Introduction
Osteoarthritis (OA), also termed degenerative
arthritis, aged arthritis or hyperplasia arthritis, is a type of
primary/secondary irreversible joint degenerative disorder caused
by various factors, and is featured with reactive bone hyperplasia
or osteophyte formation at joint ligament attachment sites or
subchondral bone (1,2). Therefore, illustration of the OA
pathogenesis mechanism from a molecular biology perspective, as
well as identification of molecular markers for evaluation of the
OA pathogenesis or progression, are critical for OA prevention,
drug development, and improving treatment efficacy and prognosis.
Matrix metalloproteinases (MMPs) are a proteinase family. It is
widely distributed in various mesenchymal tissues, is synthesized
and secreted by joint chondrocytes, fibroblasts, synovial cells and
neutrophils, and is involved in the degradation of the
extracellular matrix (ECM), embryonic development, osteogenesis and
cartilage development (3), tumor
invasion and metastasis (4).
MMP-13 is a member of the collagenase sub-family of MMPs, and
degrades type II collagen, which is the featured and abundantly
distributed protein in the cartilage matrix, with high specificity
(5). MMP-13 upregulation has been
demonstrated to be associated with OA pathogenesis (6). MicroRNA (miR) is a small non-coding
mRNA, 20–25 nucleotides in length in eukaryotes, and binds to the
3′-untranslated region (3′-UTR) of target gene mRNA to degrade mRNA
or inhibit target gene mRNA translation, thus modulating >30% of
human gene expression, and participating in the regulation of
multiple biological processes, including cell proliferation,
differentiation and tissue/organ development (7). Various studies demonstrated
significantly decreased miR-9 expression levels in cartilage
tissues in OA patients, indicating its role in OA pathogenesis
(8,9). Bioinformatics analysis identified the
complementary binding site between miR-9 and MMP-13. The current
study therefore investigated whether miR-9 is involved in
regulating MMP-13 expression levels and OA pathogenesis.
Materials and methods
Major reagents and materials
Dulbecco's modified Eagle's medium/F12 culture
medium, fetal bovine serum, penicillin-streptomycin, and 0.25%
trypsin were purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Penicillin-streptomycin was purchased from
CellGro (Corning Incorporated, Corning, NY, USA). Type II
collagenase was purchased from Sigma-Aldirch (Merck KGaA,
Darmstadt, Germany). Lipofectamine 2000 was purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). X-tremeGENE siRNA
transfection reagent was purchased from Roche Diagnostics
(Indianapolis, IN, USA). ReverTra Ace qPCR RT kit (FSQ-101) and
SYBR dye were purchased from Toyobo Life Science (Osaka, Japan).
micrON™ agomir-9, micrON™ agomir-control and miR-9 nucleotide
fragment were designed and synthesized by Ribo Life Science Co.,
Ltd. (Soochow, China). Rabbit anti-MMP-13 antibody (sc-30073),
mouse anti-collagen type II α1 chain (COL2A1) antibody (sc-52658)
and rabbit anti-COL2A1 (sc-28887) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Horseradish peroxidase
(HRP)-conjugated secondary antibody was purchased from Jackson
ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). The
dual-luciferase reporter assay system and pGL3-promoter plasmids
were purchased from Promega Corporation (Madison, WI, USA).
Experimental animals
Specific pathogen-free grade male Sprague Dawley
(SD) rats (age, 8 weeks; body weight, 220 ± 25 grams) were
purchased from shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China). A total of 30 rats were housed in a specific pathogen-free
environment with a temperature of 22±24°C, 40–70% humidity under a
12 h light/dark cycle with free access to food and water.
Clinical information
A total of 24 OA patients who received whole knee
joint replacement surgery at the No. 89 Hospital of the People's
Liberation Army of China (Weifang, China) between January 2016 and
June 2016 were recruited. Tibia samples were collected during
surgery. All cases met the diagnostic guideline of OA as stipulated
by the American Rheumatology Society (10). OA caused by infection, tumor or
rheumatoid disease were excluded. Another cohort of 21 patients,
who had undergone post-traumatic amputation, was recruited as a
control group from which tibia tissue samples were collected.
Informed consents were obtained from all patients, and the study
was reviewed and approved by the ethical committee of the No. 89
Hospital of the People's Liberation Army of China (Shandong,
China).
Generation and grouping of the OA rat
model
Eight-week-old male SD rats were anesthetized with
10% hydrate chloral via intraperitoneal injection. The skins of the
bilateral knee joints were sterilized using 75% ethanol. Knee
joints of the left and right knees were fixed at a 45° angle.
Sodium iodoacetate solution (4%; 50 µl) was injected into the right
knee joint to prepare the OA model. Focal swelling and motility of
the right knee joint indicated successful model generation. Saline
(50 µl) was injected into the left knee joint. SD rats were
sacrificed after 8 weeks of model preparation and joint cartilage
tissue samples were collected for further assays.
SD rats were further randomly divided into 3 groups
(n=10 per group). miR-9 agomir (3×103 mol/l; 20 µl) was
injected into the treated and control knee joint prior to, or 3
weeks after, OA model establishment. The negative control group
received an equal volume of agomir control in the left and right
knees. A total of 20 µl scramble negative control was used in the
blank control group at the same time points.
