Introduction
Osteoarthritis (OA) is the most common articular
disease characterized by a progressive degradation of joint
cartilage, resulting in loss of joint mobility and function at
last, which was estimated to be the 4th leading cause of disability
in Asia (1). OA affects the hands,
feet, spine and large weight-bearing joints commonly, such as the
hips and knees, while OA patients manifest joint pain, stiffness,
tenderness, limited joint movement, and joint cracking. OA is a
major disturbing in aging people as its incidence increases highly
associated with age (2).
It is reported that OA occurred in 19.2% of adults
participants age ≥45 in the Framingham Project and 27.8% in the
Johnston County Osteoarthritis Survey, ~37% of participants age
>60 years or older had radiographic knee OA in the third
National Health and Nutrition Examination Study (3,4).
According to a systematic review of Global Burden of Disease 2010
study, the global age-standardized prevalence of knee OA was 3.8%,
and hip OA was 0.85% (5).
Articular cartilage (AC) stand the mechanical distribution of loads
across the joints, while cartilage degenerated and osteophyte
formed in OA (6). Articular
chondrocytes maintain proliferation and terminal differentiation in
healthy articular cartilage. While hypertrophy, vascularization and
calcification were observed in OA cartilage (7).
Despite the global increase in the incidence of OA,
there are no effective pharmacotherapies capable of restoring the
original structure and function of damaged articular cartilage
(8). Pharmaceutical or surgical
therapies (osteotomies, microfracture) have limited efficacy in
reversing or halting OA progression, while stem cell-based
cartilage tissue engineering and cartilage regeneration that may be
an effective strategy in OA treatments (9–13).
Numerous efforts have been made to develop tissue-engineered grafts
or patches to repair focal chondral and osteochondral defects, and
to date several researchers aim to implement clinical application
of cell-based therapies for cartilage repair (14,15).
Mesenchymal stem cells (MSCs) are reported to show
promising clinical applications in articular cartilage
regeneration, and mesenchymal stem cells have a potential in
treatment of OA (16,17).
Adipose mesenchymal stem cells have been reported to
differentiate into chondrocytes in 3-dimensional culture express
lubricin, and adipose tissue derived-mesenchymal stem cells
cultured on collagen cell carrier scaffolds were to regenerated
engineered cartilage (18,19).
Human umbilical stem cell populations was reported
to be found in the umbilical cord, the cord lining, and
perivascular tissue, as well as Wharton's jelly, so they are
attractive autologous or allogenic cells to treat malignant and
non-malignant solid and soft cancers, and they also can be the
feeder layer for embryonic stem cells or other pluripotent stem
cells (20,21).
Human umbilical cord-derived MSCs (hUC-MSCs)
constitute an attractive alternative to bone marrow-derived MSCs
for potential clinical applications because of easy preparation and
lower risk of viral contamination, they can differentiate into the
three germ layers that promote tissue and organ repair and modulate
immune responses and anticancer properties (20).
However, the role of hUC-MSCs and degenerated
chondrocytes in OA progression is unclear. Therefore, we explore
the interaction between human umbilical cord stem cells and OA
degenerated chondrocytes, and the therapeutic potential of human
umbilical cord stem cells on degenerated chondrocytes.
Materials and methods
Patients
The study was approved by the Ethical Review
Committee of 455th hospital of PLA (Shanghai, China). After
obtaining informed consents from the mothers and family, the
umbilical cords were harvested from the full-term natural delivery
infants. WJ-MSC were isolated and cultured by Shanghai Omnicells
Biotechnology Co., Ltd. (Shanghai, China) as described (22): Umbilical cords were washed with
sterile phosphate-buffered saline (PBS) three times and diced into
pieces, and the blood vessels in umbilical cords were dissected.
Wharton's jelly was cut into small pieces and digested in
DMEM/Hams's F-12 (1:1 vol/vol) culture medium containing 10% FBS,
collagenase type II (1 µg/ml), penicillin (100 U/ml), streptomycin
(100 µg/ml), and amphotericin-B (2.5 µg/ml) (all from Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in a shaker for 4–6 h,
and then centrifuged at 500 × g for 15 min. The cell pellet was
resuspended in the above culture medium supplemented with 10 ng/ml
basic fibroblast growth factor (bFGF; R&D Systems, Inc.,
Minneapolis, MN, USA), and cells were subcultured using 0.05%
trypsin/0.02% EDTA (Thermo Fisher Scientific, Inc.) on reaching
80–90% confluence.
The cartilage samples were obtained from the knee
joints of OA patients (n=12, KL grade >2) undergoing joint
replacement surgery, and chondrocytes were harvested by enzymatic
digestion, chondrocytes were cultured in low glucose DMEM at 37°C
with 5% CO2 as previously described (23).
In the present study, the cells were divided into 2
groups: Experimental group (co-culture group) and control group. In
proliferation assay, the medium in experimental group was changed
with the supernatant from hUC-MSCs, while the medium in control
group was changed with normal medium. In other experiments, OA
chondrocytes and hUC-MSC were incubated in a noncontact co-culture
system: Chondrocytes were cultured in the bottom well, and hUC-MSCs
were cultured in a Transwell insert (Transwell; Corning Costar,
Corning, NY, USA) in co-culture group; while chondrocytes were
cultured alone in the bottom well in control group.
Cytoflow analysis
The expression of hUC-MSC surface markers was tested
by using flow cytometry as described previously (24). hUC-MSCs were isolated and harvested
by using 0.1% trypsin-EDTA treatment, and washed with PBS. Then the
cells were incubated with the following antibodies: Rabbit
polyclonal to CD44 (ab157107; 1/500 dilution), rabbit polyclonal to
CD73 (ab175396; 1/500 dilution), rabbit monoclonal to CD90
(ab92574; 1/1,000 dilution), mouse monoclonal to CD105 (ab11414;
1/1,000 dilution), rabbit monoclonal to CD34 (ab81289; 1/1,000
dilution), rabbit polyclonal to CD45 (ab10559; 1/1,000 dilution),
rabbit polyclonal to CD106 (ab134047; 1/1,000 dilution), rabbit
polyclonal to CD133 (ab16518; 1/1,000 dilution) (all from Abcam,
Cambridge, MA, USA), in the dark for 30 min at room temperature,
then conjugated with either fluorescein PE or FITC [Goat
Anti-Rabbit IgG H&L (FITC) (ab6717) or Goat Anti-Mouse IgG
Secondary Antibody (PE) LS-C60691; 1/1,000 dilution; LifeSpan
BioSciences, Seattle, WA, USA]. The labeled cells were washed and
tested by flow cytometry (Becton-Dickinson, Franklin Lakes, NJ,
USA).
Cell differentiation
A 6 well-plate was cultured with 5×104
cells/well. At 48 h, the medium was replaced by adipogenic medium
(high glucose DMEM containing 10% FBS, 500 µM
isobutylmethylxanthine, 5 µg/ml insulin, 200 µg/ml
ascorpate-2-phosphate, 100 U/ml penicillin, 100 U/ml streptomycin,
1 µM dexamethasone), osteogenic medium (glucose DMEM containing 10%
FBS, 100 U/ml penicillin, 100 U/ml streptomycin, 100 nM
dexamethasone, 10 mM β-glycerophosphate and 50 µg/ml
ascorbate-2-phosphate), and chondogenic medium (high glucose DMEM
containing 10% FBS, 10 ng/ml TGF-β1, 100 nM dexamethasone, 50 ng/ml
ascorbate-2-phosphate, 1 mM sodium pyruvate). All the reagents were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
The induction medium was replaced every 2 days for 3
weeks, control group were cultured without induction. After 3
weeks, RNA extractions and RT-PCR were performed to evaluate the
cell differentiation.
The special staining was further performed to show
morphological changes of differentiated cells. After 3 weeks of
adipogenic induction, cells were fixed in 4% paraformaldehyde at
room temperature for 30 min, and washed with deionized water and
then with 60% isopropanol, followed by staining with 0.5% Oil red O
(Sigma-Aldrich; Merck KGaA) in isopropanol (wt/vol) for 30 min, and
excess stain was removed and the cells were washed 3 times with
deionized water.
