Introduction
Osteoblasts are the differentiated cell types
responsible for bone formation, originating from resident bone
marrow-derived mesenchymal stem cells (BMSCs) or bone marrow
stromal cells (1,2). Regulation of osteoblast
differentiation and activity has been hypothesized as a prospective
therapy of bone injury and loss. In order to develop such
therapies, novel molecular targets that regulate osteoblast
differentiation and activity need to be identified.
Casein kinase 2-interacting protein 1 (CKIP-1) is a
negative regulator for bone formation under physiological
conditions. In CKIP-1 knockout mice, bone mineral density and mass
increased markedly, as did the activity of osteoblasts (3). CKIP-1 interacts with mothers against
decapentaplegic homolog ubiquitination regulatory factor 1 to
induce negative regulation in bone formation (4). In addition, as a result of inhibition
of CKIP-1 expression in osteoblasts, bone mass increased markedly
and the rate of bone formation accelerated (5,6).
MicroRNAs (miRs), classified as small noncoding
RNAs, regulate gene expression by targeting the 3′-untranslated
region (UTR) of mRNA (7,8). MiRs serve a role in cell development
and growth processes, including cell proliferation, apoptosis and
differentiation (9,10). Previous studies have identified
miRNAs that regulate osteoblast differentiation (11,12).
CKIP-1 is a negative regulator for osteoblast differentiation and
therefore, an miR targeting CKIP-1 may improve osteoblast
differentiation.
The authors of the present study previously
demonstrated that CKIP-1 is a putative target gene of miR-98-5p
using bioinformatics analysis (unpublished data). It has been
hypothesized that miR-98-5p may be involved in osteoblast
differentiation through regulation of CKIP-1 expression. In the
present study, mouse MC3T3-E1 pre-osteoblasts were used to
investigate the potential role and underlying mechanism of
miR-98-5p-mediated regulation of osteoblast differentiation.
Materials and methods
Cell culture
Mouse pre-osteoblast cell line, MC3T3-E1 (Institute
of Basic Medicine of Peking Union Medical College, Beijing, China),
was cultured with α-minimal essential medium (α-MEM; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 1% penicillin (Thermo Fisher Scientific,
Inc.) at 37°C in a humidified atmosphere containing 5%
CO2. For all experiments, MC3T3-E1 cells were seeded at
the density of 2.5×104 cells/cm2 in cell
culture dishes and cultivated until 80% confluence was reached. To
induce osteoblast differentiation, confluent cells were cultured at
37°C in a humidified atmosphere containing 5% CO2, in an
osteoinductive medium supplemented with 10% FBS, 50 µg/ml ascorbic
acid, 5 mM sodium b-glycerophosphate and 2 mM/l glutamine, which
was replaced once every three days (13).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cultured cells were lysed by TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and total RNA
was isolated according to the manufacturer's protocol. cDNA was
synthesized using the TIANScript II RT kit (Tiangen Biotech Co.,
Ltd., Beijing, China). For miRNA expression analysis, cDNA
synthesis was performed using the TaqMan MicroRNA reverse
transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). qPCR was performed to detect mRNA levels of osteocalcin
(OCN), collagen type I (Col I) and GAPDH or miR-98-5p levels using
SYBR Green I PCR Mix (Beijing CoWin Biotech Co., Ltd., Beijing,
China) according to the manufacturer's protocol. Primer sequences
are listed in Table I and the
primers for miR-98-5p (miRBase accession no. MIMAT0000545) were
provided by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The
following thermocycling conditions were used for the PCR: Initial
denaturation at 94°C for 180 sec; 40 cycles of 94°C for 15 sec,
annealing at 60°C for 30 sec, and extension at 72°C for 30 sec.
Relative expression was normalized to mRNA levels of GAPDH using
the 2−ΔΔCq method (14).
 | Table I.Primer sequences for quantitative
polymerase chain reactions. |
Table I.
Primer sequences for quantitative
polymerase chain reactions.
|
|
| Primer sequence |
|---|
|
|
|
|
|---|
| Gene name | Length (base
pairs) | Forward (5′→3′) | Reverse (3′→5′) |
|---|
| CKIP-1 | 116 |
AACCGCTATGTGGTGCTGAA |
CAGGGTGAACTTGCTGATTT |
| Col-I | 184 |
CACATGCGTGCAGAACGGCG |
CGCGTCTTCGGGGCAGACAG |
| OCN | 134 |
AGTCTGACAAAGCCTTCA |
AAGCAGGGTTAAGCTCACA |
| GAPDH | 118 |
CAACTCCCTCAAGATTGTCAGCAA |
GGCATGGACTGTGGTCATGA |
Detection of osteoblast
differentiation
To investigate the role of miR-98-5p expression on
osteoblast differentiation, confluent MC3T3-E1 cells were
pre-treated with 100 nM miR-98-5p agomir, antagomir, agomir control
or antagomir control (Guangzhou RiboBio Co., Ltd.) for 24 h at 37°C
in α-MEM culture medium. Control MC3T3-E1 cells were cultured in
α-MEM culture medium only, without pre-treatment. Cells were
subsequently cultured in osteoinductive medium for 3, 5, 7 and 14
days, as described above. Cells were harvested, washed with a PBS
and lysed with a lysis buffer (10 mmol/l
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 250 mmol/l
sucrose, 5 mmol/l Tris-HCl, 0.1%TritonX-100, pH 7.5). Alkaline
phosphatase (ALP) activity of lysates was measured using the ALP
Activity Assay kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) at 25°C using the p-nitrophenyl phosphate method
according to manufacturer's protocol. Matrix mineralization was
detected with Alizarin red S staining. Briefly, cells were fixed
with 70% ethanol for 30 min at room temperature and washed with
PBS. Subsequently, cells were stained with Alizarin red S solution
(40 mM) for 15 min at room temperature. Excessive stain was washed
off with PBS. Alizarin red S-stained mineral deposits were
extracted and dissolved in 0.1 N NaOH and the absorbance was
measured at a wavelength of 540 nm using an ELISA reader (Multiskan
GO 1510; Thermo Fisher Scientific, Inc.).
