Introduction
Diabetic nephropathy (DN) is the most common
complication of diabetes, as one of the main causes of renal
failure. Its incidence has increased yearly, which has become a
serious global public health problem (1). According to the estimation from the
World Trade Organization, the global diabetes incidence will reach
370 million by 2025, of which 30% will develop into DN (2). Pathological features primarily
include glomerular hypertrophy, hyperplasia, a thickened basement
membrane, increase of extracellular matrix components, and gradual
progression to glomerular sclerosis, interstitial fibrosis and loss
of function, which eventually leads to chronic renal failure,
reducing the quality of life of the patient (3). The morbidity of DN has many causes,
and the pathogenesis is complex, which remains to be fully
elucidated.
The function of a large number of microRNAs (miRNAs)
have been studied in vivo. One-third of human genes are
subject to the regulation of miRNAs (4). They are involved in various
physiological and biochemical processes, including cell growth,
differentiation, apoptosis, sugar metabolism, fat metabolism,
insulin secretion, brain formation, cardiogenesis and stem cell
differentiation, and are closely associated with the occurrence of
many diseases (including cancer) (5). Different from other genes, miRNAs
have an independent transcription method, of which the product is
not translated into a protein; they identify specific targets and
bond with them, thereby regulating target expression after
transcription (6). A large number
of studies have indicated that miRNAs are involved in the
development of DN, and form a very complex network (7). The identification of miRNAs provides
a novel way to study the pathogenesis of DN (8).
At present, the pathogenesis of DN remains to be
fully elucidated. However, it is understood that the significant
pathological features at early stages are glomerular hypertrophy
caused by glomerular mesangial cell proliferation, and the elevated
glomerular filtration rate induced by mesangial cell (MC) systolic
dysfunction (9). Oxidative stress
is enhanced in the state of high glucose, and the generation of
reactive oxygen species (ROS) is increased (10). Nicotinamide adenine dinucleotide
phosphate (NADPH) oxidase is the main source of ROS in MCs, and
NAPDH oxidase 4 (Nox4) is the most important NADPH oxidase subunit
in MCs (9). NADPH oxidase and
Nox4-derived ROS is involved in the abnormal proliferation of MCs
induced by high glucose (11).
Previous study have suggested that DN is a low-grade
inflammatory disease, proposing an ‘inflammatory theory’ of
diabetes. Inflammation is considered to promote the pathogenesis of
diabetes, with significance in the development of diabetic
complications including DN (12).
Previous have shown that during the development of DN, inflammation
is obvious, renal tissue is accompanied with the infiltration of
various inflammatory cells, including mononuclear macrophages, and
significant increase in the concentration of various
pro-inflammatory cytokines can often be detected in the tissues and
circulating blood (12,13). The control of diabetes kidney
inflammation is helpful for delaying the development of DN.
Therefore, the oxidative stress of DN and inflammation are
considered as a whole in current research, in which a large amount
of active oxygen generated by enhanced oxidative stress caused by a
high glucose environment is regarded as a major cause of DN
inflammation, and inflammation increases oxidative stress in turn,
indicating a significant interaction between them (14,15).
Therefore, the present study aimed to investigate whether
miRNA-146a/Nox4 decreases ROS generation and inflammation in a DN
model.
Materials and methods
Animal studies
The animal experimental protocol was approved by the
Institutional Ethics Committee on Animal Care and Experimentation
at the Hospital of Tianjin Nankai (Tianjin, China). Male C57BL/6
mice (age, 8 weeks; weight, 20–25 g) were obtained from the Animal
Laboratory of Tianjin Nankai and maintained in a specific pathogen
free environment with a 12-h light/dark cycle at 22–24°C and 55–60%
humidity and free access to food and water. All mice were randomly
distributed into two groups: Control (n=6) and DN model (n=6). DN
mice were given intraperitoneal injections of streptozotocin (STZ;
55 mg/kg/day; Sigma-Aldrich; Merck MGaA, Darmstadt, Germany) for 5
consecutive days as the DN model. Control mice were given
intraperitoneal injections of saline for 5 consecutive days. All
mice were sacrificed using decollation under anesthesia (35
mg/kg/day of pentobarbital sodium) and renal tissue samples were
collected, washed PBS and stores at −80°C.
Cell culture and transfection
HK-2 human kidney cells were purchased from Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China) and cultured in keratinocyte serum-free media (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
100 U/ml penicillin G, 100 μg/ml streptomycin and 0.25 μg/ml
amphotericin B, and incubated at 37°C in a 100% humidified
incubator containing 5% CO2. The groups were as follows:
DN model and D-glucose control, exposed to 45 D-glucose; control,
exposed to D-glucose; negative, exposed to 5% D-glucose and
transfected with a negative control plasmid; miR-146, exposed to
45% D-glucose and transfected with an miR-146 mimic and treated
with nacetylecysteine (NAC, 3 mM, Beyotime Institute of
Biotechnology, Haimen, China). The miR-146 mimic (30 nM) and
negative control duplex mimic (30 nM) plasmids were transfected
into HK-2 cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HK-2 cells transfected
with mimics using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and then cDNA was synthesized using a Takara RNA
PCR kit (Baoshengwu, Dalian, China). The gene expression of
microRNA-146a and Nox4 were measured by qPCR using a SYBR Select
Master mix (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
primers used were as follows: Forward, 5-CTCTGCTCCTCCTGTTCG AC-3
and reverse, 5-ACGACCAAATCCGTTGACTC-3 for GAPDH; forward,
5-GCTGACGTTGCATGTTTCAG-3 and reverse, 5-CGGGAGGGTGGGTATCTAA-3 for
Nox4. A CFX96 real-time PCR system (Bio-Rad Laboratories, Inc.) was
run at 50°C for 1 min and at 95°C for 5 min, followed by 40 cycles
at 95°C for 30 sec and 60°C for 1 min. miRNA expression was
measured using the quantitation cycle (Ct) according to the ΔΔCq
method. ΔΔCq=ΔCq (gene of interest)-ΔCq (endogenous control)
(16,17).
Assessment of ROS concentration
ROS levels were analyzed by an Oxiselect
Intracellular ROS Assay kit (Cell Biolabs, Inc., San Diego, CA,
USA). HK-2 cells were incubated with the fluorescent dye
2,7-dichlorodihydrofluorescein diacetate (DCFH-DA; Cell Biolabs,
Inc.) for 20 min at 37°C and 5% CO2 in the dark. ROS
levels were analyzed at wavelengths of 485 and 525 nm using a
fluorescence microscope (Eclipse Ti-S, Nikon Corporation, Tokyo,
Japan) and analyzed using NIS-Elements Basic Research software
version 4.10 (Nikon Corporation). Cellular levels of ROS were
measured by means of a previously described (18) semi-quantitative DCFH-DA
fluorescence technique, which can be used to track alterations in
ROS concentration over time. Intracellular ROS expression levels
were analyzed by an Oxiselect Intracellular ROS Assay kit (Cell
Biolabs, Inc.), which measures ROS by employing the cell-permeable
fluorogenic probe DCFH-DA.
