Introduction
Human cytomegalovirus (HCMV) is a ubiquitous herpes
virus with infection rate of ~50–80% in females of reproductive age
from different regions of the world, including Australia, Canada,
United States, Sweden, Finland, Spain, United Kingdom and Ghana
(1). Following an HCMV infection,
the primary clinical manifestations in healthy people are
asymptomatic recessive or latent infections. When the immune system
function is reduced, in the event of pregnancy, cancer, HIV
infection, organ transplantation and childbirth, HCMV is activated
from its latent infection status and proliferates to a primary or
secondary infection. It may also lead to serious illnesses,
including interstitial pneumonia, encephalitis, hepatitis, retinal
failure and/or nervous systems, gastrointestinal diseases and
stillbirth. Previous studies have reported that HCMV may also be
associated with the occurrence of atherosclerosis and malignant
tumors (2,3).
Currently, the pathogenesis of HCMV infection
remains to be elucidated. Previous studies revealed that the viral
genetic differences, which are associated with the virulence, the
tropism to tissues, and the ability to escape the immune system,
may be the key factors determining whether HCMV infections lead to
fetal and organ deformities (4,5).
Primary clinical isolates carry at least 19 additional genes within
the UL/b' genomic region (UL133-151 locus) that have been lost in
several commonly-used HCMV strains, which have been passaged
extensively in tissue culture (6).
The UL141 gene within the UL/b' genomic region is highly conserved
between HCMV isolates, encoding a protein containing a potential
signal peptide, a hydrophobic transmembrane domain and three
potential N-linked glycosylation sites. The UL141-encoded protein
is a glycoprotein doublet with molecular masses of 37–40 kDa that
has been localized to the endoplasmic reticulum by
immunofluorescence (7). HCMV UL141
is also requited to inhibit cell surface expression of cluster of
differentiation (CD)155, a ligand for the activating receptor CD226
(7). UL141 requires assistance
from additional HCMV-encoded functions to suppress CD112
expression, also termed nectin cell adhesion molecule 2, by
proteasome-mediated degradation (8). A previous study revealed that HCMV
UL141 protein may bind tumor necrosis factor-related
apoptosis-inducing ligand death (TRAIL)-receptor 1 (R1) and
TRAIL-receptor 2 (R2) to inhibit apoptosis of infected cells
(9). These studies revealed UL141
may effectively participate in HCMV immune escape through multiple
mechanisms (8,9). When CD155 and TRAIL-R2 accumulate in
the endoplasmic reticulum, CD112 is effectively downregulated
(8). A previous study indicated
that HCMV UL141 requisitions US2 to target CD112 for proteasome
degradation and modulates multiple immune-associated pathways
(10). It has an important effect
on cellular processes, such as antigen presentation, natural killer
(NK) cell activity, cell migration and coagulation. In addition to
acting as an immune evasion gene, UL141 is an inhibitor of HCMV
proliferation in epithelial cell culture (7). A recent study suggested that all
known mutations in the UL/b' genomic region in epithelial cells
involve loss or mutation of UL141 (11). The interactions between UL141 and
human proteins remain to be fully elucidated. Therefore, the
present study performed a yeast two-hybrid screen to identify
binary interactions that may yield important information regarding
the process of viral entry and replication.
In the present study, the human protein CUGBP
Elav-like family member 5 (CELF5) was identified as a
directly-interacting partner of HCMV-pUL141. Additionally, the
viral DNA level was increased in HCMV-infected U373 cells
overexpressing CELF5. However, reduced expression of CELF5 had no
significant effect on HCMV DNA synthesis. These findings suggest
that CELF5 has a key role in modulating viral DNA synthesis. The
interactions identified between HCMV UL141 and the CELF5 human
protein may elucidate the molecular mechanisms underlying HCMV
diseases.
Materials and methods
Oligonucleotides and plasmid
constructs
Oligonucleotide primers used for the current study
were purchased from Invitrogen; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA) and the recombinant plasmids used are presented
in Table I. To generate
pGBKT7-UL141 for the yeast two-hybrid screen, the coding sequence
of HCMV UL141 was amplified by polymerase chain reaction (PCR)
(12) using HCMV H strain DNA
(Genbank no. GQ981646) as a template with the pGBKT7-UL141 primers
(the restriction enzyme sites and protected bases are underlined):
Forward (F) 5′-CCGGGCCATGGAGGCCGACATCGCCGAAAAGATGTGGG-3′ and
reverse (R) 5′-CGCGTCGACTCACCTCTTCATCTTTCTAACAC-3′, obtained from
Invitrogen (Thermo Fisher Scientific, Inc.), and then inserted into
SfiI/SalI (Takara Biotechnology Co., Ltd., Dalian,
China)-digested plasmid pGBKT7 (Clontech Laboratories, Inc.,
Mountain View, CA, USA). The inserted sequence of pGBKT7-UL141 was
confirmed by sequencing (Shanghai Invitrogen Biotechnology Co.,
Ltd., Shanghai, China).
 | Table I.Plasmid constructs used in the
current study. |
Table I.
Plasmid constructs used in the
current study.
| Plasmid | Description |
Reference/source |
|---|
| pGEX-4T-2 | Cloning vector for
protein expression fused with GST tag in Escherichia coli
cells | Clontech
Laboratories, Inc. |
| pGEX-CELF5 | pGEX-4T-2
containing full-length human CELF5-encoding sequence | This study |
| pGBKT7 | Cloning vector for
protein expression fused with the GAL4 DNA-binding domain in
yeast | Clontech
Laboratories, Inc. |
| pGBKT7-UL141 | pGBKT7 containing
HCMV UL141 full length sequence | This study |
| pACT2 | Cloning vector for
protein expression fused with GAL4 activation domain in yeast | Clontech
Laboratories, Inc. |
| pACT2-CELF5 | pACT2 containing
full-length human CELF5-encoding sequence | This study |
| pEGFP | Cloning vector for
protein expression with green fluorescent protein(GFP) in mammalian
cell | Clontech
Laboratories, Inc. |
| pEGFP-UL141 | pEGFP containing
HCMV UL141 full length sequence | This study |
| pDsRed | Cloning vector for
protein expression with red fluorescent protein(RFP) in mammalian
cell | Clontech
Laboratories, Inc. |
| pDsRed-CELF5 | pDsRed containing
full-length human CELF5-encoding sequence | This study |
| pCMV-Myc | Cloning vector for
protein expression fused with c-Myc tag in mammalian cell | Clontech
Laboratories, Inc. |
| pCMV-Myc-UL141 | pCMV-Myc containing
HCMV UL141 full length sequence | This study |
| pCMV-HA | Cloning vector for
protein expression fused with hemagglutinin (HA) tag in mammalian
cell | Clontech
Laboratories, Inc. |
| pCMV-HA-CELF5 | pCMV-HA containing
full-length human CELF5-encoding sequence | This study |
| pACT2-cDNA | pACT2 containing a
human fetal brain cDNA library | This study |
For the glutathione S transferase (GST) pull-down
assay, the sequence of pACT2-CELF5, which was a candidate
pUL141-interacting protein, was cloned into GST-tagged pGEX-4T-2
vector (Clontech Laboratories, Inc.) in EcoRI and
XhoI (Takara Biotechnology Co., Ltd.) sites by
electroporation (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
according to the manufacturer's protocol in BL21 (DE3) cells
(Tiangen Biotech Co., Ltd., Beijing, China) at a density of
6×107 cells/ml, and expressed protein was termed
GST-CELF5. GST-labeled CELF5 (GST-CELF5) was expressed in BL21
(DE3) cells (Tiangen Biotech Co., Ltd., Beijing, China) in
logarithm growth period transfected with pGEX-4T-2-CELF5 by
electroporation (Bio-Rad Laboratories, Inc.) according to the
protocol provided by the manufacturer and induced with isopropyl
β-D-1-thiogalactopyranoside (IPTG; Clontech Laboratories, Inc.,
Mountainview, CA, USA). For the co-immunoprecipitation (co-IP)
assays, the sequences of UL141 and CELF5 were inserted into Myc-
and HA-tagged pCMV vector (Clontech Laboratories, Inc.) for
expression in 293T cells.
