Introduction
Fatigue induced by vigorous exercise results from
peripheral alterations in muscles and alterations in the central
nervous system (1). Central
fatigue has an important role in fatigue during exercise (2). The activation of central dopaminergic
systems influences exercise performance (3) and central depletion of dopamine,
which is a neurotransmitter, has been previously associated with
physical fatigue (4). It is
established that dopamine functions as an important regulator of
the immune system, behavior and movement (5). Brain-derived neurotrophic factor
(BDNF) levels alter with exercise and physical activity (6), and mediate the trophic effect of
dopamine receptor activation (7).
It has been reported that depletion of BDNF expression leads to
stress in fatigue (8). Therefore,
BDNF is considered to be an attractive therapeutic target for the
treatment of chronic fatigue (9).
High intensity exercise increases the expression of
proinflammatory cytokines in the muscle and blood (10), and these increases lead to
alterations in behavior and in basal ganglia dopamine function
(11). Various biochemical
parameters, including lactate, lactate dehydrogenase (LDH),
creatine kinase (CK) and blood urea nitrogen (BUN), are altered
during fatigue (12). In addition,
oxidative stress induces fatigue with an increase in nitrite levels
(13), and alteration of
mitochondrial functions by exposure to reactive oxygen species
induces muscular fatigue (14).
Furthermore, catalase activity is diminished in fatigue (15) and superoxide dismutase (SOD)
protects against superoxide radicals and represents a first-line
defense against oxidative stress (16).
Fatigue has been associated with an increased risk
of malnutrition (17). The
authors' previous study reported that protein-energy malnutrition
increases fatigue-associated parameters in a fatigue model
(18), and protein supplementation
was reported to improve muscle protein synthesis following exercise
(19). Also, our previous report
demonstrated that fermented placenta, which contains biologically
active compounds such as proteins and peptides, ameliorates
physical fatigue (20). Leucine
has important biochemical functions in the brain, such as the
synthesis of dopamine, which is derived from aromatic amino acids,
and leucine supplementation limits the development of fatigue
(6). In addition, glycine-rich sea
cucumber peptides were reported to exhibit an anti-fatigue activity
(21). Therefore, the present
study investigated the anti-fatigue effects of Danish porcine
placenta (DPP) and its dipeptides, leucine-glycine (LG) and
glycine-leucine (GL), in mouse models of exercise-induced fatigue
using a treadmill test and a forced swimming test (FST), which are
useful for screening anti-fatigue drugs (22,23).
In addition, the regulatory mechanisms of DPP, LG and GL on
inflammatory responses were investigated in splenocytes.
Materials and methods
Animals
A total of 40 Male ICR mice (4-weeks-old; weight,
20–25 g) were obtained from Dae-Han Experimental Animal Center
(Dajeon, Korea) and were acclimatized to a controlled room for one
week prior to experiments. The animals were kept at 22±1°C with a
relative humidity of 55±10% with a light/dark cycle of 12-h and
ad libitum food and water availability. All experimental
procedures were conducted in accordance with the ethical
regulations of and approved by the Animal Care and Use Committee of
Kyung Hee University [approval no. KHUASP (SE)-15-080].
Preparation of DPP
DPP was obtained in powder form from BIOFAC A/S
(Kastrup, Denmark) and dissolved in distilled water (DW). LG (cat.
no. G-2495; Bachem AG, Bubendorf, Switzerland) and GL (cat. no.
M1460; Bachem AG) were dissolved in DW. Liquid chromatography/mass
spectrometry/mass spectrometry demonstrated that 1 g DPP contains
240 µg LG, 1,463 µg GL, 6.05 mg leucine and 7.29 mg glycine (data
not shown). Administered doses of DPP, LG and GL were determined as
described in our previous report (20).
Treadmill exercise test
A treadmill exercise test was performed as
previously described (20,24). Briefly, all mice were familiarized
with the treadmill one week prior to the treadmill test. All mice
ran on the treadmill at 10 m/min for 10 min, followed by 16 m/min
for 10 min and 21 m/min for 10 min once a week for three weeks. The
time to exhaustion for exercise (10 m/min for 5 min, followed by
16, 18, 21, 24, 26, 29, 32, 34 and 37 m/min for 3 min each, and 40
m/min until exhaustion) was determined on the 21st day. The time to
exhaustion during treadmill running was defined as the time between
the commencement and the first failure to maintain treadmill
exercise for >3 min (24). Mice
were assigned to one of four groups (n=5/group), including the
control, DPP, LG and GL groups, according to previous reports
(24,25). Mice were orally administered DW as
a control group, DPP (0.1, 1 and 10 mg/kg), LG (1 and 10 mg/kg) or
GL (1 and 10 mg/kg) for 21 consecutive days, as previously
described (20,26).
Fatigue-associated parameter
analysis
Following the treadmill exercise test mice were
sacrificed and brains, muscle, liver and blood were obtained. A
total of 800 µl blood was taken and serum was prepared by
centrifugation at 1,900 × g at 4°C for 10 min. Dopamine
levels in the brain were determined using an ELISA kit (cat. no.
MBS732020; MyBioSource, Inc., San Diego, CA, USA). The levels of
LDH, lactate, CK, glycogen, catalase, SOD and glucose were
determined using each kit (cat. nos. ab102526, ab65331, ab155901,
ab65620, ab83464, ab65354 and ab65333, respectively; Abcam,
Cambridge, UK). Cortisol (cat. no. ADI-900-071; Enzo Life Sciences,
Inc., Farmingdale, NY, USA), BUN (cat. no. K024-H1; Arbor Assays,
Inc., Ann Arbor, MI, USA), alanine transaminase (ALT; cat. no.
MAK052; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
aspartate transaminase (AST; cat. no. MAK055; Sigma-Aldrich; Merck
KGaA) levels in the serum were also determined using commercial
kits.
Isolation of splenocytes
Splenocytes were isolated from spleen of male ICR
mice (n=6). The spleen dissected and then placed into the cell
strainer with RPMI-1640 medium at room temperature. The homogenized
cell solution was centrifuged at room temperature, 480 × g for 5
min to obtain a single cell suspension with RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.; cat. no. 31800022)
supplemented with 100 unit/ml penicillin, 100 µg/ml streptomycin
and 10% heat-inactivated fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) at 37°C 5% CO2 and 95%
humidity. Splenocytes (2×105) were treated with DPP
(0.1, 1 and 10 µg/ml), LG (0.1, 1 and 10 µg/ml) or GL (0.1, 1 and
10 µg/ml) for 24 h at 37°C, and subsequently stimulated with
immobilized anti-CD3 and soluble anti-CD28 antibodies (CD3 cat. no.
553057; 2 µg/ml; CD28 cat. no. 553294; 4 µg/ml, respectively; BD
Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA). Control cells
were treated with distilled water.
Nuclear and cytosolic protein
extraction
Splenocytes (3×105) were lysed with 400
µl ice-cold hypotonic buffer comprised of EDTA (0.1 mM),
MgCl2 (2 mM), Hepes/KOH (10 mM), PMSF (0.5 mM), DTT (1
mM) and KCl (10 mM) at (pH 7.9). Following centrifugation at 4°C, 4
min, 600 × g, supernatant aliquots were analyzed for cytoplasmic
proteins. Pelleted nuclei were lysed with 50 µl ice-cold saline
buffer comprised of EDTA (0.1 mM), HEPES/KOH (50 mM), PMSF (0.5
mM), DTT (1 mM), KCl (50 mM), NaCl (300 mM) and glycerol (10%) at
(pH 7.9). Following centrifugation at 4°C for 15 min at 16,000 × g
supernatant aliquots were analyzed for nuclear proteins. Protein
levels were measured using a bicinchoninic acid protein assay
(Sigma-Aldrich; Merck KGaA).
Western blot analysis
The protein expression of BDNF and
phosphorylated-extracellular signal-regulated kinase (pERK) in the
whole brain of mice, and the expression of caspase-1, nuclear
factor (NF)-κB, phosphorylated-NF-κB inhibitor α (pIκBα),
α-tubulin, and poly (ADP-ribose) polymerase (PARP) in splenocytes
was determined by western blot analysis, as previously described
(27). A total of 20 µl/ug protein
separated per lane. Primary antibodies (all 1:500 dilution with
PBST; pERK, cat. no. sc-7383; ERK, cat. no. sc-94; BDNF, cat. no.
sc-546; GAPDH, cat. no. sc-32233; caspase-1, cat. no. sc-56036;
α-tubulin, cat. no. sc-8035; NF-κB, cat. no. sc-8008; PARP, cat.
no. sc-8007; pIκBα, cat. no. sc-8404; all purchased from Santa Cruz
Biotechnology, Dallas, TX, USA) were incubated at room temperature
for 2 h. Secondary antibodies [all 1:5,000 with PBST; mouse
anti-rabbit immunoglobulin (Ig)G-horseradish peroxidase (HRP); cat.
no. sc-2357; Santa Cruz Biotechnology, Dallas, TX, USA; bovine
anti-mouse IgG-HRP; cat. no. sc-2371] were incubated with membranes
at room temperature for 2 h. Enhanced chemiluminescence was used
for visualization with the ECL EZ-WESTERN Lumi Pico kit (DoGenBio
Co., Ltd., Seoul, Korea). The densitometric software used to
quantify protein expression was NIH ImageJ software 1.50i (National
Institutes of Health, Bethesda, MD, USA).
