Introduction
Postoperative cognitive dysfunction (POCD) is a
clinical syndrome characterized by varying degrees of cognitive
functional decline in patients following major surgery,
particularly in the elderly (1–3).
POCD affects a considerable proportion of the surgical population
worldwide, leading to impaired postoperative rehabilitation,
decreased quality of life, and even increased mortality (1). The incidence of POCD varies
extensively, from 41–75% at 7 days to 18–45% at 3 months
postoperatively across different studies (1–4).
Considering advances in surgical and anesthetic techniques, and in
combination with the aging population, POCD has become an area of
focus for anesthesia researchers. Therefore, the prevention and
treatment of POCD has become a notable public health issue.
The mechanisms underlying surgery-induced cognitive
impairment are multifaceted, although numerous lines of evidence
implicate inflammation as a potential driving factor that serves a
central role (5–8). Surgery and tissue damage trigger an
initial peripheral innate immune response by releasing
pro-inflammatory cytokines, including nitric oxide (NO), tumor
necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and late
cytokine high-mobility group box 1 (HMGB1) (6–9).
This systemic inflammatory milieu further leads to transient
endothelial dysfunction and disrupts the integrity of the
blood-brain barrier (BBB), facilitating the migration of
macrophages into the brain parenchyma, and inducing subsequent
neuroinflammation with neuronal impairment and ensuing cognitive
dysfunction (9). Thus, from a
clinical perspective, suppressing inflammation represents a
legitimate way to reduce surgery-induced cognitive impairment. In
support of this hypothesis, it has been reported that prophylactic
administration of either a monoclonal antibody to TNF-α, or
disabling HMGB1 with an inhibitory monoclonal antibody, improves
post-surgical cognitive decline (10–12).
However, direct neutralization of these pro-inflammatory mediators
may lead to undesirable side effects. It is possible that
therapeutics directed to the early physiopathological conditions
that derive from this initial pro-inflammatory response, while not
directly antagonizing them, may be more efficient in preventing the
occurrence of POCD.
It has been demonstrated that preconditioning with
low-dose lipopolysaccharide (LPS; a bacterial endotoxin) has
protective effects against subsequent insults, as evidenced by
reduced neuroinflammation, BBB disruption and cognitive decline, a
phenomenon termed endotoxin tolerance (13–15).
On the basis of these findings, it was hypothesized that endotoxin
tolerance induced by LPS preconditioning may abolish the
exacerbated inflammation within the brain, and thus protect against
surgery-induced cognitive impairment in aging mice.
Materials and methods
Animals
A total of 172 C57BL/6 male mice (12–14 months;
24–36 g) were purchased from the Animal Center of Nanjing Medical
University (Nanjing, China). The mice were housed in pairs in a
colony room maintained at 24±1°C and 40–50% relative humidity with
a 12-h light-dark cycle (lights on at 07:00 a.m.). Mouse chow and
water were available ad libitum. All studies were approved
by the Institutional Animal Care and Use Committee of Jinling
Clinical Medical College of Nanjing Medical University, and met the
Guide for the Care and Use of Laboratory Animals from the National
Institutes of Health (NIH; Bethesda, MD, USA) guidelines for the
Use of Experimental Animals in research.
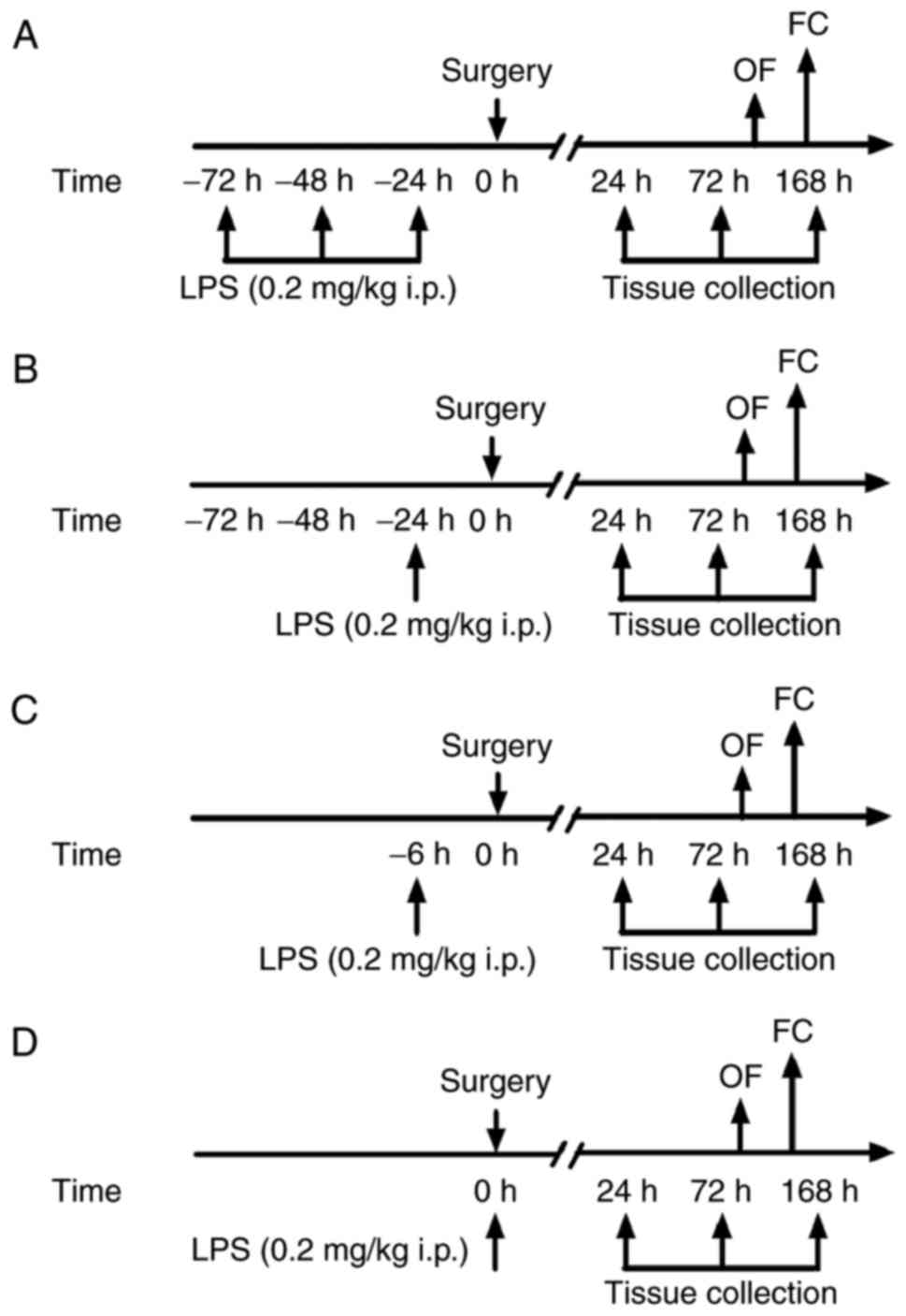
Animal grouping and surgical
model
Mice were randomly assigned to the following groups:
Control (n=22); surgery (n=30); surgery + repeated LPS (−72 h)
(n=30); surgery + LPS (−24 h) (n=30); surgery + LPS (−6 h) (n=30);
and surgery + LPS (0 h) groups (n=30). Exploratory laparotomy was
performed as previously described (16,17).