Construction of the luciferase
reporter assay gene plasmid
Using HEK 293 genomic DNA as the template, full
length fragments of wild type (wt) or mutant (mut) forms of 3′-UTR
of the MMP-13 gene were amplified and cloned into pGL-3M plasmid to
confirm the association between miR-9 and MMP-13, which was
predicted using www.microRNA.org. Recombinant plasmid was subsequently
used to transform DH5α competent cells. Positive clones with
correct sequences were screened out by sequencing, and termed
pGL3-MMP-13-3′-UTR-wt and pGT-MMP-13-3′-UTR-mut.
Luciferase reporter gene assay
Lipofectamine® 2000 was used to
co-transfect HEK 293 cells (American Type Culture Collection,
Manassas, VA, USA) with pGL3-MMP-13-3′-UTR-wt plasmid (or
pGL3-MMP-13-3′-UTR-mut) and miR-9 mimic (the wt form of miR-9).
Following 48 h of continuous incubation at 37°C, a dual-luciferase
assay was performed. Briefly, the culture medium was discarded and
cells were washed in phosphate-buffered saline (PBS) three times,
with the addition of 100 µl Passive Lysis Buffer. After a 15-min
culture, the mixture was centrifuged at 800 × g for 5 min at 4°C.
The cell lysate (50 µl) was mixed with 50 µl luciferase substrate
and the activity of luciferase was measured immediately. The
enzymatic reaction was stopped in 50 µl Stop & Glo (Promega
Corporation, Madison, WI, USA), followed by quantification of the
sea pansy luciferase activity. The relative expression level of the
reporter gene was calculated as the ratio of luciferase activity
against sea pansy luciferase activity. The following
oligonucleotide sequences were used: 5′-UUCUCCGAACGUGUCACGUUU-3′
for scramble negative control and 5′-UCUUUGGUUAUCUAGCUGUAUGA-3′ for
the miR-9 mimic.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cartilage tissue samples were homogenized in
liquid nitrogen. TRIzol reagent (Thermo Fisher Scientific, Inc.)
was added to lyse the cells and RNA was extracted. A ReverTra Ace
qPCR RT kit was used to synthesize cDNA from RNA by reverse
transcription. Using cDNA as the template, PCR amplification was
performed with the addition of SYBR fluorescent dye (Thermo Fisher
Scientific, Inc.). PCR conditions were as follows: 95°C for 15 sec,
followed by 60°C for 30 sec and 74°C for 30 sec. Forty cycles were
performed on an ABI ViiA TM7 fluorescent PCR cycler. The following
primer sequences were used for PCR: Forward,
5′-TCTTTGGTTATCTAGCTGTATGA-3′ and reverse,
5′-ACACTCCAGCTGGGTCGCCCTC-3′ for miR-9; forward,
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′ for U6; forward,
5′-TGGACGCCATGAAGGTTTTCT-3′ and reverse,
5′-TGGGAGCCAGATTGTCATCTC-3′ for COL2A1; forward,
5′-CCAGACTTCACGATGGCATTG-3′ and reverse,
5′-GGCATCTCCTCCATAATTTGGC-3′ for MMP-13; and forward,
5′-GAACCCTAAGGCCAAC-3′ and reverse, 5′-TGTCACGCACGATTTCC-3′ for
β-actin. RNA expression was quantified using the 2−ΔΔCq
method (11).
Western blot analysis
Cartilage tissues were mixed with homogenizing
buffer to obtain the tissue lysates. Protein supernatant was
prepared after centrifugation at 10,000 × g for 10 min at 4°C. The
bicinchoninic acid assay method was used to assess the protein
quantity and quality. Protein samples (80 µg) were separated in 10%
SDS-PAGE (3 h) and transferred to polyvinylidene difluoride
membrane (wet method, 300 mA current for 90 min). The membrane was
blocked in 5% skimmed milk powder for 60 min, followed by
incubation with primary antibodies (anti-MMP-13 at 1:200,
anti-COL2A1 at 1:200 or anti-β-actin at 1:500) at 4°C for 12 h.
Following washing (three times) with PBS with Tween 20 (PBST),
HRP-labelled secondary antibodies (anti-mouse or anti-rabbit;
dilution, 1:8,000) were added and incubated for 1 h at room
temperature. Subsequent to PBST rinsing (three times), the enhanced
chemiluminescence reagent was added for a 1–3 min incubation in the
dark. The membrane was then exposed in the dark and scanned for
data analysis using Quantity One software, version 4.6 (Bio-Rad
Laboratories, Hercules, California, USA).
Statistical analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA)
was used for data analysis and the data were presented as the mean
± standard deviation. Student's t-test was performed to compare
measurement data between groups and P<0.05 was considered to
indicate a statistically significant difference.