For osteogenesis, cells were fixed in 4%
paraformaldehyde at room temperature for 30 min, and washed with
deionized water and stained with Alizarin Red Solution (no. 0223,
Alizarin Red S staining kit; ScienCell Research Laboratories (San
Diego, CA, USA) at room temperature for 15 min following the
manufacturer protocol, and excess stain was removed and the cells
were washed 3 times with deionized water.
For chondrogenisis, cells were fixed with 4%
paraformaldehyde for 30 min at room temperature and then were
stained with 1% Toluidine Blue Solution (sc-206058; Santa Cruz
Biotechnology, Heidelberg, Germany), for 30 min, and excess stain
was removed and the cells were washed 3 times with deionized
water.
Reverse transcription-quantitative
polymerase chain reaction
The total RNA was isolated from samples by using TRI
Reagent® RNA Isolation Reagent (Sigma-Aldrich; Merck
KGaA) according to the manufacturer's instructions and
complementary DNA (cDNA) was prepared by using a First-Strand cDNA
Synthesis kit (Pharmacia LKB, Uppsala, Sweden). Real-time
quantitative PCR was performed using the TaqMan system (Applied
Biosystems; Thermo Fisher Scientific, Inc.), and Real-time PCR
amplification and product detection were performed using an ABI
PRISM 7300 Sequence Detection System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) (25).
Each reaction is consisted of 10 µl SYBR Green
(Applied Biosystems; Thermo Fisher Scientific, Inc.), 6 µl
molecular grade water, 1 µl each forward primer and 1 µl reverse
primer and 2 µl cDNA (Table I).
The amplification was performed under the certain conditions: 10
min at 95°C, followed by 50 cycles, 10 sec at 95°C and 60 sec at
60°C. q-PCR was performed under standard conditions and all
experiments were performed in triplicate. The quantitative gene
expression was normalized to GAPDH, and the relative expression was
determined by using the ΔΔCt method.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Sequence
(5′->3′) | Product length,
bp |
|---|
| OCN |
| 227 |
| F |
ACTCCATCTTCGTGCTCCTCA |
|
| R |
TCCGGACAGGAGAAAGGGTC |
|
| ALP |
| 281 |
| F |
CTGTGCCTCAGCCAGCTC |
|
| R |
GGAGGATTCCAGAGGGGAGT |
|
| RUNX2 |
| 225 |
| F |
CGCCTCACAAACAACCACAG |
|
| R |
TCACTGTGCTGAAGAGGCTG |
|
| Aggrecan |
| 109 |
| F |
GTTTCCACAAGGGAGAGAGGG |
|
| R |
GTAGGTGGTGGCTAGGACGA |
|
| Sox-9 |
| 110 |
| F |
AGGAGAACCCCAAGATGCAC |
|
| R |
GAGGCGTTTTGCTTCGTCAA |
|
| COL2A1 |
| 241 |
| F |
GGTCCTGCAGGTGAACCC |
|
| R |
GAGGACCTCTAGGGCCAGAA |
|
| adipoQ |
| 294 |
| F |
ATTCGGCACGAGGGATGCTA |
|
| R |
GCCCTTCAGCTCCTGTCATT |
|
| aP2 |
| 181 |
| F |
TGAAAGAAGTGGGAGTGGGC |
|
| R |
CCTTTCCTGTCATCTGCGGT |
|
| PPARγ |
| 123 |
| F |
GTGGAAGGCGAGCAGATGAT |
|
| R |
GGCAGATCTGGACTGGTAGC |
|
| MMP13 |
| 230 |
| F |
CATGAGTTCGGCCACTCCTT |
|
| R |
CCTCGGAGACTGGTAATGGC |
|
| COL10A1 |
| 143 |
| F |
CCAGCACGCAGAATCCATCTGA |
|
| R |
CTTGGTGTTGGGTAGTGGGC |
|
| COX-2 |
| 297 |
| F |
TGTGAAAGGGTGTCCCTTCG |
|
| R |
AGTACAACACAGGAATCTTCACA |
|
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc., Rockville, MD, USA) was used to
evaluate the cell proliferation. Chondrocytes from OA patients at
passage 2 were isolated and harvested by using 0.1% trypsin-EDTA
and plated into 96-well plates at a density of 103
cells/well. The plates were divided into 2 groups: Experimental
(co-culture group) and control groups. The medium in experimental
group was changed with the supernatant from hUC-MSCs, while the
medium in control group was changed with normal medium. At day 1,
3, 5, 7, the medium was change with 100 µl of fresh medium
containing 10 µl CCK-8 solution for 4 h in a 37°C incubator. The OD
at 570 nm was measured, and the assay was repeated in a
triplicate.
Western blotting
The protein expression was accessed by using western
blotting. Cells were prepared in RIPA buffer containing a protease
inhibitor cocktail, and then centrifuged at 12,000 × g for 10 min
at 4°C, and the protein concentration was tested using the BCA
protein assay kit (cat. no. 23225; Applied Biosystems; Thermo
Fisher Scientific, Inc.). The certain protein was electrophoresis
on a 10% SDS-PAGE gels and electrotransferred onto a nitrocellulose
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). And then
block with 5% non-fat milk (Sangon Biotech Co., Ltd., Shanghai,
China) in TBS for 1 h at room temperature room, the membrane was
incubated with first antibodies (mouse monoclonal to Cox-2, cat.
no. sc-19999, dilution 1:1,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) (mouse monoclonal to collagen X, cat. no. ab49945,
dilution 1:1,000; Abcam) (mouse monoclonal to MMP13, cat. no.
sc-101564, dilution 1:1,000; Santa Cruz Biotechnology, Inc.)
(anti-β-actin antibody, cat. no. ab6276, dilution 1:1,000; Abcam)
for 1 h. Then wash with TBST 3 times, the membrane was incubated
with horseradish peroxidase-conjugated goat anti-mouse IgG
(anti-mouse IgG, HRP-linked antibody, cat. no. 7076, dilution
1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) for 1 h
at RT. Then wash with TBST 3 times, a the ECL Plus detection kit
(cat. no. 32132; Thermo Fisher Scientific, Inc.) was used to
visualize the immunoreactive according to the manufacturer's
instructions, and the relative protein expression was calculated by
using ImageJ software (version 1.46; Wayne Rasband National
Institutes of Health, Bethesda, MD, USA).
Detection of total factors in
superatant
Enzyme-linked immunosorbent assay (ELISA) was
performed to measure TNF-α, IL-1β, IL-6, IL-10 in supernatant.
Briefly, antibodies specific for TNF-α (cat. no. SMTA00B), IL-1β
(cat. no. SLB50), IL-6 (cat. no. S6050), and IL-10 (cat. no.
S1000B) (all from R&D Systems, Inc.) was immobilized
onto-96-well microtiter plates, and remove unbound antibody and add
a blocking reagent. Then the sample were incubated with the solid
phase antibodies, and washing unbound molecules away, followed by
adding a detection antibody specific for TNF-α, IL-1β, IL-6, IL-10.
The incubation and washing was followed by adding HRP-conjugated
anti-mouse immunoglobulin. The plate was washed and a TMB substrate
solution (Zymed Laboratories, San Francisco, CA, USA) was added for
30 min, and measured using a Beckman Coulter DU 800
spectrophotometer (Beckman Coulter, Fullerton, CA, USA) at 450
nm.
Statistical analysis
All the data are expressed as mean ± standard
deviation. a one-way ANOVA with Tukey's HSD post hoc test was
performed to comparise differences between groups. All the
statistical evaluations were performed by using the SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). P-values <0.05 were considered
to indiacate statistically significant difference.