Western blot analysis
Following washing twice with PBS, cells were lysed
in ice-cold radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Nanjing, China). Protein concentration
was quantified using bicinchoninic acid method. Equal amounts of
protein (20 µg/lane) were separated on 10% SDS-PAGE and transferred
onto polyvinylidene difluoride (PVDF) membranes (EMD Millipore,
Billerica, MA, USA). PVDF membranes were blocked with 3% skimmed
milk for 1 h at 37°C and incubated overnight at 4°C with the
following primary antibodies: Anti-CKIP-1 (cat. no. sc-376355;
1:500), anti-runt-related transcription factor 2 (Runx 2; cat. no.
sc-390351; 1:500), anti-osteopontin (OPN; cat. no. sc-21742; 1:500)
and anti-GAPDH (cat. no. sc-59540; 1:1,000), all purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) at 4°C. Membranes
were washed with PBS with Tween-20 (PBST) and incubated with
horseradish peroxidase conjugated secondary antibodies (cat. no.
sc-2371; 1:2,000, BIOSS, Beijing, China) for 1 h at room
temperature. Following washing with PBST, bands were detected with
an enhanced chemiluminescence reagent (Wuhan Boster Biological
Technology, Ltd., Wuhan, China). Band intensities were quantified
using Image-Pro Plus software (version 6.0; Media Cybernetics,
Inc., Rockville, MD, USA). The data were presented following
normalization with the control group.
Target prediction of miR-98-5p
The target mRNA of miR-98-5p was predicted by
searching the online databases TargetScan (http://www.targetscan.org/) and miRDB (http://www.mirdb.org/). mRNAs with a 3′-untranslated
region (3′-UTR) that matched the 2–7 base of the miR-98-5p
5′-terminal region with high thermodynamic stability were selected.
Subsequently, CKIP-1 was predicted as a target mRNA of
miR-98-5p.
Dual-luciferase reporter assay
The 3′-UTR region of CKIP-1, containing the binding
site of miR-98-5p, was amplified and subcloned into a
pmiR-RB-REPORT plasmid (Guangzhou RiboBio Co., Ltd., Guangzhou,
China), according to the manufacturer's protocol. The corresponding
mutated 3′UTR of CKIP-1 was also amplified and subcloned into the
plasmid. Human 293 cells (the 293 cells in the present study were
only used for the luciferase assay) were seeded into 6-well cell
culture plates at 1×105 cells/well and subsequently
transfected with miR-98-5p mimics (sequence,
UGAGGUAGUAAGUUGUAUUGUU) or miR-98NC (sequence,
UCACAACCUCCUAGAAAGAGUAGA; Guangzhou RiboBio Co., Ltd.) at a final
concentration of 50 nM for 24 h. Subsequently, the pmiR-RB CKIP-1
3′-UTR plasmid was transfected into 293 cells at concentration of 1
ng/µl using a Lipofectamine® 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 2 days following transfection,
the luciferase activity of the cells was determined using a
dual-luciferase reporter assay kit (Promega Corporation, Madison,
WI, USA) and was normalized to Renilla luciferase activity,
according to the manufacturer's protocol.
