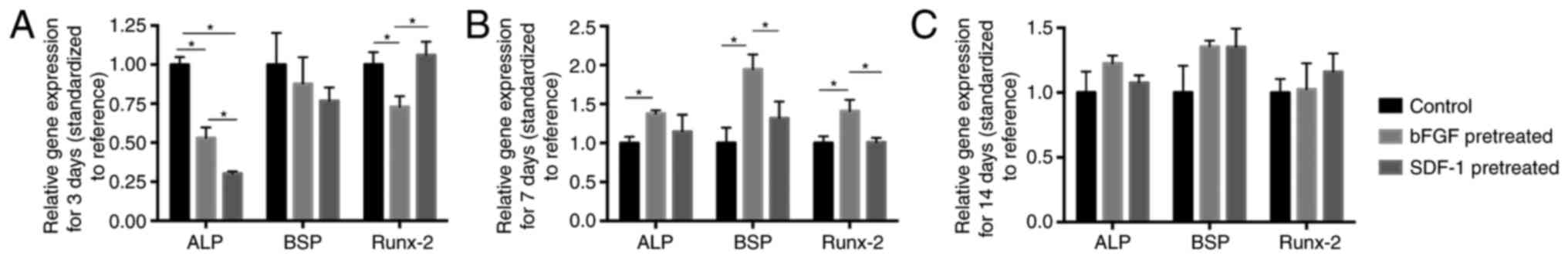 | Figure 2.RT-qPCR analysis of ALP, BSP and
Runx-2 mRNA expression levels in bFGF-, SDF-1-pretreated and
control groups. (A) At day 3, ALP expression in bFGF- and
SDF-1-pretreatment groups is significantly lower than that in
control group, and Runx-2 expression in bFGF-pretreated group is
significantly lower than control and SDF-1-pretreated group. (B) At
day 7, significantly higher levels of ALP were discernible in
bFGF-pretreated groups compared with control group, while bFGF
pretreatment significantly promoted BSP and Runx-2 expression
compared with control group and SDF-1-pretreated group. (C) At day
14, no significant difference was shown among groups. *P<0.05.
ALP, alkaline phosphatase; BSP, bone sialoprotein; Runx-2, runt
related transcription factor 2; bFGF, basic fibroblast growth
factor; SDF-1, stromal cell-derived factor-1. |
Western blot analysis
Western Blot technique was used to detect the level
of protein expression in the control group, bFGF pretreatment group
and the SDF-1 pretreatment group (Fig.
3). The result showed that, at day 3, ALP expression in bFGF-
and SDF-1-pretreated groups is significantly lower than that in
control group (P<0.05), while SDF-1 pretreatment significantly
promoted Runx-2 expression (Fig.
3B). At day 7, bFGF- and SDF-1-pretreatment groups
significantly increased ALP expression compared with control group
(P<0.05) (Fig. 3C). No
significant difference was found among groups at day 14 (Fig. 3D). However, at day 3, the ALP and
Runx-2 protein expression in SDF-1-pretreated group significantly
higher than that in bFGF-pretreated group (Fig. 3B).