Mice were allowed to acclimate for 2 weeks prior to the
experiments. The surgery was performed under 1.5% isoflurane
anesthesia to mimic exploratory abdominal surgery in humans. A
median abdominal incision (~1-cmlong vertical incision) was made to
allow for penetration of the peritoneal cavity. Thereafter, the
investigator inserted blunt forceps into the opening and explored
the viscera, intestines and musculature. Next, sterile 4-0 chromic
gut sutures were used to close the peritoneal lining and skin. The
wound was dressed with polysporin (Pfizer, Inc., New York, NY,
USA). The animals were maintained under isoflurane anesthesia
during the 10 min of the surgical procedure. Sham controls received
neither anesthesia nor surgery. In the present study, all efforts
were made to minimize animal suffering and reduce the number of
animals used.
LPS administration
All injections were performed intraperitoneally
(i.p.) at a volume of 5 ml/kg body weight. LPS (from Escherichia
coli 0111:B4, L-2630; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was dissolved in 0.9% NaCl. The dose (0.2 mg/kg) of LPS
was based on previous studies, in which it was demonstrated that
LPS preconditioning abolished the exacerbated inflammation and
protected against cognitive impairment in other animal models
(13,14). Animals in the control group
received an injection of 0.9% NaCl with the same regimen. All mice
were injected with LPS or saline between 8.00 and 9.00 a.m. Mice
were sacrificed and tissue was collected at 24, 72 and 168 h
following preconditioning. Tissue was additionally collected from
six untreated mice to include as a baseline control group. In the
present study, no animal succumbed during the observation period in
all groups. The flow chart of the experimental protocol is
presented in Fig. 1.
Open field test
An open field test was performed to evaluate the
exploratory and anxiety behaviors. Mice were placed individually in
the center of a clear Plexiglas box (50×50×40 cm) and left free to
explore the environment for 5 min. Total movements and time spent
in the center of the open field were recorded during a 5-min
exploration time period. The behavior of the mice was recorded
using a video camera (Software Technology Co., Ltd., Ningbo,
China). The apparatus was cleaned with 70% ethanol prior to testing
each mouse to avoid the presence of olfactory cues.
Fear conditioning tests
Fear conditioning tests were performed to evaluate
fear learning, as previously described (16,17).
Mice were placed into a conditioning chamber (32×25×25 cm), with a
stainless-steel shock grid floor. Mice learn to associate an
environment (context) with a conditional stimulus (CS), for
example, a tone, and an unpleasant stimulus [foot shock;
unconditional stimulus (US)]. The training paradigm was as follows:
