Introduction
Obesity is a common trophic disorder that may be
attributed to an imbalance of energy metabolism. Long-term,
high-calorie food intake overwhelms energy expenditure, leading to
excessive adipose accumulation in the body (1). Adipose tissue was originally
considered a passive reservoir for energy storage, mechanical and
heat insulation, and a regulator of thermogenesis (2); however, it is gradually becoming
recognized that it can synthesize and secrete a number of bioactive
peptides (known as adipokines) in an autocrine or paracrine manner
(3). Adipokines serve a crucial
role in regulating glucose and lipid metabolism, energy
homeostasis, insulin sensitivity, immunity and inflammation
(4). It is now widely accepted
that obesity is highly associated with chronic, low-grade systemic
inflammation in the body (5). A
large body of evidence has indicated that high-fat diets (HFDs) are
a major cause of metabolic disorders, such as obesity and
obesity-associated diseases (6–8).
Over the past few decades, our diets have altered
substantially, and in modern society the consumption of fatty foods
has increased greatly. An epidemiological report revealed that a
HFD causes hyperlipemia and is associated with detrimental effects
on the brain (9). In addition,
brain disorders induced by a HFD are usually accompanied by the
onset of neuroinflammation, which is a key component in the
progression of a number of neurological and neurodegenerative
diseases (10). A recent study
demonstrated that mice fed a HFD exhibited a greater susceptibility
to kainic acid-induced oxidative stress and neuroinflammation in
the hippocampus, and that neuroinflammation may contribute to
neuronal death following brain injury (11). Reducing the overexpression of
inflammatory cytokines in mouse brains prevents HFD-associated
blood-brain barrier disruption and glial cell activation (12). Therefore, regulating the effects of
neuroinflammation on the brain may be beneficial in decreasing the
rates of neurodegenerative diseases, including Alzheimer's disease,
Parkinson's disease and multiple sclerosis (13–15).
However, there is a lack of effective treatments for
obesity-associated neuroinflammation in the brain, which may have
wide-ranging consequences to public health (16,17).
Therefore, the identification of effective preventative and
therapeutic strategies are required.
A number of recent studies have focused on the
pharmacological properties of natural products such as
anthocyanins, which are a group of naturally occurring polyphenol
compounds that are widely distributed in flowers, fruits and
vegetables and are responsible for their intense color (18,19).
Anthocyanins have been reported to exhibit several potent
biological properties, including anti-inflammation (20), anti-oxidation (21), anti-mutagen and anti-tumor
(22). One natural anthocyanin
pigment, purple sweet potato color (PSPC; which is derived from the
storage roots of the purple sweet potato) has received increased
attention owing to its unique color, and its nutritional and
health-promoting benefits (23).
High-performance liquid chromatography (HPLC) analysis has revealed
that the main components of PSPC are acetylated cyanidin acyl
glucosides and peonidin acyl glucosides (>90%), and the
remaining components are other flavonoids (24). Compared with extracted pigments
from other plants, PSPC is more stable and is directly absorbed and
distributed in the blood (25).
Therefore, it may be a suitable candidate for further development
of physiologically functional food factors.
Previous studies have demonstrated that PSPC
possesses various biological activities, including antioxidant,
anti-inflammatory and neuroprotective effects (26–30).
PSPC has been demonstrated to ameliorate d-galactose-mediated
cognition deficits or liver injury by improving oxidative damage
and inflammatory responses in the brain or liver of aging mice
(26,27). In addition, PSPC was reported to
improve HFD-induced obesity in mice by reducing inflammation in the
liver by blocking the adenosine monophosphate-activated protein
kinase (AMPK) signaling pathway (28,31)
or by increasing the sensitivity of the liver to insulin by
blocking nuclear factor (NF)-κB signaling (29). Other recent studies have revealed
that PSPC treatment improved HFD-induced kidney damage,
d-galactose-induced premature endothelial senescence and
endothelial dysfunction by inhibiting oxidative stress-mediated
NACHT, LRR and PYD domains-containing protein 3 inflammasome
activation in mice (30,32). These results suggested that by
investigating the protective mechanisms of PSPC treatment in the
metabolic tissues associated with obesity, such as the brain, the
development of treatments for obesity may be accelerated.