To generate pEGFP-UL141 and pDsRed-CELF5 constructs,
which were used to express fluorescently-tagged fusion proteins in
human cells, the sequences of UL141 and CELF5 were amplified using
the primers UL141 F, 5′-CGGAATTCCTGCCGCCGGGAGTCGCTCCG-3′ and R,
5′-CGCGGATCCTCACCTCTTCATCTTTCTAA-3′; CELF5 F,
5′-CGGAATTCCCCTCACATTGCCCTTCAG-3′ and R
5′-CGCGGATCCACCTTCTCGCTGCTGCTG-3′, then inserted into pEGFP and
pDsRed vectors (Clontech Laboratories, Inc.), respectively. The
resultant constructs were confirmed by restriction digestion
profiles and sequencing (Shanghai Invitrogen Biotechnology Co.,
Ltd., Shanghai, China).
Viruses, antibodies and cells
In this study, 10 patients (6 male, 4 female) aged
<5 months in the Affiliated Shengjing Hospital of China Medical
University who met the CMV infection diagnostic criteria (13) were enrolled between December 2006
and March 2007. The urine of the patient was collected and treated
with an antibiotic cocktail containing 500 U/ml penicillin, 500
µg/ml streptomycin and 500 µg/ml kanamycin. Following
centrifugation at 300 × g for 15 min, the supernatant was applied
onto a human embryonic lung fibroblasts (HELFs) monolayer, and
incubated at 37°C overnight to allow virus adsorption. Then the
inoculum was removed and the cells washed with Hank's buffer. The
inoculated HELFs were maintained at 37°C in Modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 2% fetal
bovine serum (FBS), penicillin (100 U/ml)/streptomycin (100 µg/ml)
and 2 mM l-glutamine (Gibco; Thermo Fisher Scientific, Inc.). The
culture medium was refreshed every 5 days and the cells passaged
when necessary. The cultures were observed every other day to
monitor appearance of cytopathic effects (CPEs). When HCMV
characteristic CPEs appeared, the culture was cultured for an
additional 3 days and then passaged. A low-passage clinical isolate
Chinese HCMV strain Han was thus successfully isolated from these
urine samples. All participating patients gave their written
informed consent prior to enrollment in the study. This study was
approved by the Ethics Committee of the Affiliated Shengjing
Hospital of China Medical University (Shenyang, China) and
conducted in accordance with the principles of the Declaration of
Helsinki. The virus was propagated in MRC-5 cells which were
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). Monoclonal mouse anti-human antibodies
against c-Myc (1:1,000; cat. no. AHO0052; Thermo Fisher Scientific,
Inc.) or polyclonal goat anti-human antibodies against GST
(1:1,000; cat. no. PA5-18394; Thermo Fisher Scientific, Inc.) were
used for western blot analysis. Goat anti-mouse horseradish
peroxidase (HRP)-conjugated (1:2,000; cat. no. A0216; Beyotime
Institute of Biotechnology, Haimen, China) and rabbit anti-goat
HRP-conjugated immunoglobulin G secondary antibodies (1:2,000; cat.
no. abs20005; Absin Bioscience, Inc., Shanghai, China) were used
for detection. Astrocytoma U373MG cells (provided by Professor
Songya Lv, State Key Laboratory of Virology, College of Life
Sciences, Wuhan University, Wuhan, China, source American Type
Culture Collection, Manassas, VA, USA), human embryonic lung
fibroblast MRC-5 cells, and 293T cells (Shanghai Institute of
Biochemistry and Cell Biology) were cultured in Dulbecco's modified
Eagle's medium (DMEM; GE Healthcare Life Sciences, Logan, UT, USA)
containing 10% fetal calf serum (GE Healthcare Life Sciences), 1%
penicillin and 1% streptomycin. The U373MG cells may be
contaminated/mis-identified and are likely derived from U-251MG
cells. To generate a U373MG cell line that transiently expressed
CELF5 (U373-S) and control cells, pDsRed-C1-CELF5 and an empty
vector pDsRed-C1 were transfected into U373 cells using
Lipofectamine® LTX with Plus™ Reagent,
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). The two cell lines were seeded at a density of
1×106 in 6-well plates prior transfection. The levels of
CELF5 in individual cell clones were determined by western blot
analysis.
Yeast two-hybrid screening
Yeast two-hybrid experiments were performed
according to the manufacturer's protocol using the Matchmaker GAL4
Two-Hybrid System 3 (Clontech Laboratories, Inc.). Plasmid
pGBKT7-UL141 containing the GAL4 DNA-binding domain was transformed
into Saccharomyces cerevisiae AH109 (Clontech Laboratories,
Inc.,) containing a human fetal brain cDNA library, pACT2-cDNA. The
human fetal brain cDNA library was provided by Professor Gengfu
Xiao from the State Key Laboratory of Virology, College of Life
Sciences, Wuhan University (Wuhan, China), which was cloned into
pACT2 (Clontech Laboratories, Inc.) by electroporation (Bio-Rad
Laboratories, Inc.) according to the manufacturer's protocol in
BL21 (DE3) cells (Tiangen Biotech Co., Ltd., Beijing, China) at a
density of 6×107 cells/ml in logarithmic growth period.