Assessment of oxidative stress and
inflammation
Mice were anaesthetized using 35 mg/kg pentobarbital
sodium (Sigma-Aldrich; Merck KGaA) and sacrificed using
decapitation. Serum was collected. The supernatants were separated
by centrifugation at 500 × g for 5 min at 4°C and stored at −80°C.
Superoxide dismutase (SOD; cat. no. A001-3), malondialdehyde (MDA;
cat. no. A003-1), nuclear factor (NF)-κB (cat. no. H202), tumor
necrosis factor (TNF)-α (cat. no. H052), interleukin (IL)-6 (cat.
no. H007) and IL-1β (cat. no. H002) levels were measured using rat
ELISA kits (Nanjing Institute of Biological Engineering) at a
wavelength of 450 nm using a microplate reader (BMG Labtech GmbH,
Ortenberg, Germany), according to the manufacturer's protocol.
Western blot analysis
Total proteins were lysed from renal tissue in
radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology) and protein concentrations (collected by
centrifugation at 12,000 × g for 10 min at 4°C) and determined
using an Enhanced Bicinchoninic Acid Protein Assay kit (Beyotime
Institute of Biotechnology). Proteins (50 μg) were separated by
8–12% SDS-PAGE and transferred onto a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). Membranes were
blocked in TBS with Tween-20 (TBST) buffer containing 5% non-fat
dry milk. Following this, they were probed with Nox4 (cat. no.
sc-55142), vascular cell adhesion molecule (VCAM)-1 (cat. no.
sc-1504), intracellular adhesion molecule (ICAM)-1 (cat. no.
sc-1511), all at a 1:500 dilution and purchased from Sigma-Aldrich;
Merck KGaA, and GAPDH (cat. no. AG019, 1:5,000, Beyotime Institute
of Biotechnology) primary antibodies at 4°C overnight. Blots were
washed with TBST buffer and then incubated with anti-rabbit
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
cat. no. sc-2030; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 1 h at room temperature. Protein bands were detected using an
Enhanced Chemiluminescence Hyperfilm (GE Healthcare Life Sciences,
Little Chalfont, UK) and quantified using Bio-Rad gel imaging
system 4.6 software (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation
using SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA).
One-way analysis of variance and Student's Newman-Keuls test for
comparisons were used to determine differences between control and
experimental groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of microRNA-146a and Nox4
in the DN model
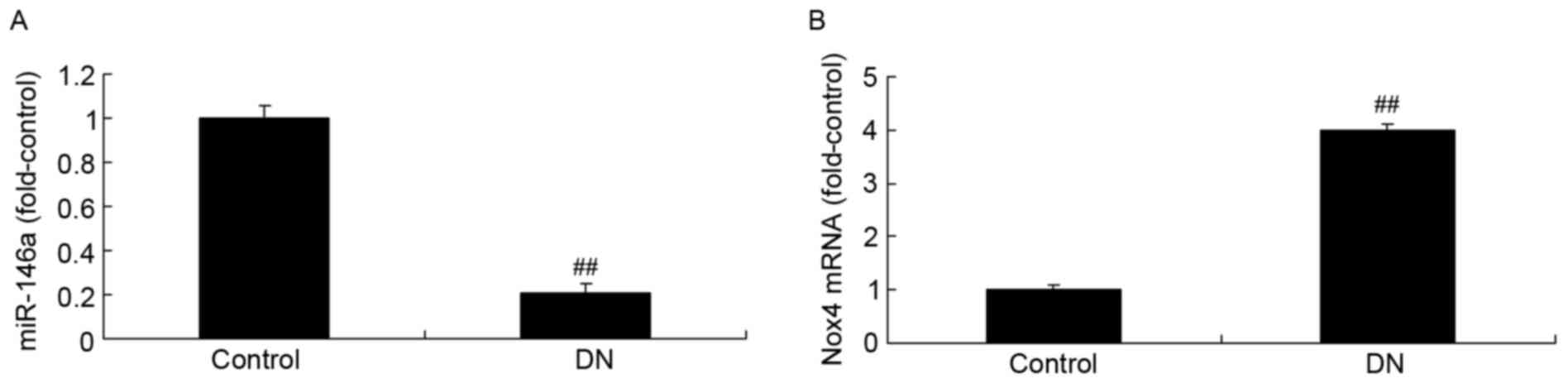
As presented in Fig.
1A, the expression of microRNA-146a in DN model mice was
reduced compared with the control group. However, the expression of
Nox4 mRNA in DN model mice was increased compared with the control
group (Fig. 1B).
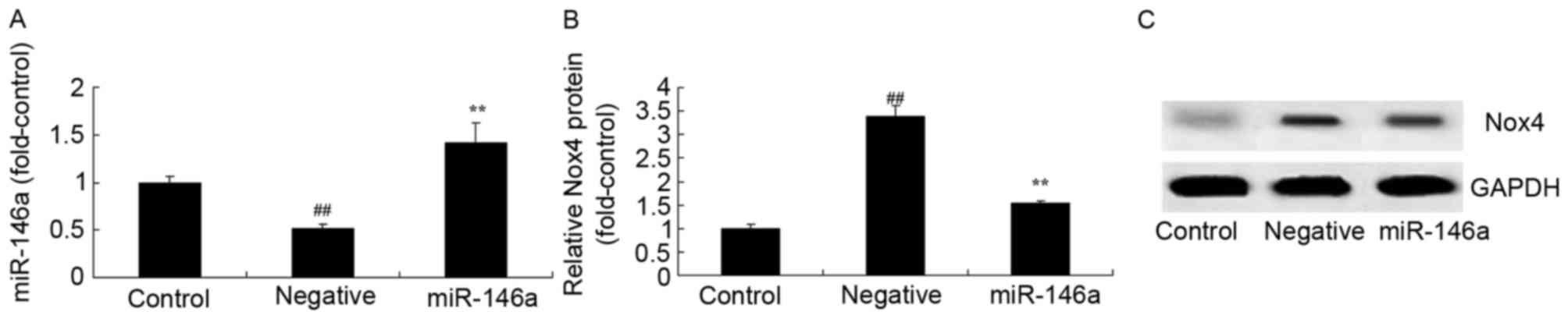
Overexpression of miR-146a on Nox4
expression in the DN model
The overexpression of miR-146a on the effect on Nox4
expression in DN mice was evaluated. As presented in Fig. 2A, there was a significant
inhibition of miR-146a expression in the negative group, compared
with the control group. An miR-146a mimic effectively increased
miR-146a expression in HK-2 cells exposed to D-glucose. Then, there
was a significant increase in Nox4 protein expression levels in
HK-2 cells exposed to D-glucose, compared with the control group
(Fig. 2B and C). Overexpression of
miR-146a significantly inhibited Nox4 protein expression in HK-2
cells exposed to D-glucose, compared with the D-glucose group
(Fig. 2B and C).