Statistical analysis
All data are presented as the mean ± standard
deviation from three separate experiments (n=3). Data was tested
for normal distribution using Shapiro-Wild test and differences
between groups were analyzed using one-way analysis of variance and
determined by Duncan's multiple range test. Statistical analysis
was performed using SPSS software (version 18; SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
MiR-98-5p promotes osteoblast
differentiation
In order to determine whether miR-98-5p is involved
in regulation of osteoblast differentiation, the expression of
miR-98-5p in MC3T3-E1 cells following osteoblast differentiation
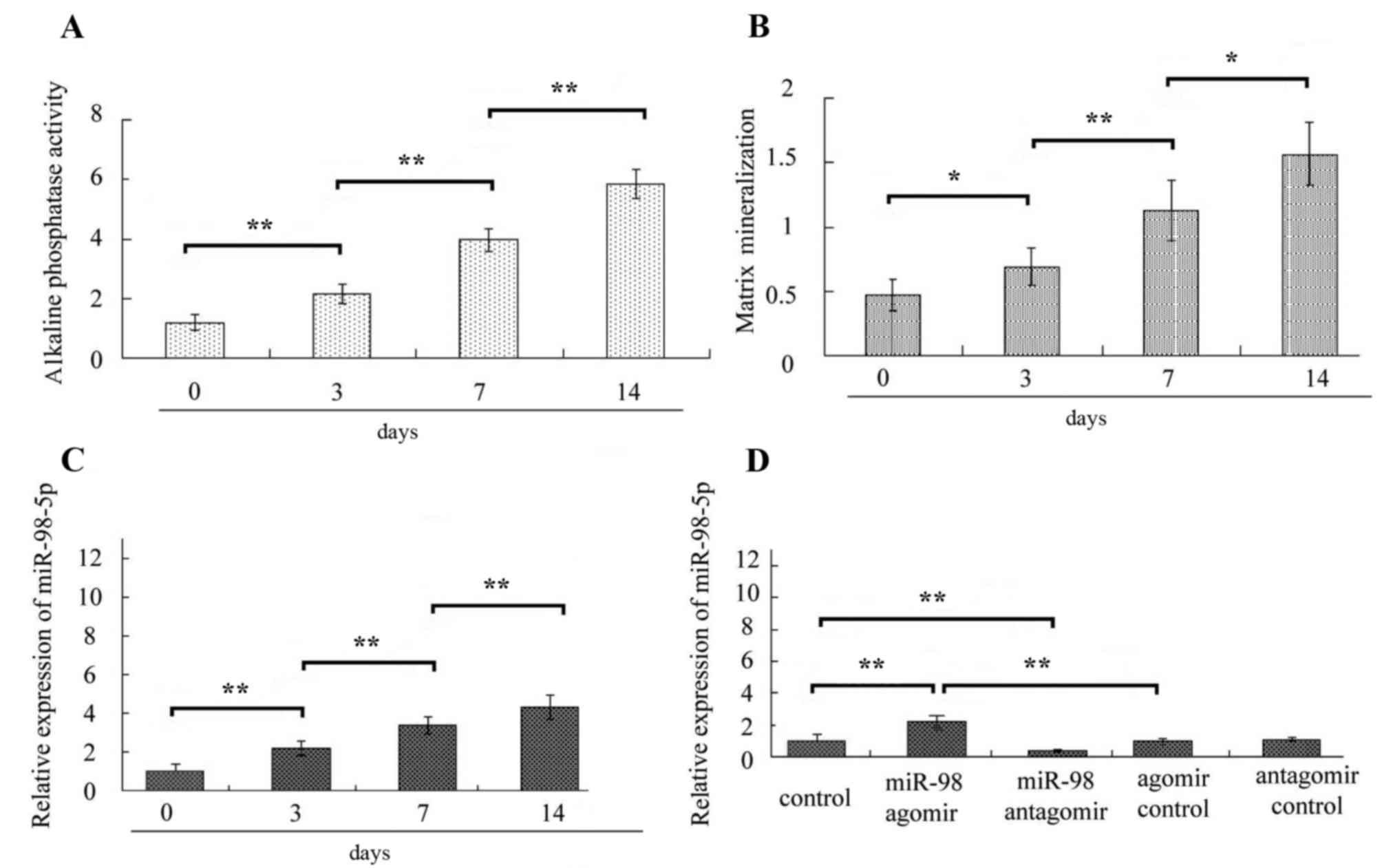
was detected by RT-qPCR. In the present study, following culture of
MC3T3-E1 cells in osteoinductive medium, alkaline phosphatase
activity and matrix mineralization of cells was elevated at each
time point (Fig. 1A and B),
indicating that MC3T3-E1 cells differentiated into osteoblasts. The
expression level of miR-98-5a was also significantly elevated at
each time point following osteoblast differentiation (all
P<0.01; Fig. 1C), which
suggested that miR-98-5p may serve a role in osteoblast
differentiation.
To confirm the role of miR-98-5p in the regulation
of osteoblast differentiation, MC3T3-E1 cells were treated with
miR-98-5p agomir or miR-98-5p antagomir and the treatments
increased and decreased the miR-98-5p expressions levels in
MC3T3-E1 cells, respectively (Fig.