Positive colonies were selected on synthetic dropout medium
(Clontech Laboratories, Inc.,) lacking tryptophan, leucine, adenine
and histidine and confirmed by detecting α-galactosidase (Clontech
Laboratories, Inc.) activity as previously described (14). The cells that produced blue signals
on a colony lift assay were identified and defined as positive
colonies. Subsequently, plasmids containing the coding sequence for
UL141-interacting partners (termed pACT2-cDNA) were extracted from
positive colonies and co-transformed into AH109 with the
pGBKT7-UL141 in order to validate the interaction. Human gene
sequences in pACT2-cDNA were determined from these selected
colonies with the pACT2 vector sequencing primer
(5′-AATACCACTACAATGGAT-3′) and matched with the Blast network
service at the National Center for Biotechnology Information
(http://www.ncbi.nlm.nih.gov/blast).
GST pull-down and western blot
analysis
GST pull-down experiments were performed using the
MagneGST™ Pull-down system following the manufacturer's
protocol (Promega Corporation, Madison, WI, USA). c-Myc-labeled
UL141 protein was synthesized as a prey protein in vitro
using pGBKT7-UL141 in a TNT (quick coupled
transcription/translation reaction) T7 Quick reaction (Promega
Corporation) according to the manufacturer's protocol (15). GST fusion protein (GST-CELE5)
expression was induced in E. coli BL21 (DE3) with 5
mmol/lisopropyl β-D-thiogalactoside (Clontech Laboratories, Inc.).
After allowing 4 h of post-induction, cell lysates were harvested
to collect the bait protein GST-CELF5. GST-CELF5 was immobilized
onto MagneGST particles and incubated with the c-Myc-labeled UL141
protein at 25°C for 1.5 h on a rotating platform. After washing
three times with a buffer, proteins binding the MagneGST particles
were eluted and solubilized in SDS sample buffer (Beyotime
Institute of Biotechnology).
To perform western blot analysis, denatured proteins
were subjected to electrophoresis on a 12% SDS-polyacrylamide
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and transferred
electronically onto polyvinylidene fluoride membranes
(Sigma-Aldrich, Merck Millipore, Darmstadt, Germany). Membranes
were blocked in 5% non-fat dried milk in tris-buffered saline (TBS)
and 0.05% Tween-20. Detection of tagged proteins was performed
following incubation overnight at 4°C with 1:1,000-diluted mouse
anti-Myc or 1:500-diluted mouse anti-GST primary antibodies
followed by incubation for 2 h at room temperature with
1:2,000-diluted goat anti-mouse IgG secondary antibody conjugated
with HRP (Beyotime Institute of Biotechnology). Proteins were
subsequently visualized using enhanced chemiluminescence detection
reagent kits (Thermo Fisher Scientific, Inc.) and signals were
detected with the Molecular Imager ChemiDoc XRS system (Bio-Rad
Laboratories, Inc.).
Co-IP and western blot analysis
293T cells at a density of 2×106
cells/plate were cultured in DMEM containing 10% fetal calf serum
in 60 mm plates prior transfection. At ~90% confluence, cells were
co-transfected with pCMV-Myc-UL141 and pCMV-HA-CELF5 with using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, cell lysates were
harvested and proteins were extracted with M-PER™
Mammalian Protein Extraction Reagent (Thermo Fisher Scientific,
Inc.). Co-immunoprecipitation experiments were performed using the
ProFound mammalian c-Myc tag
immunoprecipitation/co-immunoprecipitation (IP/co-IP) and HA tag
IP/co-IP kits, following the manufacturer's protocols (cat. nos.
23620 and 23610 respectively; Pierce; Thermo Fisher Scientific,
Inc.). Myc-UL141 and HA-CELF5 were detected by western blotting as
aforementioned using mouse anti-Myc or rabbit anti-HA antibodies
(cat. no. 71–5500; 1:100; Thermo Fisher Scientific, Inc.) incubated
overnight at 4°C and corresponding peroxidase-conjugated goat
anti-mouse and goat anti-rabbit (cat. no. A0208; 1:2,000; Beyotime
Institute of Biotechnology) IgG secondary antibodies incubated at
room temperature for 2 h. The immunoreactive bands were visualized
by the diaminobenzidine coloration method. The semi-quantitation of
proteins was surveyed with a Tanon GIS gel imager System.
Immunofluorescent co-localization
293T cells (2×106) were cultured on glass
coverslips in DMEM containing 10% fetal calf serum. They were
seeded into a 35-mm confocal microscope dish (Nest Biotechnology
Co., Ltd., Jiangsu, China) 24 h prior to transfection. At ~75%
confluence, cells were co-transfected with 4 µg pEGFP-UL141 and 4
µg pDsRed-CELF5 using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). At 48 h post-transfection, cells
were subjected to DAPI staining (Invitrogen; Thermo Fisher
Scientific, Inc.) for 5 min at room temperature, and the expression
levels of EGFP-UL141 and DsRed-CELF5 were detected using a TCS SP2
Leica laser scanning confocal microscope (Nikon Eclipse C1 Plus;
Nikon Corporation, Tokyo, Japan) with a 488 nm and a 543 nm
excitation beam.
Transfection small interfering (si)RNA
into cells
U373-S cells (2×105 cells/well) were
seeded in 12-well plates and transfected with anti-CELF5 siRNA
(CELF5-siRNA; Ambion; Thermo Fisher Scientific, Inc.) and control
siRNA (C-siRNA; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
For each well, 3 µl of 20 nM siRNA and 3 µl Lipofectamine RNAi MAX
(Invitrogen; Thermo Fisher Scientific, Inc.) were diluted in 50 and
12 µl Opti-MEM medium (Invitrogen; Thermo Fisher Scientific, Inc.),
respectively. Following a 5 min incubation at room temperature,
both solutions were mixed. After 20 min, 400 µl pre-warmed Opti-MEM
medium (Invitrogen; Thermo Fisher Scientific, Inc.) was added to
each transfection reaction mixture, which was subsequently added to
cells. At 10 h post-transfection, siRNA-containing medium was
removed, and cells were washed and incubated with 10% DMEM. The
transfection procedure was subsequently repeated. Cells were either
prepared for analysis by western blotting or HCMV infection 48 h
after the second transfection.
Viral infection and HCMV DNA level
analysis
To assay viral growth, the cells (1×105)
were infected with HCMV at a multiplicity of infection=3,
subsequently the cells and medium were collected at 5 days
post-infection and viral stocks were prepared. The titers of the
viral stocks were determined by infecting 1×105 human
foreskin fibroblasts and the number of plaques 10–14 days following
the infection were counted. The values obtained were averages from
triplicate experiments. The levels of intracellular viral DNA were
quantified by amplifying the HCMV UL83 sequence with its primers
(forward, 5′-GTCAGCGTTCGTGTTTCCCA-3′ and reverse,
5′-GGGACACAACACCGTAAAGC-3′) using quantitative PCR (qPCR). The
number of viral genomes was normalized to the number of cellular
copies of β-actin as determined using β-actin primers (forward,
5′-CGGAACCGCTCATTGCC-3′ and reverse, 5′-ACCCACACTGTGCCCATCTA-3′).