Results
Reduced levels of miR-9 expression and
elevated MMP-13 expression levels in cartilage tissue samples of OA
patients
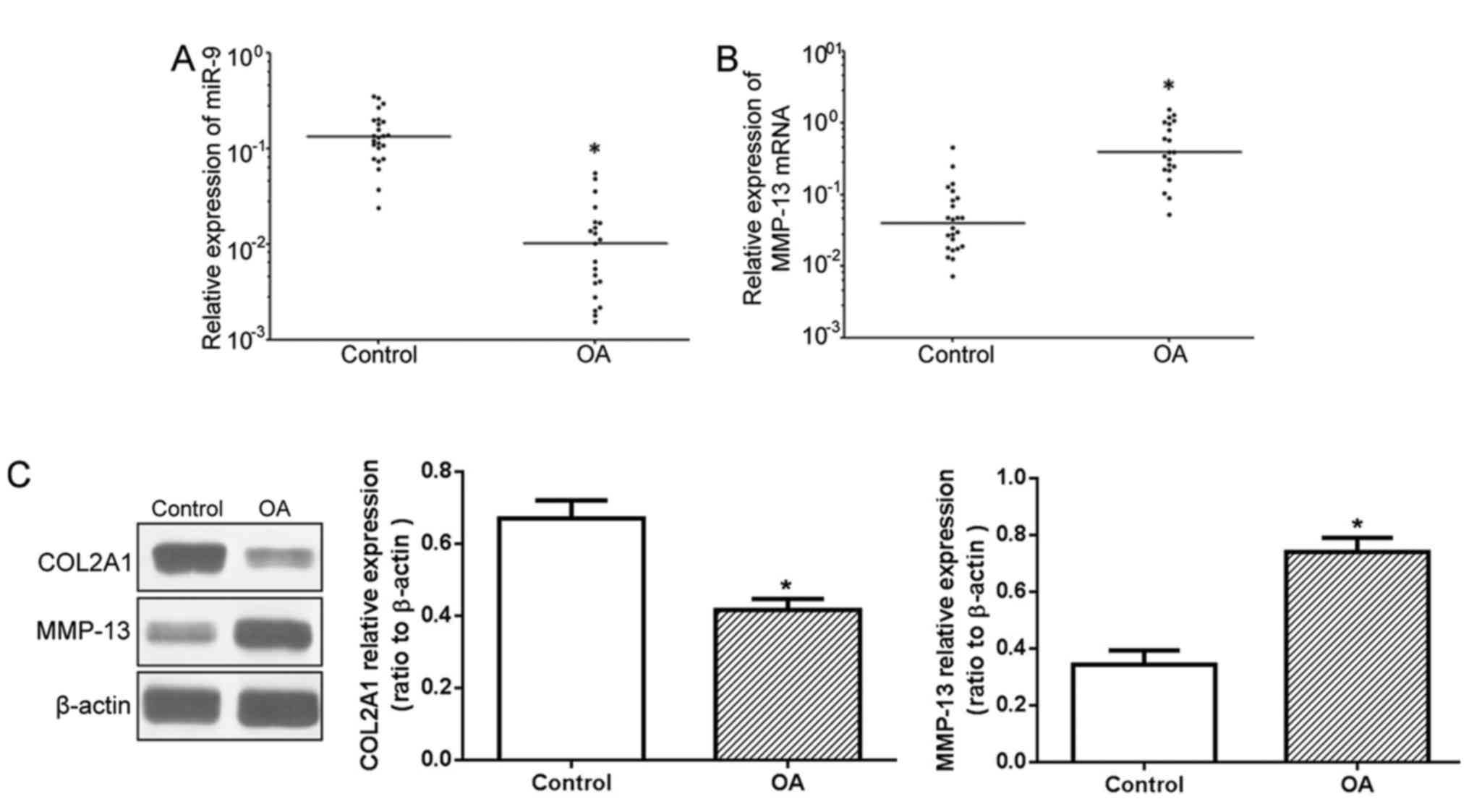
The RT-qPCR results demonstrated significantly
reduced miR-9 expression levels in cartilage tissue samples of OA
patients when compared with the control group (Fig. 1A), whilst the MMP-13 mRNA
expression level was significantly higher (Fig. 1B). Western blot analysis (Fig. 1C) identified higher MMP-13 protein
expression levels in OA patients compared with the control group,
whereas the COL2A1 protein expression levels were significantly
decreased.