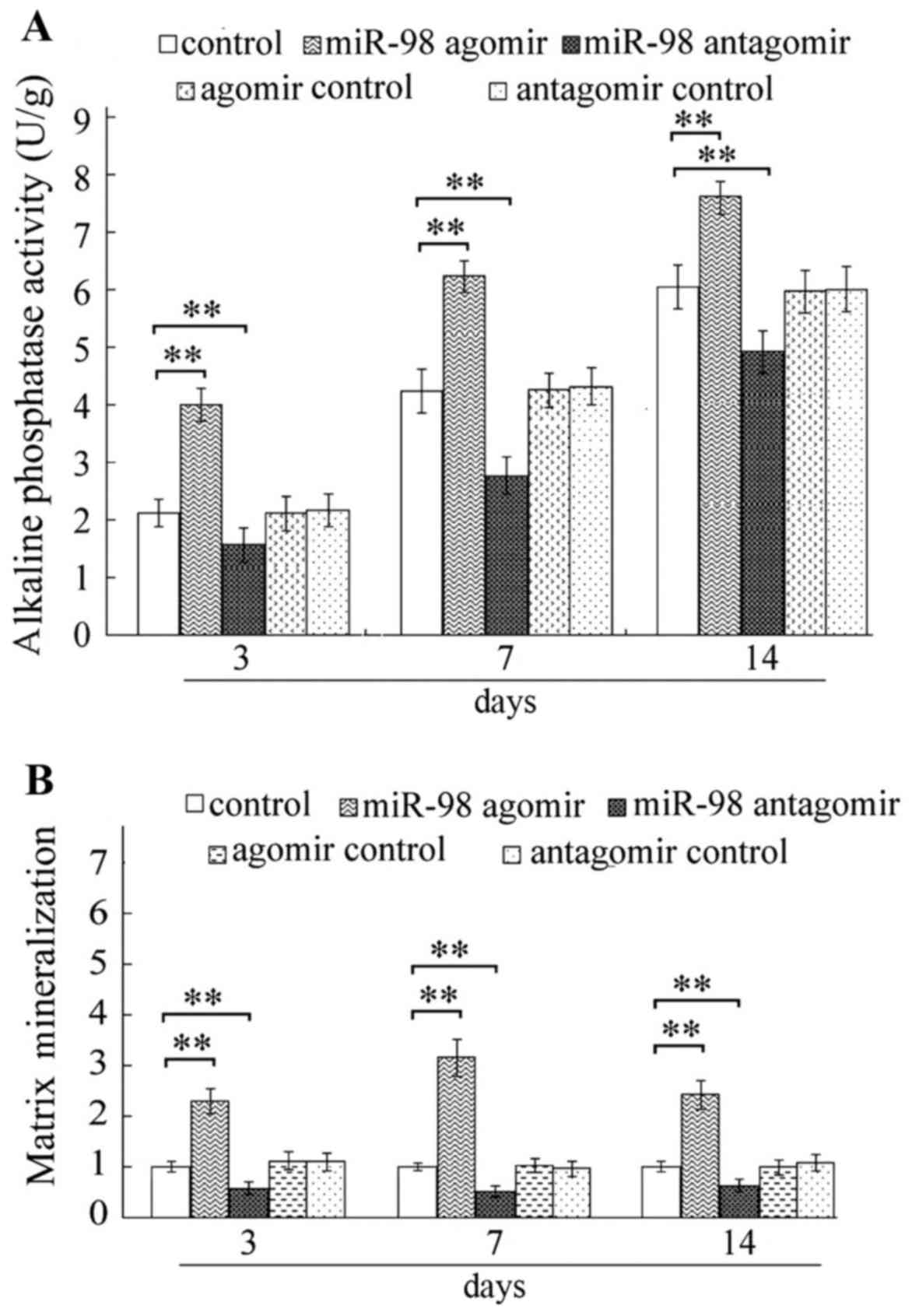
1D). Furthermore, the present study demonstrated that miR-98-5p
agomir markedly increased ALP activity (Fig. 2A) and matrix mineralization
(Fig. 2B), at each time point
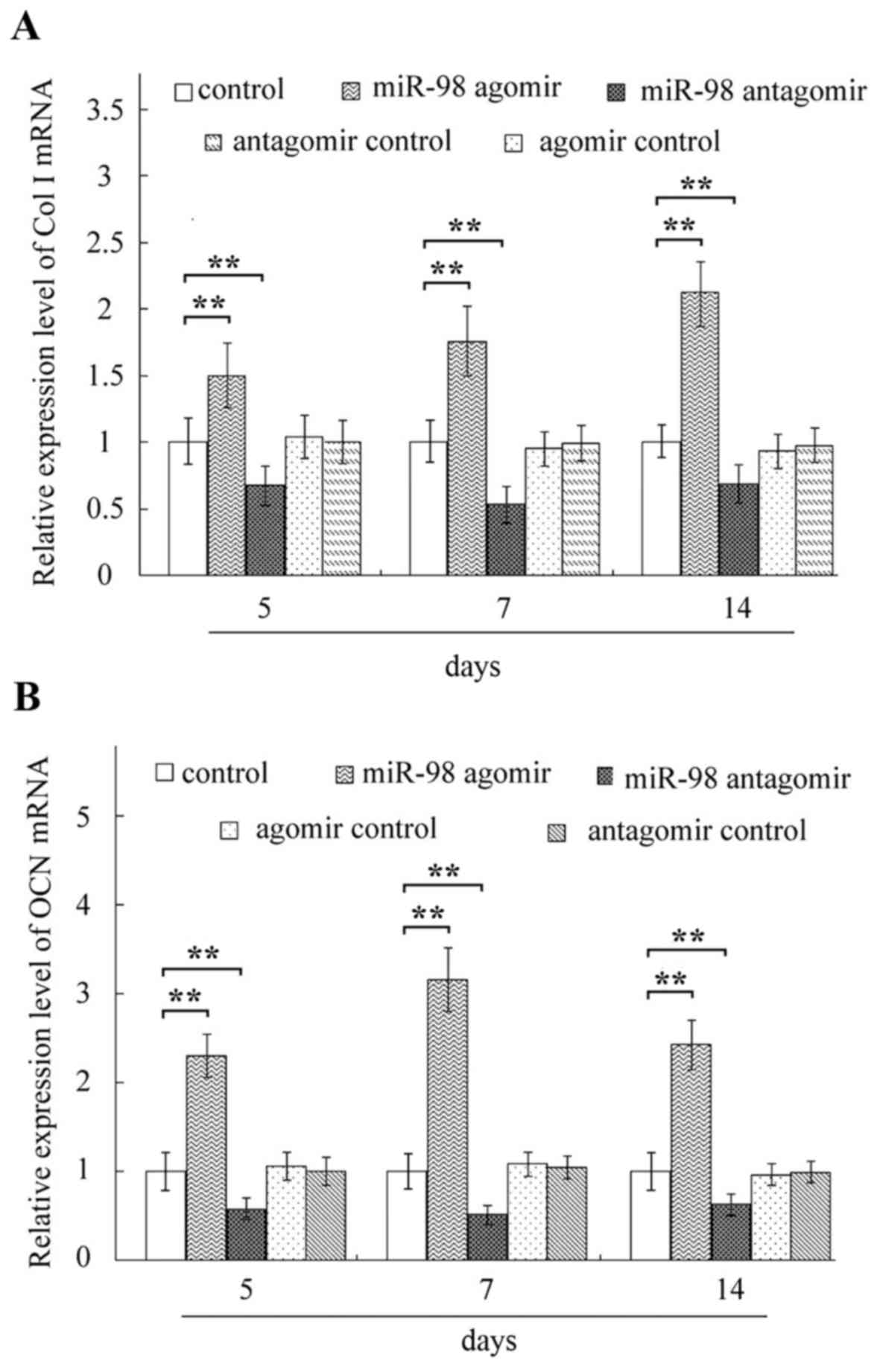
compared with the respective control groups. Elevated mRNA
expressions of Col I and OCN (Fig.