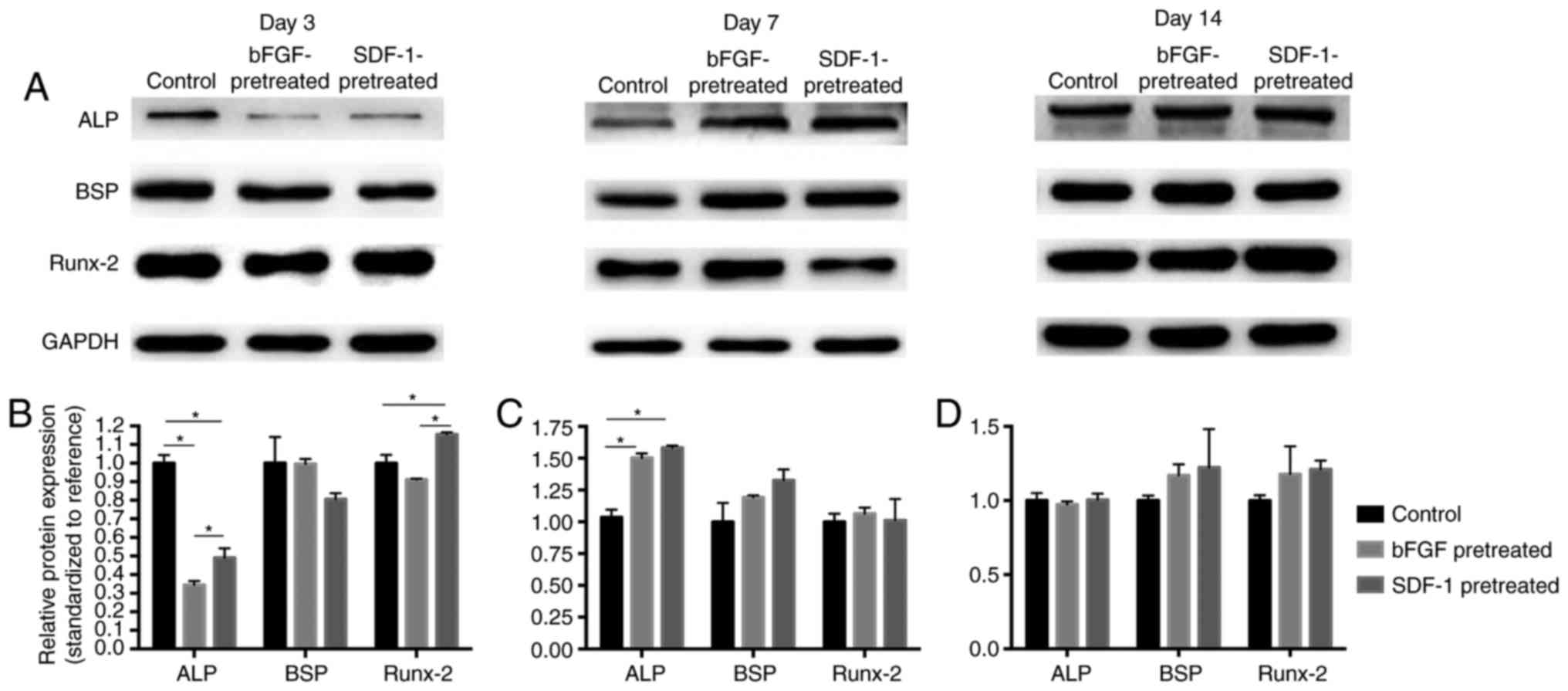 | Figure 3.Western-blot analysis of ALP, BSP and
Runx-2 protean expression levels in bFGF-, SDF-1-pretreated and
control groups. (A) Representative blots. (B) At day 3, ALP
expression in bFGF- and SDF-1-pretreatment groups is significantly
lower than that in control group (P<0.05), while SDF-1
pretreatment significantly promoted Runx-2 expression. (C) At day
7, significantly higher levels of ALP were discernible in bFGF- and
SDF-1-pretreated groups compared with control group. (D) At day 14,
no significant difference was shown among groups, *P<0.05. ALP,
alkaline phosphatase; BSP, bone sialoprotein; Runx-2, runt related
transcription factor 2; bFGF, basic fibroblast growth factor;
SDF-1, stromal cell-derived factor-1. |
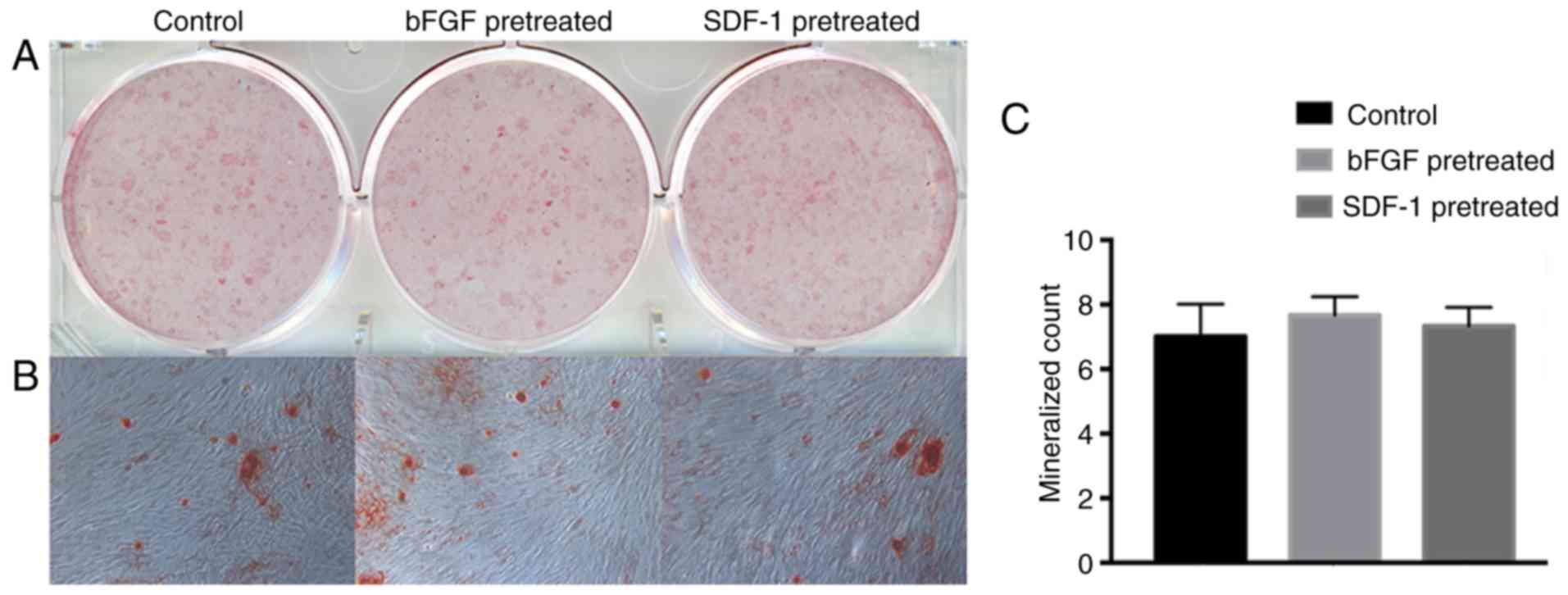
Mineral nodules formation
After 28 days of mineralized induced culture,
mineral nodules were stained and evaluated by macroscopic and the
microscopic observation. The bFGF- and SDF-1-pretreated groups were
able to form similar number of calcified nodules to the control
group (Fig. 4).
Discussion
This study set three groups: negative control,
bFGF-pretreated group and SDF-1-pretreated group. First, the effect
of bFGF and SDF-1 on morphology and numbers of BMMSCs were
observed. Results showed that morphology of BMMSCs did not change
apparently after treated by bFGF and SDF-1 for 24 h, while number
of BMMSCs obviously increased. The number of cells treated by bFGF
increased more apparently in relation to SDF-1. This suggests that
both bFGF and SDF-1 promote BMMSC proliferation and bFGF has