Amplification was performed in a 25 µl reaction mixture, which
contained 4 µl DNA extract, 12.5 µl Power SYBR-Green PCR master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and 0.5 µl
each primer at 10 µM using an ABI 7300 device (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Thermal cycling conditions were as
follows: 95°C for 10 min, 40 cycles of 95°C for 15 sec and 60°C for
1 min, 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. qPCR
results were derived from three independent experiments using the
2−ΔΔCq method (16).
Statistical analysis
Statistical significance between different groups
was determined by one-way analysis of variance test with post hoc
Bonferroni correction. All data were collected and analyzed with
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA). GraphPad Prism
version 5 (GraphPad Software, Inc., La Jolla, CA, USA) was used to
produce the correlation graphs. P<0.05 was considered to
indicate a statistically significant difference.
Results
Interaction between UL141 and CELF5
identified by yeast two-hybrid analysis
Yeast two-hybrid analysis was used to identify
potential protein partners that interact with HCMV UL141. The
candidate, PACT2-CELF5, was screened to examine its interaction
with UL141 and the nucleotide sequence of pACT2-CELF5 was 100%
identical to that of the human CELF5 sequence in NCBI.
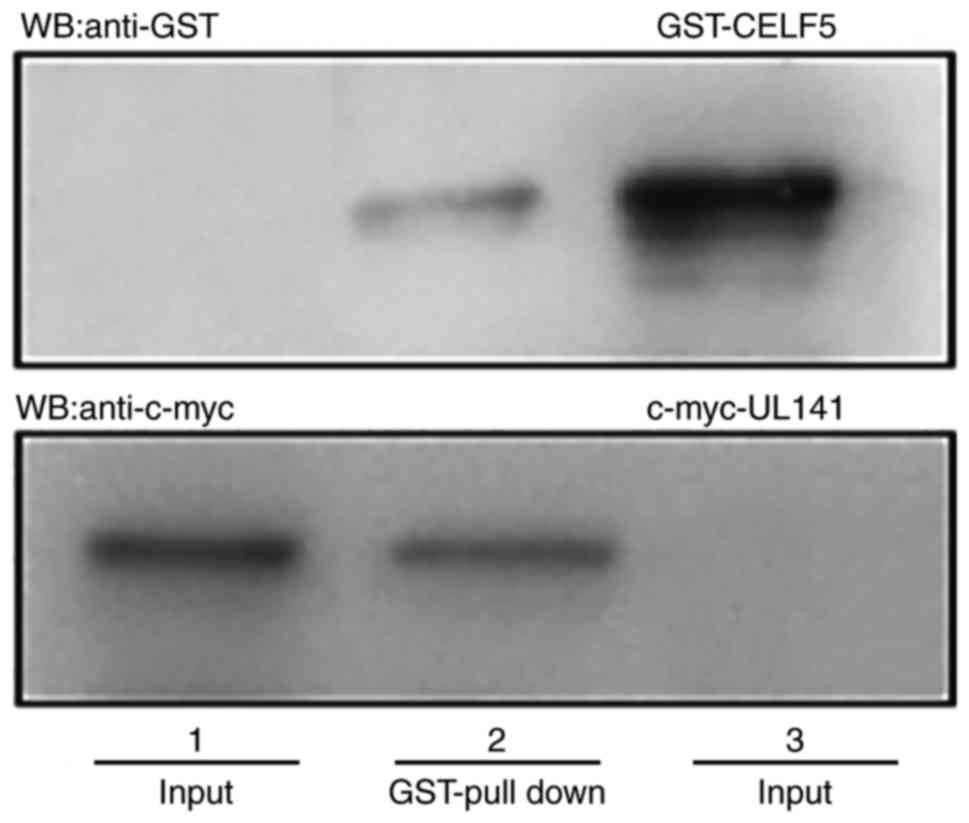
Interaction between HCMV pUL141 and
CELF5 identified by GST pull-down assay
GST pull-down experiments were performed in order to
validate whether there is a direct interaction between HCMV pUL141
and CELF5 in vitro. In these experiments, GST-tagged CELF5
was used as a bait protein and c-Myc-tagged pUL141 as the prey
protein. UL141 and CELF5 were detected as proteins of ~37 and 50
kDa, respectively by western blotting (Fig. 1). According to these findings, the
UL141 protein has the ability to interact with the CELF5 protein in
the absence of another medium in vitro.
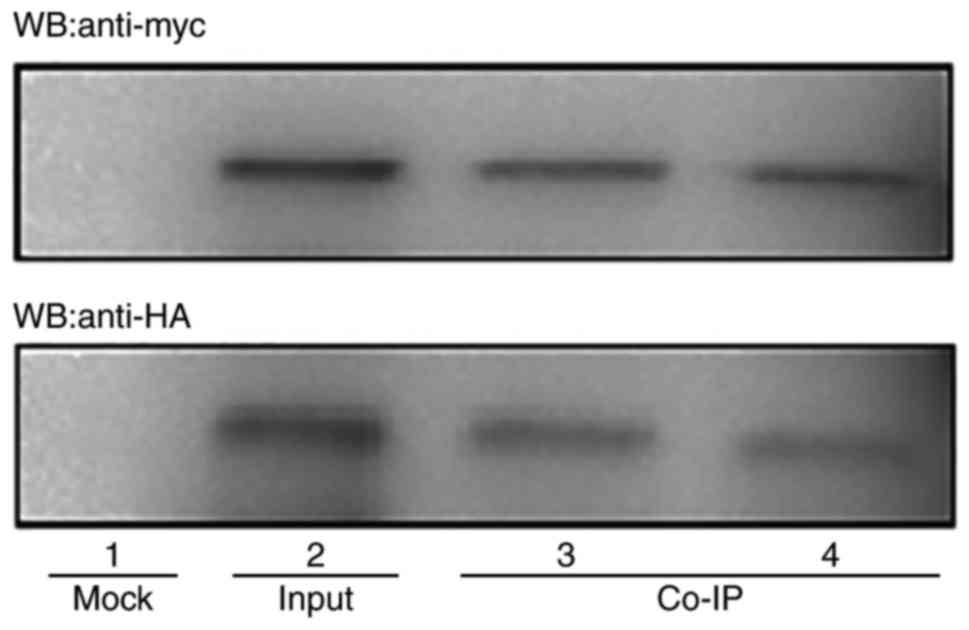
Identification of the interaction
between HCMV pUL141 and CELF5 in human cells by co-IP
In order to confirm the identified interaction
between pUL141 and CELF5 in human cells, a co-IP assay was
performed. In these experiments, the pCMV-Myc-UL141 and
pCMV-HA-CELF5 plasmids, which expressed fusion proteins with an
amino-terminal Myc or HA epitope tag, respectively were constructed
by cloning the coding sequences for UL141 and CELF5 into the
expression vectors pCMV-Myc and pCMV-HA, respectively. 293 cells
were transfected with the expression constructs and were harvested
after 48 h. Protein lysates from the cells were initially
immunoprecipitated with anti-Myc or anti-HA and subsequently
immunoblotted with antibodies against the Myc and HA epitope tags.