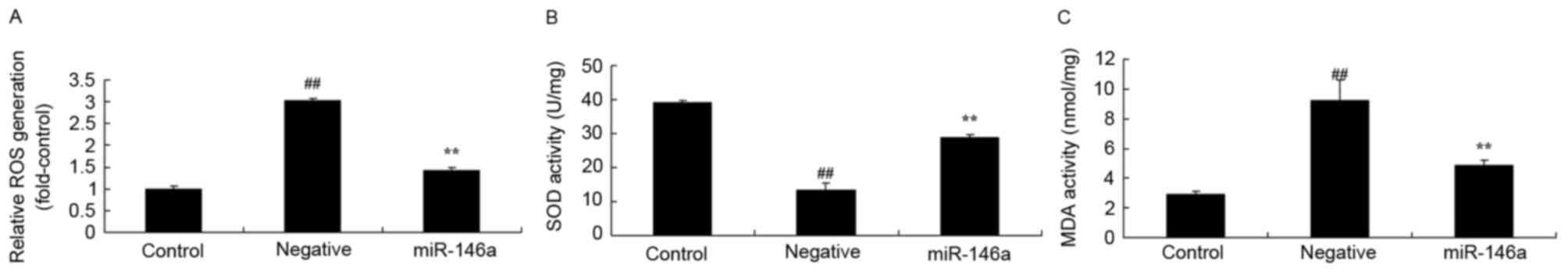
Overexpression of miR-146a on ROS
generation and oxidative stress in the DN model
Whether overexpression of miR-146a affected ROS
generation and oxidative stress in the DN model was investigated.
As presented in Fig. 3A and B,
there was a significant increase of ROS generation and MDA
activity, respectively, in HK-2 cells exposed to D-glucose,
compared with the control group. However, there was a significant
decrease in SOD activity in HK-2 cells exposed to D-glucose,
compared with the control group (Fig.
3C). However, overexpression of miR-146a significantly reversed
these effects (Fig. 3).
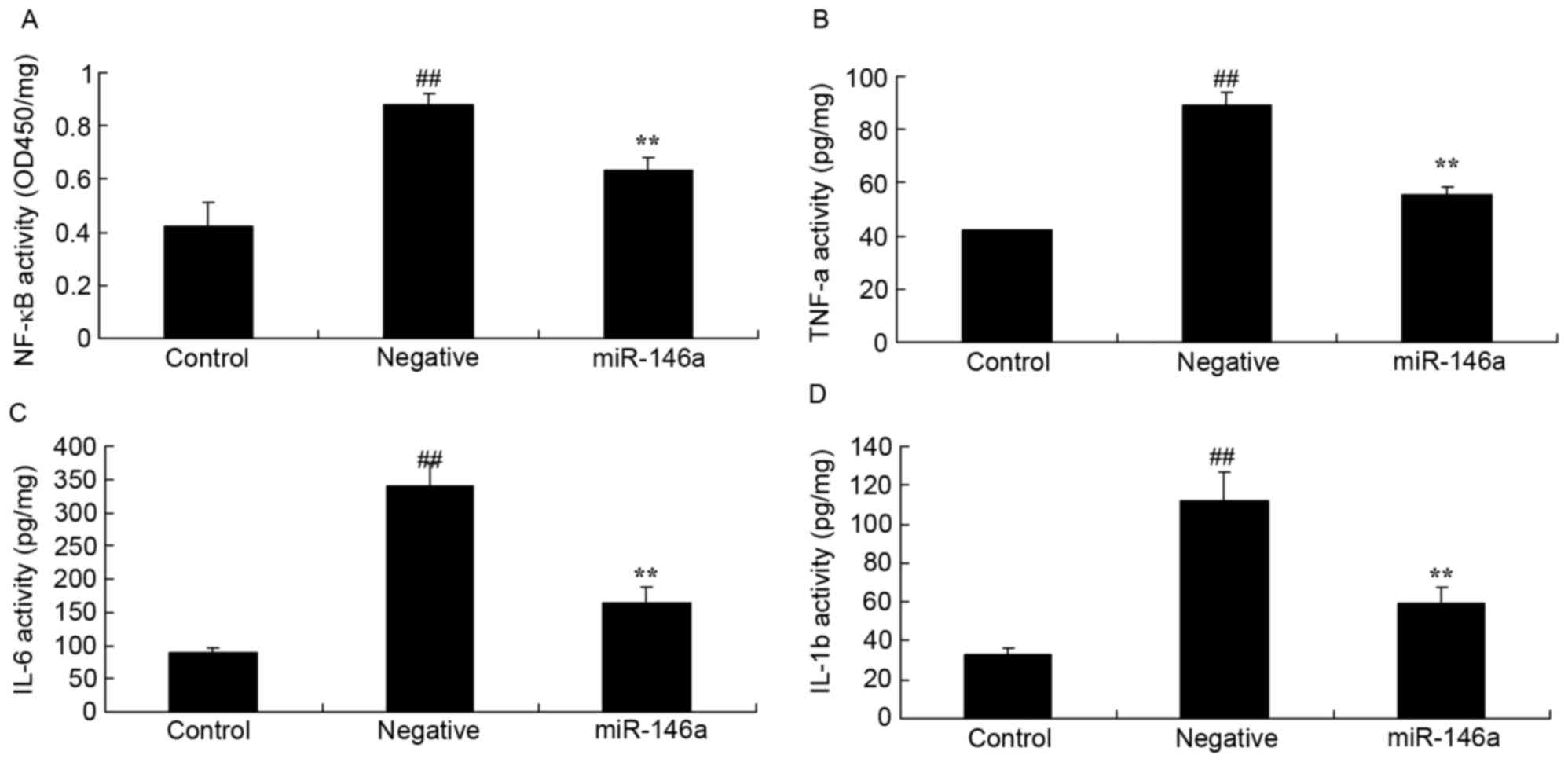
Overexpression of miR-146a on
inflammation in the DN model
To confirm whether overexpression of miR-146a
affected inflammation in the DN model, NF-κB, TNF-α, IL-6 and IL-1β
activity were measured using ELISA kits. There was a significant
increase of NF-κB (Fig. 4A), TNF-α
(Fig. 4B), IL-6 (Fig. 4C) and IL-1β (Fig. 4D) activity in HK-2 cells exposed to
D-glucose, compared with the control group. Overexpression of
miR-146a significantly reversed this effect (Fig. 4).