Reduced miR-9 and increased MMP-13
expression levels in OA model rats
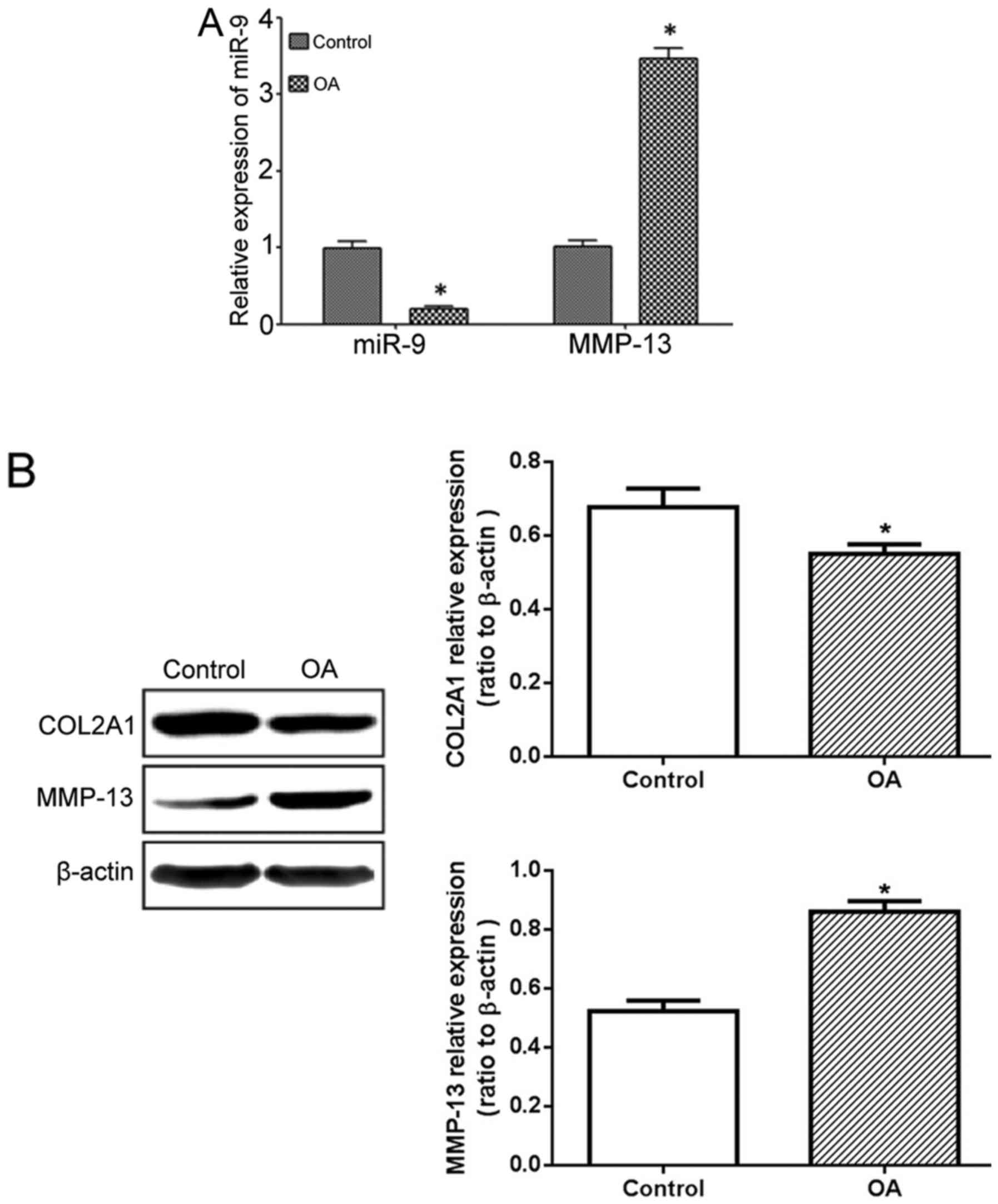
The RT-qPCR results demonstrated significantly lower
miR-9 expression levels in the treated side of the cartilage in the
OA model rats, compared with those in the control side, whilst the
MMP-13 mRNA expression level was significantly elevated (Fig. 2A). The western blot results
demonstrated similar results, as the OA model exhibited higher
MMP-13 protein expression levels on the drug treated site compared
with the other side, whilst COL2A1 protein expression was
downregulated (Fig. 2B).
miR-9 targets and regulates MMP-13
expression
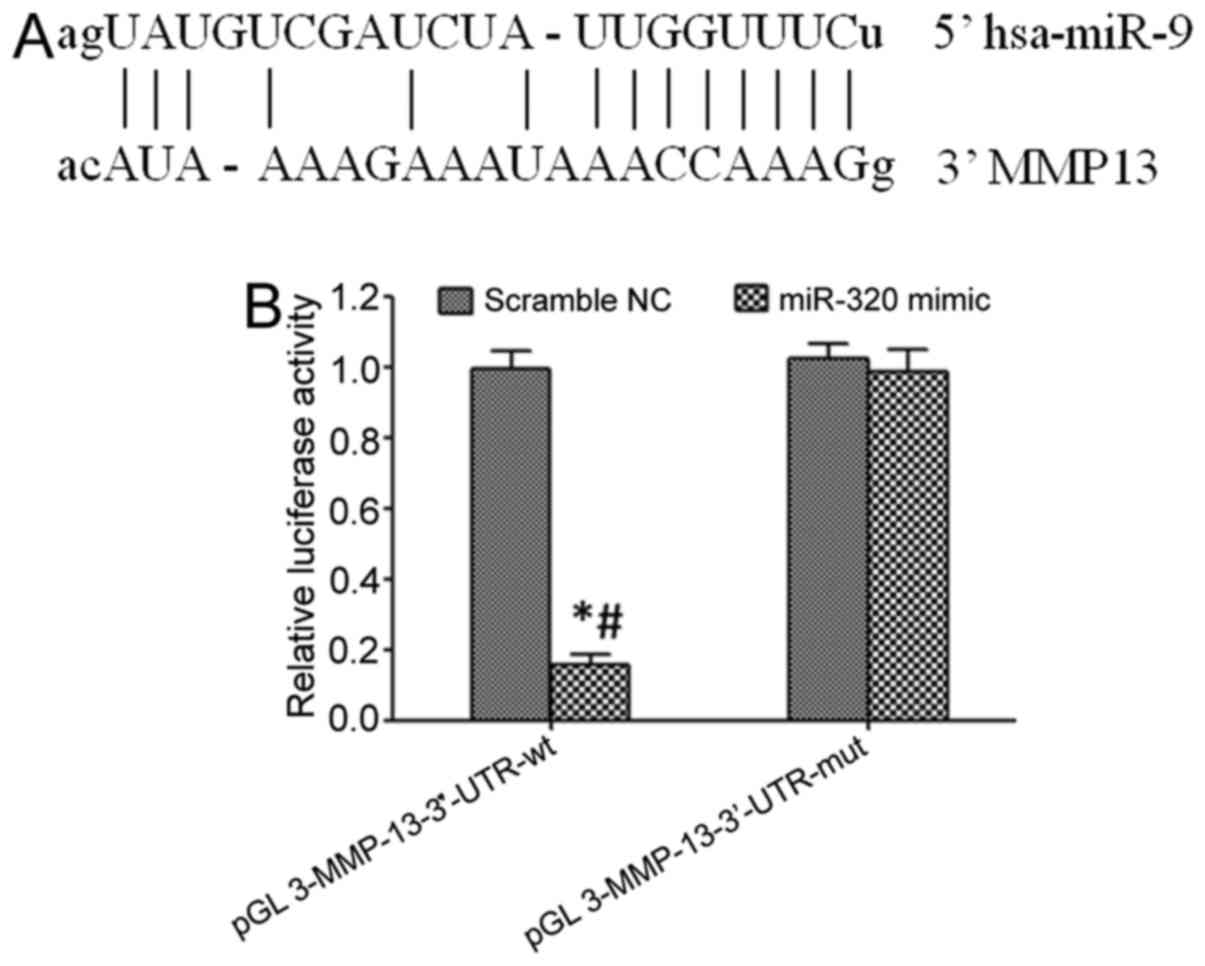
Online prediction identified the targeted binding
site between miR-9 and the 3′-UTR of MMP-13 mRNA (Fig. 3A). Transfection of an miR-9 mimic
significantly decreased the relative luciferase activity in the HEK
293 cells following transfection with the pGL3-MMP-13-3′-UTR-wt
plasmid (P<0.05), but did not exert a significant effect in the
HEK 293 cells transfected with pGL3-MMP-13-3′-UTR-mut (P>0.05).
These results indicated that miR-9 may target the 3′-UTR of
pGL3-MMP-13-3′-UTR-mut and regulate its expression (Fig. 3B).
miR-9 agomir injection decreased
MMP-13 expression levels and collagen degradation in cartilage
tissue samples of OA model rats
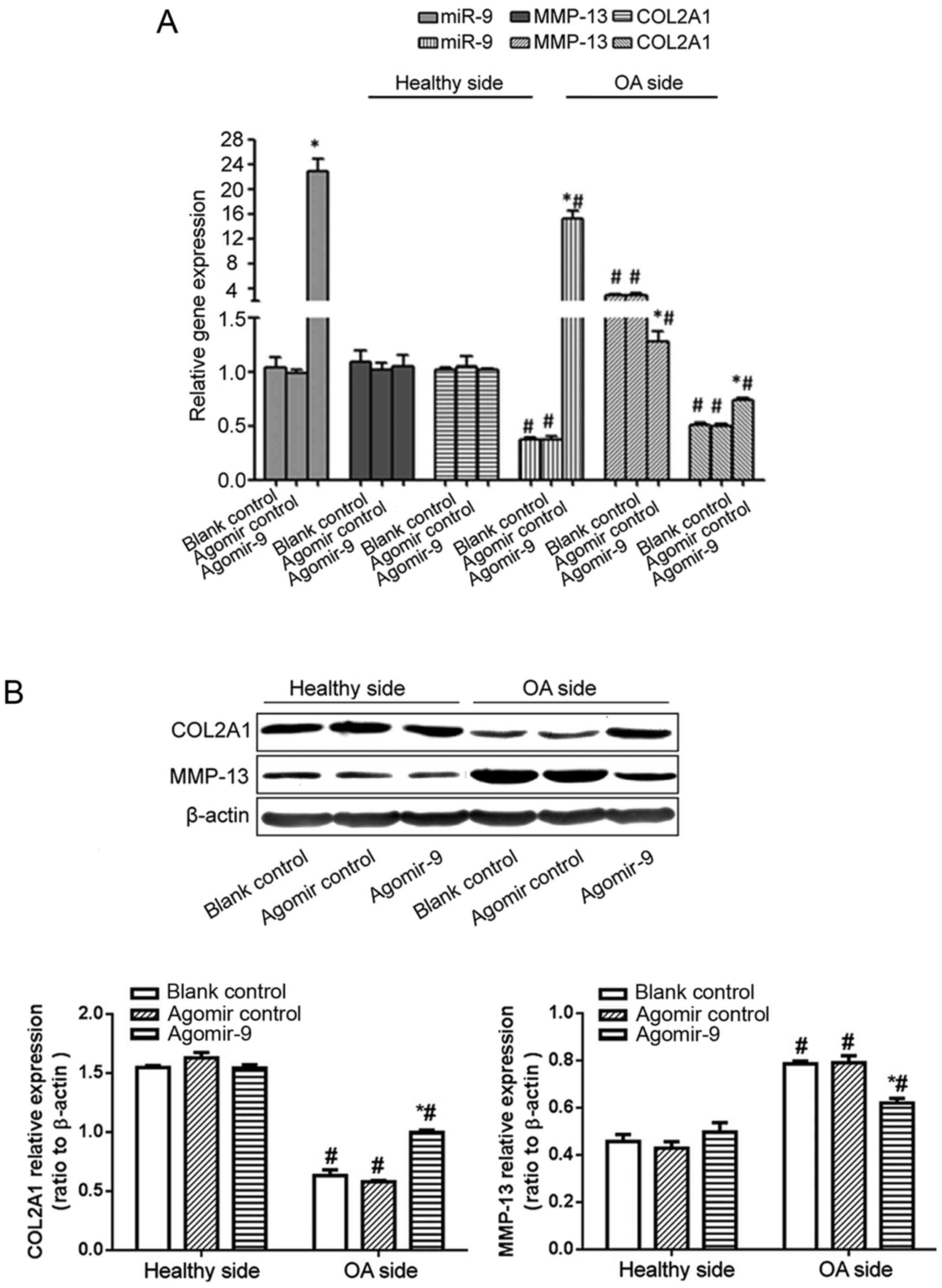
Injection of miR-9 agomir inside the knee joint
significantly decreased MMP-13 expression levels in the cartilage
tissue samples of OA model rats, with reduced collagen degradation,
thus significantly elevating COL2A1 expression levels (Fig. 4A and B).
Discussion
OA is the most common type of degenerative disease
in human axial and peripheral motor joints. It affects the joint
cartilage, subchondral bone, synovial tissues, joint capsule and
peripheral muscular tissues, causing a series of clinical symptoms,