Multiple pharmacological effects of PSPC have been
studied; however, the detailed effects of PSPC treatment on
HFD-induced neuroinflammation have not yet been identified. The aim
of the present study was to investigate whether PSPC treatment was
able to improve spontaneous behavior, learning and memory functions
in HFD-challenged mice. In addition, the present study aimed to
determine whether PSPC has a protective role in HFD-associated
neuroinflammation in the brain and to provide novel insight into
the mechanisms of action.
Materials and methods
Reagents and drugs
PSPC (purity, >90%) was purchased from Qingdao
Pengyuan Natural Pigment Research Institute (Qingdao, China).
Unless otherwise noted, all reagents and drugs were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Bioreagents for
western blotting were purchased from Promega Corporation (Madison,
WI, USA) or Cell Signaling Technology, Inc. (Danvers, MA, USA).
Animal procedures
A total of 60 male C57BL/6 mice (age, 8 weeks;
weight, 20–24 g; Jackson Laboratory, Ben Harbor, ME, USA) were used
in the present study. Mice were housed in a ventilated and
temperature-controlled room (humidity, 60%; temperature, 23±1°C)
with a 12 h light-dark cycle and free access to rodent chow and tap
water; 3 mice were housed per cage. Following acclimation to the
laboratory conditions, mice were randomly divided into the
following 4 groups (15 mice/group): i) Control group, which were
fed a normal diet containing 10 kcal% fat, 20 kcal% protein and 70
kcal% carbohydrate (cat. no. D12450B; Research Diets, Inc., New
Brunswick, NJ, USA); ii) HFD group, which were fed a HFD containing
60 kcal% fat, 20 kcal% protein and 20 kcal% carbohydrate (cat. no.
D12492; Research Diets, Inc.); iii) HFD + PSPC group, which were
fed a HFD and were given PSPC (700 mg/kg body weight/day; in 0.9%
normal saline) by oral gavage for 20 weeks, based as previously
described (21,23); and iv) PSPC group, which were fed a
normal diet and were given the same daily dose of PSPC as the HFD +
PSPC group. Following 20 weeks, mice were sacrificed and whole
brain tissues were collected for experimental use or stored at
−80°C. All animal care and experimental procedures were in
accordance with the Chinese legislation on the use and care of
laboratory animals, and experiments were approved by the Animal
Supervision Committee of Chengdu Medical College (Sichuan, China).
All studies involving animals are reported in accordance with the
ARRIVE guidelines for reporting experiments involving animals
(33).
Behavioral tests
Open field testing
Open field testing was performed as previously
described (27), with minor
modifications, to evaluate the effects of HFD and PSPC treatment on
mouse behavior. Locomotor activity and exploratory behavior were
examined in a circular arena (diameter, 50 cm; height, 30 cm) with
a 40 W bulb (3,000 lux). The floor of the arena was divided into 21
equal units by white lines. Mice were allowed to acclimate for 1
min at the center of the arena prior to the experiment, the
spontaneous behavioral variables were recorded within 5 min and
were classified into 4 mutually exclusive categories, including: i)
The distance mice travelled; ii) the speed mice travelled; iii) the
number of rearing and leaning events; and iv) the number of
grooming sessions.