HCMV UL141 and CELF5 were detected as proteins of 37 and 25 kDa
with anti-c-Myc and anti-HA antibodies, respectively, by western
blotting (Fig. 2). These findings
confirm the interaction between viral UL141 and host CELF5 proteins
in human cells.
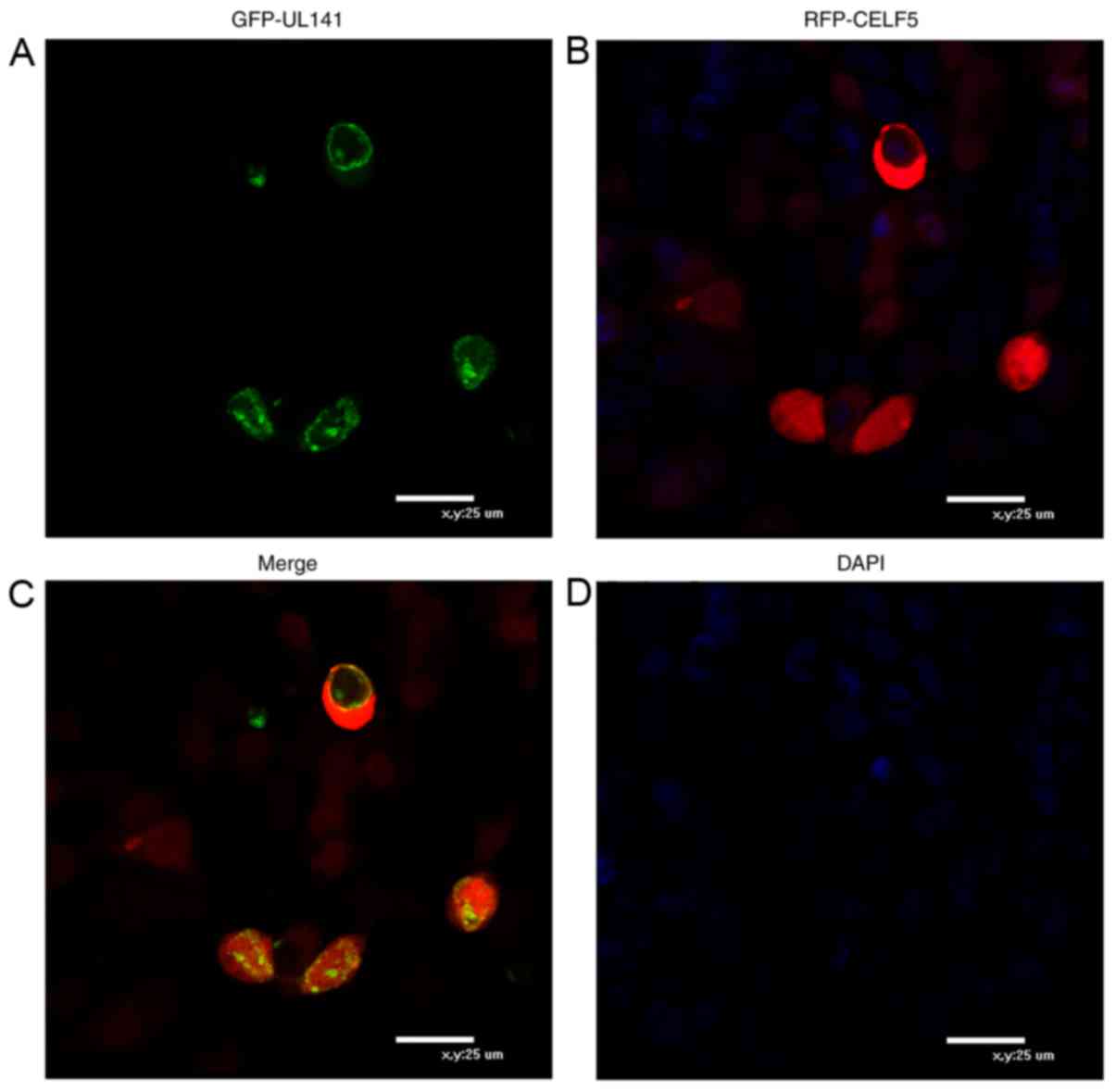
Identification of pUL141 and CELF5
co-localization in human cells by fluorescence confocal
microscopy
If UL141 is associated with CELF5 in cells, it was
expected that these proteins would localize within the same
cellular compartments. To determine whether UL141 co-localizes with
CELF5, 293 cells were transfected with pEGFP-UL141 and/or
pDsRed-CELF5 plasmids. The cellular localization of those expressed
proteins was investigated using fluorescence confocal microscopy.
In cells transfected with construct GFP-UL141 alone, UL141 was
found to be primarily expressed in the cytoplasm (Fig. 3A), whereas RPF-CELF5 was primarily
localized in the cytoplasm and nucleus of 293 cells (Fig. 3B). Co-localization in the cytoplasm
of 293 cells was observed for these proteins in a diffuse pattern
and within the nucleus in a mottled pattern (Fig. 3C). At 48 h post transfection,
nuclear DNA was stained with DAPI (blue image; Fig. 3D).
Effect of up and downregulation of
CELF5 expression on HCMV DNA synthesis and lytic infection
In order to investigate the effect of CELF5 on HCMV
DNA replication, U373MG cells were transfected with pCMV-HA-CELF5
to induce overexpression of exogenous CELF5 (U373-Celf5) or
transfected with siRNA-CELF5 to downregulate the expression of
endogenous CELF5 (Celf5-siRNA). Different cells, including U373,
U373-Celf5, Celf5-siRNA, and C-siRNA groups were infected with HCMV
and then harvested 48 h post-infection. CELF5 expression levels
were confirmed by western blot analysis. The expression of
exogenous CELF5 in U373-Celf5 cells was higher compared with other
cells (Fig. 4A). The expression
level of the endogenous CELF5 protein in Celf5-siRNA cells
collected 48 h post-HCMV infection was reduced by >60% (data not
shown) when compared with the cells transfected with control siRNA
(C-siRNA; Fig. 4A).