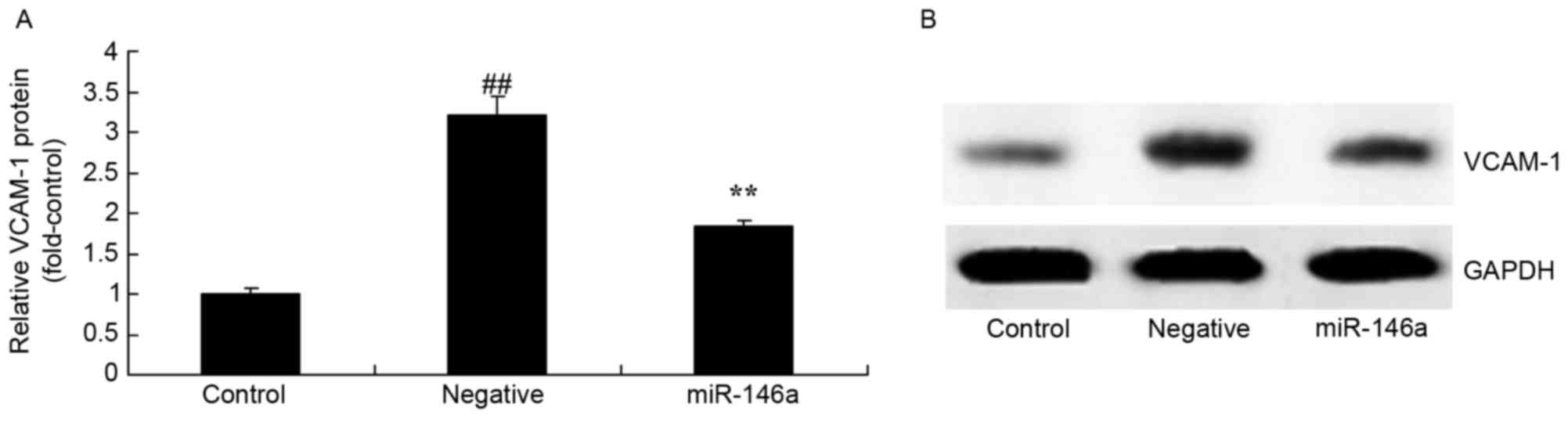
Overexpression of miR-146a on VCAM-1
protein expression in the DN model
Overexpression of miR-146a on VCAM-1 protein
expression in the DN model was examined by western blot analysis.
As presented in Fig. 5, VCAM-1
protein expression levels were significantly increased in the
negative group compared with the control group. Overexpression of
miR-146a significantly reversed this effect.
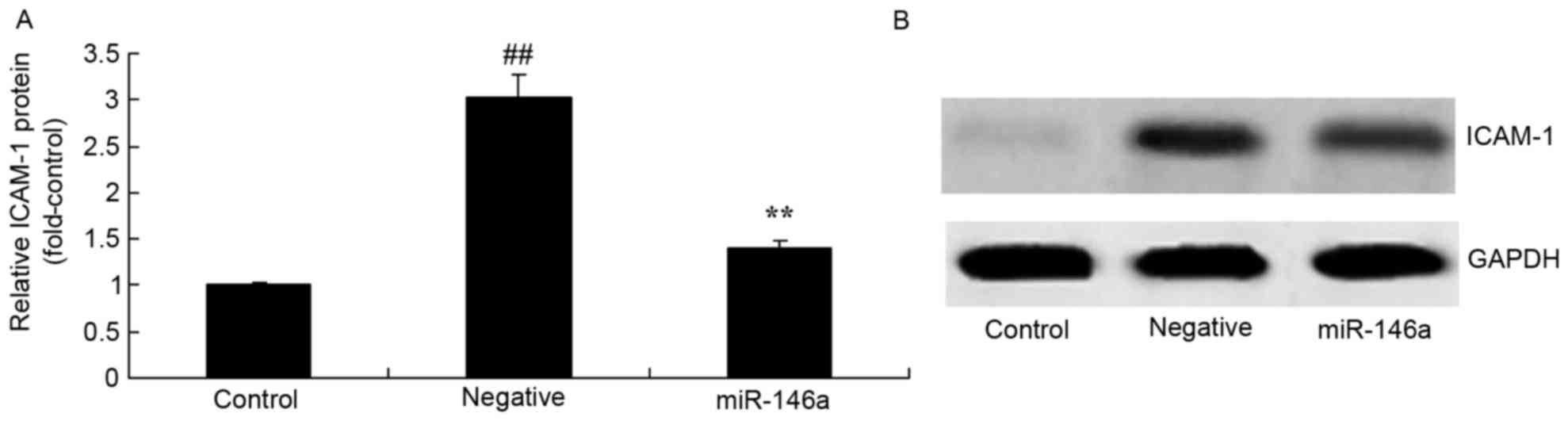
Overexpression of miR-146a on ICAM-1
protein expression in the DN model
Overexpression of miR-146a on ICAM-1 protein
expression in the DN model was examined by western blot analysis.
As presented in Fig. 6, ICAM-1
protein expression levels were significantly increased in the
negative group compared with the control group. Overexpression of
miR-146a significantly reversed this effect.
Inhibition of Nox4 on ROS generation
and oxidative stress in DN following overexpression of
miR-146a
Whether inhibition of Nox4 affects ROS generation
and oxidative stress in DN following overexpression of miR-146a was
examined. ROS generation was significantly increased in the
negative group compared with the control group; however,
overexpression of miR-146a ameliorated this effect. NAC
significantly decreased ROS generation (Fig. 7A), increased SOD activity (Fig. 7B) and decreased MDA activity
(Fig. 7C) in HK-2 cells exposed to
D-glucose following overexpression of miR-146a, compared with the
miR-146a group.