Tone at 75 dB for 30 sec; shock at 0.75 mA for 2 sec. Contextual
memory was tested 24 h subsequent to the training. The animals were
placed back in the original training chamber to monitor freezing
behavior, which was defined as an absence of any movement for >3
sec. The cued fear memory was tested 2 h subsequently in a novel
context with a continuous 3-min training tone presentation to
monitor freezing behavior.
ELISA analysis
The concentrations of TNF-α (cat. no. 22351), IL-1β
(cat. no. 23107), IL-6 (cat. no. 21724), and IL-10 (cat. no. 26271)
in the hippocampus were determined using ELISA kits (North China
Institute of Science and Technology, Beijing, China), according to
the manufacturer's instructions, as previously described (16,17).
Mice were sacrificed with an i.p. injection of 2% sodium
pentobarbital (60 mg/kg) and the hippocampus was collected,
separated, and placed in a homogenizer. Homogenates were
centrifuged at 10,000 × g for 10 min at 4°C. The quantity of TNF-α,
IL-1β, IL-6 and IL-10 in each sample was standardized to its
protein content.
Immunofluorescence
Under deep isoflurane anesthesia, mice were perfused
transcardially with normal saline, followed by 4% paraformaldehyde
(PFA) in PBS for 15 min. Brains were harvested and post-fixed in 4%
PFA overnight at room temperature and then with 30% sucrose for 24
h at 4°C. Brains were freeze-mounted in optimal cutting temperature
(−20°C) embedding medium, cut into 10-µm-thick sections using a
cryostat, and mounted on slides. Slices were blocked with 3% bovine
serum albumin (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
for 1 h at room temperature. The sections were incubated with a rat
anti-allograft inflammatory factor 1 [IBA1 (a marker of microglial
activation), 1:200, cat. no. ab48004; Abcam, Cambridge, UK]
antibody overnight at 4°C, followed by a 1 h incubation with the
secondary antibodies [Cy3-conjugated donkey anti-rat immunoglobulin
G (1:300, cat. no. sc53682; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA] at room temperature. Following three washes for 5 min at
room temperature using PBS, sections were counterstained with DAPI
for 15 min at room temperature, mounted on glass slides and
coverslipped with fluorescence mounting medium. A total of three
independent microscopic fields in each section were randomly
acquired in the hippocampal CA1, CA3 and dentate gyrus regions, and
three sections per mouse were imaged. Images were processed, and
the area of the microglia was quantified using ImageJ software
(version 1.50i; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS 16.0
for Windows (SPSS, Inc., Chicago, IL, USA). Data are presented as
the mean ± standard error of the mean. Multiple comparisons were
analyzed using one-way analysis of variance followed by the post
hoc Tukey test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Single low-dose LPS preconditioning at