Measurement of caspase-1, pIκBα and
NF-κB levels
Splenocytes (3×105) were treated with
DPP, LG and GL and stimulated with CD3 and CD28 antibodies for 45
min at 37°C. The cytosolic expression of caspase-1 and pIκBα was
determined, while nuclear levels of NF-κB were measured with
western blotting as described above.
Caspase-1 activity assay
Splenocytes (3×105) were treated with
DPP, LG and GL and stimulated with CD3 and CD28 antibodies for 45
min at 37°C. The caspase-1 activity in cytoplasm was measured using
a caspase-1 kit according to the manufacturer's protocol (cat. no.
K110-100; R&D Systems, Minneapolis, Minnesota, USA).
Cytokine levels
Cytokines levels in the serum and spleen following
the treadmill exercise test at day 21, and cytokine production by
splenocytes (2×105) treated with DPP, LG and GL and
stimulated with CD3 and CD28 antibodies for 24 h at 37°C, were
measured by ELISA [kits used: IL-1β, mouse IL-1β antibody (cat. no.
MAB401; R&D Systems, Inc.), mouse IL-1β biotinylated antibody
(cat. no. BAF401; R&D Systems, Inc.), TNF-α-purified rat
anti-mouse/rat TNF (cat. no. 551225; BD Pharmingen), biotin rat
anti-mouse TNF (cat. no. 554415; BD Pharmingen) IL-6-purified rat
anti-mouse IL-6 (cat. no. 554400; BD Pharmingen), biotin rat
anti-mouse IL-6 (cat. no. 554402; BD Pharmingen), IL-4-purified rat
anti-mouse IL-4 (cat. no. 554387; BD Pharmingen), biotin rat
anti-mouse (cat. no. 554390, BD Pharmingen) IFN-γ-purified rat
anti-mouse IFN-γ (cat. no. 551216; BD Pharmingen) and biotin rat
anti-mouse (cat. no. IFN-γ 554410; BD Pharmingen)], as previously
described (27).
Nitric oxide assay
Following the treadmill exercise test at day 21,
nitric oxide levels in the serum of mice were measured using the
Griess method (27).
L6 cell culture
L6 cells, originally derived from rat skeletal
muscle, have been previously employed in studies concerning muscle
in animal models (28). In the
present study, L6 cells were obtained from the Korean Cell Line
Bank (Seoul, Korea). Cells were cultured in Dulbecco's modified
Eagle medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in 5% CO2/95% air and 95%
humidity.
MTT assay
L6 cells (1×104) or splenocytes
(2×105) were treated with DPP (0.1, 1 and 10 µg/ml), LG
(0.1, 1 and 10 µg/ml) or GL (0.1, 1 and 10 µg/ml) for 24 h,
followed by incubation with MTT solution (5 mg/ml; Sigma-Aldrich;
Merck KGaA) for 4 h at 37°C in a 5% CO2 atmosphere.
Following dissolving formazan with dimethyl sulfoxide, cell
viability was measured at 540 nm using an ELISA reader.
Proliferation assay
Splenocytes (2×105) were treated with
DPP, LG and GL and stimulated with CD3 and CD28 antibodies for 48 h
at 37°C. Splenocyte proliferation was determined by measuring
bromodeoxyuridine (BrdU) incorporation into cellular DNA during
cell proliferation using a Cell Proliferation ELISA, BrdU
(colorimetric; cat. no. 11647229001; Roche Diagnostics GmbH,
Mannheim, Germany), which was performed according to the
manufacturer's protocol.
Quantitative polymerase chain reaction
(qPCR)
Total RNA from splenocytes was isolated using an
easy-BLUE™ RNA extraction kit (Intron Biotechnology,
Inc., Sungnam, Korea). Total RNA concentrations were evaluated
using a NanoDrop spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc., Wilmington, DE, USA).
AccuPower® RT-PCR PreMix from Bioneer (Daejeon, Republic
of Korea) was used for RT at 42°C for 1 h. qPCR was performed on an
ABI StepOne Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using SYBR-Green master mix (Applied Biosystems,
cat. no. 4368577) for the detection of marker of proliferation
Ki-67 (Ki-67) mRNA following synthesis of cDNA. The following PCR
primers were used: Mouse Ki-67 forward, 5′CTTCACTCTTACTTTCCACA'3
and reverse, 5′AACACCTACAAAATGACTTC'3; and mouse GAPDH forward,
5′TCGACAGTCAGCCGCATCTTCTTT'3 and reverse,
5′ACCAAATCCGTTGACTCCGACCTT'3. Cycling parameters were 95°C for 2
min, following by 40 cycles of 95°C for 30 sec, 60°C for 20 sec,
and 72°C for 20 sec. The ratio of Ki-67 mRNA to GAPDH mRNA was
calculated. Data were analyzed using the 2−ΔΔCq method
(29). This experiment was
repeated three times.
Forced swim test (FST)
A total of 35 male ICR mice were familiarized with
forced swimming prior to administration of each sample, such as
DPP, LG, and GL. When mice were placed into the cylinders for the
first time, the mice swam around vigorously. Following 2–3 min, the
mice cease the vigorous activity and exhibit a characteristic
immobility in which the mouse moves only to maintain its head above
water. The mice with the shortest and longest immobility times were
excluded. Mice were assigned to one of seven groups (n=5/group),
including the control, DPP (1 and 10 mg/kg), LG (1 and 10 mg/kg),
and GL (1 and 10 mg/kg) groups based on the first recorded
immobility times in pre-FST that performed prior to administration
of each sample. Mice were orally administered DW as a control
group, DPP (1 and 10 mg/kg), LG (1 and 10 mg/kg) or GL (1 and 10
mg/kg) for 21 consecutive days, and FST immobility times were
determined for 6 min on the 21st day. The immobility times were
calculated by measuring the duration of the period of vigorous
activity during which the mice try to escape. This is an indication
of physical immobility in FST (30). The FST immobility times were
considered to indicate the degree of fatigue on the 21st day
(23).
Statistical analysis
Sample size (n=5 mice per group, power 95%) was
determined in a pilot study using two independent sample t-tests
and a power analysis. In vivo data are presented as the mean
± standard error of the mean (SEM; n=5 per group). In vitro
data are presented as the mean ± SEM of at least three independent
experiments performed in duplicate or triplicate. Data normality
was checked using the Shapiro-Wilk test and statistical analysis
was performed by one-way analysis of variance with Fisher's least
significant difference post-hoc test using SPSS statistical
software 23 (IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
DPP, LG and GL ameliorate fatigue
following treadmill exercise
To determine the anti-fatigue effects following
treadmill exercise, dopamine levels in the brain were measured
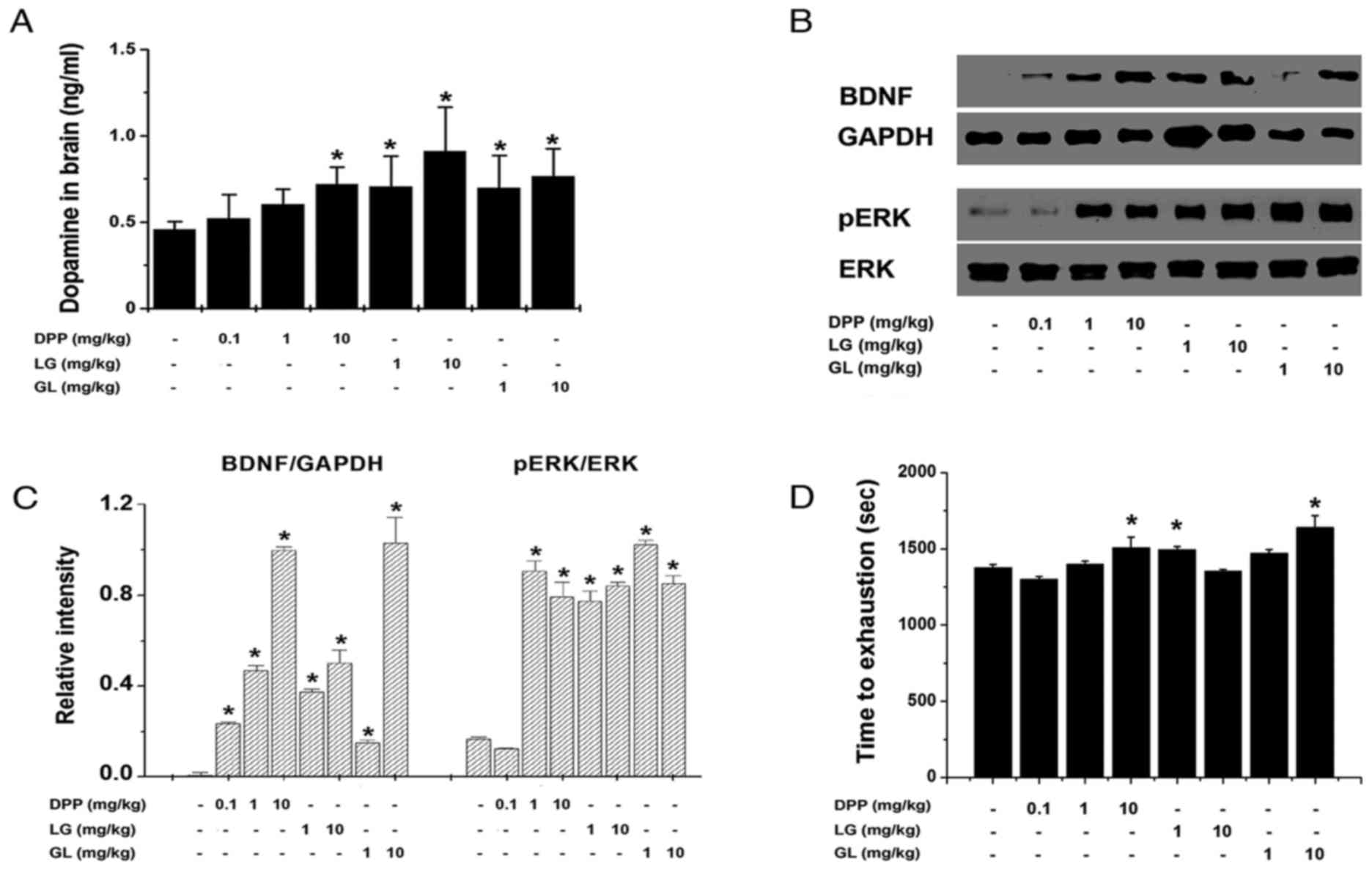
following treadmill exercise. As demonstrated in Fig. 1A, DPP (10 mg/kg), LG (1 and 10
mg/kg) and GL (1 and 10 mg/kg) significantly increased the dopamine
levels in the brain, compared with the control group (P<0.05).