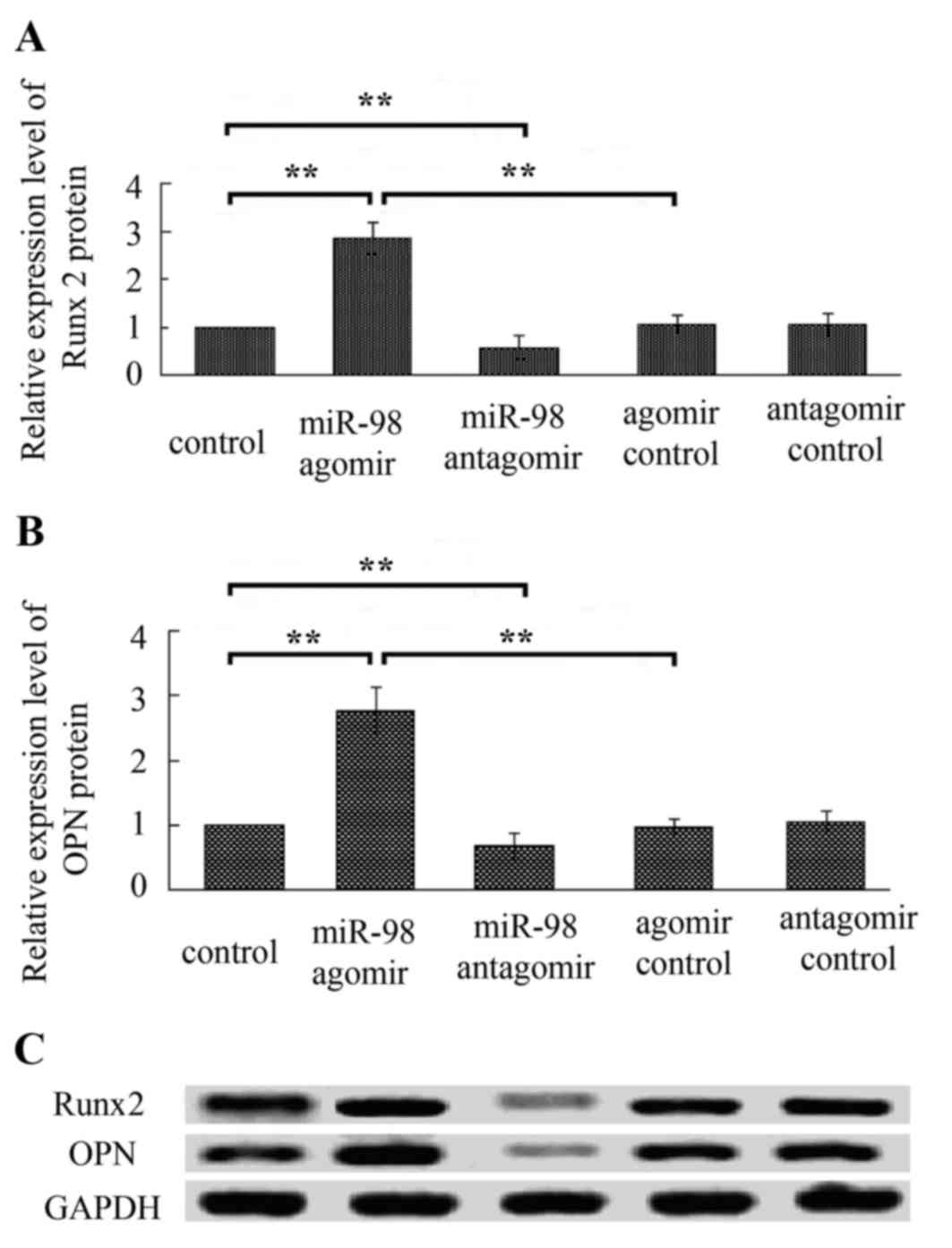
3), and increased protein levels of Runx2 and OPN (Fig. 4) were observed in cells following
treatment with miR-98-5p, at each time point compared with the
respective control groups. Conversely, miR-98-5p antagomir
significantly inhibited ALP activity and matrix mineralization, and
decreased expression levels of OCN, Col I, Runx2 and OPN (Figs. 2–4).