including joint swelling, pain, aching, stiffness, atrophy,
motility reduction and limitation, thus severely affecting patient
quality of life. OA is a degenerative cartilage disease whose
incidence increases with age. The number of subchondral feeding
vessels rapidly decreases in aged people, causing a loss of
elasticity, friction and structural destruction of cartilage
(12). The core pathological
change of OA is impaired joint cartilage plus osteophyte formation
and involves multiple factors, including catabolism/anabolism
imbalance of cartilage matrix and joint chondrocytes, plus focal
inflammation (13–15). Globally, OA has a high incidence;
the overall incidence of OA is ~15%, with a significantly increased
incidence in aged individuals from 55 to 74-years-old (16). OA is prevalent in China, with
>150 million OA patients, which severely affects the quality of
life and working capacity of the aged population (17). The eventual rate of morbidity of OA
is >50%, making it a major factor for deprivation of working
capacity and immobility in aged individuals (18,19).
There is currently no highly effective method of reversing OA
progression; therefore, the predominant treatment approaches
include pain management, deformity correction and recovery or
improvement of joint functions (20). Artificial joint replacement surgery
is usually required for patients with severe OA. However, the
lifespan of artificial joints is only ~10 years, therefore it is
unfavorable for younger OA patients (21). Thus, further investigation into the
pathogenesis of OA is critical for improving treatment efficacy and
the quality of life of patients.
Healthy joint cartilage tissues include large
regions of ECM, which occupy 99% of all tissues, leaving just 1% as
joint chondrocytes. Major components of the ECM include water
(70–85%), collagen (10–25%) and proteoglycan (5–10%). Type II