stronger effect than SDF-1. Then, osteogenic differentiation
markers in bFGF- and SDF-1-pretreated BMMSCs were detected after
osteogenic induction culture. Results revealed a differential
effect of bFGF- and SDF-1-pretreatment on osteogenic
differentiation potency of BMMSCs.
BMMSCs pretreated in maintenance medium containing
20 ng/ml bFGF or 200 ng/ml SDF-1 for 48 h were re-cultured in an
osteogenic medium. The ALP and Runx-2 mRNA expression and ALP
protein expression in bFGF pretreatment group were significantly
lower than in the control group after 3 days of cultivation.
However, at day 7, expressions of ALP, BSP and Runx-2 mRNA and ALP
protein were significantly higher than control. No significant
difference in osteogenic markers was proved on day 14 and 28. This
result suggests that direct stimulation by bFGF temporally inhibit
osteogenic differentiation of BMMSCs but bFGF pretreatment enhance,
at least maintain, medium and later osteogenic differentiation
potency. Direct inhibition of osteogenic differentiation of BMMSC
by bFGF was consistent with the experiment conducted by Tasso
(34), who proved that bFGF could
select MSCs with higher proliferation activity. Cells pretreated by
bFGF had higher proliferation activity, lower degree of
differentiation, and significantly lower ALP level than the control
group. The results of enhancing later osteogenic differentiation
potency by bFGF were similar to the basic pattern proved by our
previous experiment: on the day 7 after pretreated by bFGF, rat
BMMSCs showed the strongest osteogenic differentiation capability.