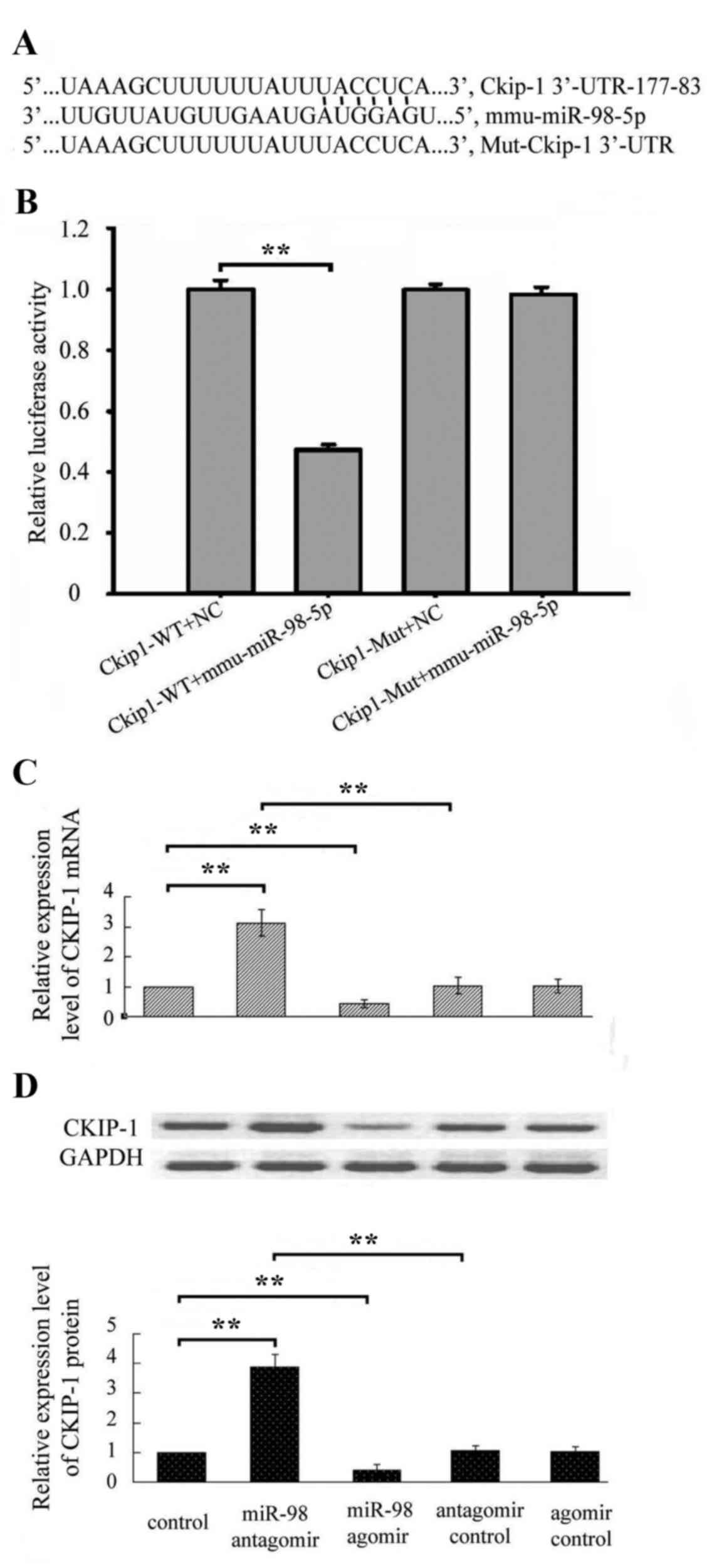
CKIP-1 is a target gene of
miR-98-5p
Subsequently, the expression of the target gene of
miR-98-5p that contributed to the regulation of osteoblast
differentiation was investigated and CKIP-1. To validate the
interaction between miR-98-5p and CKIP-1, a dual-luciferase
reporter assay was performed in the present study. The results
demonstrated that overexpression of miR-98-5p significantly
decreased the luciferase activity in cells transfected with pmiR-RB
CKIP-1 3′-UTR, whereas it demonstrated no effect on pmiR-RB-mut
CKIP-1 3′-UTR (Fig. 5B).
Following treatment with miR-98-5p agomir or
miR-98-5p antagomir, qPCR and western blot analysis demonstrated
that miR-98-5p overexpression decreased mRNA and protein levels of
CKIP-1, whereas inhibition of miR-98-5p increased mRNA and protein
expression of CKIP-1 (Fig. 5C).
The aforementioned results indicate that CKIP-1 is the target gene
of miR-98-5p.
miR-98-5p regulates osteoblast
differentiation by targeting CKIP-1
The present study investigated the mechanism
underlying miR-98-5p-mediated regulation of osteoblast
differentiation. The association between overexpression and
inhibition of miR-98-5p and osteoblast differentiation was
investigated. In the present study, following treatment with
miR-98-5p agomir, osteoblast differentiation-associated markers,
including ALP, matrix mineralization, OCN, Col I, Runx2 and OPN
were markedly elevated in MC3T3-E1 cells (Figs. 2–4). Conversely, treatment with miR-98-5p
antagomir decreased the levels of osteoblast
differentiation-associated markers (Figs. 2–4).