Results
Expression of the MSCs-surface
markers
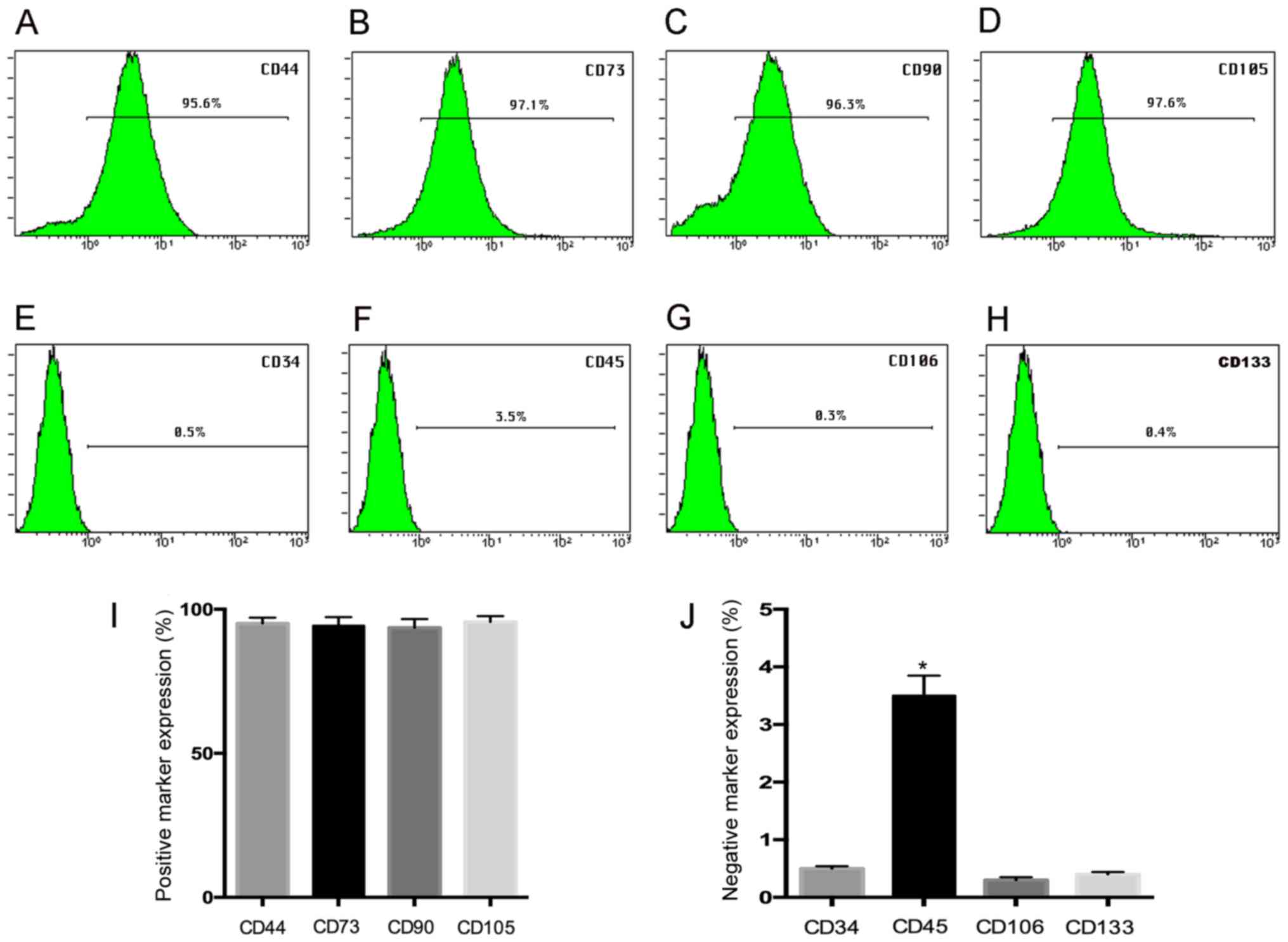
Flow cytometry assay demonstrated that hUC-MSC at
passage 3 show a high expression (>95%) for the mesenchymal stem
positive markers CD44 (95.6%), CD73 (97.1%), CD90 (96.3%), CD105
(97.6), while the negative expression of CD34 (0.5%), CD45 (3.5%),
CD106 (0.3%), and CD133 (0.4%) was observed (Fig. 1A-H), and the histogram gives a
clear picture of the expression ratio (Fig. 1I and J) suggesting our results was
in agreement with previous studies (21).
Multi-differentiation assay
The multi-differentiation (chondrogenic, osteogenic,
adipogenic differentiation) assay was used to evaluate the
differentiation potential of hUC-MSC. After 3 weeks of induction
toward the multi-lineage differentiation, the RNA was extracted,
and quantitative RT-PCR was performed to evaluate the cell
differentiation. The result indicated that compared with the cells
in the control group, cells in the induction group have high
expression of chondrogenic genes (aggrecan, collagen II, sox-9),
osteogenic genes (OCN, ALP, RUNX2), and adipogenic genes (adipoq,
aP2, PPARr) following 3 weeks of chondrogenic, osteogenic, and
adipogenic differentiation (P<0.05) (Fig. 2).
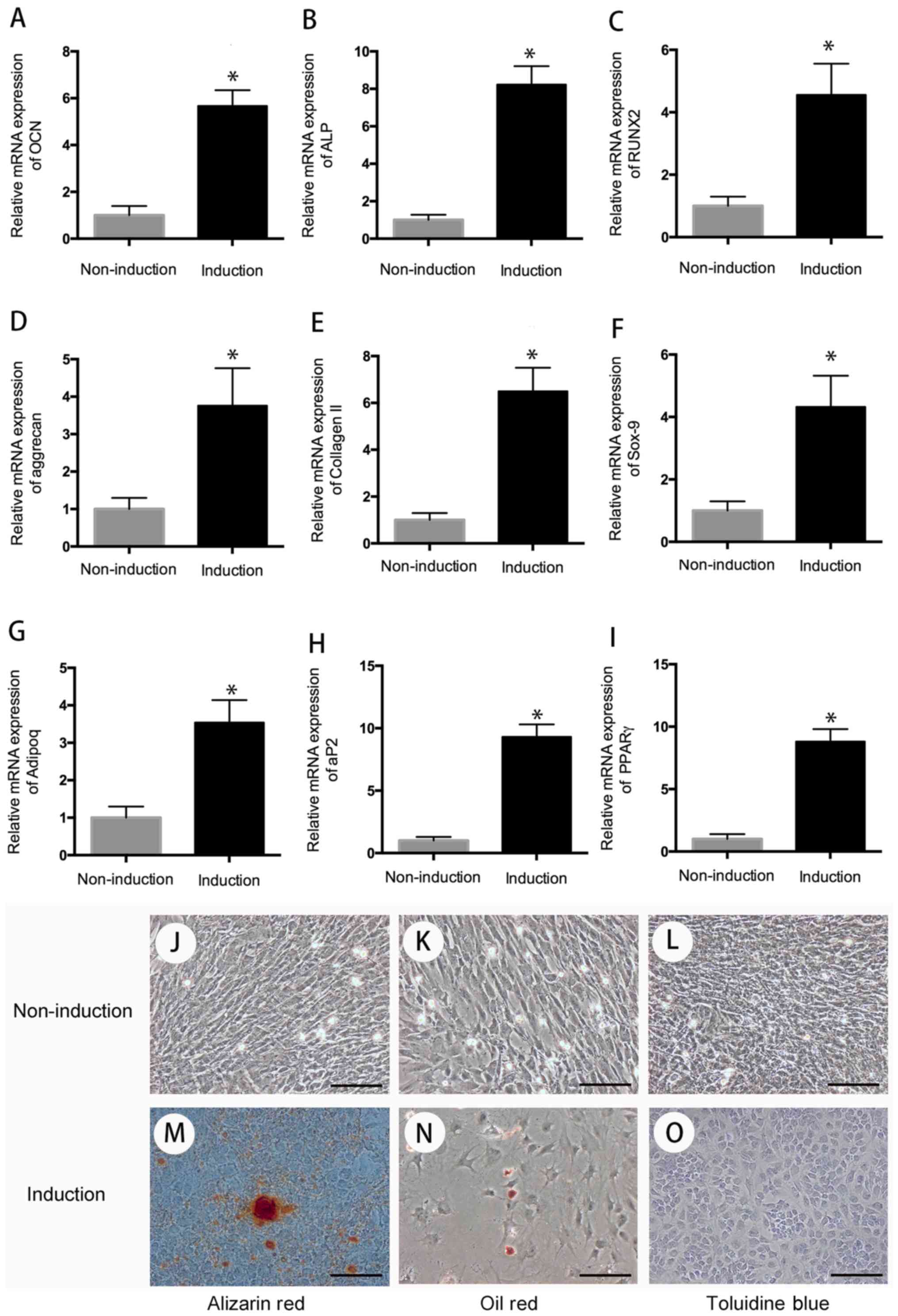 | Figure 2.Cell differentiation. After 3 weeks
of multi-differentiation induction, cells in the experiment group
have higher expression of osteogenic genes, (A) OCN, (B) ALP, (C)
RUNX2, chondrogenic genes, (D) aggrecan, (E) collagen II, (F)
sox-9, and adipogenic genes, (G) adipoq, (H) aP2, (I) PPARγ than
that in control group (P<0.05). There is no positive staining in
control group (J-L), wheread, positive Alizarin Red staining was
observed in osteogenic differentiation group for 21 days (M); Oil
Red O-positive staining of lipid droplets were detected in
induction group after 3 weeks of chondrogenic differentiation (N);
Cells in chondrogenic induction group showed remarkable changes in
cellular morphology and were stain positive for Toluidine Blue (O).