In addition, DPP (1 and 10 mg/kg), LG (1 and 10 mg/kg) and GL (1
and 10 mg/kg) significantly increased the protein expression of
BDNF and pERK in the brain following treadmill exercise, compared
with the control group (P<0.05; Fig. 1B and C). Furthermore, the time to
exhaustion in response to treadmill running was significantly
extended by treatment with DPP (10 mg/kg), LG (1 mg/kg) and GL (10
mg/kg), compared with the control group (P<0.05; Fig. 1D). However, DPP (0.1 and 1 mg/kg),
LG (10 mg/kg), and GL (1 mg/kg) did not significantly extend the
time to exhaustion in treadmill running, compared with the control
group.
DPP, LG and GL reduce inflammatory
responses following treadmill exercise
As dopamine has been reported to control
inflammatory responses in fatigue (31,32),
the present study investigated whether DPP, LG and GL regulates
levels of proinflammatory cytokines in the serum and spleen
following treadmill exercise. As demonstrated in Table I, DPP, LG and GL significantly
inhibited treadmill exercise-induced serum IL-1β, TNF-α, IL-6 and
IL-4 levels, compared with the control group (P<0.05). In
addition, DPP, LG and GL also significantly suppressed the IL-1β,
TNF-α, IL-6 and IL-4 levels in the spleen compared with the control
group (P<0.05; Table I).
However, compared with the control group, IFN-γ levels in the serum
and spleen were significantly increased by DPP, LG and GL treatment
(P<0.05; Table I).
 | Table I.DPP, LG and GL reduce inflammatory
responses following treadmill exercise. |
Table I.
DPP, LG and GL reduce inflammatory
responses following treadmill exercise.
| A, Levels of
inflammatory cytokines in the serum of mice following treadmill
exercise |
|---|
|
|---|
|
|
| DPP, mg/kg | LG, mg/kg | GL, mg/kg |
|---|
|
|
|
|
|
|
|---|
| Cytokine | Control | 0.1 | 1.0 | 10.0 | 1.0 | 10.0 | 1.0 | 10.0 |
|---|
| IL-1β | 0.76±0.02 |
0.44±0.01a |
0.37±0.21a |
0.27±0.02a |
0.37±0.01a |
0.31±0.03a |
0.33±0.03a |
0.34±0.07a |
| TNF-α | 1.13±0.05 |
0.80±0.04a |
0.46±0.05a |
0.50±0.05a |
0.87±0.02a |
0.67±0.03a |
0.89±0.03a |
0.57±0.02a |
| IL-6 | 0.14±0.00 |
0.09±0.02a |
0.09±0.00a |
0.08±0.01a |
0.06±0.02a |
0.04±0.01a |
0.09±0.01a |
0.07±0.02a |
| IL-4 | 0.94±0.02 |
0.69±0.01a |
0.47±0.02a |
0.51±0.02a |
0.75±0.04a |
0.63±0.01a |
0.76±0.01a |
0.56±0.01a |
| IFN-γ | 0.70±0.02 |
0.90±0.03a |
1.14±0.03a |
1.32±0.05a |
0.86±0.01a |
1.27±0.09a |
0.97±0.01a |
1.21±0.07a |
|
| B, Levels of
inflammatory cytokines in the spleen of mice following treadmill
exercise |
|
|
|
| DPP,
mg/kg | LG,
mg/kg | GL,
mg/kg |
|
|
|
|
|
|
|
Cytokine | Control | 0.1 | 1.0 | 10.0 | 1.0 | 10.0 | 1.0 | 10.0 |
|
| IL-1β | 3.81±0.10 |
2.16±0.29a |
2.17±0.27a |
1.75±0.37a |
2.51±0.25a |
1.90±0.10a |
2.40±0.13a |
2.04±0.11a |
| TNF-α | 0.35±0.01 |
0.24±0.01a |
0.14±0.04a |
0.19±0.05a |
0.27±0.05a |
0.22±0.01a |
0.26±0.02a |
0.20±0.01a |
| IL-6 | 0.11±0.00 | 0.10±0.01 |
<0.01a |
<0.01a | 0.10±0.01 |
0.08±0.01a |
0.08±0.01a |
0.07±0.01a |
| IL-4 | 0.60±0.07 |
0.45±0.05a |
0.04±0.01a |
0.03±0.01a |
0.34±0.04a |
0.31±0.01a |
0.45±0.04a |
0.43±0.02a |
| IFN-γ | 0.85±0.10 |
2.39±0.29a | 1.50±0.28 |
2.78±0.61a |
2.19±0.54a |
3.07±0.54a | 1.78±0.12 |
3.37±0.27a |
DPP, LG and GL regulate
fatigue-associated muscle biochemical parameters following
treadmill exercise
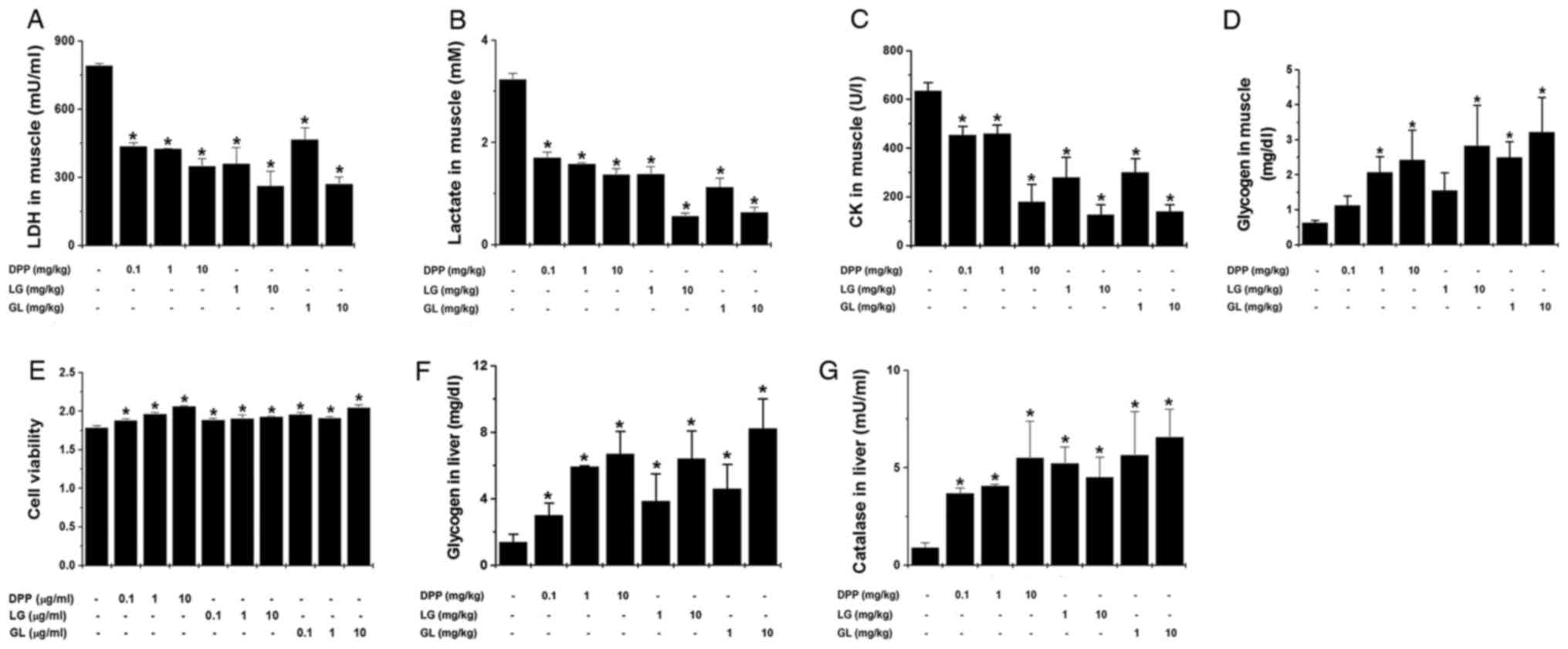
The present study also investigated whether DPP, LG
and GL regulates muscle fatigue-associated biochemical parameters
following treadmill exercise. The results demonstrated that DPP, LG
and GL significantly reduced muscle LDH, lactate and CK levels, and
significantly increased muscular glycogen levels, compared with the
control group (P<0.05; Fig.