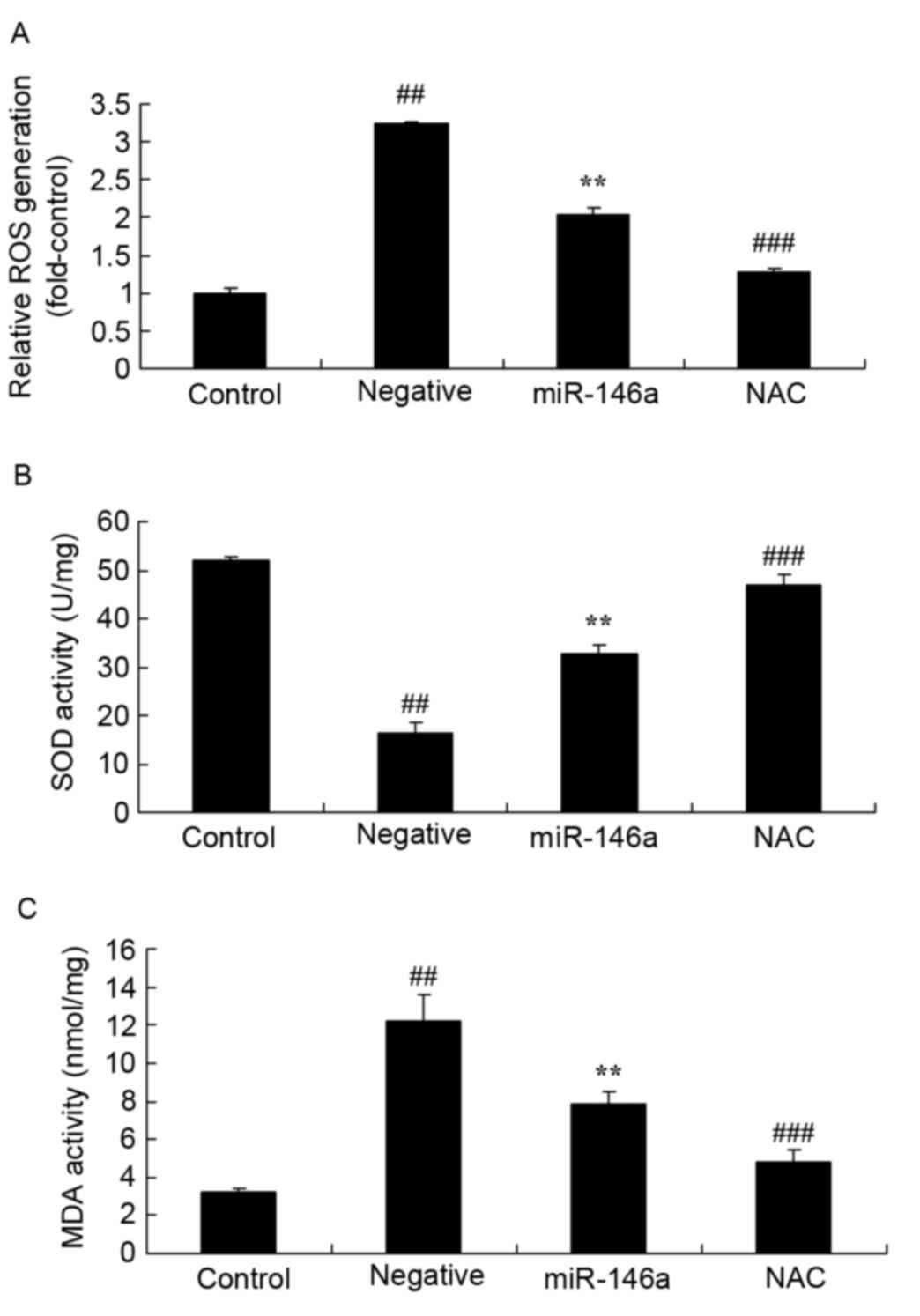 | Figure 7.Inhibition of Nox4 on ROS generation
and oxidative stress in DN following overexpression of miR-146a.
Effect of treatment with the Nox4 inhibitor, NAC, on (A) ROS
generation, and (B) SOD and (C) MDA activity in a DN model
following overexpression of miR-146a. Data are presented as the
mean ± standard deviation. ##P<0.01 vs. control
group; **P<0.01 vs. negative group; ###P<0.01 vs.
miR-146a group. DN, diabetic nephropathy; miR, microRNA; NAC,
nacetylcysteine; ROS, reactive oxygen species, SOD, superoxide
dismutase; MDA, malondialdehyde; Nox4, NAPDH oxidase 4. |
Inhibition of Nox4 on inflammation in
the DN model following overexpression of miR-146a
Whether inhibition of Nox4 would affect inflammation
in the DN model following overexpression of miR-146a was examined.
Inhibition of Nox4 by NAC significantly inhibited NF-κB (Fig. 8A), TNF-α (Fig. 8B), IL-6 (Fig. 8C) and IL-1β (Fig. 8D) activity in HK-2 cells exposed to
D-glucose following overexpression of miR-146a, compared with the
compared with the miR-146a group.
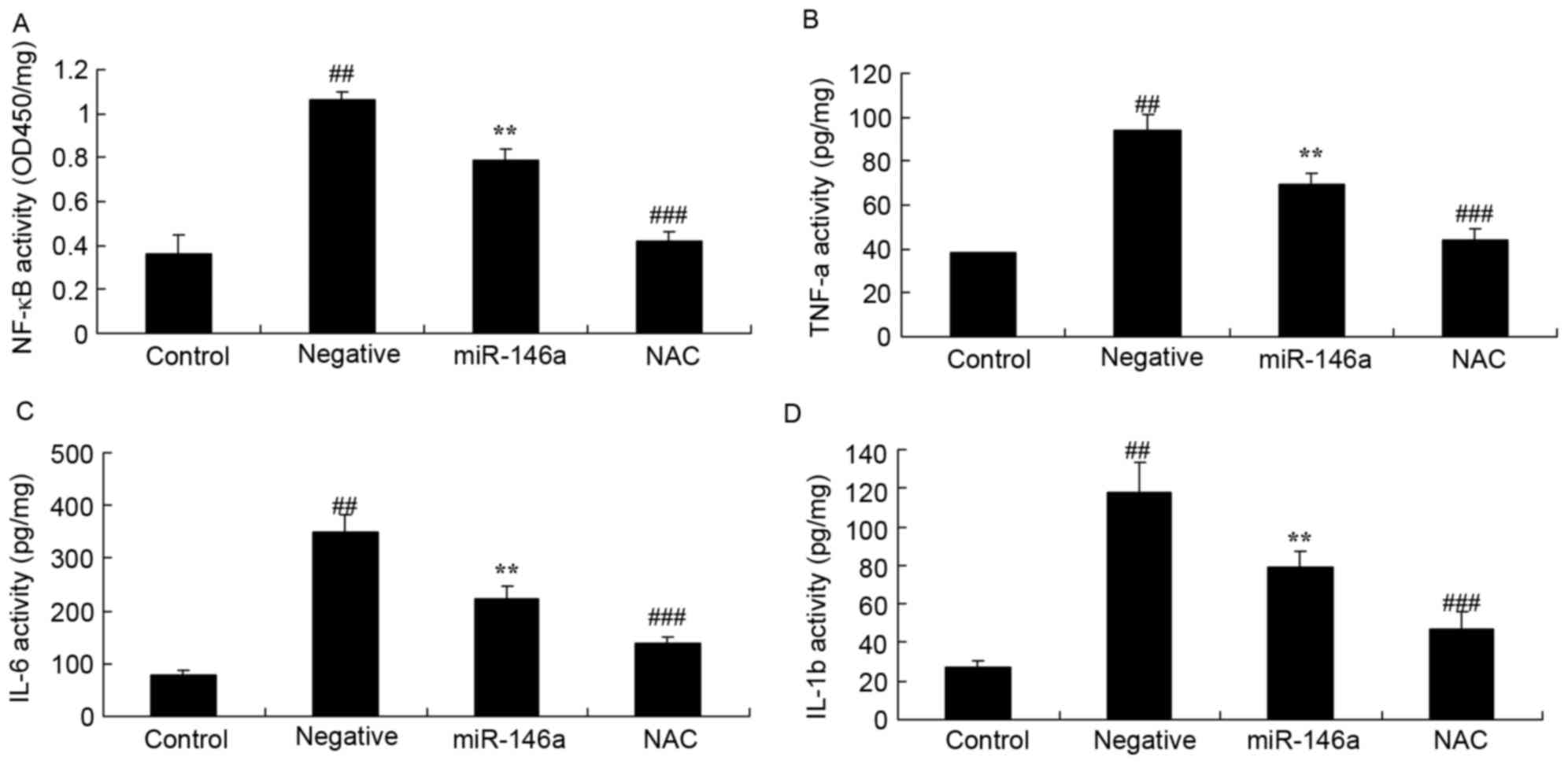 | Figure 8.Inhibition of Nox4 affect on
inflammation in a DN model following overexpression of miR-146a.