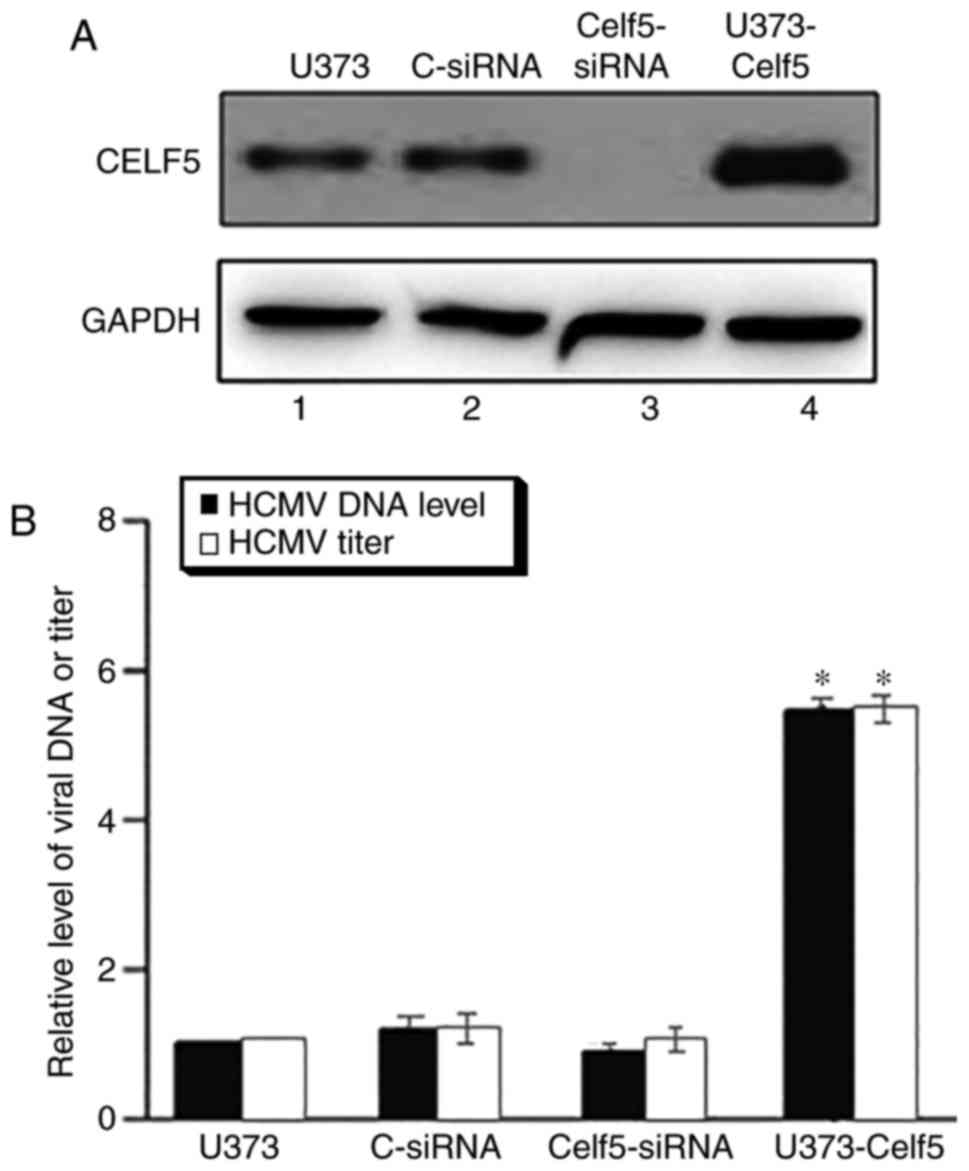 | Figure 4.CELF5 levels affect HCMV DNA synthesis
by quantitative polymerase chain reaction. U373MG cells were
transfected with C-siRNA, CELF5-siRNA, or U373-CELF5. (A) Western
blot analysis of the levels of CELF5 in the parental U373 cells,
U373 cells that were transfected with C-siRNA or Celf5-siRNA, cells
overexpressing exogenous CELF5. The expression of GAPDH was used as
the internal reference. At 48 h post-transfection, cells were
infected with HCMV, multiplicity of infection=3. CELF5 protein
expression level was detected by western blot analysis with an
anti-CELF5 antibody. (B) Total infection cultures were collected 4
days post-infection to assay the level of viral production. The
values of the relative HCMV DNA levels and HCMV titer represent the
ratios of the levels of viral DNA in different cells, to that in
the parental U373MG cells. Data are presented as the mean ±
standard deviation of two independent experiments performed in
triplicate. *P<0.001 vs. Celf5-siRNA and vs. C-siRNA.
CELF5-siRNA and control U373 cells. C, control; siRNA, small
interfering RNA; U373-CELF5, pCMV-HA-CELF5; HCMV, human
cytomegalovirus. CELF5, CUGBP Elav-like family member 5. |
To determine the effect of altered CELF5 expression
on HCMV infection, total intracellular DNA from different cell
types was extracted 48 h post-infection and the level of viral DNA
synthesis was analyzed. The number of viral genomes present in each
sample was detected by qPCR. Cellular β-actin was used as an
internal reference to normalize the copy number of the viral UL83
sequence. Viral DNA copy numbers (UL83/β-actin) were significantly
increased by ~3-fold in the U373-Celf5 cells when compared with
C-siRNA (P<0.001) and parental U373MG cells (P<0.001) at 48 h
post-infection (Fig. 4B). These
findings suggest that upregulating CELF5 expression in
HCMV-infected cells may promote HCMV DNA synthesis. However, no
significant difference was identified in the number of viral DNA
copies (UL83/β-actin) among cells treated with anti-CELF5-siRNA
(Celf5-siRNA) and control-siRNA or parental U373MG cells. This may
be due to the low expression level of CELF5 in U373MG cells during
viral infection. The interaction between HCMV pUL141 and CELF5 is
limited in infected cells; therefore, interference in CELF5
expression levels may not have a significant effect on HCMV
replication in U373MG cells.
Discussion
Previous studies on HCMV infection in newborns and
infants, the patient's clinical symptoms, diagnosis, treatment and
the influence of HCMV infection on immune function have attracted
widespread attention (17–19). As with other herpes viruses, HCMV
may establish a lifelong relationship with the host through active
and latent infection. Previous studies demonstrated that in order
to be able to survive and maintain a latent infection status for a
long time, the virus encodes a variety of immune escape-associated
proteins to target different antigen-presenting molecules, such as
major histocompatibility complex (MHC)-I, MHC-II and inhibitory and
activating receptors on NK cells following HCMV infection (20,21).
For example, HCMV proteins, such as US2, US3, US6, US10, US11, UL82
and UL83 may all suppress the expression of MHC-I and MHC-II on the
cell surface through different mechanisms and thus regulate antigen
presentation (20). UL141 promotes
HCMV survival by blocking CD155, CD122, TRAIL-R1 and TRAIL-R2
surface expression, subsequently inhibiting apoptosis and NK
cell-mediated host cell cytotoxicity (22,23).
These actions may be mediated by interactions between viral and
human proteins. Understanding these interactions is crucial for the
identification of the mechanisms of viral latent and proliferative
infections.