2A-D). Subsequently, the present study investigated whether
DPP, LG and GL may regulate muscle damage in an in vitro
model using L6 cells. The results of the MTT assay demonstrated
that DPP, LG and GL significantly increased L6 cell viability
compared with the control group (P<0.05; Fig. 2E). In addition, DPP, LG and GL
significantly increased liver glycogen and catalase levels,
compared with the control group (P<0.05; Fig. 2F and G).
DPP, LG and GL reduce
fatigue-associated biochemical parameters in the serum following
treadmill exercise
As fatigue results from LDH or lactate increases in
the serum following exercise, serum LDH and lactate levels were
also measured in DPP, LG and GL-treated mice following treadmill
exercise. As demonstrated in Fig. 3A
and B, DPP, LG and GL significantly inhibited serum LDH and
lactate levels compared with the control group (P<0.05). In
addition, DPP, LG and GL significantly reduced serum nitric oxide
levels and increased serum SOD levels, compared with the control
group (P<0.05; Fig. 3C and D).
Furthermore, DPP, LG and GL significantly inhibited serum BUN, ALT
and AST levels (P<0.05; Table
II), while only DPP significantly inhibited serum cortisol
levels (P<0.05; Table II),
compared with the control group. In addition, DPP, LG and GL
significantly reduced serum glucose levels compared with the
control group (P<0.05; Table
II).
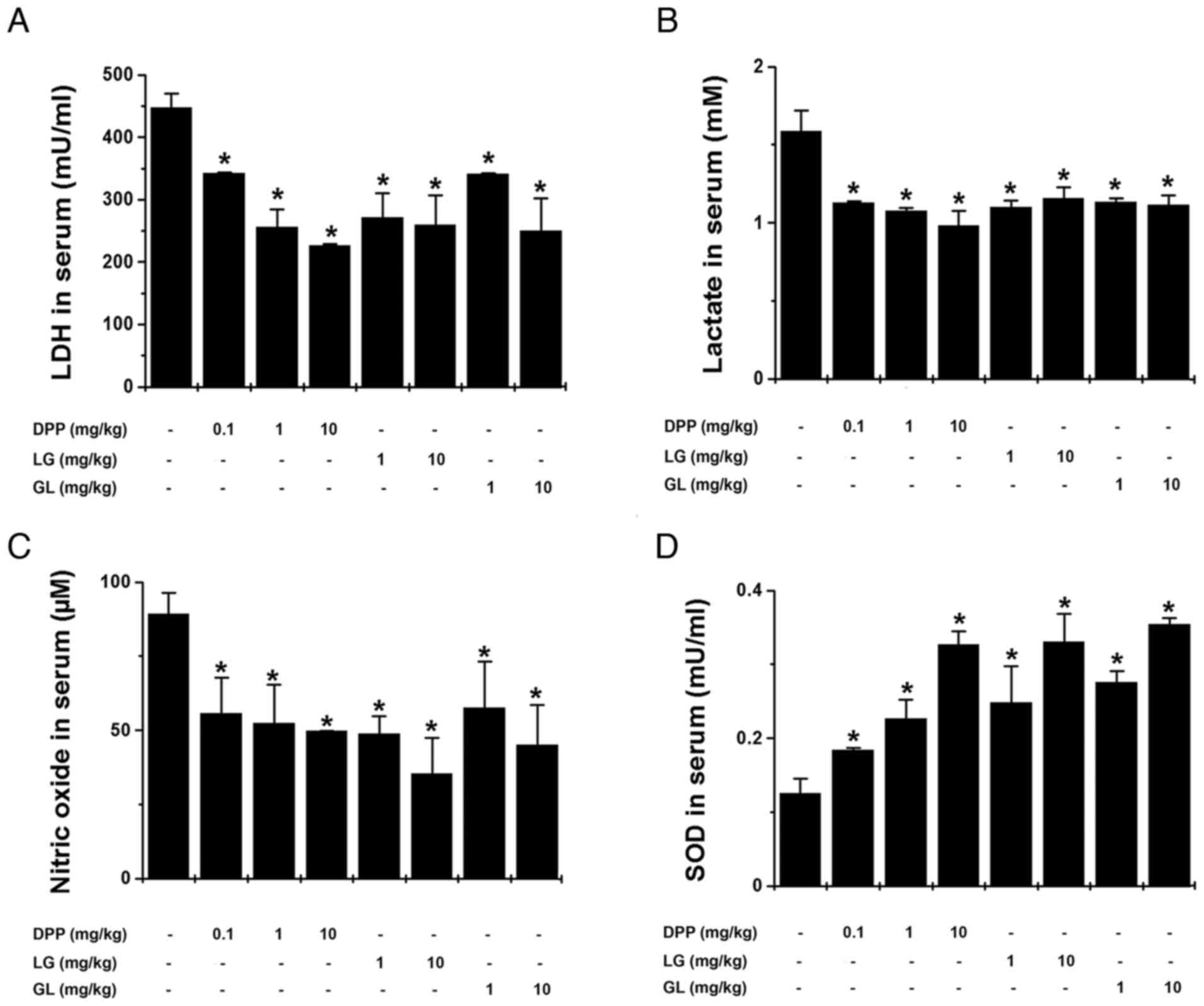 | Figure 3.DPP, LG and GL reduced
fatigue-associated biochemical parameters in the serum following
treadmill exercise. The levels of (A) LDH, (B) lactate, (C) nitric
oxide and (D) SOD in the serum of mice were determined following
treadmill exercise. LDH, lactate and SOD levels were measured using
kits, while nitric oxide levels were measured using the Griess
method. Data are presented as the mean ± standard error of the
mean, n=5 per group. *P<0.05 vs. control group. DPP, Danish
porcine placenta; LG, leucine-glycine dipeptide; GL,
glycine-leucine dipeptide; LDH, lactate dehydrogenase; SOD,
superoxide dismutase. |
 | Table II.DPP, LG and GL regulate
fatigue-associated biochemical parameters following treadmill
exercise. |
Table II.
DPP, LG and GL regulate
fatigue-associated biochemical parameters following treadmill
exercise.