Discussion
ALP, OPN, Runx 2, OCN and matrix mineralization are
markers of osteogenic differentiation and therefore they have been
previously assayed to evaluate osteoblast differentiation (15–19).
Col I, a collagen identified in bone matrices, is also a maker of
osteogenesis (20). Therefore, in
the present study, ALP, OPN, Runx 2, OCN, Col I and matrix
mineralization were selected as indices of osteoblast
differentiation.
In the present study, miR-98-5p served the role of
regulator of osteoblast differentiation by downregulating the
expression of CKIP-1, a negative regulator of osteoblast
differentiation. The results of the present study indicated that
the expression of miR-98-5p was increased during osteoblast
differentiation and miR-98-5p overexpression significantly promoted
osteoblast differentiation of MC3T3-E1 cells. Downregulated
expression of miR-98-5p negatively regulated osteoblast
differentiation. Using a dual-luciferase reporter assay, a direct
interaction between miR-98-5p and the 3′-UTR region of CKIP-1 was
verified. In the present study, miR-98-5p negatively regulated mRNA
and protein expression levels of CKIP-1 in MC3T3-E1 cells.
Transfection with miR-98-5p agomir or antagomir disrupted the
interaction between CKIP-1 and osteoblast differentiation. The
present study demonstrated that miR-98-5p regulates CKIP-1
expression.
The role of miR-98 in different cellular processes
in various cell types has been previously investigated. miR-98
repressed the expression of cytokine-inducible Src homology
2-containing protein in cholangiocytes and was involved in
responses to microbial challenge and inflammation (21). MiR-98 also negatively regulated
interleukin-10 production and endotoxin tolerance in macrophages
following stimulation with lipopolysaccharide (22). miR-98 is a tumor suppressive miRNA,
knockdown of which leads to a reduction of 1,25-vitamin D
anti-growth effect of prostate cancer and overexpression of miR-98
suppressed prostate cancer cell growth (23). miR-98 inhibits human ovarian cancer
stem cell proliferation via retinoblastoma-associated protein-E2F
transcription factor pathway and suppresses hepatocellular
carcinoma by targeting Sal-like protein 4 and collagen triple helix
repeat containing 1 (24–26). Currently, a limited amount of data
is available regarding miR-98-mediated regulation of osteoblast
differentiation.
In the present study, miR-98-5p was involved in the
regulation of osteoblast differentiation through targeting and
regulating CKIP-1 expression. The results of the present study
indicated that CKIP-1 was a target gene of miR-98-5p, through which
miR-98-5p regulated osteoblast differentiation in MC3T3-E1
cells.
CKIP-1 regulates a number of processes, including
cell proliferation, differentiation and apoptosis. CKIP-1 also
serves a role in numerous types of cancer, including colon and
breast cancers, and human osteosarcoma. Knockdown of CKIP-1
promotes proliferation of NHL cells by altering the interaction
with RAC-alpha serine/threonine-protein kinase (Akt) (27). CKIP-1 inhibits macrophage
proliferation through activation of Akt (28). Furthermore, CKIP-1 is a negative
regulator of bone formation. In a mouse model, CKIP-1 knockout
counteracted osteoporosis induced by stimulation with microgravity
(29). CKIP-1 silencing with small
interfering RNA promotes bone formation in a rat model, inhibits
apoptosis, however promotes osteogenic differentiation of cultured
BMSCs (30).
In conclusion, the present study demonstrated that
inhibition of CKIP-1 by miR-98-5p overexpression significantly
promoted osteoblast differentiation. Overexpression of CKIP-1
induced by inhibition of miR-98-5p suppressed osteoblast
differentiation. The results of the present study indicated that
miR-98-5p regulated osteoblast differentiation in MC3T3-E1 cells by
targeting CKIP-1.
Acknowledgements
The present study was supported by grants from the
National Nature Science Foundation of China (grant nos. 11372351,
31660261 and 31370942) and the Natural Science Foundation of
Guangxi (grant no. 2016GXNSFAA380322).
References
|
1
|
Lerner UH: Osteoblasts, osteoclasts, and
osteocytes: Unveiling their intimate associated responses to
applied orthodontic forces. Semin in Orthodon. 18:237–248. 2012.
View Article : Google Scholar
|
|
2
|
Rolfe R, Roddy K and Murphy P: Mechanical
regulation of skeletal development. Curr Osteoporos Rep.
11:107–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu K, Yin X, Weng T, Xi S, Li L, Xing G,
Cheng X, Yang X, Zhang L and He F: Targeting WW domains linker of
HECT-type ubiquitin ligase Smurf1 for activation by CKIP-1. Nat
Cell Biol. 10:994–1002. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nie J, Liu L, He F, Fu X, Han W and Zhang
L: CKIP-1: A scaffold protein and potential therapeutic target
integrating multiple signaling pathways and physiological
functions. Ageing Res Rev. 12:276–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang G, Guo B, Wu H, Tang T, Zhang BT,
Zheng L, He Y, Yang Z, Pan X, Chow H, et al: A delivery system
targeting bone formation surfaces to facilitate RNAi-based anabolic
therapy. Nat Med. 18:307–314. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo B, Zhang B, Zheng L, Tang T, Liu J, Wu
H, Yang Z, Peng S, He X, Zhang H, et al: Therapeutic RNA
interference targeting CKIP-1 with a cross-species sequence to
stimulate bone formation. Bone. 59:76–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ranganathan K and Sivasankar V:
MicroRNAs-biology and clinical applications. J Oral Maxillofac
Pathol. 18:229–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vimalraj S and Selvamurugan N: MicroRNAs:
Synthesis, gene regulation and osteoblast differentiation. Curr
Issues Mol Biol. 15:7–18. 2013.PubMed/NCBI
|
|
12
|
Huang J, Zhao L, Xing L and Chen D:
MicroRNA-204 regulates Runx2 protein expression and mesenchymal
progenitor cell differentiation. Stem Cells. 28:357–364.