*P<0.05 when compared to control group. Scale bar, 100 µm. |
For special staining, Oil Red O-positive staining of
lipid droplets were observed in adipogenic differentiation after 3
weeks of adipogenic induction. On day 21 of osteogenic
differentiation, cells were detected with positive Alizarin Red
staining. After 3 weeks of chondrogenic differentiation, cells
showed remarkable changes in cellular morphology and were stain
positive for toluidine blue (Fig.
2).
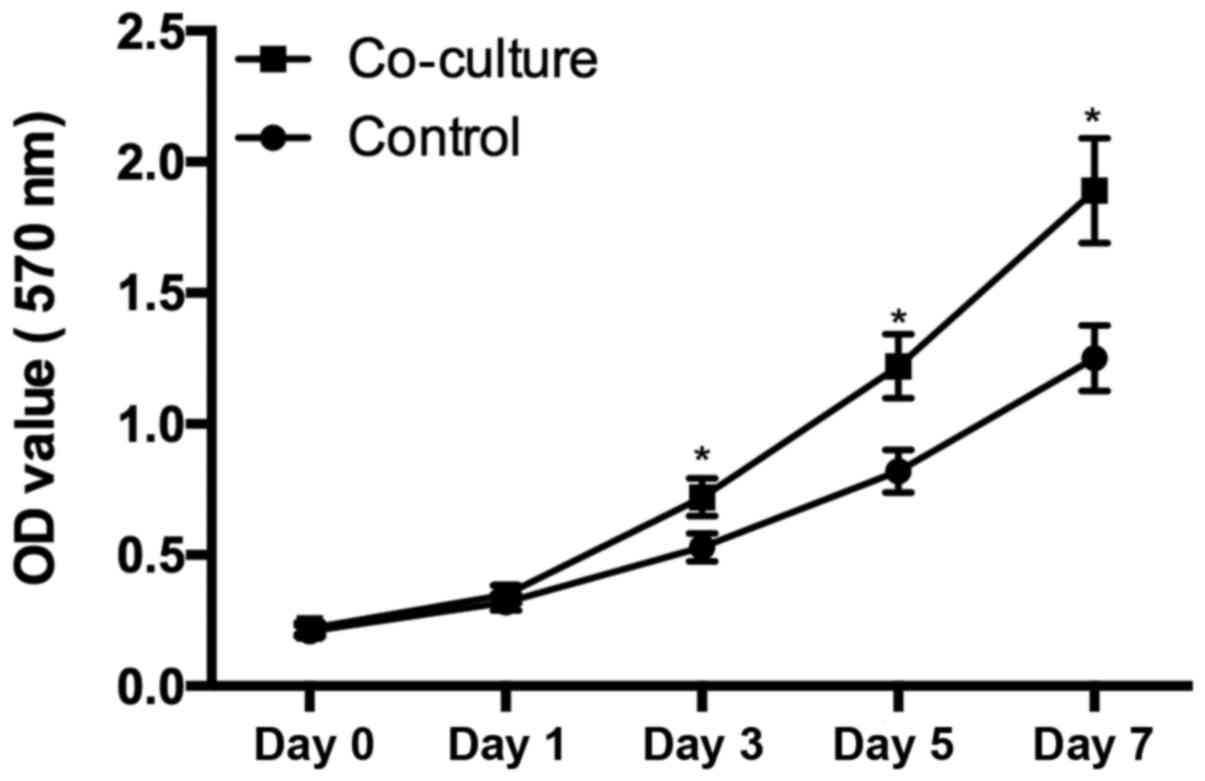
Cell proliferation
Chondrocytes harvested from OA patients seeded at a
density of 1,000 cells/wells were cultured in the supernatant from
hUC-MSC. From day 3, the cells cultured in the supernatant from
hUC-MSC demonstrated significant higher proliferation rates than
control group (P<0.05), suggested that the secretion of hUC-MSC
enhanced the chondrocytes proliferation (Fig. 3).
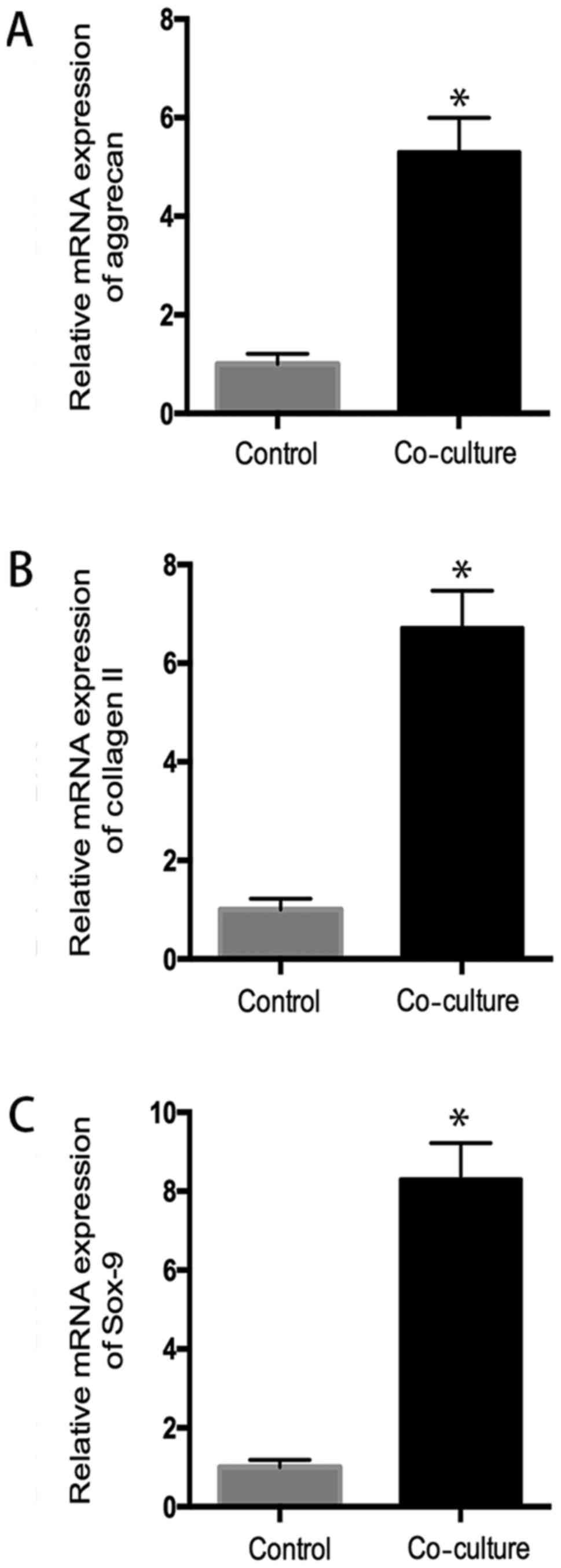
The quantitative RT-PCR assay
The quantitative RT-PCR assay was performed to
evaluate the mRNA of hUC-MSC and chondrocytes in coculture system.