Passive avoidance performance
testing
Passive avoidance performance testing was performed
as previously described (34),
using an apparatus composed of two compartments, one illuminated
and one dark, with a small hole in the wall separating the two. The
illuminated compartment was lit with a 25 W bulb and the dark one
contained a metal floor to conduct foot shocks (36 V; 50 Hz). Each
mouse was placed in the dimly lit room 3 min prior to the
experiment to acclimate to the novel environment within the
apparatus. Latency was defined as the interval between the initial
entry into the hole and fully entering the dark compartment. Mice
were placed individually into the illuminated compartment facing
away from the hole in the wall. Once the mouse escaped to the dark
compartment, a mild foot shock was administered for 5 sec and the
mouse was immediately removed from the apparatus. Following 24 h,
the mouse was placed in the illuminated compartment once more,
following the aforementioned method, to carry out the retention
test. The step-through latency within 5 min was recorded. The test
was conducted on 2 consecutive days after the open-field
testing.
Biochemical analysis
Body fat content (FC) was assessed using nuclear
magnetic resonance-magnetic resonance imaging-based technology
(EchoMRI LLC, Houston, TX, USA). At 20 weeks after the
administration, blood samples were taken by tail venipuncture using
heparin-coated capillary tube, and all rats were sacrificed by
cervical dislocation without the use of an anesthetic. Blood
samples were centrifuged at 1,000 × g for 10 min at 4°C to remove
cell debris, and the serum was stored at −20°C until measurements
were taken. Serum levels of total cholesterol (TC), triglyceride
(TG), high-density lipoprotein (HDL) and low-density lipoprotein
(LDL) cholesterol were determined using the following reagent kits
according to the manufacturer's protocol. TC assay kit (cat. no.
A111-2), TG assay kit (cat. no. A110-2), HDL assay kit (cat. no.
A112-2) and LDL assay kit (cat. no. A113-2), were obtained from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All
experiments were performed in triplicate.
Enzyme-linked immunosorbent assay
(ELISA)
Following sacrifice, mice were perfused with 50 mM
chilled phosphate-buffered saline solution (PBS; pH 7.40). Mouse
brains were harvested and the tissues were homogenized in PBS
containing 0.1% phenylmethylsulfonyl fluoride (Sigma-Aldrich; Merck
KGaA). Following centrifugation at 16,000 × g for 15 min at 4°C,
the supernatant was collected. Quantitative measurements of the
expression levels of inflammatory cytokines interleukin (IL)-6,
IL-1β, tumor necrosis factor (TNF)-α and IL-10 in mouse brains were
performed with the following commercially ELISA kits according to
the manufacturer's protocol: IL-6 (cat. no. R6000B), IL-1β (cat.
no. RLB00), TNF-α (cat. no. RTA00), IL-10 (cat. no. R1000),
obtained from R&D Systems, Inc. (Minneapolis, MN, USA). ELISAs
were conducted using an Infinite M200 PRO automated microplate
reader (Tecan Group Ltd., Männedorf, Switzerland) at a wavelength
of 540 nm. All samples were tested in triplicate.
Western blot analysis
For western blot analysis, whole brain tissues were
homogenized with CelLytic M lysis reagent and a protease inhibitor
cocktail (Sigma-Aldrich; Merck KGaA). Nuclear and cytoplasmic
extracts for western blotting were obtained using the NE-PER
Nuclear and Cytoplasmic Extraction reagents according to the
manufacturer's protocol (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Protein concentrations were determined by the
Bradford assay method. Samples were prepared with 5X loading
buffer, then equal amounts (40 µg) of total protein were separated
by 10% SDS-PAGE and transferred to nitrocellulose membranes, which
were rinsed with Tris-buffered saline containing 0.1% Tween-20
(TBS-T) and then blocked in TBS-T containing 5% nonfat dry milk for
1 h at room temperature. Membranes were incubated with the
anti-iNOS rabbit mAb (cat. no. 13120; dilution, 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-COX-2 rabbit
mAb (cat. no. 12282; dilution, 1:1,000; Cell Signaling Technology,
Inc.), anti-IL-6 rabbit mAb (cat. no. 12153; dilution, 1:1,000;
Cell Signaling Technology, Inc.), anti-IL-1β rabbit mAb (cat. no.