On contrast, our previous study also showed enhanced osteogenic
differentiation capability after 3 and 14 days pretreated by bFGF
(32). It is difficult to explain
the exact reason of the difference, while difference of cells used
in two studies may be main causes. Nevertheless, it is a fact that
bFGF can strengthen the osteogenic potential of MSCs.
SDF-1-pretreated group showed similar result to
bFGF-pretreated group, that is, osteogenesis was first inhibited
and then promoted. But there were also some differences between two
groups. Expressions of ALP mRNA on day 3 and BSP and Runx-2 mRNA on
day 7 in bFGF pretreatment group were significantly higher than
those in SDF-1 pretreatment group; expressions of Runx-2 mRNA and
ALP, Runx-2 protein on day 3 in SDF-1 pretreatment group were
higher than those in bFGF pretreatment group. The results suggest
that SDF-1-pretreated group put up lower inhibition in the early
period, but also weaker promotion in the later stage for osteogenic
differentiation compared with bFGF.
The transcription and translation process itself is
influenced by many factors. Protein regulation is multi-level,
multi-temporal and multi-type and RNA alone does not determine the
proteins. Other factors such as protein half-life and synthesis
speed also affect the levels of protein expression. Many studies
have found this phenomenon (35,36).
This may be the reason why inconsistent expressions of mRNA and
protean of some markers in BMMSCs pretreated by bFGF or SDF-1
(Figs. 2B and 3B).
SDF-1 has received a lot of attention in relation to
its biological activities such as cell recruitment, migration, and
proliferation, and also the effect to increases the periodontal
regeneration. Our previous study showed that SDF-1 can promote stem
cell recruitment, migration and proliferation, and promotes
periodontal regeneration (10). At
cell level, studies have shown that many stem cells have the
expression of CXCR4 (SDF-1 receptor), indicating that SDF-1/CRCX4
signal axis plays an important role in the migration and homing of
stem cell (20). In addition,
SDF-1 can significantly promote proliferation of osteoblasts; in
the region of the bone reconstruction, osteoblast can express
CXCL12/CXCR4, and also induce the expression of collagen type I
(37). Some studies suggest that
SDF-1 regulate BMP2-induced differentiation of primary MSCs into
osteoblastic cells. Blocking SDF-1/CXCR4 signal axis can inhibit
differentiation of MSCs which mediated by BMP-2 (38), suggesting that SDF-1 play a role in
osteogenic differentiation and promotion of bone regeneration.
Meanwhile, the extensive studies have shown that
bFGF can regulate cell proliferation, migration and
differentiation, and is able to promote angiogenesis (27) and nerve regeneration (28,29).
There have been numerous studies showing that bFGF can sustain
self-renewal and multidirectional potential of embryonic stem cells
and various forms of adult stem cell. Studies based on rabbit
embryonic stem cells (rESCs) show that signaling pathways involved
in stemness maintenance of rESCs includes TGF-β, FGF and Wnt.
Blocking the FGF downstream MAPK/ERK signal and PI3K/AKT pathway
leads to differentiation of rESCs (39). In addition, bFGF plays an important
role in maintaining stemness of stem cells isolated from human
exfoliated deciduous teeth and apical papilla to enhance colony
forming unit capacity and promote gene expression level of
pluripotent markers such as OCT4, REX1 and NANOG (40,41).
In the process of bone regeneration promoted by
MSCs, firstly, a sufficient cell number is necessary; secondly,
primary cells differentiate to functional cells like osteoblast,
cementoblast, osteoclast and so on. Thus, both homing and
proliferation and following osteogenic differentiation are
important events for bone tissue regeneration. Comparative studies
have shown that bFGF has greater chemotaxis ability to MSCs than
SDF-1 (31,42). The present study suggests that bFGF
has an advantage over SDF-1 in promoting proliferation and
maintaining the osteogenic potential for BMMSCs.