As shown in Fig. 4, hUC-MSC
demonstrated a significant increased mRNA expression of aggrecan,
sox-9, collagen II, compared to the control group (P<0.05),
indicating chondrocytes promoted chondrogenic differentiation of
hUC-MSC (Fig. 4).
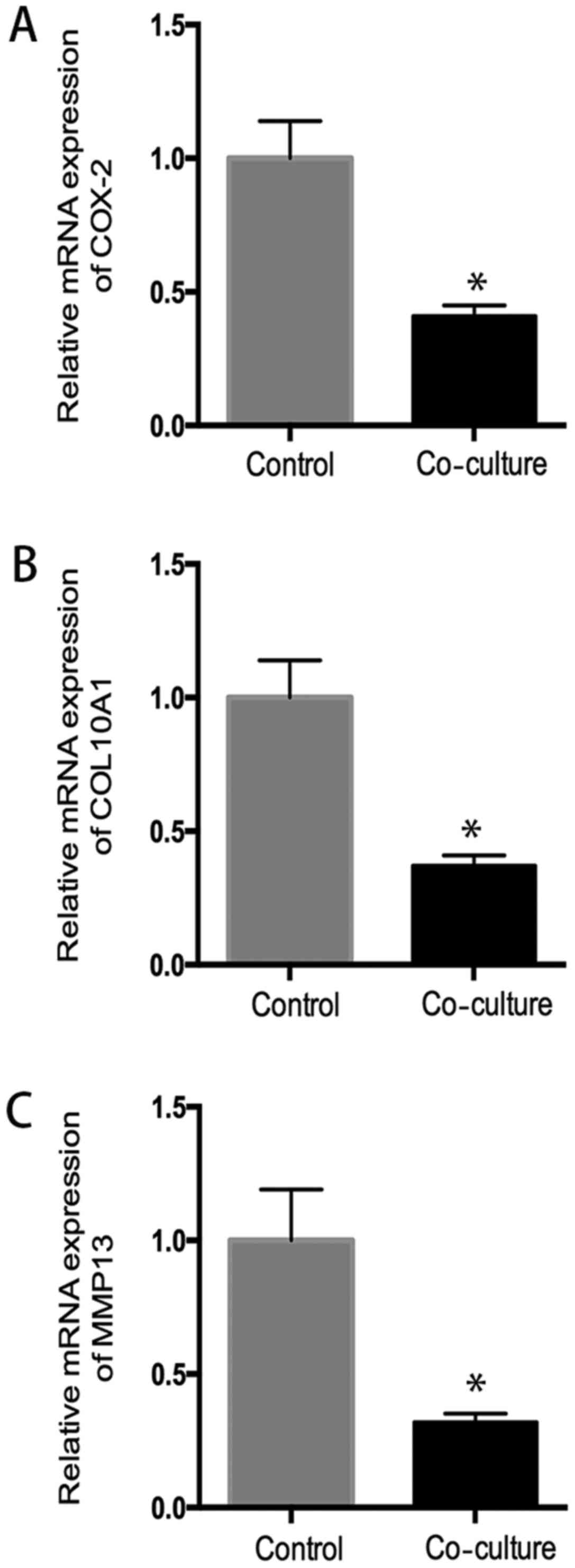
After coculture with hUC-MSC, chondrocytes from OA
patients showed a significant decreased mRNA expression of cox2,
collagen X, MMP13, compared to the control group (P<0.05), which
suggested hUC-MSC inhibited inflammatory activity in OA
chondrocytes (Fig. 5).
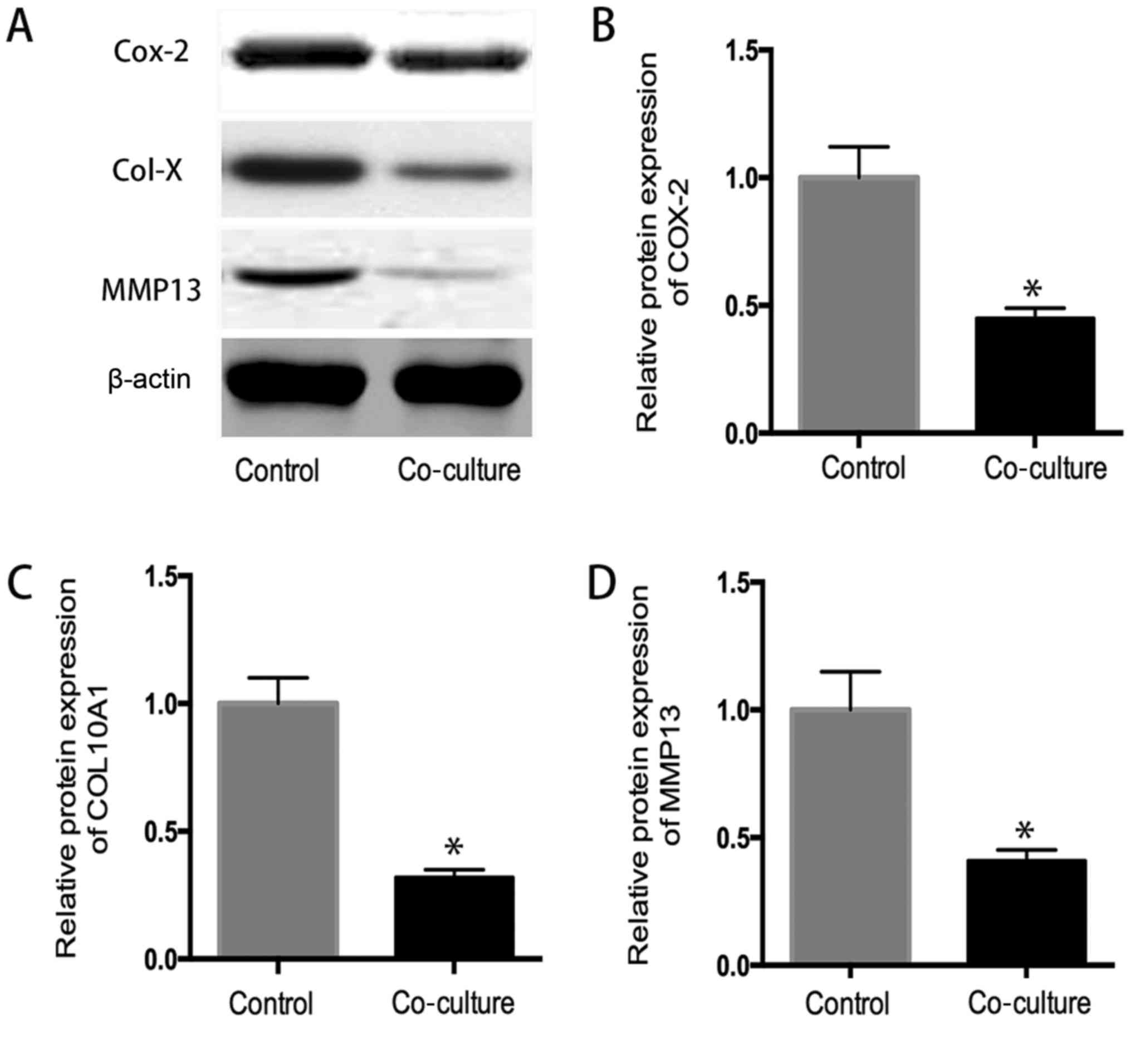
Western blot analysis
As shown in Fig. 6,
the western blot analysis indicated that the protein expression of
COX-2, collagen 10A1 and MMP13 in coculture system significantly
decreased in comparison to that in control group determined by
western blot analysis (P<0.05), suggested that hUC-MSC inhibit
the expression inflammatory related protein in OA chondrocytes
(Fig. 6).