Effect of treatment with the Nox4 inhibitor, NAC, on (A) NF-κB, (B)
TNF-α, (C) IL-6 and (D) IL-1β activity in a DN model following
overexpression of miR-146a. Data are presented as the mean ±
standard deviation. ##P<0.01 vs. control group;
**P<0.01 vs. negative group; ###P<0.01 vs.
miR-146a group. DN, diabetic nephropathy; miR, microRNA; NAC,
nacetylcysteine; NF-κB, nuclear factor κB; TNF-α, tumor necrosis
factor-α; IL, interleukin; OD, optical density. |
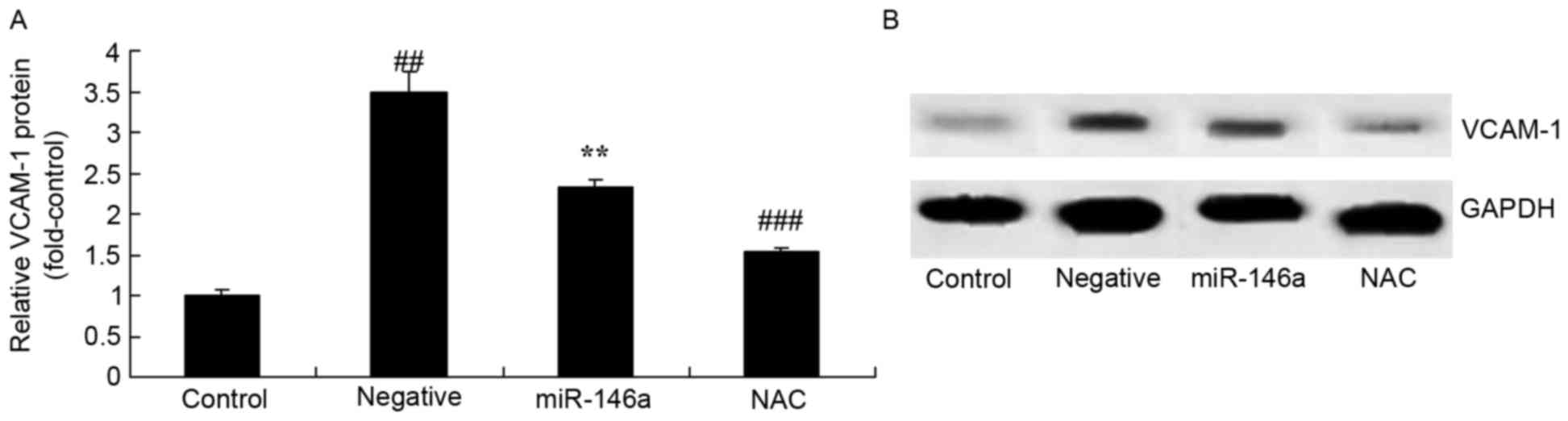
Inhibition of Nox4 on VCAM-1 protein
expression in the DN model following overexpression of
miR-146a
Whether inhibition of Nox4 would affect VCAM-1
protein expression in the DN model following overexpression of
miR-146a was examined. As presented in Fig. 9, inhibition of Nox4 significantly
inhibited VCAM-1 protein expression in HK-2 cells exposed to
D-glucose following overexpression of miR-146a, compared with the
miR-146a group.
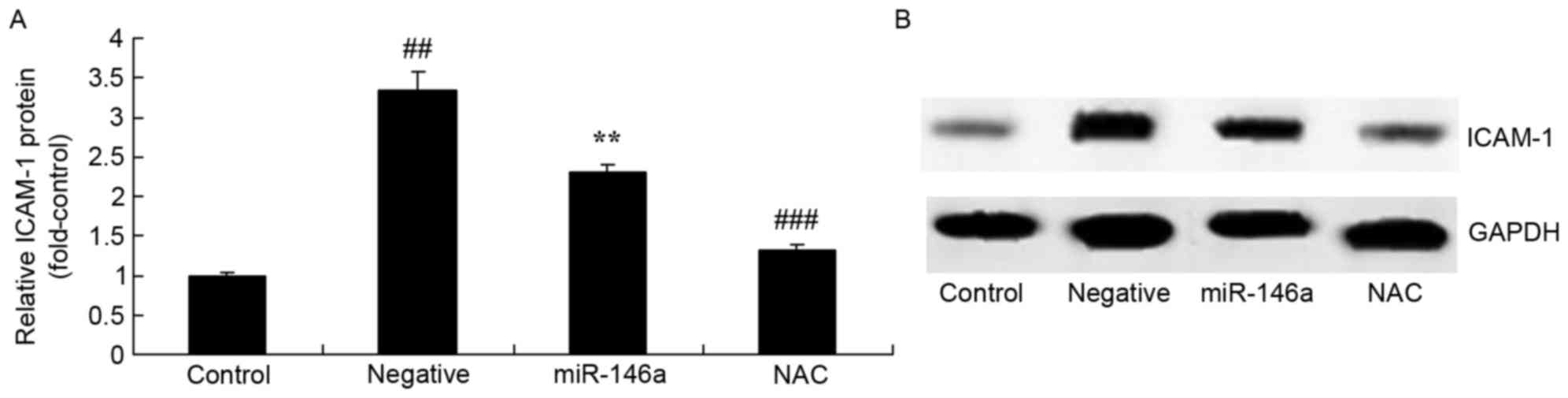
Inhibition of Nox4 on ICAM-1 protein
expression in the DN model following overexpression of
miR-146a
Whether inhibition of Nox4 would affect ICAM-1
protein expression in the DN model following overexpression of
miR-146a was examined. As presented in Fig. 10, inhibition of Nox4 significantly
suppressed the protein expression of ICAM-1 in HK-2 cells exposed
to D-glucose following overexpression of miR-146a, compared with
compared with the miR-146a group.