24 h prior to surgery reverses surgery-induced cognitive
impairment
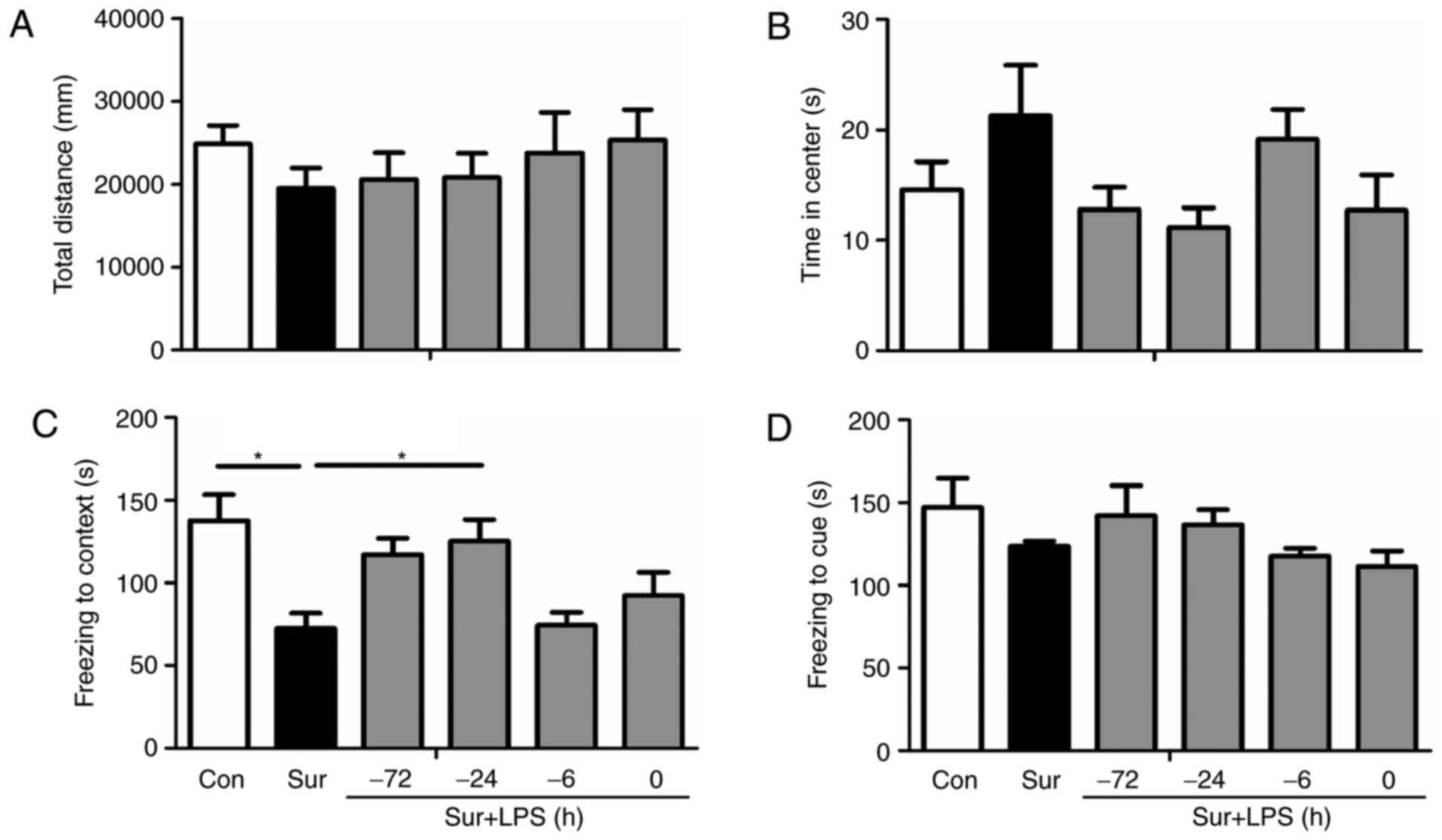
To first exclude possible impairments in locomotor
activity and exploratory behavior following surgery, mice were
evaluated in an open field arena prior to the contextual
assessment. As presented in Fig. 2A
and B, no alterations were observed in the total distance and
time spent in the center of the arena between any group (total
distance: F(5,66) = 0.546, P=0.740; time spent in the
center: F(5,66) = 1.871, P=0.111).
To assess whether surgery induced cognitive
impairments, cognitive function was assessed by fear conditioning
tests. As revealed in Fig. 2C and
D, the contextual fear response was significantly decreased in
mice subjected to surgery compared with the control group. Notably,
a single LPS preconditioning treatment at 24 h prior to surgery
restored freezing behavior, an indicator of memory retention in
rodents when performed at day 7 following surgery
(F(5,66) = 5.274, P<0.001; Fig. 2C). Repeated low-dose LPS
administration did not reverse surgery-induced cognitive impairment
when compared with the control group (P=0.099). However, no
significant difference was observed in post-tone freezing time in
the auditory-cued fear test between groups (F(5,66) =
1.460, P=0.215; Fig. 2D),
suggesting that surgery impaired hippocampal-dependent memory
(5).
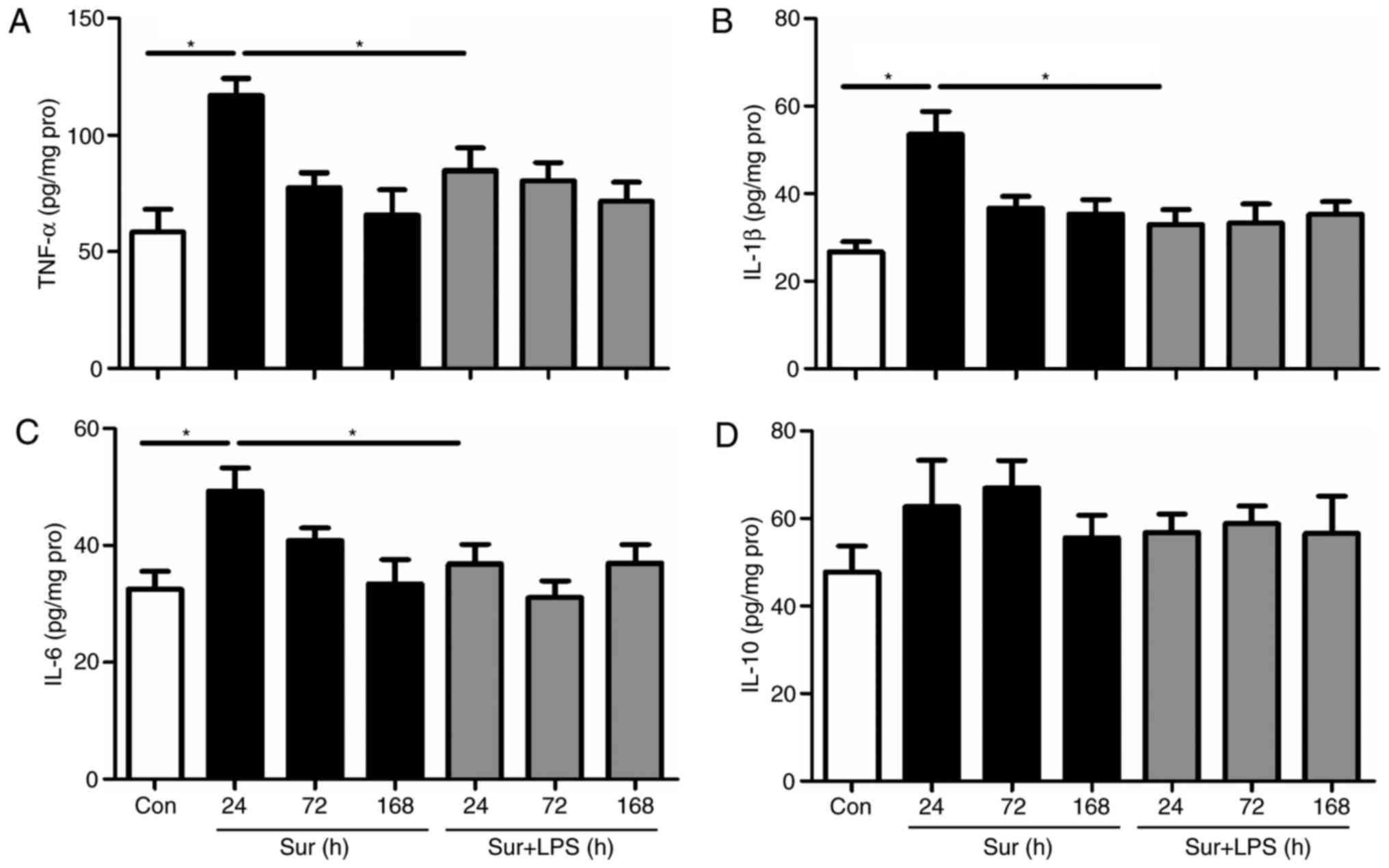
Low-dose LPS preconditioning reverses
the signs of neuroinflammation, depending on the regime of LPS
administration
In general, repeated injections of LPS at an
increasing dose may provide greater suppression of pro-inflammatory
responses. Therefore, LPS was first administered at 72 h prior to
surgery for 3 consecutive days to investigate whether this regime
may decrease the signs of neuroinflammation and consequently
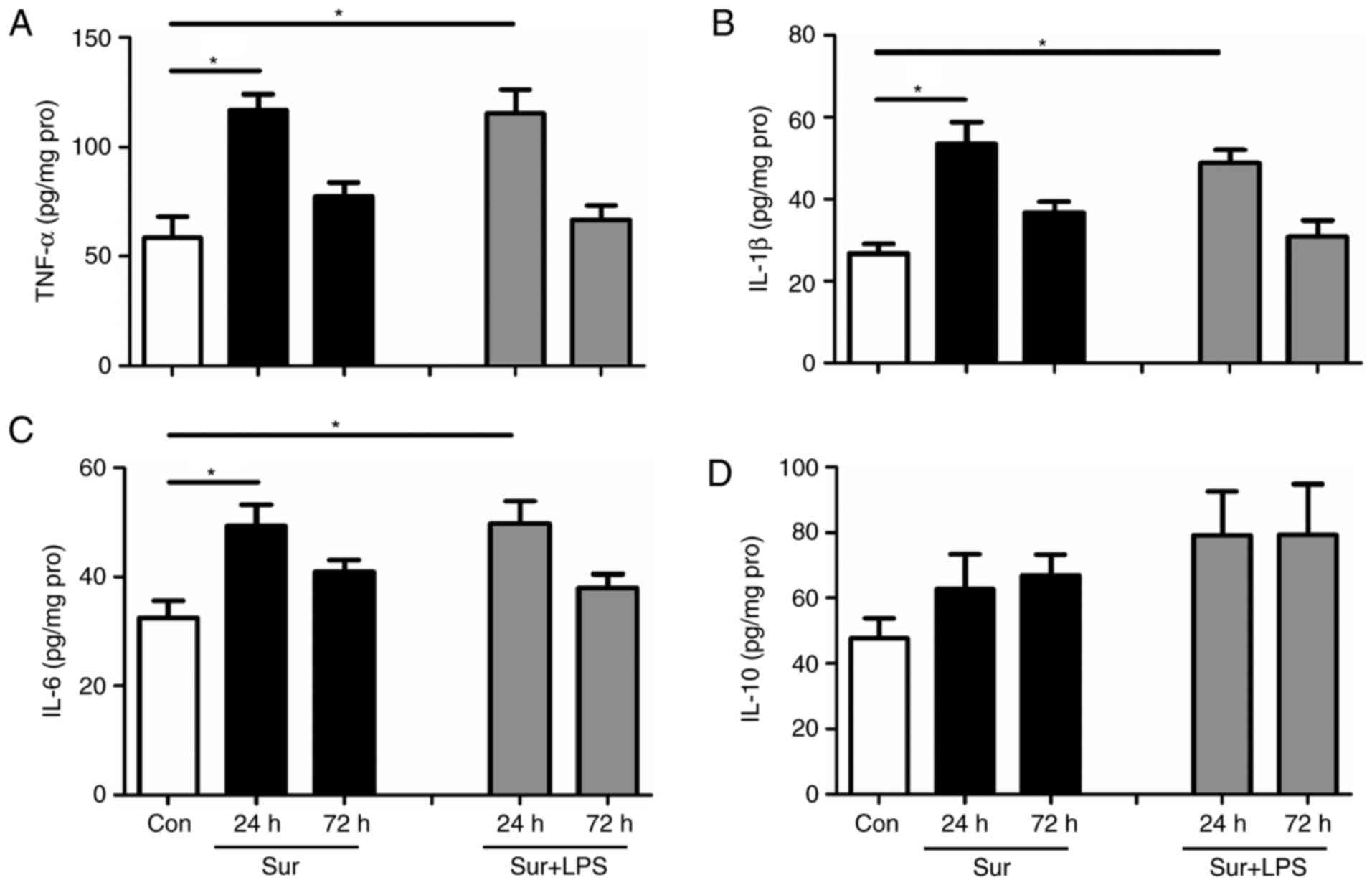
improve cognitive impairment following surgery. As presented in
Fig. 3, repeated LPS
administration significantly decreased the hippocampal expression
levels of pro-inflammatory mediators, including: TNF-α, IL-1β and
IL-6, at 24 h post-surgery (TNF-α: F(4,25) = 4.730,
P=0.001; IL-1β: F(4,25) = 5.435, P<0.001; IL-6:
F(4,25) = 3.534, P=0.008). However, repeated LPS
administration did not significantly alter the hippocampal levels
of the anti-inflammatory mediator IL-10 at 24 h post-surgery
(IL-10: F(4,25) = 0.785, P=0.587). In addition, it was
observed that there was no difference in the hippocampal levels of
TNF-α, IL-1β, IL-6 and IL-10 among the groups at 72 h post-surgery,
returning to the baseline levels as in the control mice. Based on
these results, and to minimize the number of animals used, cytokine
levels were only measured at 24 and 72 h post-surgery in the
subsequent experiments.