In the present study, a yeast two-hybrid screen was
used to identify novel human proteins that may interact with the
HCMV UL141 protein. Preliminary findings indicated 6 candidates
(Table II), one of which was
CELF5. CELF5 is a member of the CELF or Bruno-like family and is
involved in the control of mRNA splicing and translation. Human
CELF5 expression is primarily located in the fore-, mid- and
hindbrain, but is low in the corpus callosum and pons (24). However, its specific function
remains to be determined. GST pull-down analysis indicated a
physical interaction between CELF5 and HCMV pUL141 proteins
(Fig. 1). A co-IP assay was used
to validate this interaction in a cellular milieu. Myc-tagged UL141
proteins specifically immunoprecipitated with HA-tagged CELF5
(Fig. 2). Co-localization of CELF5
and UL141 protein was confirmed by confocal microscopy analysis.
These proteins were primarily co-localized in the cytoplasm of 293
cells (Fig. 3). To the best of our
knowledge, the present study is the first to report of a physical
interaction between CELF5 and any HCMV protein.
 | Table II.Homologous genes that interacted with
HCMV UL141 protein were analyzed by comparing the gene sequences
with the human genome from Genebank. |
Table II.
Homologous genes that interacted with
HCMV UL141 protein were analyzed by comparing the gene sequences
with the human genome from Genebank.
| Homologous
genes | Homology (%) |
|---|
| Homo sapiens
chromodomain helicase DNA binding protein 3 (CHD3) | 98 |
| Homo sapiens
DnaJ (Hsp40) homolog (DNAJB1) | 98 |
| Homo sapiens
dachshund homolog 1 (DACH1) | 96 |
| Homo sapiens
transducin-like enhancer of split1 (TLE1) | 100 |
| Homo sapiens
wilms tumor 1 interacting protein (WTIP) | 100 |
| Homo sapiens
CUGBP, Elav-like family member 5 (CELF5) | 100 |
The current study also demonstrated that an increase
in CELF5 expression in HCMV-infected cells promoted HCMV DNA
synthesis. The interaction between UL141 and CELF5 may be involved
in modulating viral DNA synthesis and progeny production. The role
of UL141 in gene expression has been previously suggested to be
that UL141 promotes HCMV survival by inhibiting apoptosis and NK
cell-mediated host cell cytotoxicity (7). It may be the reason for the findings
observed in the current study. Conversely, reducing CELF5 mRNA
expression via specific siRNA did not affect viral DNA copy number
or titer in HCMV-infected cells (Fig.
4). This phenomenon may be due to the low basal expression
level of CELF5 in U373MG cells during viral infection.
The CELF protein family contains 6 members termed
CELF1 to CELF6. CELF proteins have 3 conserved RNA recognition
motifs (RNP-1)-RRM1, RRM2 in the N-terminus of the protein, and
RRM3 in the C-terminus. RRM3 is separated from RRM1 and RRM2 by a
non-conserved or divergent domain that is unique in each family
member (25). The CELF family of
RNA binding proteins regulate several steps of RNA processing in
the nucleus and cytoplasm, including pre-mRNA alternative splicing,
C to U RNA editing, deacetylation, mRNA decay and translation. CELF
proteins have been have been previously revealed to have roles in
gametogenesis and early embryonic development, heart and skeletal
muscle function, and neurosynaptic transmission in vivo
(26). In a study of pathogenesis
of nervous system diseases, CLEF RNA processing was demonstrated to
have a role in a variety of nervous system diseases, such as
ankylosing muscular dystrophy, amyotrophic lateral sclerosis,
ataxia and multiple neurofibromatosis type 1 (NF-1). CELF proteins
are involved regulating a neurofibromin exon that may be important
for regulating higher cognitive functions (27). Previous studies revealed that
overexpression and siRNA knockdown of CELF1-5 in cell transfection
experiments lead to a decrease or increase of NF1 exon 23a
inclusion, respectively (28,29).
CELF1 has an important role in promoting the responses of immune
cells to external stimulation and coordinating gene networks
involved in cell activation (30).
CELF5 has been previously described as a refined differentially
expressed gene in a cross-species comparison including
neurologically normal humans, rats and mice, and its transcript it
was enriched in axons and synapses (31). It is possible that different CELF5
levels may impact upon the anatomy and physiology of motor neurons.
However, CELF5-associated research is limited at the mRNA level,
and investigation of the host protein CELF5 also uncommon.
CELF proteins exhibit differential localizations
that may be associated with specific neuronal subtype,
developmental stage or pathogenic condition. CELF1 has been
primarily identified in the nuclei of cultured neurons (32), whereas CELF5 was located
predominantly in the cytoplasm (33). This is in line with the findings of
the current study, that CELF5 and UL141 proteins are primarily
co-localized in the cytoplasm of 293 cells. Although few functional
models of CELF5 have been reported, CELF5 has been hypothesized to
have an important role in neural development and function from its
embryonic and postnatal expression patterns (25). It may also be associated with
central nervous system damage (25,28).
However, a specific functional mechanism remains to be
elucidated.
CELF proteins are also associated with apoptosis.
The present study determined that CELF5 may promote HCMV
replication, which is a key step during persistent infections. It
has been previously hypothesized that CELF5 as a member of CELF
family proteins may also inhibit apoptotic factors. For example,
CELF1 overexpression may inhibit apoptosis in tumor cells and its
downregulation may lead to sensitivity in tumor cells, resulting in
death receptor-mediated apoptosis (31). Therefore, the current study
suggests that future should aim to identify the impact of CELF5
expression in specific cellular pathways associated with nervous
system damage caused by HCMV. However, reducing the expression of
endogenous CELF5 had no significant effect on HCMV replication in
U373MG cells, which may be associated with low endogenous
expression of CELF5 in U373 cells. Therefore, its specific
mechanism of action requires further investigation. The current
findings suggest that the interaction between UL141 and CELF5 may
be involved in the modulation of viral DNA synthesis and progeny
production. Therefore, CELF5 may represent a possible mechanism for
regulating HCMV genomic DNA synthesis, which is a key step during
HCMV infection leading to neurological disease. Future studies are
required to elucidate the mechanism of the interaction between
pUL141 and CELF5 in the regulation of HCMV DNA replication.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant no. 81171581) and the
Outstanding Scientific Fund of Shengjing Hospital.
References
|
1
|
Manicklal S, Emery VC, Lazzarotto T,
Boppana SB and Gupta RK: The ‘silent’ global burden of congenital
cytomegalovirus. Clin Microbiol Rev. 26:86–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang ZR, Yu LP, Yang XC, Zhang F, Chen
YR, Feng F, Qian XS and Cai J: Human cytomegalovirus linked to
stroke in a Chinese population. CNS Neurosci Ther. 18:457–460.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dziurzynski K, Chang SM, Heimberger AB,
Kalejta RF, McGregor Dallas SR, Smit M, Soroceanu L and Cobbs CS:
HCMV and Gliomas Symposium: Consensus on the role of human
cytomegalovirus in glioblastoma. Neuro Oncol. 14:246–255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sinzger C, Digel M and Jahn G:
Cytomegalovirus cell tropism. Curr Top Microbiol Immunol.