Discussion
DN is one of the most common chronic microvascular
complications of diabetes, of which the incidence rate increases
with diabetes duration (2).
Patients with partial DN may develop into end-stage renal disease,
causing kidney failure and requiring a kidney transplant, and may
lead to mortality (19). The
results demonstrated that overexpression of miR-146a significantly
decreased NF-κB, TNF-α, IL-6 and IL-1β activities in HK2 cells
exposed to D-glucose.
Increasing evidence suggests that there is an
obvious enhancement in oxidative stress response under DM state
(20). ROS produced in the
oxidative stress reaction may be a major cause of vascular
complications and DM (21). The
excessive generation of ROS destroys the dynamic equilibrium of
normal oxidation/reduction reaction, which results in oxidative
damage of biological macromolecules, including proteins, lipids and
nucleic acids, and interferes with regular biological activities
(21). Certain studies have
suggested that excessive generation of ROS is a direct result of
hyperglycemia, and ROS generated from mitochondria by the induction
of chronic hyperglycemia is the main initiator of multiple
pathological pathways for DN (22,23).
The results of the present study suggested that overexpression of
miR-146a significantly reversed ROS generation, SOD and MDA
activity in HK-2 cells exposed to D-glucose.
NADPH oxidase is originally found in neutrophils,
and also exists in other cell types such as endothelial, vascular
smooth muscle, mesangial and renal tubular epithelial cells,
similar to phagocytic cells, which also produce ROS under
hyperglycemic conditions. In physiological conditions, NADPH
oxidase activity is reduced, resulting in small amounts of ROS, and
kidneys can tolerate this without injury (24). However, under the stimulation of
various factors, including diabetes DNA oxidative damage, its
activity is increased significantly. When hemodynamic alterations
occur, excessive ROS can cause kidney cell dysfunction and even
more serious injuries (25). Nox
is a homolog of the catalytic subunit gp91phox, mainly including
Noxl, Nox3, Nox4 and Nox5, and exist in a variety of non-phagocytic
cells, with cell and tissue specificity, and can generate ROS
through catalysis (26). As a
second messenger, ROS generated by Nox is involved in the
regulation of cell proliferation, differentiation and apoptosis.
Nox4 is the main type present in MCs in Nox subunits (27). The endogenous ROS of Nox4 is
closely associated with glomerular injury associated with diabetes,
and upregulation of Nox4 expression can induce glomerular
hypertrophy in diabetic rat models (9). The results of the present study
demonstrated that overexpression of miR-146a significantly
inhibited Nox4 mRNA expression in HK-2 cells exposed to
D-glucose.
As the members of the immunoglobulin superfamily,
VCAM-1 and ICAM-1 have increased expressions in DN, which are
important in the DN immune response and inflammatory reaction, and
their effect in DN has gained increasing attention (28). VCAM-1 and ICAM-1 are a class of
glycoproteins distributed on the cell surface or extracellular
matrix, with adhesive functions. They act by ligand-receptor
interactions, which serves an important role in many physiological
and pathological processes, including embryonic differentiation,
maintenance of healthy structure, inflammation and immune response,
blood coagulation and thrombosis, wound healing, and tumor spread
and metastasis (29,30). In the present study, overexpression
of miR-146a significantly suppressed upregulated VCAM-1 and ICAM-1
protein expression levels in HK-2 cells exposed to D-glucose.
The early stage of DN primarily includes
monocyte/macrophage infiltration, in which monocytes gathered in
the kidney are activated and proliferate into macrophages, further
inducing the production of the inflammatory cytokines, including
TNF-α, IL-6 and matrix metalloproteinase (15). Therefore, a series of circulating
inflammatory markers such as TNF-α and IL-6 have increased
expression levels in DN, which are closely associated with disease
progression and proteinuria (31).
In addition, hyperglycemia and angiotensin II stimulate vascular
endothelial growth factor production, causing the release of
endothelial nitric oxide, blood vessel dilation and increased
glomerular filtration. Hyperglycemia also induces the synthesis of
TGF-β1, IV collagen and fibronectin, so as to increase the
extracellular matrix (32). The
results of the present study indicated that NAC, a ROS inhibitor,
suppressed VCAM-1 and ICAM-1 protein expression, and decreased
oxidative stress and inflammation of HK-2 cells following
overexpression of miR-146a.
In conclusion, the present study demonstrated that
overexpression of miR-146a significantly decreased NF-κB, TNF-α,
IL-6 and IL-1β activities, inhibited ROS generation and MDA
activity, and increased SOD activity via Nox4/VCAM-1 and ICAM-1
expression in HK-2 cells exposed to D-glucose. Therefore,
miR-146a/Nox4 may represent a potential therapeutic target for
patients with DN.
References
|
1
|
Zilişteanu DS, Atasie T and Voiculescu M:
Efficacy of long-term low-dose sulodexide in diabetic and
non-diabetic nephropathies. Rom J Intern Med. 53:161–169.
2015.PubMed/NCBI
|
|
2
|
Shima A, Miyamoto M, Kubota Y, Takagi G
and Shimizu W: Beraprost sodium protects against diabetic
nephropathy in patients with arteriosclerosis obliterans: A
prospective, randomized, open-label study. J Nippon Med Sch.
82:84–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Buren PN, Adams-Huet B, Nguyen M,
Molina C and Toto RD: Potassium handling with dual
renin-angiotensin system inhibition in diabetic nephropathy. Clin J
Am Soc Nephrol. 9:295–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng R, Liu H, Peng H, Zhou J, Zha H, Chen
X, Zhang L, Sun Y, Yin P, Wen L, et al: Promoter hypermethylation
of let-7a-3 is relevant to its down-expression in diabetic
nephropathy by targeting UHRF1. Gene. 570:57–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kato M, Castro NE and Natarajan R:
MicroRNAs: Potential mediators and biomarkers of diabetic
complications. Free Radic Biol Med. 64:85–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conserva F, Pontrelli P, Accetturo M and
Gesualdo L: The pathogenesis of diabetic nephropathy: Focus on
microRNAs and proteomics. J Nephrol. 26:811–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alvarez ML and DiStefano JK: Towards
microRNA-based therapeutics for diabetic nephropathy. Diabetologia.
56:444–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silambarasan M, Tan JR, Karolina DS,
Armugam A, Kaur C and Jeyaseelan K: MicroRNAs in hyperglycemia
induced endothelial cell dysfunction. Int J Mol Sci. 17:5182016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmad A, Mondello S, Di Paola R, Mazzon E,
Esposito E, Catania MA, Italiano D, Mondello P, Aloisi C and
Cuzzocrea S: Protective effect of apocynin, a NADPH-oxidase
inhibitor, against contrast-induced nephropathy in the diabetic
rats: A comparison with n-acetylcysteine. Eur J Pharmacol.