Angiogenesis and nerve regeneration play an
important role in periodontal/bone regeneration. Previous study has
also shown that angiogenic factors are expressed during the early
phases of bone formation and remodeling for reestablishment of the
vasculature, while osteogenic growth factors can continuously
expressed in the later phases (43,44),
which is in favor of bone regeneration. It has been demonstrated
that a dose of SDF-1 released from gelatin hydrogels enhanced
angiogenesis because of the recruitment of cells which are
effective in angiogenesis (45).
The bFGF has already been extensively studied that it can regulate
cell proliferation, cell migration and cell differentiation, able
to promote angiogenesis and nerve regeneration. Angiogenesis is an
important process of early bone formation and remodeling. At the
same time, nerve regeneration can improve the quality of newbone.
Potential to improve fracture healing in animal models of bFGF has
been studied already (46).
All in all, from the aspect of migration and
proliferation, or maintaining stemness and differentiation
potential, bFGF possesses some advantage over SDF-1 in the in
situ periodontal tissue engineering. The differential effect on
angiogenesis and nerve regeneration, activated different signal
pathways in MSCs and the periodontal regeneration effects in
vivo by bFGF and SDF-1still need to be further evaluated.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81271141 to P. Y.) and the
Construction Engineering Special Fund of ‘Taishan Scholars’.
References
|
1
|
Flemming TF: Periodontitis. Ann
Periodontol. 4:32–38. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villar CC and Cochran DL: Regeneration of
periodontal tissues: Guided tissue regeneration. Dent Clin North
Am. 54:73–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Melcher AH: On the repair potential of
periodontal tissues. J Periodontol. 47:256–260. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Langer R and Vacanti JP: Tissue
engineering. Science. 260:920–926. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sengupta D, Waldman SD and Li S: From in
vitro to in situ tissue engineering. Ann Biomed Eng. 42:1537–1545.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakahara T, Nakamura T, Kobayashi E, Inoue
M, Shigeno K, Tabata Y, Eto K and Shimizu Y: Novel approach to
regeneration of periodontal tissues based on in situ tissue
engineering: Effects of controlled release of basic fibroblast
growth factor from a sandwich membrane. Tissue Eng. 9:153–162.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shao Z, Zhang X, Pi Y, Wang X, Jia Z, Zhu
J, Wang X, Jia Z, Zhu J, Dai L, et al: Polycaprolactone electrospun
mesh conjugated with an MSC affinity peptide for MSC homing in
vivo. Biomaterials. 33:3375–3387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shafiq M, Lee SH, Jung Y and Kim SH:
Strategies for recruitment of stem cells to treat myocardial
infarction. Curr Pharm Des. 21:1584–1597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee CH, Cook JL, Mendelson A, Moioli EK,
Yao H and Mao JJ: Regeneration of the articular surface of the
rabbit synovial joint by cell homing: A proof of concept study.
Lancet. 376:440–448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Li M, Du L, Yang P and Ge S: Local
administration of stromal cell-derived factor-1 promotes stem cell
recruitment and bone regeneration in a rat periodontal bone defect
model. Mater Sci Eng C Mater Biol Appl. 53:83–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vanden Berg-Foels WS: In situ tissue
regeneration: Chemoattractants for endogenous stem cell
recruitment. Tissue Eng Part B Rev. 20:28–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du L, Yang P and Ge S: Stromal
cell-derived factor-1 significantly induces proliferation,
migration, and collagen type I expression in a human periodontal
ligament stem cell subpopulation. J Periodontol. 83:379–388. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rehimi R, Khalida N, Yusuf F, Dai F,
Morosan-Puopolo G and Brand-Saberi B: Stromal-derived factor-1
(SDF-1)expression during early chick development. Int J Dev Biol.
52:87–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baggiolini M: Chemokines and leukocyte
traffic. Nature. 392:565–568. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu X, Huang Y, Collin-Osdoby P and Osdoby
P: Stromal cell-derived factor-1 (SDF-1) recruits osteoclast
precursors by inducing chemotaxis, matrix metalloproteinase-9
(MMP-9) activity, and collagen transmigration. J Bone Miner Res.