|
|
| DPP, mg/kg | LG, mg/kg | GL, mg/kg |
|---|
|
|
|
|
|
|
|---|
| Parameter | Control | 0.1 | 1.0 | 10.0 | 1.0 | 10.0 | 1.0 | 10.0 |
|---|
| BUN, mg/dl | 11.66±0.87 |
7.17±0.98a |
5.82±0.57a |
5.40±0.95a |
6.30±0.58a |
5.26±2.46a |
6.18±0.66a |
5.23±0.64a |
| ALT, mU/ml | 5.17±0.79 |
2.67±0.44a | 5.22±0.15 |
2.38±0.71a |
2.63±0.20a |
2.35±0.77a |
2.45±0.46a |
2.44±0.36a |
| AST, mU/ml | 540.2±29.28 |
268.37±78.88a | not tested |
361.23±66.28a |
234.29±71.86a |
200.61±152.82a |
280.40±42.93a |
234.11±42.39a |
| Cortisol,
ng/ml | 328.75±9.73 | 275.15±37.90 |
161.54±30.37a |
174.39±29.93a | 296.80±16.32 | 287.60±25.80 | 270.96±30.17 | 284.91±4.12 |
| Glucose, mg/dl | 0.67±0.01 |
0.57±0.02a |
0.41±0.04a |
0.39±0.05a |
0.53±0.07a |
0.28±0.06a |
0.58±0.01a |
0.31±0.04a |
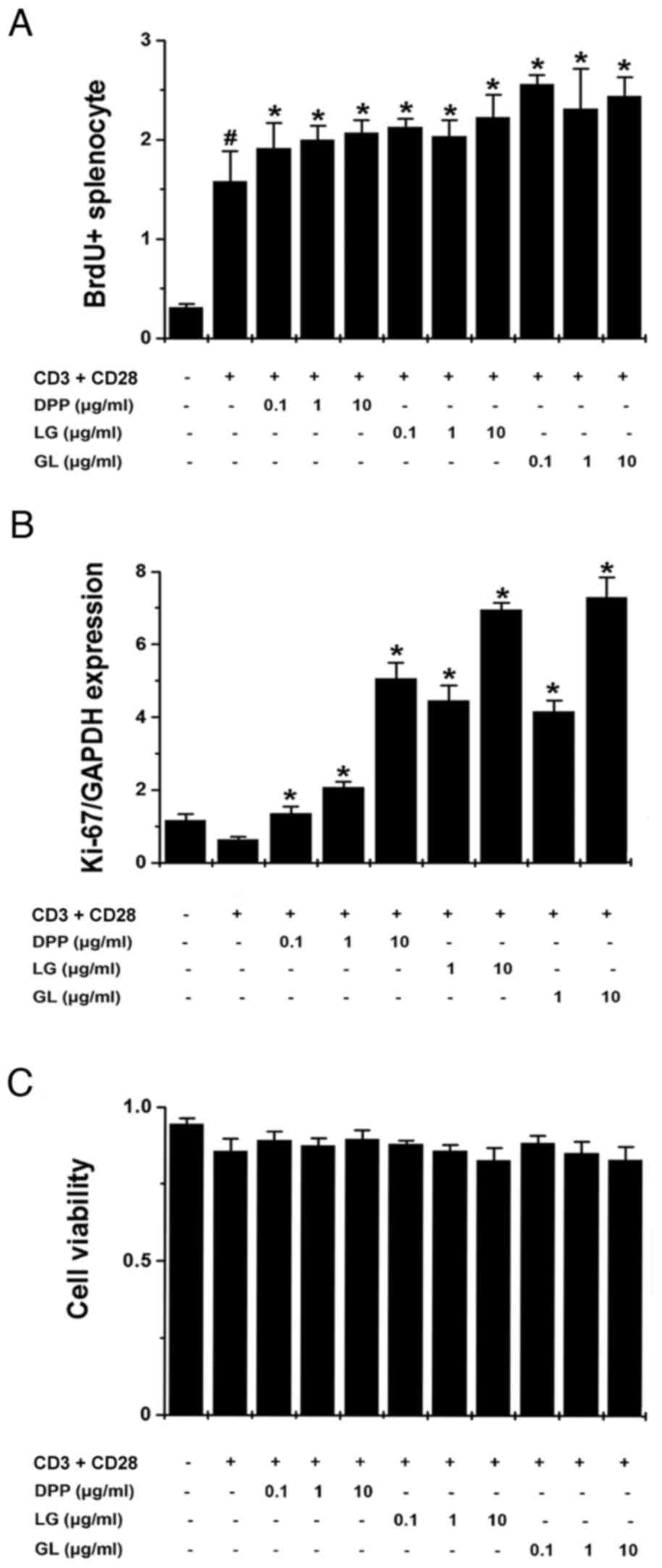
DPP, LG and GL increase splenocyte
proliferation
To assess the regulatory effects of DPP, LG and GL
on splenocyte proliferation, splenocytes were stimulated with
anti-CD3 and anti-CD28 antibodies, and BrdU incorporation and Ki-67
mRNA expression was subsequently determined. The results
demonstrated that treatment with DPP, LG and GL significantly
promoted BrdU incorporation and increased Ki-67 mRNA expression in
activated splenocytes, compared with control splenocytes that were
stimulated with CD3 and CD28 antibodies (P<0.05; Fig. 4A and B). MTT assay results
demonstrated that DPP, LG and GL exhibited no cytotoxic effect on
activated splenocytes (Fig.
4C).
DPP, LG and GL regulate
fatigue-associated cytokine levels in splenocytes
Based on the observations that DPP, LG and GL
reduced proinflammatory cytokine levels in the serum and spleen of
mice following treadmill exercise, the present study further
investigated the anti-inflammatory effects of DPP, LG and GL in
activated splenocytes. The results of ELISA demonstrated that all
three treatments significantly inhibited the production of IL-1β,
TNF-α, IL-6 and IL-4 by activated splenocytes (P<0.05; Fig. 5A-D). In addition, the current study
also investigated whether DPP, LG and GL regulates the expression
of upstream regulators of proinflammatory cytokines. The results
demonstrated that DPP, LG and GL significantly suppressed caspase-1
activity and the protein expression of caspase-1 in activated
splenocytes (P<0.05; Fig.
5E-G), and also inhibited the nuclear translocation of NF-κB
and reduced the levels of pIκBα in the cytoplasm (P<0.05;
Fig. 5H and I).
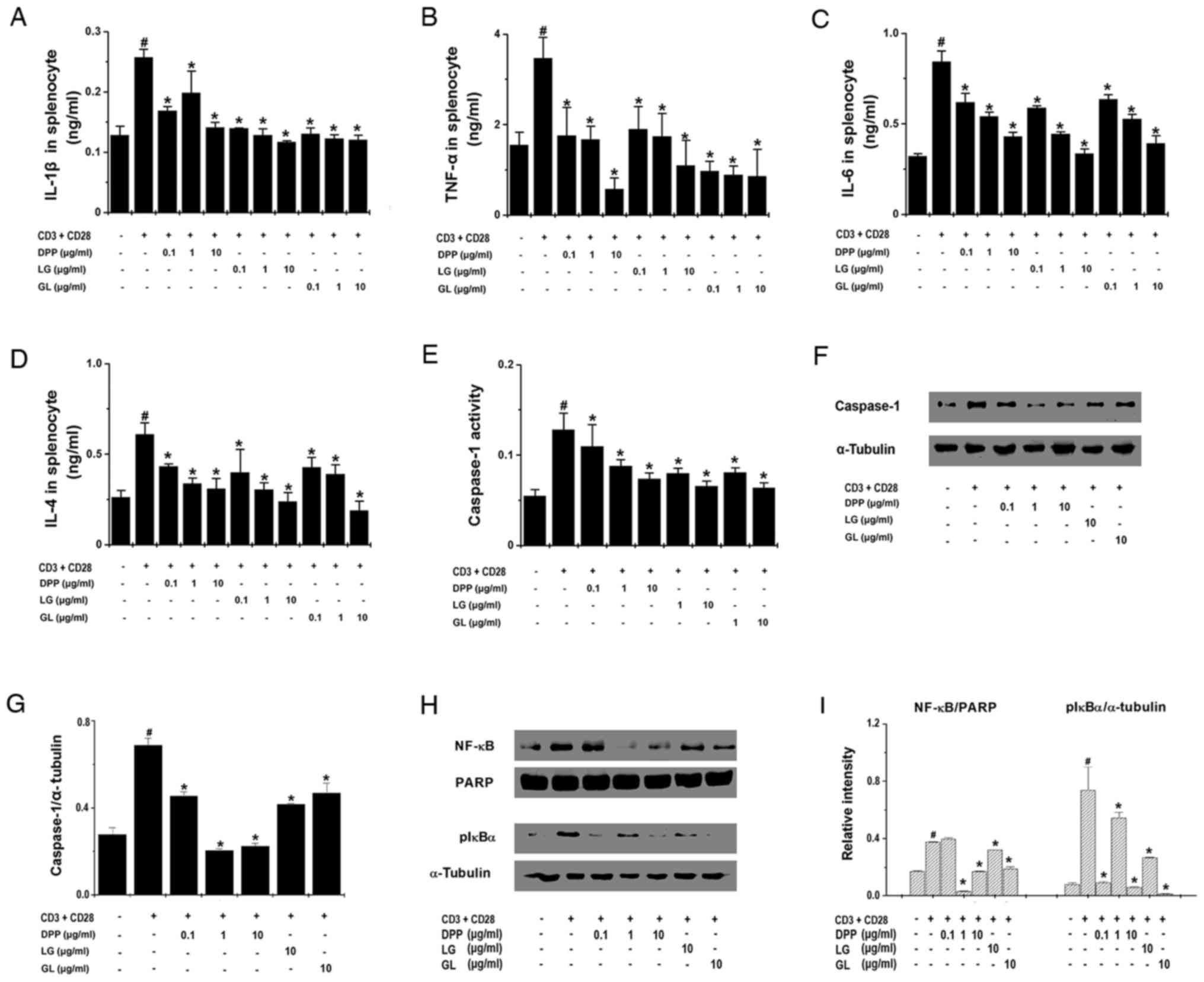 | Figure 5.DPP, LG and GL regulated
fatigue-associated cytokine levels in splenocytes. Splenocytes
(2×105) were treated with DPP, LG and GL for 24 h and
stimulated with CD3 and CD28 antibodies. The production of (A)
IL-1β, (B) TNF-α, (C) IL-6 and (D) IL-4 was determined by ELISA.