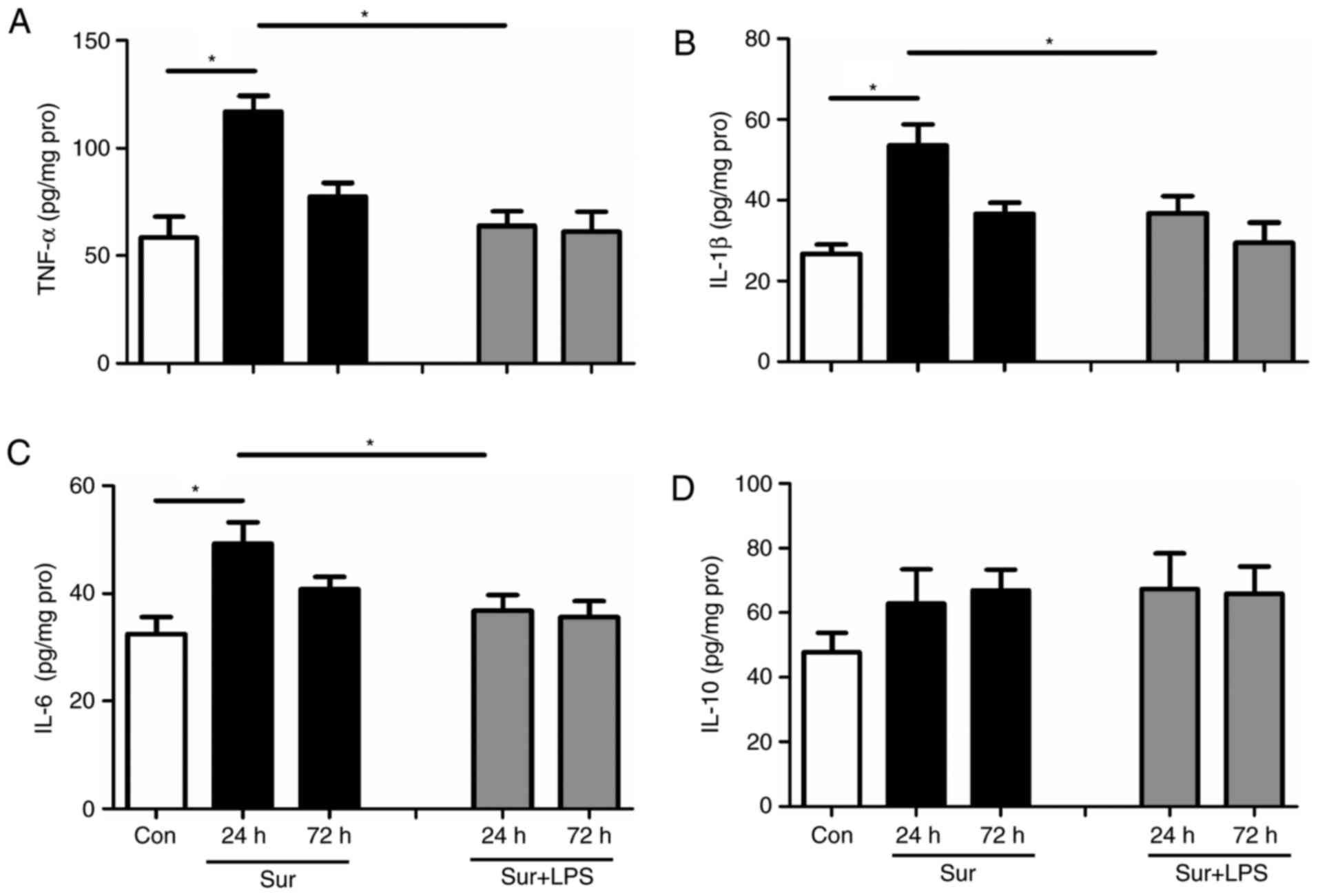
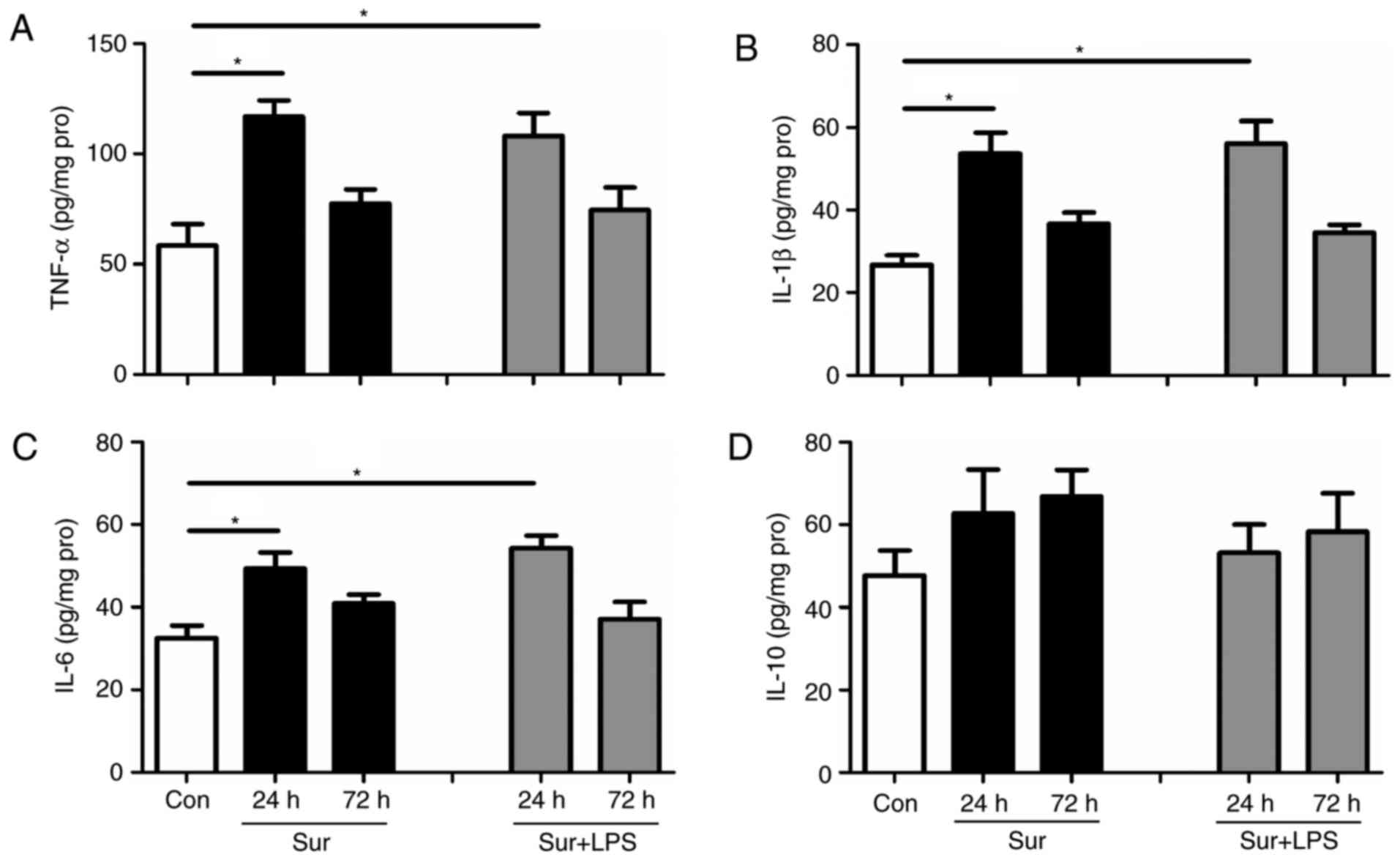
Although repeated LPS administration downregulated
the signs of neuroinflammation, it was observed that this regime
did not confer beneficial cognitive outcomes. Subsequently, the
present study tested the hypothesis that a single low-dose LPS
administration at 24 h prior to surgery may decrease the levels of
pro-inflammatory mediators, in addition to reversing cognitive
impairment. As expected, one single low-dose treatment with LPS at
24 h prior to surgery significantly reduced the hippocampal levels
of TNF-α, IL-1β and IL-6 at 24 h post-surgery (TNF-α:
F(4,25) = 9.254, P<0.001; IL-1β: F(4,25) =
6.598, P=0.001; IL-6: F(4,25) = 4.410, P=0.008; Fig. 4A-C). Similarly, a single low dose
of LPS at 24 h prior to surgery did not significantly alter the
hippocampal level of the anti-inflammatory mediator IL-10 following
surgery (IL-10: F(4,25) = 0.890, P=0.484; Fig. 4D).