18:1404–1418. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wynn RF, Hart CA, Corradi-Perini C,
O'Neill L, Evans CA, Wraith JE, Fairbairn LJ and Bellantuono I: A
small proportion of mesenchymal stem cells strongly expresses
functionally active CXCR4 receptor capable of promoting migration
to bone marrow. Blood. 104:2643–2645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kucia M, Ratajczak J, Reca R,
Janowska-Wieczorek A and Ratajczak MZ: Tissue-specific muscle,
neural and liver stem/progenitor cells reside in the bone marrow,
respond to an SDF-1 gradient and are mobilized into peripheral
blood during stress and tissue injury. Blood Cells Mol Dis.
32:52–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abbott JD, Huang Y, Liu D, Hickey R,
Krause DS and Giordano FJ: Stromal cell-derived factor-1alpha plays
a critical role in stem cell recruitment to the heart after
myocardial infarction but is not sufficient to induce homing in the
absence of injury. Circulation. 110:3300–3305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji JF, He BP, Dheen ST and Tay SS:
Interactions of chemokines and chemokine receptors mediate the
migration of mesenchymal stem cells to the impaired site in the
brain after hypoglossal nerve injury. Stem Cells. 22:415–427. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji W, Yang F, Ma J, Bouma MJ, Boerman OC,
Chen Z, van den Beucken JJ and Jansen JA: Incorporation of stromal
cell-derived factor-1alpha in PCL/gelatin electrospun membranes for
guided bone regeneration. Biomaterials. 34:735–745. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J, Chen Y, Liu Y, Zhang J, Kang Q, Ho
K, Chai Y and Li G: Effect of SDF-1/Cxcr4 signaling antagonist
AMD3100 on bone mineralization in distraction osteogenesis. Calcif
Tissue Int. 100:641–652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ratanavaraporn J, Furuya H, Kohara H and
Tabata Y: Synergistic effects of the dual release of stromal
cell-derived factor-1 and bone morphogenetic protein-2 from
hydrogels on bone regeneration. Biomaterials. 32:2797–2811. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thevenot PT, Nair AM, Shen J, Lotfi P, Ko
CY and Tang L: The effect of incorporation of SDF-1alpha into PLGA
scaffolds on stem cell recruitment and the inflammatory response.
Biomaterials. 31:3997–4008. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grzibovskis M, Urtane I and Pilmane M:
Specific signaling molecule expression in periodontal ligaments in
different age groups: Pilot study. Stomatologija. 13:117–122.
2011.PubMed/NCBI
|
|
25
|
Osathanon T, Nowwarote N and Pavasant P:
Basic fibroblast growth factor inhibits mineralization but induces
neuronal differentiation by human dental pulp stem cells through a
FGFR and PLCgamma signaling pathway. J Cell Biochem. 112:1807–1816.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Narong S and Leelawat K: Basic fibroblast
growth factor induces cholangio-carcinoma cell migration via
activation of the MEK1/2 pathway. Oncol Lett. 2:821–825.
2011.PubMed/NCBI
|
|
27
|
Horikoshi-Ishihara H, Tobita M, Tajima S,
Tanaka R, Oshita T, Tabata Y and Mizuno H: Coadministration of
adipose-derived stem cells and control-released basic fibroblast
growth factor facilitates angiogenesis in a murine ischemic hind
limb model. J Vasc Surg. 64:1825–1834.e1. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu H, Yang A, Du J, Li D, Liu M, Ding F,
Gu X and Liu Y: Basic fibroblast growth factor is a key factor that
induces bone marrow mesenchymal stem cells towards cells with
Schwann cell phenotype. Neurosci Lett. 559:82–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikeda M, Uemura T, Takamatsu K, Okada M,
Kazuki K, Tabata Y, Ikada Y and Nakamura H: Acceleration of
peripheral nerve regeneration using nerve conduits in combination
with induced pluripotent stem cell technology and a basic
fibroblast growth factor drug delivery system. J Biomed Mater Res
A. 102:1370–1378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Tsai YT, Hsu CW, Erol D, Yang J, Wu
WH, Davis RJ, Egli D and Tsang SH: Long-term safety and efficacy of
human-induced pluripotent stem cell (iPS) grafts in a preclinical
model of retinitis pigmentosa. Mol Med. 18:1312–1319. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levenstein ME, Ludwig TE, Xu RH, Llanas
RA, VanDenHeuvel-Kramer K, Manning D and Thomson JA: Basic
fibroblast growth factor support of human embryonic stem cell
self-renewal. Stem Cells. 24:568–574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du M, Zhu T, Duan X, Ge S, Li N, Sun Q and
Yang P: Acellular dermal matrix loading with bFGF achieves similar
acceleration of bone regeneration to BMP-2 via differential effects
on recruitment, proliferation and sustained osteodifferentiation of
mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 70:62–70.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmidt A, Ladage D, Schinköthe T,
Klausmann U, Ulrichs C, Klinz FJ, Brixius K, Arnhold S, Desai B,
Mehlhorn U, et al: Basic fibroblast growth factor controls
migration in human mesenchymal stem cells. Stem Cells.