Levels of inflammentary factors
The supernatant was also collected for ELISA assay,
and the result showed that the levels of TNF-α, IL-1β, IL-6, IL-10
were: 22.38±3.12, 13.23±2.32, 16.53±1.92, and 20.89±3.54 pg/ml in
the supernatant of co-culture system, respectively which were
significantly lower than those in the control group: 32.28±4.21,
21.37±3.31, 26.75±3.24, and 31.23±4.53 pg/ml (P<0.05) (Fig. 7).
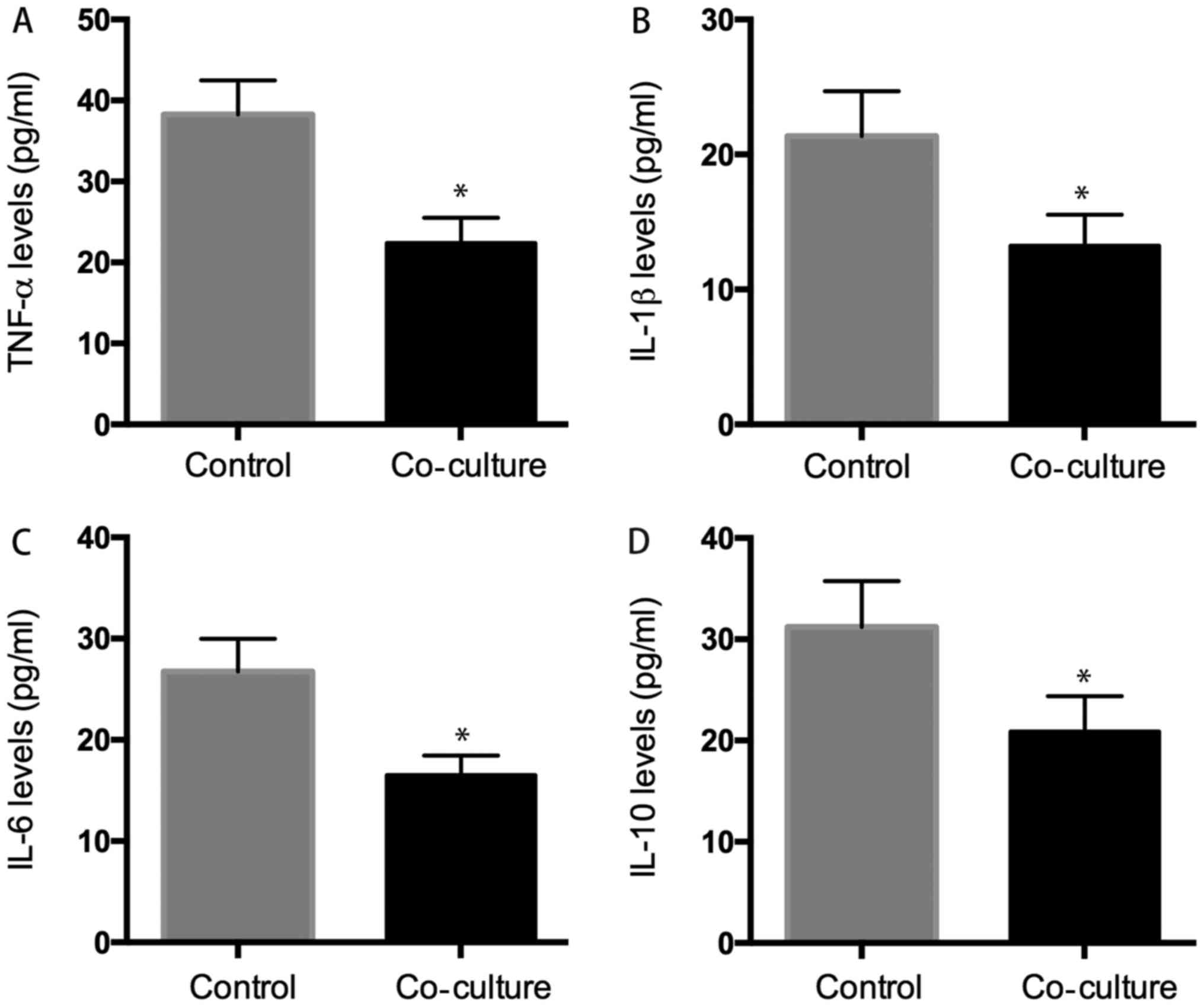 | Figure 7.ELISA analysis. We evaluated whether
hUC-MSC affected inflammatory cytokines secretion of osteoarthritic
chondrocytes, the results indicated that the level of inflammatory
cytokines (TNF-α, IL-1β, IL-6, IL-10) was 22.38±3.2 pg/ml (A),
13.23±2.3 pg/ml (B), 16.53±1.9 pg/ml (C), 20.89±3.5 pg/ml (D) in
co-culture group decreased significant than that was 38.28±4.3
pg/ml (A), 21.37±3.3 pg/ml (B), 26.75±3.6 pg/ml (C), 31.23±4.5
pg/ml (D) in control group. *P<0.05 when compared to control
group. |
Discussion
OA is caused by degeneration of the joint cartilage
and growth of new bone, cartilage and connective tissue, leading to
pain disability and impaired quality of life (26). Knee OA is the most common type of
OA, which results in major disability in senior people and more
health care problems (27).
There is currently no best treatment for OA
symptoms, while the application of stem cell as a novel therapy for
discogenic pain, neuropathic pain, and OA becomes more common
practice (28,29). It is reported that the application
of MSCs, such as bone marrow mesenchymal stem cells,
adipose-derived mesenchymal stem cells, synovial-derived
mesenchymal stem cells and peripheral blood-derived mesenchymal
stem cells, ameliorated the overall outcomes of patients with knee
osteoathritis, including pain relief and functional improvement
from basal evaluations, particularly at 12 and 24 months after
follow-up (30).
It is indicated that mesenchymal stem cells isolated
from the synovial membrane are reported that a candidate cell
source in cartilage and menisci regenerative medicine and OA
(31). Feng et al reported
that intra-articular injection of allogeneic adipose-derived
mesenchymal stem cells combined with hyaluronic acid could
efficiently block OA progression and promote cartilage regeneration
and allogeneic adipose-derived mesenchymal stem cells combined with
HA might survive at least 14 weeks after intra-articular injection
(32). Zhang et al the
coculture of bone marrow stem cells with chondrocytes from patients
with OA increases cell proliferation of chondrocytes and inhibits
inflammatory activity in OA (33).
Due to a painless collection procedure and
self-renewal properties, the human umbilical cord provides a
promising source of mesenchymal stem cells, although the use of
umbilical cord-derived stem cells in cell therapy was reported in
other diseases (34–36), the effect of human umbilical cord
stem cell for OA treatment has not been reported in the literature.
In the present study, we explored the effect of human umbilical
cord mesenchymal stem cells on chondrocytes from patients with OA
was observed in a co-culture system, we found human umbilical cord
mesenchymal stem cells and chondrocytes have mutual effect on each
other, and human umbilical cord stem cell significantly attenuated
OA in a co-culture system.
Human umbilical cord mesenchymal stem cells are
reported to be positive for CD13, CD29, CD73, CD90, CD105 and
HLA-ABC and are negative for CD34, CD45, CD133 and HLA-DR (37,38).
As analyzed using flow cytometry in our study, we also found the
umbilical cord mesenchymal stem cells have a high expression of
CD29, CD73, CD90, CD105, and less expression of CD34, CD45 and
CD133. In addition, human umbilical cord mesenchymal stem cells are
pluripotent stem cells, they can differentiate into chondrogenic,
osteogenic and adipogenic lineage. In our study, the cells have
high expression of chondrogenic genes (aggrecan, collagen II and
sox-9), osteogenic genes (OCN, ALP and RUNX2) and adipogenic genes
(adipoq, aP2 and PPARr) after multi-lineage induction.