12242; dilution, 1:1,000; Cell Signaling Technology, Inc.),
anti-IL-10 rabbit mAb (cat. no. 12163; dilution, 1:1,000; Cell
Signaling Technology, Inc.), anti-TNF-α rabbit mAb (cat. no. 11948;
dilution, 1:1,000; Cell Signaling Technology, Inc.), anti-β-actin
rabbit mAb (cat. no. 4970; dilution, 1:5,000; Cell Signaling
Technology, Inc.) overnight at 4°C, followed by washes in TBS-T and
incubation with the horseradish peroxidase-conjugated secondary
antibodies (cat. no. sc-2357; dilution, 1:10,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature.
Membranes were washed with TBS-T and bands were visualized using an
enhanced chemiluminescence reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
intensity of the bands was analyzed using Quantity One software
(version 4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
expression levels of all proteins in each sample were normalized to
β-actin levels or K-70 protein, and the experiment was repeated
three times.
Statistical analysis
Statistical analysis was performed using the
Statistical Package for Social Sciences (SPSS, Inc., Chicago, IL,
USA) software, version 16.0 for Windows. Data are presented as the
mean ± standard error of the mean. One-way analysis of variance
followed by a Student-Newman-Keuls post hoc test, or an unpaired
Student's t-test was used where appropriate for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of PSPC treatment on the
general parameters of HFD-treated mice
A number of studies have demonstrated that a HFD is
a risk factor for obesity or obesity-associated disease (6–8). In
the present study, mice fed a HFD for 20 weeks exhibited a
significant increase in body weight gain (BWG) and FC compared with
mice in the Control group that were fed a normal diet (Table I). In addition, serum levels of TG,
TC and LDL were significantly elevated, whereas serum levels of HDL
were significantly decreased in HFD-treated mice compared with the
Control group.
 | Table I.Effects of purple sweet potato color
on the different parameters in each group of mice. |
Table I.
Effects of purple sweet potato color
on the different parameters in each group of mice.
| Parameter | Control | HFD | HFD + PSPC | PSPC |
|---|
| Body weight gain
(g) | 15.62±2.54 |
28.23±3.81b |
19.78±3.66c | 14.83±3.04 |
| FCa (% of body weight) | 9.78±1.33 |
20.45±1.98b |
13.57±1.19d | 10.15±1.28 |
| Serum TC
(mmol/l) | 2.98±0.48 |
3.84±0.37b |
3.21±0.24d | 3.15±0.33 |
| Serum TG
(mmol/l) | 1.04±0.15 |
1.75±0.25b |
1.29±0.18d | 1.20±0.17 |
| Serum HDL
(mmol/l) | 1.58±0.36 |
0.96±0.28b |
1.34±0.42c | 1.46±0.45 |
| Serum LDL
(mmol/l) | 1.37±0.26 |
2.67±0.42b |
1.75±0.28d | 1.58±0.37 |
The HFD-induced increases in BWG, FC and serum TG,
TC, LDL and LPS levels were all reduced, and serum HDL levels were
increased in HFD + PSPC-treated mice, compared with mice in the HFD
group. No significant differences were identified between the
Control group and mice treated with PSPC alone and fed a normal
diet.