Splenocytes (3×105) were stimulated and treated with
DPP, LG and GL for 45 min to analyze caspase-1 activity and
expression. (E) Caspase-1 activity was determined using a caspase-1
kit. (F) Cytoplasmic caspase-1 protein expression was analyzed by
western blotting. (G) Caspase-1 protein expression was quantified
by densitometry. (H) Splenocytes (3×105) were stimulated
and treated with DPP, LG and GL for 45 min. NF-κB protein
expression in the nucleus and pIκBα expression in the cytoplasm
were analyzed by western blotting. (I) Densitometric analysis was
performed to quantify nuclear NF-κB and cytoplasmic pIκBα
expression. Data are presented as the mean ± standard error of the
mean of at least three independent experiments performed in
duplicate or triplicate. #P<0.05 vs. unstimulated
splenocytes; *P<0.05 vs. stimulated splenocytes without DPP, LG
or GL treatment. DPP, Danish porcine placenta; LG, leucine-glycine
dipeptide; GL, glycine-leucine dipeptide; IL, interleukin; TNF,
tumor necrosis factor; NF-κB, nuclear factor-κB; pIκBα,
phosphorylated-NF-κB inhibitor α; PARP, Poly (ADP-ribose)
polymerase. |
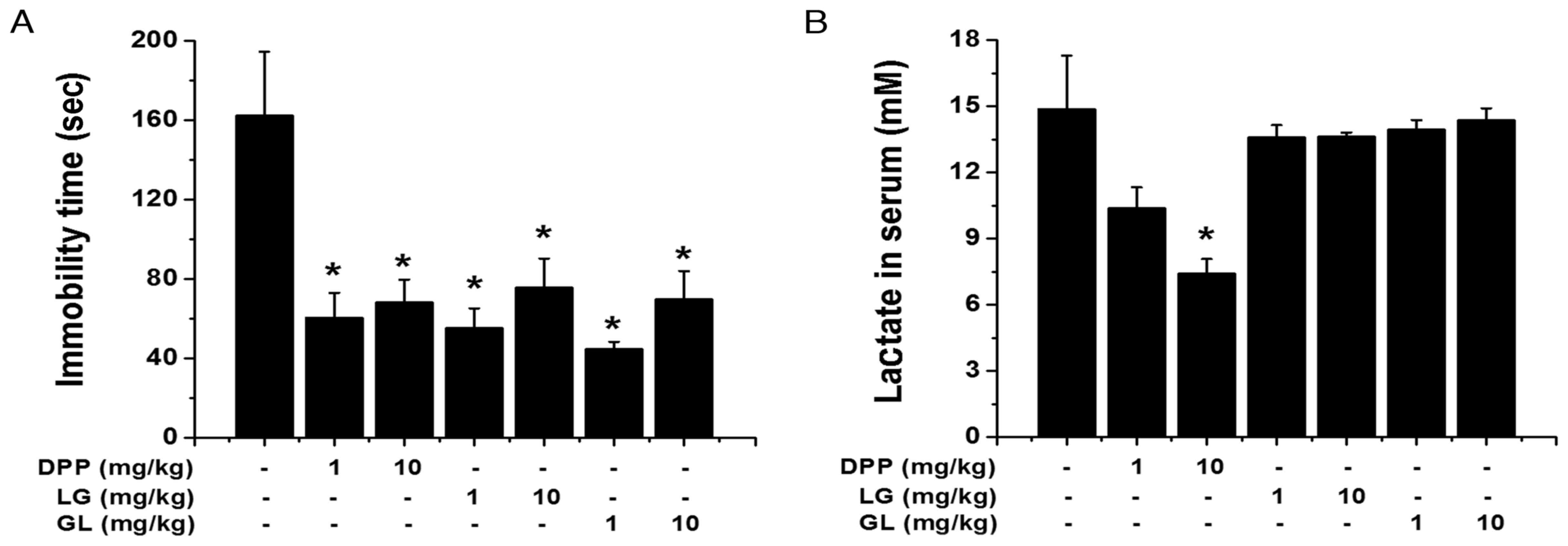
DPP, LG and GL regulate fatigue
following FST
To further define the anti-fatigue activity of DPP,
LG and GL, another fatigue model, FST, was employed. As
demonstrated in Fig. 6A, the
immobility times during the FST were significantly reduced by
treatment with DPP, LG and GL during the FST, compared with the
control group (P<0.05). Furthermore, DPP significantly
attenuated serum lactate levels following the FST, while LG and GL
exhibited no significant effect, compared with the control group
(P<0.05; Fig. 6B).
Discussion
In the present study, DPP, LG and GL improved
treadmill exercise-induced exhaustive time through enhancing
dopamine, BDNF and pERK levels in the brain, and decreasing LDH,
lactate, CK, BUN, ALT and AST levels in the muscles or serum.
Furthermore, DPP, LG and GL enhanced glycogen levels in the
muscles, glycogen levels and catalase activity in the liver, and
SOD activity in serum, and inhibited proinflammatory cytokine
levels in the serum and spleen. The results indicated that DPP, LG
and GL may inhibit proinflammatory cytokine production by reducing
the activation of caspase-1 and NF-κB in activated splenocytes.
Additionally, DPP, LG and GL also reduced immobility time during
the FST.
Endurance exercise induces neurochemical
alterations, which include disruption of dopamine function in the
brain (33). Fatigue during
prolonged exercise in rats was reported to be associated with
reduced dopamine levels in the brain (34), and increased dopamine levels
improved endurance treadmill exercise performance in animals
(35), while reduced dopamine
levels in the brain impaired treadmill running time to exhaustion
in animals (36). BDNF is reported
to be involved in the maintenance and maturation of peripheral and
central neurons in the developing nervous system (37). It was demonstrated that
dopamine-secreting neurons involved in movement generation express
BDNF (38). Furthermore, BDNF
levels were depressed in chronic fatigue syndrome (37). A previous study demonstrated that
BDNF levels may be associated with the activation of the ERK
signaling pathway (39), and ERK
becomes activated by phosphorylation in response to various
neurotransmitters, including dopamine (40). It was previously demonstrated that
enhancing dopamine levels by dopaminergic medication reduced
fatigue in patients with chronic fatigue syndrome (41). Additional studies reported that
creatine supplement increased brain dopamine synthesis during
exercise and diminished central fatigue, with reduced accumulation
of lactate in humans (42,43). Furthermore, rutin attenuated
physical fatigue by upregulating the expression of pERK (44). In the present study, DPP, LG and GL
enhanced dopamine, BDNF and pERK levels in the brain following
treadmill exercise-induced fatigue. These findings indicate that
DPP, LG and GL may exhibit anti-physical fatigue activity through
regulation of central dopaminergic systems. However, the time to
exhaustion did not change in parallel with dopamine levels in the
present study. Thus, further investigation is required to clarify
specific functions of DPP, LG and GL on dopaminergic systems of
fatigue. Additionally, further experiments at lower doses of LG and
GL are required as the dopamine levels were enhanced in mice that
were treated with 1 and 10 mg/kg of LG and GL.
The effects of proinflammatory cytokines on ganglia
dopamine are particularly relevant to fatigue (31). In patients with psychological
stress, proinflammatory cytokine expression was associated with
fatigue (32). In addition,
proinflammatory cytokines were reported to lead to a significant
reduction of BDNF expression (45). The low levels of dopamine observed
in the brains of patients with chronic fatigue were reported to be
associated with inflammatory responses or oxidative stress
(46), and oxidative stress
induced by excessive exercise was associated with the production of
nitric oxide metabolites (47).
Antioxidant enzymes, including SOD and catalase, remove free
radicals and constitute an intracellular defense system (48); Wu et al (49) reported that an antioxidant
regulated fatigue-associated factors and functioned as an
anti-fatigue agent. In the present study, DPP, LG and GL inhibited
IL-1β, TNF-α, IL-6, IL-4 and nitric oxide levels in the serum
and/or spleen of fatigued mice, and enhanced SOD and catalase
levels. In addition, proinflammatory cytokine production has been
previously reported to occur via the activation of caspase-1 and
the NF-κB/IκBα pathway in splenocytes (50). In the present study, DPP, LG and GL
reduced the production of IL-1β, TNF-α, IL-6 and IL-4, and the
activation of caspase-1 and NF-κB nuclear translocation, in
activated splenocytes. Therefore, the results of the present study
demonstrate that DPP, LG and GL may prevent inflammatory responses
and oxidative stress through blocking caspase-1/NF-κB signaling and
regulating dopaminergic systems, subsequently moderating
fatigue-associated factors.
The association between physical exercise and
fatigue may be investigated by examining muscle metabolites.