collagen is the major component of the matrix collagen of
cartilage, occupying 80–90% of total proteins. MMPs are a family of
proteinase superfamily and are important in ECM degradation. It is
further divided into five groups based on protein structure and
reaction substrates, including collagenase (MMP-1, −8 and −13),
gelatinase (MMP-2 and −9), tromelysins (MMP-3, −10 and −11), model
MMPs (MMP-14, −15, −16, −17, −24 and −25) and others (MMP-7, −12,
−20 and −23). Collagenase is an important family of MMPs throughout
OA pathogenesis, and exerts lysis effects at specific sites.
Previous findings demonstrated that abnormally elevated expression
levels and activity of collagenase in focal cartilage tissues were
important reasons causing the imbalance of catabolism/anabolism of
cartilage ECM, and OA pathogenesis (5). MMP-13 is a powerful enzyme with high
specificity for degrading type II collagen in the cartilage matrix.
Certain studies identified that MMP-13 has approximately 5–10 folds
of activity for degrading type II collagen compared with another
member of the collagenase family, such as MMP-1 (5). Therefore, MMP-13 performs a major
role in degrading the cartilage matrix. Various studies
demonstrated significantly enhanced expression levels and activity
of MMP-13 in OA cartilage tissue samples (22–25).
The degradation of type II collagen (the major component of joint
cartilage tissues) by MMP-13 is a major reason causing OA
pathogenesis (6). In addition,
multiple studies demonstrated significantly reduced miR-9
expression levels in cartilage tissues of OA patients, indicating
that miR-9 downregulation is associated with OA onset. Furthermore,
bioinformatics analysis demonstrated complementary binding sites
between miR-9 and MMP-13. The current study therefore investigated
whether miR-9 was involved in regulating MMP-13 expression levels
and OA pathogenesis.