Effects of PSPC treatment on the behavior
of HFD-treated mice
Open field testing
To confirm whether locomotor activity and
exploratory behavior were affected by HFD, an open field experiment
was performed with mice following different treatments. As
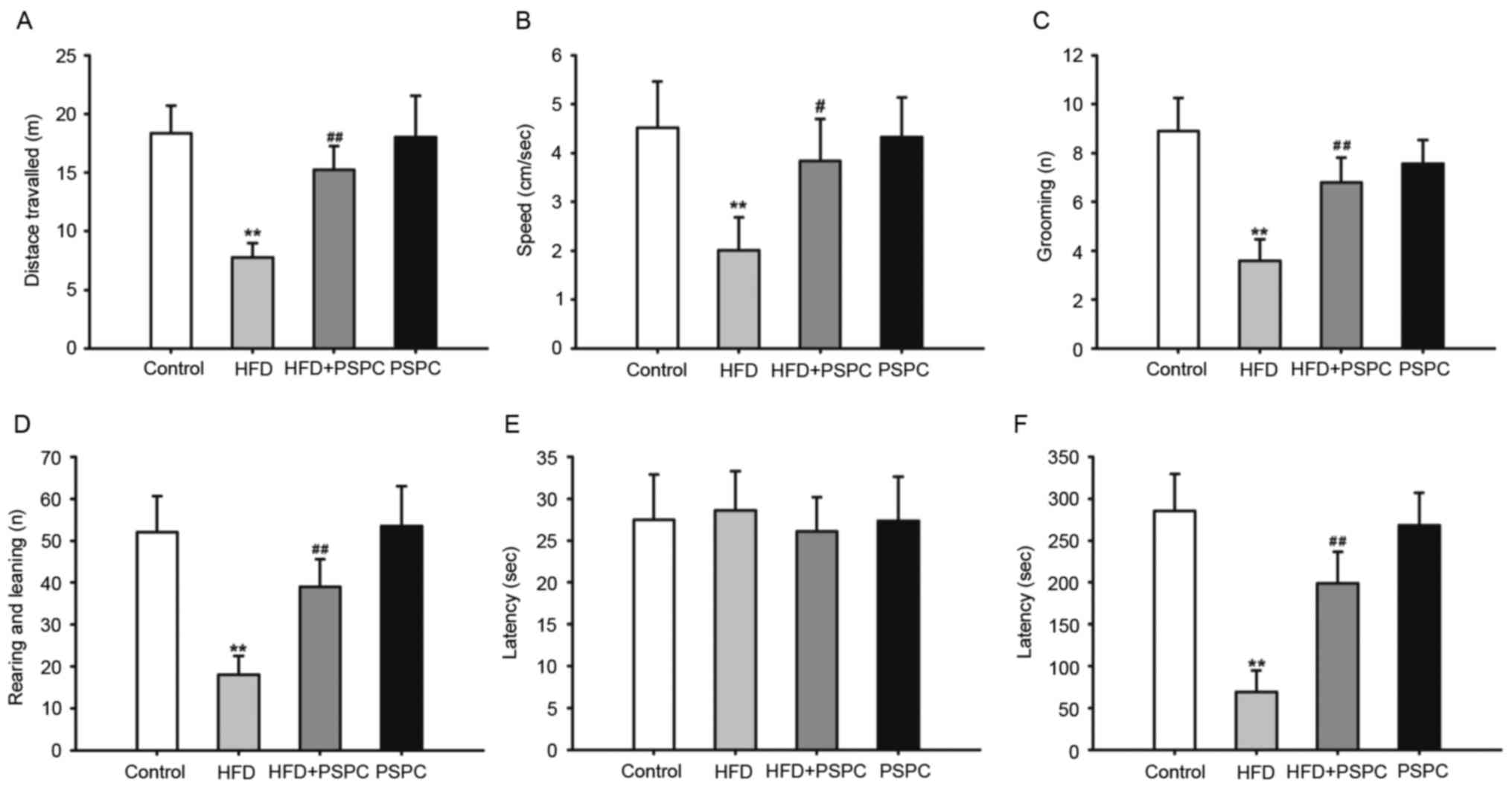
presented in Fig. 1, mice
challenged with HDF for 20 weeks had a significant decrease in the
distance and speed travelled (Fig. 1A
and B), the number of grooming sessions (Fig. 1C) and the number of rearing and
leaning events (Fig. 1D) compared
with mice in the Control group, indicating that mice challenged
with HFD had impaired locomotor activity and exploratory behavior.
When HFD-treated mice were co-treated with PSPC the impaired
activities and behaviors significantly improved (Fig. 1A-D). There were no significant
differences identified between the Control and PSPC-only groups for
all parameters measured.
Passive avoidance performance
testing
In the initial part of the step-through test, no
significant differences were identified between the latencies among
the four groups (Fig. 1E).