325:63–83. 2008.PubMed/NCBI
|
|
5
|
Gredmark-Russ S and Söderberg-Nauclér C:
Dendritic cell biology in human cytomegalovirus infection and the
clinical consequences for host immunity and pathology. Virulence.
3:621–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dolan A, Cunningham C, Hector RD,
Hassan-Walker AF, Lee L, Addison C, Dargan DJ, McGeoch DJ, Gatherer
D, Emery VC, et al: Genetic content of wild-type human
cytomegalovirus. J Gen Virol. 85:1301–1312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomasec P, Wang EC, Davison AJ, Vojtesek
B, Armstrong M, Griffin C, McSharry BP, Morris RJ, Llewellyn-Lacey
S, Rickards C, et al: Downregulation of natural killer
cell-activating ligand CD155 by human cytomegalovirus UL141. Nat
Immunol. 6:181–188. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prod'homme V, Sugrue DM, Stanton RJ,
Nomoto A, Davies J, Rickards CR, Cochrane D, Moore M, Wilkinson GW
and Tomasec P: Human cytomegalovirus UL141 promotes efficient
downregulation of the natural killer cell activating ligand CD112.
J Gen Virol. 91:2034–2039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nemčovičová I, Benedict CA and Zajonc DM:
Structure of human cytomegalovirus UL141 binding to TRAIL-R2
reveals novel, non-canonical death receptor interactions. PLoS
Pathog. 9:e10032242013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu JL, Van den Boomen DJ, Tomasec P,
Weekes MP, Antrobus R, Stanton RJ, Ruckova E, Sugrue D, Wilkie GS,
Davison AJ, et al: Plasma membrane profiling defines an expanded
class of cell surface proteins selectively targeted for degradation
by HCMV US2 in cooperation with UL141. PLoS Pathog.
11:e10048112015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murrell I, Wilkie GS, Davison AJ, Statkute
E, Fielding CA, Tomasec P, Wilkinson GW and Stanton RJ: Genetic
stability of bacterial artificial chromosome-derived human
cytomegalovirus during culture in vitro. J Virol. 90:3929–3943.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Ren G, Cui X, Lu Z, Ma Y, Qi Y,
Huang Y, Liu Z, Sun Z and Ruan Q: Human cytomegalovirus RL13
protein interacts with host NUDT14 protein affecting viral DNA
replication. Mol Med Rep. 13:2167–2174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ross SA and Boppana SB: Congenital
cytomegalovirus infection: Outcome and diagnosis. Semin Pediatr
Infect Dis. 16:44–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang G, Ren G, Cui X, Lu Z, Ma Y, Qi Y,
Huang Y, Liu Z, Sun Z and Ruan Q: Host protein Snapin interacts
with human cytomegalovirus pUL130 and affects viral DNA
replication. J Biosci. 41:173–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McMahon TP and Anders DG: Interactions
between human cytomegalovirus helicase-primase proteins. Virus Res.
86:39–52. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zavattoni M, Rustico M, Tassis B, Lombardi
G, Furione M, Piralla A and Baldanti F: Risk of congenital disease
in 46 infected fetuses according to gestational age of primary
human cytomegalovirus infection in the mother. J Med Virol.
88:120–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu LL, Mou WF, Yang L and Wang YC:
Application of HCMV DNA detection in infants' blood, urine and
mothers' breast milk in the diagnosis of HCMV infection among
infants. Zhongguo Dang Dai Er Ke Za Zhi. 15:748–750. 2013.(In
Chinese). PubMed/NCBI
|
|
19
|
Choudhary A, Pati SK, Patro RK, Deorari AK
and Dar L: Comparison of conventional, immunological and molecular
techniques for the diagnosis of symptomatic congenital human
cytomegalovirus infection in neonates and infants. Indian J Med
Microbiol. 33 Suppl:15–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noriega V, Redmann V, Gardner T and
Tortorella D: Diverse immune evasion strategies by human
cytomegalovirus. Immunol Res. 54:140–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilkinson GW, Tomasec P, Stanton RJ,
Armstrong M, Prod'homme V, Aicheler R, McSharry BP, Rickards CR,
Cochrane D, Llewellyn-Lacey S, et al: Modulation of natural killer
cells by human cytomegalovirus. J Clin Virol. 41:206–212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith W, Tomasec P, Aicheler R, Loewendorf
A, Nemčovičová I, Wang EC, Stanton RJ, Macauley M, Norris P, Willen
L, et al: Human cytomegalovirus glycoprotein UL141 targets the
TRAIL death receptors to thwart host innate antiviral defenses.
Cell Host Microbe. 13:324–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
David R: Immune evasion: UL141 keeps HCMV
in charge. Nat Rev Microbiol. 11:2972013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loria PM, Duke A, Rand JB and Hobert O:
Two neuronal, nuclear-localized RNA binding proteins involved in
synaptic transmission. Curr Biol. 13:1317–1323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ladd AN, Charlet N and Cooper TA: The CELF
family of RNA binding proteins is implicated in cell-specific and
developmentally regulated alternative splicing. Mol Cell Biol.
21:1285–1296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dasgupta T and Ladd AN: The importance of
CELF control: Molecular and biological roles of the CUG-BP,
Elav-like family of RNA-binding proteins. Wiley Interdiscip Rev
RNA. 3:104–121. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gallo JM and Spickett C: The role of CELF
proteins in neurological disorders. RNA Biol. 7:474–479. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barron VA, Zhu H, Hinman MN, Ladd AN and
Lou H: The neurofibromatosis type I pre-mRNA is a novel target of
CELF protein-mediated splicing regulation. Nucleic Acids Res.
38:253–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalsotra A, Xiao X, Ward AJ, Castle JC,
Johnson JM, Burge CB and Cooper TA: A postnatal switch of CELF and
MBNL proteins reprograms alternative splicing in the developing
heart. Proc Natl Acad Sci USA. 105:pp. 20333–20338. 2008;
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vlasova-St Louis I, Dickson AM, Bohjanen
PR and Wilusz CJ: CELFish ways to modulate mRNA decay. Biochim
Biophys Acta. 1829:695–707. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kline RA, Kaifer KA, Osman EY, Carella F,
Tiberi A, Ross J, Pennetta G, Lorson CL and Murray LM: Comparison
of independent screens on differentially vulnerable motor neurons
reveals alpha-synuclein as a common modifier in motor neuron
diseases. PLoS Genet. 13:e10066802017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anderson KN, Baban D, Oliver PL, Potter A
and Davies KE: Expression profiling in spinal muscular atrophy
reveals an RNA binding protein deficit. Neuromuscul Disord.
14:711–722. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu J, Li C, Zhao S and Mao B: Differential
expression of the Brunol/CELF family genes during Xenopus laevis
early development. Int J Dev Biol. 54:209–214. 2010. View Article : Google Scholar : PubMed/NCBI
|