To further investigate whether a shorter interval
between LPS administration and surgery may produce similar effects
to the 24 h regime, LPS was administered 6 h prior to surgery. A
single low-dose LPS administration at 6 h prior to surgery did not
alter the cytokine levels (TNF-α: F(4,25) = 10.713,
P<0.001; IL-1β: F(4,5) = 9.9655, P<0.001; IL-6:
F(4,25) = 5.058, P=0.004; IL-10: F(4,25) =
1.434, P=0.252; Fig. 5).
To evaluate whether LPS administration can aggravate
the inflammatory response to surgical trauma, low-dose LPS was
administered immediately prior to surgery. Unexpectedly, low-dose
LPS administration did not further increase the expression levels
of the assessed cytokines in the hippocampus, measured at 24 and 72
h post-surgery, when compared with the surgery group (TNF-α:
F(4,25) = 7.596, P<0.001; IL-1β: F(4,25) =
11.135, P<0.001; IL-6: F(4,25) = 7.028, P=0.001;
IL-10: F(4,25) = 0.896, P=0.481; Fig. 6).
Low-dose LPS preconditioning reverses
surgery-induced microglial activation, depending on the regime of
LPS administration
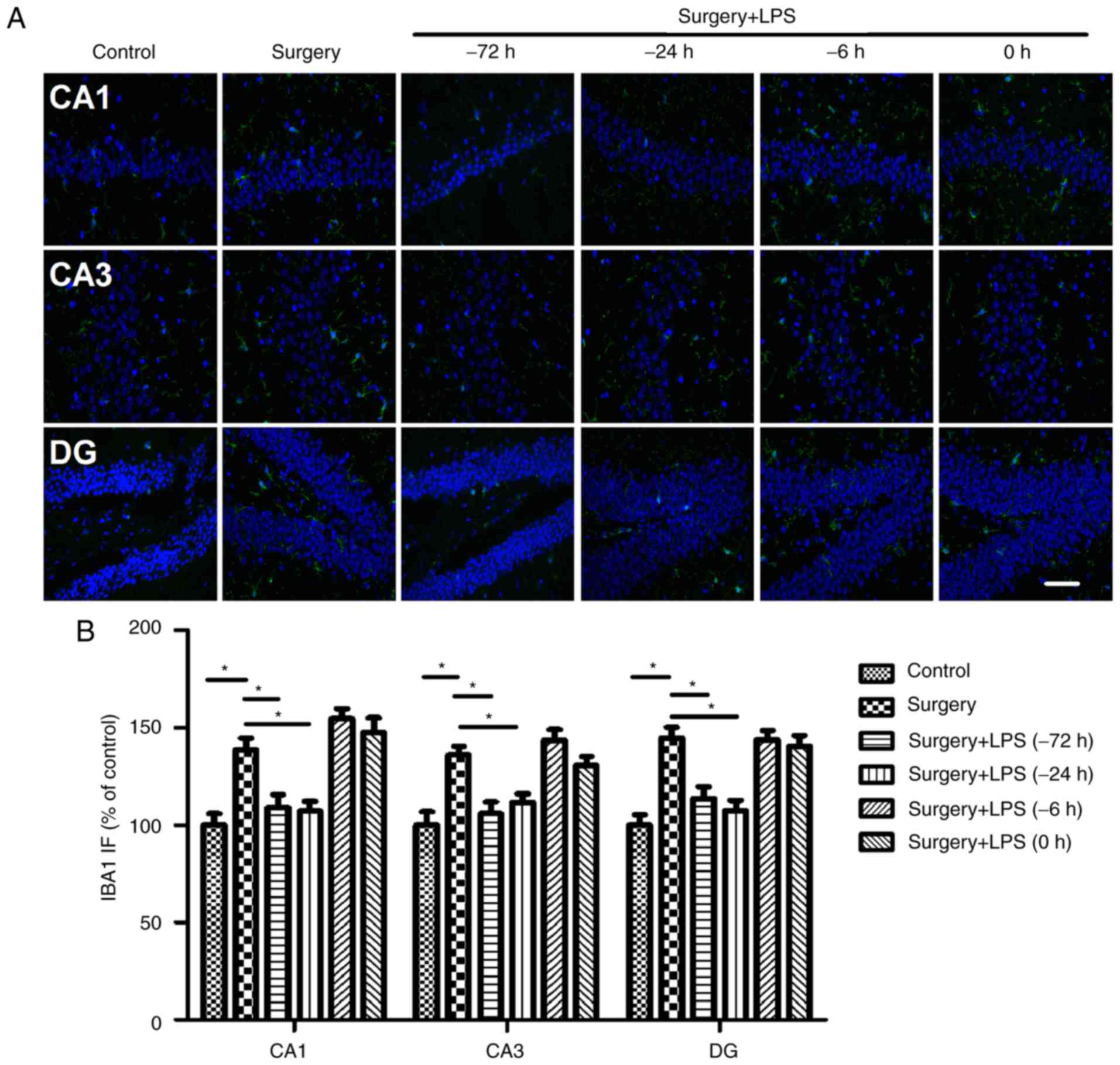
Since microglial cells are among the principal
immune cells of the brain involved in the amplified
neuroinflammatory response during the development of POCD, the
present study evaluated whether low-dose LPS preconditioning was
able to modulate the activity of microglia. Similar to the results
of the analysis of cytokine expression, surgery induced a
significant increase in the number of hippocampal IBA1-positive
cells in the hippocampus, which was reversed by repeated LPS
administration at 72 h or a single LPS administration at 24 h prior
to surgery. Consistent with the above results, one single low-dose
LPS administration at 6 h or immediately prior to surgery did not
further increase the number of IBA1-positive cells in the
hippocampus (Fig. 7).
Discussion
Substantial evidence has indicated that a
dysregulated inflammatory response is causally linked to
surgery-induced cognitive impairment (5–9).
However, there remains no effective strategy to prevent or treat
cognitive impairment resulting from surgery. In the present study,
further evidence was produced to suggest that low-dose LPS
preconditioning may protect against surgery-induced cognitive
impairment in aging mice, as reflected by an improvement in
hippocampal-dependent memory impairment and reduced production of
pro-inflammatory mediators in the hippocampus.
Acute inflammation, as induced by aseptic trauma,
including surgery, is important for optimal antimicrobial defense
and healing, although an excessive inflammatory response may lead
to exaggerated tissue damage (10). Peripheral surgery triggers an
inflammatory response that may affect the central nervous system,
contributing to neuroinflammation and POCD (11). Using a mouse model of
surgery-induced cognitive decline (16,17),
which frequently leads to cognitive dysfunctions in humans, the
results of the present study demonstrated that surgery induced
significant cognitive impairment, concomitant with increased
hippocampal levels of inflammatory cytokines. These data confirm
previous findings demonstrating the role of neuroinflammation in
the development of POCD (11). For
example, inhibition of IL-1 receptors, or prophylactic treatment of
mice with either a monoclonal antibody to TNF-α or by disabling
HMGB1 with an inhibitory monoclonal antibody, may improve
post-surgical cognitive decline (10–12,18).