674:397–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeong SI, Kim SJ, Kwon TH, Yu KY and Kim
SY: Schizandrin prevents damage of murine mesangial cells via
blocking NADPH oxidase-induced ROS signaling in high glucose. Food
Chem Toxicol. 50:1045–1053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Winiarska K, Dzik JM, Labudda M, Focht D,
Sierakowski B, Owczarek A, Komorowski L and Bielecki W: Melatonin
nephroprotective action in Zucker diabetic fatty rats involves its
inhibitory effect on NADPH oxidase. J Pineal Res. 60:109–117. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jangale NM, Devarshi PP, Bansode SB,
Kulkarni MJ and Harsulkar AM: Dietary flaxseed oil and fish oil
ameliorates renal oxidative stress, protein glycation, and
inflammation in streptozotocin-nicotinamide-induced diabetic rats.
J Physiol Biochem. 72:327–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borsting E, Patel SV, Decleves AE,
Declèves AE, Lee SJ, Rahman QM, Akira S, Satriano J, Sharma K,
Vallon V and Cunard R: Tribbles homolog 3 attenuates mammalian
target of rapamycin complex-2 signaling and inflammation in the
diabetic kidney. J Am Soc Nephrol. 25:2067–2078. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deliyanti D, Zhang Y, Khong F, Berka DR,
Stapleton DI, Kelly DJ and Wilkinson-Berka JL: FT011, a novel
cardiorenal protective drug, reduces inflammation, gliosis and
vascular injury in rats with diabetic retinopathy. PLoS One.
10:e01343922015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donate-Correa J, Martín-Núñez E,
Muros-de-Fuentes M, Mora-Fernández C and Navarro-González JF:
Inflammatory cytokines in diabetic nephropathy. J Diabetes Res.
2015:9484172015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang TF, Lei Z, Li YX, Wang YS, Wang J,
Wang SJ, Hao YJ, Zhou R, Jin SJ, Du J, et al: Oxysophoridine
protects against focal cerebral ischemic injury by inhibiting
oxidative stress and apoptosis in mice. Neurochem Res.
38:2408–2417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muluye RA, Bian Y, Wang L, Alemu PN, Cui
H, Peng X and Li S: Placenta peptide can protect mitochondrial
dysfunction through inhibiting ROS and TNF-α generation, by
maintaining mitochondrial dynamic network and by increasing il-6
level during chronic fatigue. Front Pharmacol. 7:3282016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schneider MP, Schneider A, Jumar A,
Kistner I, Ott C and Schmieder RE: Effects of folic acid on renal
endothelial function in patients with diabetic nephropathy: Results
from a randomized trial. Clin Sci (Lond). 127:499–505. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moon JY, Tanimoto M, Gohda T, Hagiwara S,
Yamazaki T, Ohara I, Murakoshi M, Aoki T, Ishikawa Y, Lee SH, et
al: Attenuating effect of angiotensin-(1–7) on angiotensin
II-mediated NAD(P)H oxidase activation in type 2 diabetic
nephropathy of KK-A(y)/Ta mice. Am J Physiol Renal Physiol.
300:F1271–F1282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang JC, Zhao Y, Chen SJ, Long J, Jia QQ,
Zhai JD, Zhang Q, Chen Y and Long HB: AOPPs induce MCP-1 expression
by increasing ROS-mediated activation of the NF-κB pathway in rat
mesangial cells: Inhibition by sesquiterpene lactones. Cell Physiol
Biochem. 32:1867–1877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pessôa BS, Peixoto EB, Papadimitriou A,
Lopes de Faria JM and Lopes de Faria JB: Spironolactone improves
nephropathy by enhancing glucose-6-phosphate dehydrogenase activity
and reducing oxidative stress in diabetic hypertensive rat. J Renin
Angiotensin Aldosterone Syst. 13:56–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thallas-Bonke V, Thorpe SR, Coughlan MT,
Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME and
Forbes JM: Inhibition of NADPH oxidase prevents advanced glycation
end product-mediated damage in diabetic nephropathy through a
protein kinase C-alpha-dependent pathway. Diabetes. 57:460–469.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He T, Guan X, Wang S, Xiao T, Yang K, Xu
X, Wang J and Zhao J: Resveratrol prevents high glucose-induced
epithelial-mesenchymal transition in renal tubular epithelial cells
by inhibiting NADPH oxidase/ROS/ERK pathway. Mol Cell Endocrinol.
402:13–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Towler DA: Mitochondrial ROS deficiency
and diabetic complications: AMP [K]-lifying the adaptation to
hyperglycemia. J Clin Invest. 123:4573–4576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Pang S, Deng B, Qian L, Chen J,
Zou J, Zheng J, Yang L, Zhang C, Chen X, et al: High glucose
induces renal mesangial cell proliferation and fibronectin
expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is
inhibited by resveratrol. Int J Biochem Cell Biol. 44:629–638.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jha JC, Gray SP, Barit D, Okabe J, El-Osta
A, Namikoshi T, Thallas-Bonke V, Wingler K, Szyndralewiez C, Heitz
F, et al: Genetic targeting or pharmacologic inhibition of NADPH
oxidase nox4 provides renoprotection in long-term diabetic
nephropathy. J Am Soc Nephrol. 25:1237–1254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu JJ, Yeoh LY, Sum CF, Tavintharan S, Ng
XW, Liu S, Lee SB, Tang WE and Lim SC: SMART2D study: Vascular cell
adhesion molecule-1, but not intercellular adhesion molecule-1, is
associated with diabetic kidney disease in Asians with type 2
diabetes. J Diabetes Complications. 29:707–712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsui T, Nishino Y, Maeda S, Takeuchi M
and Yamagishi S: Irbesartan inhibits advanced glycation end product
(AGE)-induced up-regulation of vascular cell adhesion molecule-1
(VCAM-1) mRNA levels in glomerular endothelial cells. Microvasc
Res. 81:269–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alkhalaf A, Kleefstra N, Groenier KH, Bilo
HJ, Gans RO, Heeringa P, Scheijen JL, Schalkwijk CG, Navis GJ and
Bakker SJ: Effect of benfotiamine on advanced glycation endproducts
and markers of endothelial dysfunction and inflammation in diabetic
nephropathy. PLoS One. 7:e404272012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jheng HF, Tsai PJ, Chuang YL, Shen YT, Tai
TA, Chen WC, Chou CK, Ho LC, Tang MJ, Lai KT, et al: Albumin
stimulates renal tubular inflammation through an HSP70-TLR4 axis in
mice with early diabetic nephropathy. Dis Model Mech. 8:1311–1321.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yaghobian D, Don AS, Yaghobian S, Chen X,
Pollock CA and Saad S: Increased sphingosine 1-phosphate mediates
inflammation and fibrosis in tubular injury in diabetic
nephropathy. Clin Exp Pharmacol Physiol. 43:56–66. 2016. View Article : Google Scholar : PubMed/NCBI
|