24:1750–1758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tasso R, Gaetani M, Molino E, Cattaneo A,
Monticone M, Bachi A and Cancedda R: The role of bFGF on the
ability of MSC to activate endogenous regenerative mechanisms in an
ectopic bone formation model. Biomaterials. 33:2086–2096. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Sousa Abreu R, Penalva LO, Marcotte EM
and Vogel C: Global signatures of protein and mRNA expression
levels. Mol Biosyst. 5:1512–1526. 2009.PubMed/NCBI
|
|
36
|
de Klerk E and 't Hoen PA: Alternative
mRNA transcription, processing, and translation: Insights from RNA
sequencing. Trends Genet. 31:128–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kitamura M, Nakashima K, Kowashi Y, Fujii
T, Shimauchi H, Sasano T, Furuuchi T, Fukuda M, Noguchi T,
Shibutani T, et al: Periodontal tissue regeneration using
fibroblast growth factor-2: Randomized controlled phase II clinical
trial. PLoS One. 3:e26112008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hosogane N, Huang Z, Rawlins BA, Liu X,
Boachie-Adjei O, Boskey AL and Zhu W: Stromal derived factor-1
regulates bone morphogenetic protein 2-induced osteogenic
differentiation of primary mesenchymal stem cells. Int J Biochem
Cell Biol. 42:1132–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S, Shen Y, Yuan X, Chen K, Guo X,
Chen Y, Niu Y, Li J, Xu RH, Yan X, et al: Dissecting signaling
pathways that govern self-renewal of rabbit embryonic stem cells. J
Biol Chem. 283:35929–35940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sukarawan W, Nowwarote N, Kerdpon P,
Pavasant P and Osathanon T: Effect of basic fibroblast growth
factor on pluripotent marker expression and colony forming unit
capacity of stem cells isolated from human exfoliated deciduous
teeth. Odontology. 102:160–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu J, Huang GT, He W, Wang P, Tong Z, Jia
Q, Dong L, Niu Z and Ni L: Basic fibroblast growth factor enhances
stemness of human stem cells from the apical papilla. J Endod.
38:614–622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ponte AL, Marais E, Gallay N, Langonné A,
Delorme B, Hérault O, Charbord P and Domenech J: The in vitro
migration capacity of human bone marrow mesenchymal stem cells:
Comparison of chemokine and growth factor chemotactic activities.
Stem Cells. 25:1737–1745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen FM, Zhang M and Wu ZF: Toward
delivery of multiple growth factors in tissue engineering.
Biomaterials. 31:6279–6308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kempen DH, Lu L, Heijink A, Hefferan TE,
Creemers LB, Maran A, Yaszemski MJ and Dhert WJ: Effect of local
sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone
regeneration. Biomaterials. 30:2816–2825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kimura Y and Tabata Y: Controlled release
of stromal-cell-derived factor-1 from gelatin hydrogels enhances
angiogenesis. J Biomater Sci Polym Ed. 21:37–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hankenson KD, Dishowitz M, Gray C and
Schenker M: Angiogenesis in bone regeneration. Injury. 42:556–561.
2011. View Article : Google Scholar : PubMed/NCBI
|