Although specific neutralization of the pro-inflammatory mediators
appears promising in animal studies, anti-cytokine drugs are not
selective to particular tissues and frequently produce undesirable
side effects in clinical practice. In septic patients, the failure
of anti-TNF-α- or IL-1β-based therapies to decrease mortality has
generated doubts as to whether cytokine-based treatments maybe
effective (19). Moreover,
cytokines have dual roles in sustaining the innate immune response
and key physiological processes, including synaptic plasticity and
tissue regeneration/healing (20).
Therefore, the requirement for novel, selective treatment options
for inflammation is urgent.
LPS is a surface component of the Gram-negative
bacteria Toll-like receptor-4 ligand that activates the immune
response to infections or other insults, leading to the induction
of pro-inflammatory gene expression via the activation of a number
of transcriptional pathways, including nuclear factor-κB (21). Notably, animals that survive a
sub-lethal exposure to the endotoxin are resistant to subsequent
challenges with otherwise lethal doses of LPS during the first few
days following the initial exposure, a phenomenon termed endotoxin
tolerance (21). Increasing
evidence indicates that endotoxin tolerance is a mechanism through
which to limit the inflammatory response to subsequent stimuli to
prevent excessive tissue damage (22). Although it has been suggested that
long-term endotoxin tolerance may be a potentially detrimental
condition, as it may hamper the ability to elicit a required immune
response and thus lead to susceptibility to infection, it has been
reported that immune function during LPS tolerance actually
improves, as reflected by enhanced bacterial clearance and
decreased mortality in LPS-tolerant animals challenged with live
Gram-negative bacteria (21,23).
In addition, endotoxin tolerance confers resistance to inflammation
and injury induced by a variety of other challenges, including
myocardial infarction (24),
kidney ischemia/reperfusion (25),
neural ischemia (26) and
traumatic spinal cord injury (14).
In the present study, it was demonstrated that
repeated (72 h prior to surgery) and single low-dose LPS
preconditioning (24 h prior to surgery) abolished the signs of
neuroinflammation. However, only single low-dose LPS
preconditioning at 24 h prior to surgery improved the subsequent
cognitive impairment following surgery. A possible explanation for
these data maybe that repeated LPS administration itself may induce
cognitive decline, which may offset the beneficial effects produced
by endotoxin tolerance. Although the surgery-induced increased
expression of hippocampal TNF-α, IL-1β and IL-6 did not coincide
with a time-point when animals exhibited significant
hippocampus-dependent cognitive impairment, it is possible that the
anti-inflammatory effects of endotoxin tolerance produced by the
LPS preconditioning in the early phase prevented the occurrence of
subsequent events leading to delayed cognitive dysfunction. In
addition, it was observed that LPS administration at 6 h or
immediately prior to surgery did not exert marked beneficial
effects. It has been suggested that the development of tolerance
requires de novo protein synthesis (27), providing a possible explanation for
the requirement of a time lag for tolerance to develop. In
addition, it has been reported that LPS may induce sensitization
and tolerance to subsequent oxygen-glucose deprivation (OGD),
depending on the time interval between the insults, in organotypic
hippocampal slice cultures: At a 0 h time interval between insults,
LPS sensitized all neuronal regions of the hippocampus to
subsequent OGD, whereas a time interval of 72 h resulted in
ischemic tolerance (28). These
observations confirm previous findings that the biological effects
of LPS preconditioning depend on the time of preconditioning, the
type of tissue challenged and the dose of LPS administered.
Although the mechanisms underlying LPS-induced
tolerance remain unclear, accumulating evidence has implicated a
role for pro-inflammatory cytokines, mediated by the activation of
counter-regulatory mechanisms that result in an immune-suppressed
state, characterized by lower levels of released cytokines upon a
second challenge (21–24). In the present study, LPS
preconditioning prevented the elevation of IL-1β, TNF-α and IL-6
expression in the hippocampus of mice at 1 day post-surgery. In
addition, LPS preconditioning facilitates an inflammatory response
to surgical trauma, albeit a less pronounced response, indicating
that it may be a means to limit excessive inflammatory responses
and tissue damage. In general, repeated and increased LPS dose
injections may provide a greater suppression of the
pro-inflammatory response (29).
Notably, the present study suggested that one single dose of LPS
administration at 24 h prior to surgery may be sufficient to induce
a certain level of tolerance to subsequent surgical trauma, as
evidenced by markedly reduced hippocampal pro-inflammatory cytokine
expression. In addition, increased reactivity to an immune insult
has been well characterized in aged subjects (30,31),
providing one explanation for the capacity of a lower dose of LPS
to induce endotoxin tolerance. Other mechanisms, including reduced
gliosis and antioxidant properties, have been reported to be
involved in the mechanisms underlying endotoxin tolerance (13,15).
It was previously demonstrated that LPS preconditioning selectively
inhibited the M1 response in an animal model of traumatic brain
injury (13). Since LPS
preconditioning regulates microglial phenotypic alterations, it
provides a novel direction for future investigation of the
mechanisms underlying the protective effects of endotoxin
tolerance. Despite great clinical interest in LPS tolerance, the
molecular events that underlie LPS tolerance remain to be
completely understood, although Toll-like receptor desensitization
and the suppression of the inflammatory signaling pathways via
epigenetic reprogramming have been implicated (29). In order to have a comprehensive
view of this phenomenon, an improved understanding of the
mechanisms that underlie LPS tolerance ought to be determined.
In conclusion, the results of the present study
suggested that LPS preconditioning may result in a reduced,
although not inhibited, inflammatory response to subsequent
surgical trauma. Since the majority of operations are planned,
modulation of the dysregulated inflammatory response with LPS or
other immune stimulators may afford protection against
surgery-induced brain injury in patients who are at a higher risk
of POCD and other neurodegenerative conditions.
Acknowledgements
The present study was financially supported by
grants from the National Basic Research Program of China (grant no.
2013CB835100) and the National Natural Science Foundation of China
(grant no. 81222013).
References
|
1
|
Steinmetz J, Christensen KB, Lund T, Lohse
N and Rasmussen LS; ISPOCD Group, : Long-term consequences of
postoperative cognitive dysfunction. Anesthesiology. 110:548–555.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monk TG, Weldon BC, Garvan CW, Dede DE,
van der Aa MT, Heilman KM and Gravenstein JS: Predictors of
cognitive dysfunction after major noncardiac surgery.