Some studies showed that chondrocytes promoted that
chondrogenic differentiation of human umbilical cord blood-derived
MSCs (39–42). Similar to previous study, our
studies indicated that chondrocytes from patients with OA could
promote the chondrogenesis of human umbilical cord stem cell. The
mRNA analysis demonstrated that expression of collagen II, SOX-9,
aggrecan, the specific marker of cartilage in human umbilical cord
blood-derived MSCs, was increased in the co-culture with
chondrocytes.
Some studies have shown that chondrocytes secrete
the same cytokines and induce human stem cells to differentiate
into chondrocytes (43,44). The present data was consistent with
the previous study by Zheng et al that found chondrogenic
differentiation of human umbilical cord blood-derived MSCs by
co-culture with rabbit chondrocytes (41).
It is reported that intra-articular injection of
mesenchymal stem cells significantly attenuated OA, as mesenchymal
stem cells could downregulate some intrachondrogenic osteogenic
genes and proteins (45). The
present study indicated that human umbilical cord stem cell
decreased the osteogenic genes (COX2, COL10A1 and MMP13) and
production of some inflammatory factors (TNF-α, IL-1β, IL-6,
IL-10), indicating human umbilical cord stem cell attenuated
inflammatory response of OA. Zhu et al reported that human
umbilical cord blood MSC transplantation suppresses inflammatory
responses during early stage of focal cerebral ischemia in rabbits,
ischemia-induced increases of IL-1β, IL-6 and TNF-α levels in the
serum and peri-ischemic brain tissues within 6 h MCAO-reperfusion
were markedly suppressed human umbilical cord stem cell
transplantation (46). In another
study, human umbilical cord mesenchymal stem cells were reported to
decrease expression of MDA, GSSG, TNF-α, IFN-γ, TGF-β, IL-1, IL-2,
IL-6, collagen type 1 mRNA and MMPs (47).
In addition, further experiments demonstrated that
human umbilical cord stem cell significantly promoted chondrocyte
proliferation, that may be explained by that human umbilical cord
stem cell secreted many soluble factors, such as G-CSF, PDGF-BB and
bFGF (48,49). In addition, human umbilical cord
stem cell may inhibit apoptosis, and then promoted proliferaration,
Zhang et al found human umbilical cord stem cell promoted
proliferation and inhibited apoptosis of skin cells after
heat-stress in vitro by secreting exosomes (50).
In conclusion, human umbilical cord stem cell could
promote the proliferation of chondrocytes from patients with OA and
downregulate inflammatory activity in OA, they would be promising
autologous or allogenic cells in the treatment of OA, and this is a
preliminary study and further studies are need to strenghten the
presented results, such as the cell cycle changes in cell
proliferation assay, and western blots in the differentiation
assay, as well as COX-2 expression in inflammatory cells should be
performed to strengthen the experiments. In addition, the
isolation, purification, and expansion of human umbilical cord stem
cell in vitro should be optimized before clinical
application.
Acknowledgements
This study was supported by Medical and Health
Research Foundation of PLA (grant no. 14ZD09), and Medical Science
Foundation of Nanjing Military Area (grant nos. 14MS024 and
12MA019).
References
|
1
|
Fransen M, Bridgett L, March L, Hoy D,
Penserga E and Brooks P: The epidemiology of osteoarthritis in
Asia. Int J Rheum Dis. 14:113–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Kraan PM and van den Berg WB:
Chondrocyte hypertrophy and osteoarthritis: Role in initiation and
progression of cartilage degeneration? Osteoarthritis Cartilage.
20:223–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y and Jordan JM: Epidemiology of
osteoarthritis. Clin Geriatr Med. 26:355–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neogi T and Zhang Y: Epidemiology of
osteoarthritis. Rheum Dis Clin North Am. 39:1–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cross M, Smith E, Hoy D, Nolte S, Ackerman
I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al:
The global burden of hip and knee osteoarthritis: Estimates from
the global burden of disease 2010 study. Ann Rheum Dis.
73:1323–1330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akkiraju H and Nohe A: Role of
chondrocytes in cartilage formation, progression of osteoarthritis
and cartilage regeneration. J Dev Biol. 3:177–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dreier R: Hypertrophic differentiation of
chondrocytes in osteoarthritis: The developmental aspect of
degenerative joint disorders. Arthritis Res Ther. 12:2162010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mobasheri A, Kalamegam G, Musumeci G and
Batt ME: Chondrocyte and mesenchymal stem cell-based therapies for
cartilage repair in osteoarthritis and related orthopaedic
conditions. Maturitas. 78:188–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bennell KL, Hunter DJ and Hinman RS:
Management of osteoarthritis of the knee. BMJ. 345:e49342012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jakob R: The management of early
osteoarthritis. Knee. 21:799–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kon E, Filardo G, Drobnic M, Madry H,
Jelic M, van Dijk N and Della Villa S: Non-surgical management of
early knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc.
20:436–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Musumeci G, Loreto C, Castorina S, Imbesi
R, Leonardi R and Castrogiovanni P: Current concepts in the
treatment of cartilage damage. A review. Ital J Anat Embryol.
118:189–203. 2013.PubMed/NCBI
|
|
13
|
Musumeci G, Mobasheri A, Trovato FM,
Szychlinska MA, Graziano AC, Lo Furno D, Avola R, Mangano S,
Giuffrida R and Cardile V: Biosynthesis of collagen I, II, RUNX2
and lubricin at different time points of chondrogenic
differentiation in a 3D in vitro model of human mesenchymal stem
cells derived from adipose tissue. Acta Histochem. 116:1407–1417.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Musumeci G, Castrogiovanni P, Leonardi R,
Trovato FM, Szychlinska MA, Di Giunta A, Loreto C and Castorina S:
New perspectives for articular cartilage repair treatment through
tissue engineering: A contemporary review. World J Orthop. 5:80–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szychlinska MA, Stoddart MJ, D'Amora U,
Ambrosio L, Alini M PhD and Musumeci G: Mesenchymal stem cell-based
cartilage regeneration approach and cell senescence: Can we
manipulate cell aging and function? Tissue Eng Part B Rev. May
17–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Savkovic V, Li H, Seon JK, Hacker M, Franz
S and Simon JC: Mesenchymal stem cells in cartilage regeneration.
Curr Stem Cell Res Ther. 9:469–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pers YM, Ruiz M, Noël D and Jorgensen C:
Mesenchymal stem cells for the management of inflammation in
osteoarthritis: State of the art and perspectives. Osteoarthritis
Cartilage. 23:2027–2035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Musumeci G, Lo Furno D, Loreto C,
Giuffrida R, Caggia S, Leonardi R and Cardile V: Mesenchymal stem
cells from adipose tissue which have been differentiated into
chondrocytes in three-dimensional culture express lubricin. Exp
Biol Med (Maywood). 236:1333–1341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szychlinska MA, Castrogiovanni P, Nsir H,
Di Rosa M, Guglielmino C, Parenti R, Calabrese G, Pricoco E,
Salvatorelli L, Magro G, et al: Engineered cartilage regeneration
from adipose tissue derived-mesenchymal stem cells: A
morphomolecular study on osteoblast, chondrocyte and apoptosis
evaluation. Exp Cell Res. 357:222–235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding DC, Chang YH, Shyu WC and Lin SZ:
Human umbilical cord mesenchymal stem cells: A new era for stem
cell therapy. Cell Transplant. 24:339–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gauthaman K, Yee FC, Cheyyatraivendran S,
Biswas A, Choolani M and Bongso A: Human umbilical cord Wharton's
jelly stem cell (hWJSC) extracts inhibit cancer cell growth in
vitro. J Cell Biochem. 113:2027–2039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ali H, Al-Yatama MK, Abu-Farha M,
Behbehani K and Al Madhoun A: Multi-lineage differentiation of
human umbilical cord Wharton's Jelly Mesenchymal Stromal Cells
mediates changes in the expression profile of stemness markers.