Results of the present study demonstrated
significantly reduced miR-9 expression levels in cartilage tissue
samples from OA patients when compared with the healthy control
group, whilst the MMP-13 mRNA expression level was greater. Western
blotting indicated markedly elevated MMP-13 expression levels in OA
patient chondrocytes, whilst the COL2A1 protein expression level
was significantly lower. In addition, animal experiments showed
reduced miR-9 expression levels in cartilage tissue samples during
OA onset, with greater levels of MMP-13 expression. Blaney et
al (21) identified that the
level of MMP-13 expression was significantly potentiated in
cartilage tissue samples from OA model mice, indicating that MMP-13
may act as a biomarker during OA pathogenesis. Pelletier et
al (23) demonstrated that
MMP-13 expression levels were increased in OA model dogs. Li et
al (24) demonstrated only a
minimal quantity of MMP-13 in healthy cartilage tissue samples,
whilst OA cartilage tissue samples exhibited potentiated MMP-13
expression levels. The current study observed significantly
elevated MMP-13 expression levels in human and rat OA cartilage
tissue samples, which is consistent with Blaney et al
(23) and Li et al
(26). Gu et al (8) demonstrated significantly reduced
miR-9 expression levels in cartilage tissue samples of OA patients
when compared with those of healthy tissue samples. In addition, Gu
et al (8) performed an
animal study and identified lower miR-9 expression levels in
cartilage tissue samples of OA model rats. Song et al
(9) observed significantly reduced
miR-9 expression levels in chondrocytes from OA-derived tissues
compared with those with a normal cell origin (9). The current study observed
significantly reduced miR-9 expression levels in cartilage tissue
samples from OA patients, which were consistent with the findings
of Gu et al (8) and Song
et al (9). A dual
luciferase gene reporter assay demonstrated that transfection of
miR-9 mimic significantly decreased relative luciferase activity in
HEK 293 cells transfected with pGL3-MMP-13-3′-UTR-wt plasmid,
indicating that MMP-13 is the target gene of miR-9. The injection
of miR-9 agomir into the knee joint at the disease site of OA model
rats significantly impaired the elevation of MMP-13 expression
levels in cartilage tissue samples from the OA model, in addition
to a smaller decrease in the expression level of COL2A1. These
results demonstrated that application of miR-9 agomir into the knee
joint effectively inhibits MMP-13 expression in the cartilage
tissues of OA model rats, and reduces collagen lysis. Gu et
al (8) identified that miR-9
inhibits the expression of nuclear factor (NF)-κB1 by targeted
binding to its 3′-UTR, further inhibiting activation of the NF-κB
signaling pathway and expression levels of the downstream
inflammatory factor, interleukin (IL)-6, thus inhibiting the
secretion of inflammatory factor IL-6 on MMP-13. miR-9
downregulation contributes to enhancing NF-κB signaling pathway
activity, upregulating inflammatory factor and MMP-13 expression
levels, and facilitating the pathogenesis of OA. Song et al
(9) demonstrated that miR-9
decreased the activation of caspase-3 and the pro-apoptotic effect
on chondrocytes by protogenin (PRTG) via targeted binding to
3′-UTR, leading to expression inhibition. miR-9 downregulation is
therefore involved in facilitating chondrocyte apoptosis and
inducing OA pathogenesis. The current study revealed the role of
miR-9 in targeted inhibition of MMP-13 expression and suppression
of OA onset, which is consistent with previous studies conducted by
Gu et al (8) and Song et
al (9).
In conclusion, the level of miR-9 expression is
suppressed whilst MMP-13 expression levels are elevated in OA
cartilage tissues. miR-9 inhibits the expression level of MMP-13,
thus suppressing its inhibitory effects on COL2A1 and enhancing
COL2A1 expression levels, which consequently antagonizes the
pathogenesis of OA. The results of the present study suggested that
the therapeutic targeting miR-9 or MMP-13 may be beneficial for the
treatment of OA. However, due to limited number of patients
enrolled in the present study, large-cohort clinical studies are
required to confirm these findings in the future.
Acknowledgements
This study was supported by grant no. CJN13J002.
References
|
1
|
Coudeyre E, Byers Kraus V and Rannou F:
Osteoarthritis in physical medicine and rehabilitation. Ann Phys
Rehabil Med. 59:1332016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Queen RM, Sparling TL and Schmitt D: Hip,
knee, and ankle osteoarthritis negatively affects mechanical energy
exchange. Clin Orthop Relat Res. 474:2055–2063. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Zhao X and Tang S: Inhibitory
effects of EGb761 on the expression of matrix metalloproteinases
(MMPs) and cartilage matrix destruction. Cell Stress Chaperones.