Lactate is considered to be an active metabolite that has a primary
role in the induction of muscle fatigue (51). In addition, physical
fatigue-associated biomarkers, including serum lactate, BUN and
glucose, and tissue damage markers, such as AST, ALT and CK, have
been previously investigated in fatigue models (52). Glutamine supplementation attenuated
increases in prolonged exercise-induced CK levels and inflammatory
markers levels (53). In addition,
curcumin supplementation decreased lactate, BUN, AST and ALT levels
following physical challenge, improved exercise performance and
prevented fatigue (54). In the
present study, DPP, LG and GL reduced LDH, lactate, CK, BUN, ALT
and AST levels in the serum and/or muscle following treadmill
exercise, and reduced lactate levels and immobility time following
intensive swimming, which indicates that DPP, LG and GL may exhibit
anti-fatigue effects. In addition, DPP, LG and GL enhanced L6
viability in the current study, indicating the potential
amelioration of muscle damage.
CD28 molecule and CD3 T-cell receptors trigger
T-lymphocyte proliferation and amplify antigen-specific immune
responses (55). Arginine was
reported to promote splenocyte proliferation and improve immunity
in a mouse model (56). Serum
IFN-γ levels and blood CD4-positive T-cell counts were lower in
fatigued athletes compared with healthy controls, indicating a
potential T-cell deficiency (57).
Furthermore, INF-γ null mice exhibited impaired muscle healing
associated with muscle fibrosis (58). In the present study, DPP, LG and GL
enhanced CD3 and CD28 antibody-induced splenocyte proliferation
without any cytotoxic effect. In addition, in vivo
experiments demonstrated that DPP, LG and GL enhanced IFN-γ levels
in the serum and spleen of fatigued mice. These results indicate
that DPP, LG and GL may exert beneficial effects on the immune
system by increasing T-lymphocyte proliferation and IFN-γ levels,
subsequently ameliorating physical fatigue.
Nutritional deficiencies have been reported to
induce alterations in brain neurochemistry in conjunction with
various psychological disorders or fatigue (59), which highlights the role of
nutrition in fatigue induced by exercise (60). Leucine supplementation reduced
inflammatory reactions and muscle pain following intensive exercise
(61) and a supplement that
included glycine improved exercise performance and reduced
oxidative stress (62). In
addition, peptides from Pseudosciaena crocea prolonged
exhaustive swimming time in mice and exhibited notable anti-fatigue
effects (23). The peptide
glutamine is considered to be an essential nutrient for the
prevention of fatigue in athletes (63). Therefore, oral intake of LG, GL and
DPP may be considered promising treatment strategies for fatigue.
An effective dose of DPP as a health food for fatigue prevention
may be 10 mg/kg based on the present study. However, further
investigation is required to determine the clinical applications of
DPP in humans.
DPP contains various compounds, including active and
inactive compounds. LG and GL are active compounds, and the doses
of LG and GL used in the present study were higher than those that
are present within DPP, as demonstrated by the results; overall, in
the present study, the DPP group did not exhibit an increased
effect compared with the LG or GL groups at the same dose, which
may be explained by the difference between the amount of LG and GL
present within DPP and the doses of LG and GL used in the present
study.
In conclusion, the present study investigated the
regulatory effects of DPP, LG and GL on fatigue-associated factors
using treadmill exercise-induced and forced swimming-induced models
of fatigue, in addition to the inhibitory effects of DPP, LG and GL
on fatigue-associated inflammatory factors in splenocytes. Overall,
the results of the present study demonstrated that DPP, LG and GL
may prevent physical fatigue by inhibiting inflammatory responses
through increasing dopaminergic systems following intensive
exercise, indicating that DPP may be considered a potential
treatment for fatigue.
References
|
1
|
Gandevia SC: Spinal and supraspinal
factors in human muscle fatigue. Physiol Rev. 81:1725–1789. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meeusen R and Watson P: Amino acids and
the brain: Do they play a role in ‘central fatigue’? Int J Sport
Nutr Exerc Metab. 17 Suppl:S37–S46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balthazar CH, Leite LH, Rodrigues AG and
Coimbra CC: Performance-enhancing and thermoregulatory effects of
intracerebroventricular dopamine in running rats. Pharmacol Biochem
Behav. 93:465–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chaouloff F: Physical exercise and brain
monoamines: A review. Acta Physiol Scand. 137:1–13. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Basu S and Dasgupta PS: Dopamine, a
neurotransmitter, influences the immune system. J Neuroimmunol.
102:113–124. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meeusen R: Exercise, nutrition and the
brain. Sports Med. 44 Suppl 1:S47–S56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Razgado-Hernandez LF, Espadas-Alvarez AJ,
Reyna-Velazquez P, Sierra-Sanchez A, Anaya-Martinez V,
Jimenez-Estrada I, Bannon MJ, Martinez-Fong D and Aceves-Ruiz J:
The transfection of BDNF to dopamine neurons potentiates the effect
of dopamine D3 receptor agonist recovering the striatal
innervation, dendritic spines and motor behavior in an aged rat
model of Parkinson's disease. PLoS One. 10:e01173912015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmidt HD and Duman RS: The role of
neurotrophic factors in adult hippocampal neurogenesis,
antidepressant treatments and animal models of depressive-like
behavior. Behav Pharmacol. 18:391–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saligan LN, Lukkahatai N, Holder G, Walitt
B and Machado-Vieira R: Lower brain-derived neurotrophic factor
levels associated with worsening fatigue in prostate cancer
patients during repeated stress from radiation therapy. World J
Biol Psychiatry. 27:608–614. 2016.
|
|
10
|
Liburt NR, Adams AA, Betancourt A, Horohov
DW and McKeever KH: Exercise-induced increases in inflammatory
cytokines in muscle and blood of horses. Equine Vet J Suppl.
38:280–288. 2010. View Article : Google Scholar
|
|
11
|
Felger JC and Miller AH: Cytokine effects
on the basal ganglia and dopamine function: The subcortical source
of inflammatory malaise. Front Neuroendocrinol. 33:315–327. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koo HN, Lee JK, Hong SH and Kim HM:
Herbkines increases physical stamina in mice. Biol Pharm Bull.
27:117–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pall ML: Elevated, sustained peroxynitrite
levels as the cause of chronic fatigue syndrome. Med Hypotheses.
54:115–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coombes JS, Rowell B, Dodd SL, Demirel HA,
Naito H, Shanely RA and Powers SK: Effects of vitamin E deficiency
on fatigue and muscle contractile properties. Eur J Appl Physiol.
87:272–277. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Borah M, Sarma P and Das S: A study of the
protective effect of triticum aestivum L. in an experimental animal
model of chronic fatigue syndrome. Pharmacognosy Res. 6:285–291.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Finaud J, Lac G and Filaire E: Oxidative
stress: Relationship with exercise and training. Sports Med.
36:327–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tennant KF, Takacs SE, Gau JT, Clark BC
and Russ DW: A preliminary study of symptomatic fatigue in rural
older adults. Aging Clin Exp Res. 24:324–330. 2012.PubMed/NCBI
|
|
18
|
Han NR, Kim KY, Kim MJ, Kim MH, Kim HM and
Jeong HJ: Porcine placenta mitigates protein-energy
malnutrition-induced fatigue. Nutrition. 29:1381–1387. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wolfe RR: Protein supplements and
exercise. Am J Clin Nutr. 72 2 Suppl:551S–557S. 2000.PubMed/NCBI
|
|
20
|
Kim HY, Han NR, Kim NR, Lee M, Kim J, Kim
CJ, Jeong HJ and Kim HM: Effect of fermented porcine placenta on
physical fatigue in mice. Exp Biol Med (Maywood). 241:1985–1996.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye J, Shen C, Huang Y, Zhang X and Xiao M:
Anti-fatigue activity of sea cucumber peptides prepared from
Stichopus japonicus in an endurance swimming rat model. J Sci Food
Agric. 97:4548–4556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Buel EM, Sigrist H, Seifritz E, Fikse
L, Bosker FJ, Schoevers RA, Klein HC, Pryce CR and Eisel UL: Mouse
repeated electroconvulsive seizure (ECS) does not reverse social
stress effects but does induce behavioral and hippocampal changes
relevant to electroconvulsive therapy (ECT) side-effects in the
treatment of depression. PLoS One. 12:e01846032017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao YQ, Zeng L, Yang ZS, Huang FF, Ding
GF and Wang B: Anti-fatigue effect by peptide fraction from protein
hydrolysate of croceine croaker (pseudosciaena crocea) swim bladder
through inhibiting the oxidative reactions including DNA damage.