PLoS One. 10:e01224652015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fernandes AM, Herlofsen SR, Karlsen TA,
Küchler AM, Fløisand Y and Brinchmann JE: Similar properties of
chondrocytes from osteoarthritis joints and mesenchymal stem cells
from healthy donors for tissue engineering of articular cartilage.
PLoS One. 8:e629942013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang ZH, Li XL, He XJ, Wu BJ, Xu M, Chang
HM, Zhang XH, Xing Z, Jing XH, Kong DM, et al: Delivery of the Sox9
gene promotes chondrogenic differentiation of human umbilical cord
blood-derived mesenchymal stem cells in an in vitro model. Braz J
Med Biol Res. 47:279–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hata K, Watanabe Y, Nakai H, Hata T and
Hoshiai H: Expression of the vascular endothelial growth factor
(VEGF) gene in epithelial ovarian cancer: An approach to anti-VEGF
therapy. Anticancer Res. 31:731–737. 2011.PubMed/NCBI
|
|
26
|
Puljak L, Marin A, Vrdoljak D, Markotic F,
Utrobicic A and Tugwell P: Celecoxib for osteoarthritis. Cochrane
Database Syst Rev. 5:CD0098652017.PubMed/NCBI
|
|
27
|
Xu Q, Chen B, Wang Y, Wang X, Han D, Ding
D, Zheng Y, Cao Y, Zhan H and Zhou Y: The effectiveness of manual
therapy for relieving pain, stiffness, and dysfunction in knee
osteoarthritis: A systematic review and meta-analysis. Pain
Physician. 20:229–243. 2017.PubMed/NCBI
|
|
28
|
Fellows CR, Matta C, Zakany R, Khan IM and
Mobasheri A: Adipose, bone marrow and synovial joint-derived
mesenchymal stem cells for cartilage repair. Front Genet.
7:2132016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chakravarthy K, Chen Y, He C and Christo
PJ: Stem cell therapy for chronic pain management: Review of uses,
advances, and adverse effects. Pain Physician. 20:293–305.
2017.PubMed/NCBI
|
|
30
|
Cui GH, Wang YY, Li CJ, Shi CH and Wang
WS: Efficacy of mesenchymal stem cells in treating patients with
osteoarthritis of the knee: A meta-analysis. Exp Ther Med.
12:3390–3400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kohno Y, Mizuno M, Ozeki N, Katano H,
Komori K, Fujii S, Otabe K, Horie M, Koga H, Tsuji K, et al: Yields
and chondrogenic potential of primary synovial mesenchymal stem
cells are comparable between rheumatoid arthritis and
osteoarthritis patients. Stem Cell Res Ther. 8:1152017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng C, Luo X, He N, Xia H, Lv X, Zhang X,
Li D, Wang F, He J, Zhang L, et al: Efficacy and persistence of
allogeneic adipose-derived mesenchymal stem cells combined with
hyaluronic acid in osteoarthritis after intra-articular injection
in a sheep model. Tissue Eng Part A. Sep 27–2017.(Epub ahead of
print). PubMed/NCBI
|
|
33
|
Zhang Q, Chen Y, Wang Q, Fang C, Sun Y,
Yuan T, Wang Y, Bao R and Zhao N: Effect of bone marrow-derived
stem cells on chondrocytes from patients with osteoarthritis. Mol
Med Rep. 13:1795–1800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon
JH, Lee YS, Lee KS, Park HK and Kang KS: Successful stem cell
therapy using umbilical cord blood-derived multipotent stem cells
for Buerger's disease and ischemic limb disease animal model. Stem
Cells. 24:1620–1626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baker CD and Abman SH: Umbilical cord stem
cell therapy for bronchopulmonary dysplasia: Ready for prime time?
Thorax. 68:402–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu Z, Akiyama K, Ma X, Zhang H, Feng X,
Yao G, Hou Y, Lu L, Gilkeson GS, Silver RM, et al: Transplantation
of umbilical cord mesenchymal stem cells alleviates lupus nephritis
in MRL/lpr mice. Lupus. 19:1502–1514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nagamura-Inoue T and He H: Umbilical
cord-derived mesenchymal stem cells: Their advantages and potential
clinical utility. World J Stem Cells. 6:195–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mennan C, Brown S, McCarthy H,
Mavrogonatou E, Kletsas D, Garcia J, Balain B, Richardson J and
Roberts S: Mesenchymal stromal cells derived from whole human
umbilical cord exhibit similar properties to those derived from
Wharton's jelly and bone marrow. FEBS Open Bio. 6:1054–1066. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li X, Duan L, Liang Y, Zhu W, Xiong J and
Wang D: Human umbilical cord blood-derived mesenchymal stem cells
contribute to chondrogenesis in coculture with chondrocytes. Biomed
Res Int. 2016:38270572016.PubMed/NCBI
|
|
40
|
Wang J, Li J, Deng N, Zhao X, Liu Y, Wang
X and Zhang H: Transfection of hBMP-2 into mesenchymal stem cells
derived from human umbilical cord blood and bone marrow induces
cell differentiation into chondrocytes. Minerva Med. 105:283–288.
2014.PubMed/NCBI
|
|
41
|
Zheng P, Ju L, Jiang B, Chen L, Dong Z,
Jiang L, Wang R and Lou Y: Chondrogenic differentiation of human
umbilical cord blood-derived mesenchymal stem cells by co-culture
with rabbit chondrocytes. Mol Med Rep. 8:1169–1182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mennan C, Wright K, Bhattacharjee A,
Balain B, Richardson J and Roberts S: Isolation and
characterisation of mesenchymal stem cells from different regions
of the human umbilical cord. Biomed Res Int. 2013:9161362013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu L, Prins HJ, Helder MN, van
Blitterswijk CA and Karperien M: Trophic effects of mesenchymal
stem cells in chondrocyte co-cultures are independent of culture
conditions and cell sources. Tissue Eng Part A. 18:1542–1551. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Meretoja VV, Dahlin RL, Kasper FK and
Mikos AG: Enhanced chondrogenesis in co-cultures with articular
chondrocytes and mesenchymal stem cells. Biomaterials.
33:6362–6369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jo CH, Lee YG, Shin WH, Kim H, Chai JW,
Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, et al: Intra-articular
injection of mesenchymal stem cells for the treatment of
osteoarthritis of the knee: A proof-of-concept clinical trial. Stem
Cells. 32:1254–1266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu Y, Guan YM, Huang HL and Wang QS:
Human umbilical cord blood mesenchymal stem cell transplantation
suppresses inflammatory responses and neuronal apoptosis during
early stage of focal cerebral ischemia in rabbits. Acta Pharmacol
Sin. 35:585–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Min F, Gao F, Li Q and Liu Z: Therapeutic
effect of human umbilical cord mesenchymal stem cells modified by
angiotensin-converting enzyme 2 gene on bleomycin-induced lung
fibrosis injury. Mol Med Rep. 11:2387–2396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Amable PR, Teixeira MV, Carias RB,
Granjeiro JM and Borojevic R: Protein synthesis and secretion in
human mesenchymal cells derived from bone marrow, adipose tissue
and Wharton's jelly. Stem Cell Res Ther. 5:532014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen J, Liu Z, Hong MM, Zhang H, Chen C,
Xiao M, Wang J, Yao F, Ba M, Liu J, et al: Proangiogenic
compositions of microvesicles derived from human umbilical cord
mesenchymal stem cells. PLoS One. 9:e1153162014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang B, Wang M, Gong A, Zhang X, Wu X,
Zhu Y, Shi H, Wu L, Zhu W, Qian H and Xu W: HucMSC-exosome
mediated-Wnt4 signaling is required for cutaneous wound healing.
Stem Cells. 33:2158–2168. 2015. View Article : Google Scholar : PubMed/NCBI
|