2010.PubMed/NCBI
|
|
13
|
Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang
W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL,
et al: miR-218 directs a Wnt signaling circuit to promote
differentiation of osteoblasts and osteomimicry of metastatic
cancer cells. J Biol Chem. 287:42084–42092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beck GR Jr, Zerler B and Moran E:
Phosphate is a specific signal for induction of osteopontin gene
expression. Proc Natl Acad Sci USA. 97:pp. 8352–8357. 2000;
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu M, Hesse E, Morvan F, Zhang JP, Correa
D, Rowe GC, Kiviranta R, Neff L, Philbrick WM, Horne WC and Baron
R: Zfp521 antagonizes Runx2, delays osteoblast differentiation in
vitro and promotes bone formation in vivo. Bone. 44:528–536. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mahalingam CD, Datta T, Patil RV, Kreider
J, Bonfil RD, Kirkwood KL, Goldstein SA, Abou-Samra AB and Datta
NS: Mitogen-activated protein kinase phosphatase 1 regulates bone
mass, osteoblast gene expression, and responsiveness to parathyroid
hormone. J Endocrinol. 211:145–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo Y, Liu L, Hao Q, Li R, Zhang X, Wang L
and Ning B: Effects of extracellular matrix produced in vitro on
growth and differentiation of MC3T3-E1 cells. Sheng Wu Gong Cheng
Xue Bao. 27:1606–1612. 2011.PubMed/NCBI
|
|
19
|
Guo Y, Zhang CQ, Zeng QC, Li RX, Liu L,
Hao QX, Shi CH, Zhang XZ and Yan YX: Mechanical strain promotes
osteoblast ECM formation and improves its osteoinductive potential.
Biomed Eng Online. 11:802012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhatt KA, Chang EI, Warren SM, Lin SE,
Bastidas N, Ghali S, Thibboneir A, Capla JM, McCarthy JG and
Gurtner GC: Uniaxial mechanical strain: An in vitro correlate to
distraction osteogenesis. J Surg Res. 143:329–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu G, Zhou R, Liu J, Gong AY, Eischeid AN,
Dittman JW and Chen XM: MicroRNA-98 and let-7 confer cholangiocyte
expression of cytokine-inducible Src homology 2-containing protein
in response to microbial challenge. J Immunol. 183:1617–1624. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Chen Q, Song Y, Lai L, Wang J, Yu
H, Cao X and Wang Q: MicroRNA-98 negatively regulates IL-10
production and endotoxin tolerance in macrophages after LPS
stimulation. FEBS Lett. 585:1963–1968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ting HJ, Messing J, Yasmin-Karim S and Lee
YF: Identification of microRNA-98 as a therapeutic target
inhibiting prostate cancer growth and a biomarker induced by
vitamin D. J Biol Chem. 288:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Hou L and Huang Y: EZH2-specific
microRNA-98 inhibits human ovarian cancer stem cell proliferation
via regulating the pRb-E2F pathway. Tumour Biol. 35:7239–7247.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou W, Zou B, Liu L, Cui K, Gao J, Yuan S
and Cong N: MicroRNA-98 acts as a tumor suppressor in
hepatocellular carcinoma via targeting SALL4. Oncotarget.
7:74059–74073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang CY, Zhang JJ, Hua L, Yao KH, Chen JT
and Ren XQ: MicroRNA-98 suppresses cell proliferation, migration
and invasion by targeting collagen triple helix repeat containing 1
in hepatocellular carcinoma. Mol Med Rep. 13:2639–2644. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu X, Ouyang Y, Zhong F, Wang Q, Ding L,
Zhang P, Chen L, Liu H and He S: Silencing of CKIP-1 promotes tumor
proliferation and cell adhesion-mediated drug resistance via
regulating AKT activity in non-Hodgkin's lymphoma. Oncol Rep.
37:622–630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Wang Y, Xiao F, Wang S, Xing G,
Li Y, Yin X, Lu K, Wei R, Fan J, et al: CKIP-1 regulates macrophage
proliferation by inhibiting TRAF6-mediated Akt activation. Cell
Res. 24:742–761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Wang Q, Wan Z, Li J, Liu L and
Zhang X: CKIP-1 knockout offsets osteoporosis induced by simulated
microgravity. Prog Biophys Mol Biol. 122:140–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou ZC, Che L, Kong L, Lei DL, Liu R and
Yang XJ: CKIP-1 silencing promotes new bone formation in rat
mandibular distraction osteogenesis. Oral Surg Oral Med Oral Pathol
Oral Radiol. 123:e1–e9. 2017. View Article : Google Scholar : PubMed/NCBI
|