20:781–786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jabłońska-Trypuć A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31:177–183.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li P, Deng J, Wei X, Jayasuriya CT, Zhou
J, Chen Q, Zhang J, Wei L and Wei F: Blockade of hypoxia-induced
CXCR4 with AMD3100 inhibits production of OA-associated catabolic
mediators IL-1beta and MMP-13. Mol Med Rep. 14:1475–1482. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen YT, Hou CH, Hou SM and Liu JF: The
effects of amphiregulin induced MMP-13 production in human
osteoarthritis synovial fibroblast. Mediators Inflamm.
2014:7590282014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asahara H: Current status and strategy of
microrna research for cartilage development and osteoarthritis
pathogenesis. J Bone Metab. 23:121–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu R, Liu N, Luo S, Huang W, Zha Z and
Yang J: MicroRNA-9 regulates the development of knee osteoarthritis
through the NF-kappaB1 pathway in chondrocytes. Medicine
(Baltimore). 95:e43152016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song J, Kim D, Chun CH and Jin EJ:
MicroRNA-9 regulates survival of chondroblasts and cartilage
integrity by targeting protogenin. Cell Commun Signal. 11:662013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peat G, Thomas E, Duncan R, Wood L, Hay E
and Croft P: Clinical classification criteria for knee
osteoarthritis: performance in the general population and primary
care. Ann Rheum Dis. 65:1363–1367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dziri C, Aloulou I, Loubiri I, Rekik M,
Zohra Ben Salah F and Abdallah A: Assessment of disability in
osteoarthritis of the knee. Annals of physical and rehabilitation
medicine. 59s:e1152016. View Article : Google Scholar
|
|
13
|
Kim YH, Dorj A, Han A, Kim K and Nha KW:
Improvements in spinal alignment after high tibial osteotomy in
patients with medial compartment knee osteoarthritis. Gait Posture.
48:131–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mayorga AJ, Wang S, Kelly KM and
Thipphawong J: Efficacy and safety of fulranumab as monotherapy in
patients with moderate to severe, chronic knee pain of primary
osteoarthritis: a randomised, placebo- and active-controlled trial.
Int J Clin Pract. 70:493–505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van der Kraan PM, Berenbaum F, Blanco FJ,
Cosimo de B, Lafeber F, Hauge E, Higginbottom A, Ioan-Facsinay A,
Loughlin J, Meulenbelt I, et al: Translation of clinical problems
in osteoarthritis into pathophysiological research goals. RMD Open.
2:e0002242016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cushnaghan J and Dieppe P: Study of 500
patients with limb joint osteoarthritis. I. Analysis by age, sex,
and distribution of symptomatic joint sites. Ann Rheum Dis.
50:8–13. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arden N and Nevitt MC: Osteoarthritis:
Epidemiology. Best Prac Res Clin Reheum. 20:3–25. 2006. View Article : Google Scholar
|
|
18
|
Montero A, Mulero JF, Tornero C, Guitart J
and Serrano M: Pain, disability and health-related quality of life
in osteoarthritis-joint matters: An observational, multi-specialty
trans-national follow-up study. Clin Rheumatol. 35:2293–2305. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frioui Mahmoudi S, Toulgui E, Ben Jeddou
K, Gaddour M, Jemni S and Khachnaoui F: Quality of life for patient
with knee osteoarthritis. Ann Phys Rehabil Med. 59S:e158–e159.
2016. View Article : Google Scholar
|
|
20
|
Wainwright TW, Immins T and Middleton RG:
A cycling and education programme for the treatment of hip
osteoarthritis: A quality improvement study. Int J Orthop Trauma
Nurs. 23:14–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu D, Zhao Y, Shen J, Cai Z and Hua Y:
Comparison of venous thromboembolism after total artificial joint
replacement between musculoskeletal tumors and osteoarthritis of
the knee by a single surgeon. PLoS One. 11:e01582152016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neuhold LA, Killar L, Zhao W, Sung ML,
Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, et
al: Postnatal expression in hyaline cartilage of constitutively
active human collagenase-3 (MMP-13) induces osteoarthritis in mice.
J Clin Invest. 107:35–44. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blaney Davidson EN, Remst DF, Vitters EL,
van Beuningen HM, Blom AB, Goumans MJ, van den Berg WB and van der
Kraan PM: Increase in ALK1/ALK5 ratio as a cause for elevated
MMP-13 expression in osteoarthritis in humans and mice. J Immunol.
182:7937–7945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim NH, Meinjohanns E, Meldal M,
Bou-Gharios G and Nagase H: In vivo imaging of MMP-13 activity in
the murine destabilised medial meniscus surgical model of
osteoarthritis. Osteoarthritis Cartilage. 22:862–868. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pelletier JP, Boileau C, Martin Boily,
Brunet J, Mineau F, Geng C, Reboul P, Laufer S, Lajeunesse D and
Martel-Pelletier J: The protective effect of licofelone on
experimental osteoarthritis is correlated with the downregulation
of gene expression and protein synthesis of several major cartilage
catabolic factors: MMP-13, cathepsin K and aggrecanases. Arthritis
Res Ther. 7:R1091–1102. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li NG, Shi ZH, Tang YP, Wang ZJ, Song SL,
Qian LH, Qian DW and Duan JA: New hope for the treatment of
osteoarthritis through selective inhibition of MMP-13. Curr Med
Chem. 18:977–1001. 2011. View Article : Google Scholar : PubMed/NCBI
|