Anesthesiology. 108:18–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moller JT, Cluitmans P, Rasmussen LS, Houx
P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD,
et al: Long-term postoperative cognitive dysfunction in the elderly
ISPOCD1 study. ISPOCD investigators. International study of
post-operative cognitive dysfunction. Lancet. 351:857–861. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krenk L, Rasmussen LS and Kehlet H: New
insights into the pathophysiology of postoperative cognitive
dysfunction. Acta Anaesthesiol Scand. 54:951–956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiu LL, Ji MH, Zhang H and Yang JJ, Sun
XR, Tang H, Wang J, Liu WX and Yang JJ: NADPH oxidase 2-derived
reactive oxygen species in the hippocampus might contribute to
microglial activation in postoperative cognitive dysfunction in
aged mice. Brain Behav Immun. 51:109–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan H, Cao J, Zhang J and Zuo Z: Critical
role of inflammatory cytokines in impairing biochemical processes
for learning and memory after surgery in rats. J Neuroinflammation.
11:932014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hovens IB, Schoemaker RG, van der Zee EA,
Absalom AR, Heineman E and van Leeuwen BL: Postoperative cognitive
dysfunction: Involvement of neuroinflammation and neuronal
functioning. Brain Behav Immun. 38:202–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Liu F, Ma H, White PF, Yumul R,
Jiang Y, Wang N and Cao X: Age exacerbates surgery-induced
cognitive impairment and neuroinflammation in Sprague-Dawley rats:
The role of IL-4. Brain Res. 1665:65–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li N, Zhang X, Dong H, Hu Y and Qian Y:
Bidirectional relationship of mast cells-neurovascular unit
communication in neuroinflammation and its involvement in POCD.
Behav Brain Res. 322:60–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Terrando N, Monaco C, Ma D, Foxwell BM,
Feldmann M and Maze M: Tumor necrosis factor-alpha triggers a
cytokine cascade yielding postoperative cognitive decline. Proc
Natl Acad Sci USA. 107:pp. 20518–20522. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vacas S, Degos V, Tracey KJ and Maze M:
High-mobility group box 1 protein initiates postoperative cognitive
decline by engaging bone marrow-derived macrophages.
Anesthesiology. 120:1160–1167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terrando N, Yang T, Wang X, Fang J, Cao M,
Andersson U, Erlandsson HH, Ouyang W and Tong J: Systemic HMGB1
neutralization prevents postoperative neurocognitive dysfunction in
aged rats. Front Immunol. 7:4412016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turner RC, Naser ZJ, Lucke-Wold BP,
Logsdon AF, Vangilder RL, Matsumoto RR, Huber JD and Rosen CL:
Single low-dose lipopolysaccharide preconditioning: Neuroprotective
against axonal injury and modulates glial cells. Neuroimmunol
Neuroinflamm. 4:6–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stevens SL, Leung PY, Vartanian KB,
Gopalan B, Yang T, Simon RP and Stenzel-Poore MP: Multiple
preconditioning paradigms converge on interferon regulatory
factor-dependent signaling to promote tolerance to ischemic brain
injury. J Neurosci. 31:8456–8463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Jiang D, Li Q, Yao S, Sun X, Yang Y,
Meng Z and Liu W: Lipopolysaccharide-induced preconditioning
protects against traumatic spinal cord injury by upregulating Nrf2
expression in rats. Life Sci. 162:14–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schaafsma W, Zhang X, van Zomeren KC,
Jacobs S, Georgieva PB, Wolf SA, Kettenmann H, Janova H, Saiepour
N, Hanisch UK, et al: Long-lasting pro-inflammatory suppression of
microglia by LPS-preconditioning is mediated by RelB-dependent
epigenetic silencing. Brain Behav Immun. 48:205–221. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Z, Yuan H, Zhao H, Qi B, Li F and An
L: PPARγ activation ameliorates postoperative cognitive decline
probably through suppressing hippocampal neuroinflammation in aged
mice. Int Immunopharmacol. 43:53–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Z, Li X, Li F and An L: Berberine
alleviates postoperative cognitive dysfunction by suppressing
neuroinflammation in aged mice. Int Immunopharmacol. 38:426–433.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barrientos RM, Hein AM, Frank MG, Watkins
LR and Maier SF: Intracisternal interleukin-1 receptor antagonist
prevents postoperative cognitive decline and neuroinflammatory
response in aged rats. J Neurosci. 32:14641–14648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dinarello CA: Anti-cytokine therapeutics
and infections. Vaccine. 21 Suppl 2:S24–S34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Terrando N, Gómez-Galán M, Yang T,
Carlström M, Gustavsson D, Harding RE, Lindskog M and Eriksson LI:
Aspirin-triggered resolvin D1 prevents surgery-induced cognitive
decline. FASEB J. 27:3564–3571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murphey ED, Fang G and Sherwood ER:
Endotoxin pretreatment improves bacterial clearance and decreases
mortality in mice challenged with Staphylococcus aureus. Shock.
29:512–518. 2008.PubMed/NCBI
|
|
23
|
Arbibe L and Sansonetti PJ: Epigenetic
regulation of host response to LPS: Causing tolerance while
avoiding Toll errancy. Cell Host Microbe. 1:244–246. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fensterheim BA, Guo Y, Sherwood ER and
Bohannon JK: The cytokine response to lipopolysaccharide does not
predict the host response to infection. J Immunol. 198:3264–3273.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fernández-Ruiz I, Arnalich F,
Cubillos-Zapata C, Hernández-Jiménez E, Moreno-González R, Toledano
V, Fernández-Velasco M, Vallejo-Cremades MT, Esteban-Burgos L, de
Diego RP, et al: Mitochondrial DAMPs induce endotoxin tolerance in
human monocytes: An observation in patients with myocardial
infarction. PLoS One. 9:e950732014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai Y, Jia P, Fang Y, Liu H, Jiao X, He JC
and Ding X: miR-146a is essential for lipopolysaccharide
(LPS)-induced cross-tolerance against kidney ischemia/reperfusion
injury in mice. Sci Rep. 6:270912016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vartanian KB, Stevens SL, Marsh BJ,
Williams-Karnesky R, Lessov NS and Stenzel-Poore MP: LPS
preconditioning redirects TLR signaling following stroke: TRIF-IRF3
plays a seminal role in mediating tolerance to ischemic injury. J
Neuroinflammation. 8:1402011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eklind S, Mallard C, Arvidsson P and
Hagberg H: Lipopolysaccharide induces both a primary and a
secondary phase of sensitization in the developing rat brain.
Pediatr Res. 58:112–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Markus T, Cronberg T, Cilio C, Pronk C,
Wieloch T and Ley D: Tumor necrosis factor receptor-1 is essential
for LPS-induced sensitization and tolerance to oxygen-glucose
deprivation in murine neonatal organotypic hippocampal slices. J
Cereb Blood Flow Metab. 29:73–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seeley JJ and Ghosh S: Molecular
mechanisms of innate memory and tolerance to LPS. J LeukocBiol.
101:107–119. 2017. View Article : Google Scholar
|
|
31
|
Kohman RA, Crowell B and Kusnecov AW:
Differential sensitivity to endotoxin exposure in young and
middle-age mice. Brain Behav Immun. 24:486–492. 2010. View Article : Google Scholar : PubMed/NCBI
|