Mar Drugs. 14:pii: E2212016. View Article : Google Scholar
|
|
24
|
Seo JH, Sung YH, Kim KJ, Shin MS, Lee EK
and Kim CJ: Effects of Phellinus linteus administration on
serotonin synthesis in the brain and expression of monocarboxylate
transporters in the muscle during exhaustive exercise in rats. J
Nutr Sci Vitaminol (Tokyo). 57:95–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumar V, Aneesh KA, Kshemada K, Ajith KGS,
Binil RSS, Deora N, Sanjay G, Jaleel A, Muraleedharan TS, Anandan
EM, et al: Amalaki rasayana, a traditional Indian drug enhances
cardiac mitochondrial and contractile functions and improves
cardiac function in rats with hypertrophy. Sci Rep. 7:85882017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Motaghinejad M, Motevalian M,
Asadi-Ghalehni M and Motaghinejad O: Attenuation of morphine
withdrawal signs, blood cortisol and glucose level with forced
exercise in comparison with clonidine. Adv Biomed Res. 3:1712014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rim HK, Kim KY and Moon PD: Evidence of
hydrolyzed traditional Korean red ginseng by malted barley on
activation of receptor interacting proteins 2 and IkappaB
kinase-beta in mouse peritoneal macrophages. TANG. 2:e372015.
|
|
28
|
Jung HY, Lee AN, Song TJ, An HS, Kim YH,
Kim KD, Kim IB, Kim KS, Han BS, Kim CH, et al: Korean mistletoe
(Viscum album coloratum) extract improves endurance capacity in
mice by stimulating mitochondrial activity. J Med Food. 15:621–628.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Teng YS and Wu D: Anti-fatigue effect of
green tea polyphenols (−)-Epigallocatechin-3-Gallate (EGCG).
Pharmacogn Mag. 13:326–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azzinnari D, Sigrist H, Staehli S, Palme
R, Hildebrandt T, Leparc G, Hengerer B, Seifritz E and Pryce CR:
Mouse social stress induces increased fear conditioning,
helplessness and fatigue to physical challenge together with
markers of altered immune and dopamine function. Neuropharmacology.
85:328–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Flachenecker P, Bihler I, Weber F,
Gottschalk M, Toyka KV and Rieckmann P: Cytokine mRNA expression in
patients with multiple sclerosis and fatigue. Mult Scler.
10:165–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marin H and Menza MA: Specific treatment
of residual fatigue in depressed patients. Psychiatry (Edgmont).
1:12–18. 2004.PubMed/NCBI
|
|
34
|
Bailey SP, Davis JM and Ahlborn EN:
Neuroendocrine and substrate responses to altered brain 5-HT
activity during prolonged exercise to fatigue. J Appl Physiol
(1985). 74:3006–3012. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gerald MC: Effects of (+)-amphetamine on
the treadmill endurance performance of rats. Neuropharmacology.
17:703–704. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heyes MP, Garnett ES and Coates G:
Nigrostriatal dopaminergic activity is increased during exhaustive
exercise stress in rats. Life Sci. 42:1537–1542. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sorenson M, Jason L, Peterson J,
Herrington J and Mathews H: Brain derived neurotrophic factor is
decreased in chronic fatigue syndrome and multiple sclerosis. J
Neurol Neurophysiol. 12 Suppl:S2–S13. 2014.
|
|
38
|
Foley TE and Fleshner M: Neuroplasticity
of dopamine circuits after exercise: Implications for central
fatigue. Neuromolecular Med. 10:67–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen X, Li A, Zhang Y, Dong X, Shan T, Wu
Y, Jia J and Hu Y: The effect of different intensities of treadmill
exercise on cognitive function deficit following a severe
controlled cortical impact in rats. Int J Mol Sci. 14:21598–21612.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng P, Guan Z, Yang X and Fang J:
Impairments of ERK signal transduction in the brain in a rat model
of depression induced by neonatal exposure of clomipramine. Brain
Res. 991:195–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dobryakova E, Genova HM, DeLuca J and
Wylie GR: The dopamine imbalance hypothesis of fatigue in multiple
sclerosis and other neurological disorders. Front Neurol. 6:522015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rahimi E, Moghadasi M, Mahani MN, Torkfar
A and Yadolazadeh A: Central and peripheral fatigue factors after
an exhaustive aerobic exercise following creatine supplementation.
Ann Biol Res. 3:4209–4214. 2012.
|
|
43
|
Watanabe A, Kato N and Kato T: Effects of
creatine on mental fatigue and cerebral hemoglobin oxygenation.
Neurosci Res. 42:279–285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Su KY, Yu CY, Chen YW, Huang YT, Chen CT,
Wu HF and Chen YL: Rutin, a flavonoid and principal component of
saussurea involucrata, attenuates physical fatigue in a forced
swimming mouse model. Int J Med Sci. 11:528–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Calabrese F, Rossetti AC, Racagni G, Gass
P, Riva MA and Molteni R: Brain-derived neurotrophic factor: A
bridge between inflammation and neuroplasticity. Front Cell
Neurosci. 8:4302014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jason LA, Porter N, Herrington J, Sorenson
M and Kubow S: Kindling and oxidative stress as contributors to
myalgic encephalomyelitis/chronic fatigue syndrome. J Behav
Neurosci Res. 7:1–17. 2009.PubMed/NCBI
|
|
47
|
Suárez A, Guillamó E, Roig T, Blázquez A,
Alegre J, Bermúdez J, Ventura JL, García-Quintana AM, Comella A,
Segura R and Javierre C: Nitric oxide metabolite production during
exercise in chronic fatigue syndrome: A case-control study. J
Womens Health (Larchmt). 19:1073–1077. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu J, Yeo HC, Overvik-Douki E, Hagen T,
Doniger SJ, Chu DW, Brooks GA and Ames BN: Chronically and acutely
exercised rats: Biomarkers of oxidative stress and endogenous
antioxidants. J Appl Physiol (1985). 89:21–28. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu C, Chen R, Wang XS, Shen B, Yue W and
Wu Q: Antioxidant and anti-fatigue activities of phenolic extract
from the seed coat of Euryale ferox Salisb. and identification of
three phenolic compounds by LC-ESI-MS/MS. Molecules.
18:11003–11021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lamkanfi M, Sarkar A, Vande Walle L,
Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD and Dixit
VM: Inflammasome-dependent release of the alarmin HMGB1 in
endotoxemia. J Immunol. 185:4385–4392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sola-Penna M: Metabolic regulation by
lactate. IUBMB Life. 60:605–608. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang CC, Lin TJ, Lu YF, Chen CC, Huang CY
and Lin WT: Protective effects of L-arginine supplementation
against exhaustive exercise-induced oxidative stress in young rat
tissues. Chin J Physiol. 52:306–315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cruzat VF, Rogero MM and Tirapegui J:
Effects of supplementation with free glutamine and the dipeptide
alanyl-glutamine on parameters of muscle damage and inflammation in
rats submitted to prolonged exercise. Cell Biochem Funct. 28:24–30.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang WC, Chiu WC, Chuang HL, Tang DW, Lee
ZM, Wei L, Chen FA and Huang CC: Effect of curcumin supplementation
on physiological fatigue and physical performance in mice.
Nutrients. 7:905–921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
June CH, Ledbetter JA, Gillespie MM,
Lindsten T and Thompson CB: T-cell proliferation involving the CD28
pathway is associated with cyclosporine-resistant interleukin 2
gene expression. Mol Cell Biol. 7:4472–4481. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Suarez Butler MF, Langkamp-Henken B,
Herrlinger-Garcia KA, Klash AE, Szczepanik ME, Nieves C Jr, Cottey
RJ and Bender BS: Arginine supplementation enhances mitogen-induced
splenocyte proliferation but does not affect in vivo indicators of
antigen-specific immunity in mice. J Nutr. 135:1146–1150. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Clancy RL, Gleeson M, Cox A, Callister R,
Dorrington M, D'Este C, Pang G, Pyne D, Fricker P and Henriksson A:
Reversal in fatigued athletes of a defect in interferon gamma
secretion after administration of Lactobacillus acidophilus. Br J
Sports Med. 40:351–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cheng M, Nguyen MH, Fantuzzi G and Koh TJ:
Endogenous interferon-gamma is required for efficient skeletal
muscle regeneration. Am J Phys Cell Phys. 294:C1183–C1191. 2008.
View Article : Google Scholar
|
|
59
|
Fernstrom JD: Dietary amino acids and
brain function. J Am Diet Assoc. 94:71–77. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Davis JM, Alderson NL and Welsh RS:
Serotonin and central nervous system fatigue: Nutritional
considerations. Am J Clin Nutr. 72 2 Suppl:573S–578S.
2000.PubMed/NCBI
|
|
61
|
Cruzat VF, Krause M and Newsholme P: Amino
acid supplementation and impact on immune function in the context
of exercise. J Int Soc Sports Nutr. 11:612014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Smith WA, Fry AC, Tschume LC and Bloomer
RJ: Effect of glycine propionyl-L-carnitine on aerobic and
anaerobic exercise performance. Int J Sport Nutr Exerc Metab.
18:19–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Favano A, Santos-Silva PR, Nakano EY,
Pedrinelli A, Hernandez AJ and Greve JM: Peptide glutamine
supplementation for tolerance of intermittent exercise in soccer
players. Clinics (Sao Paulo). 63:27–32. 2008. View Article : Google Scholar : PubMed/NCBI
|