However, following a 5 sec mild foot shock in the dark compartment,
the latency in the 24-h retention test was significantly decreased
in HFD-treated mice compared with the Control group. By contrast,
PSPC treatment markedly increased the latency in HFD + PSPC mice
compared with mice only fed a HFD (Fig. 1F). These results suggest that PSPC
may be able to improve the deficits in memory function of mice to a
certain degree. However, no significant differences were identified
between the mice challenged with PSPC alone and the Control
group.
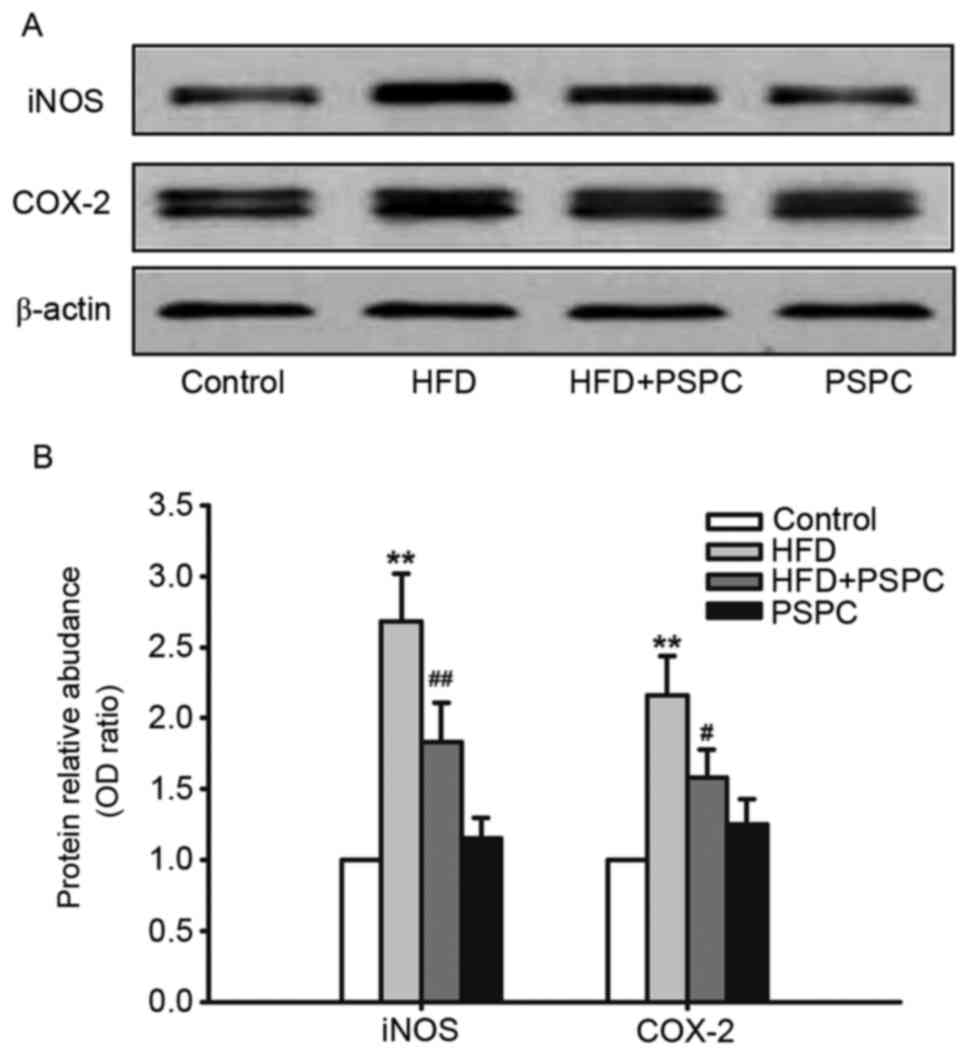
Effects of PSPC treatment on
cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS)
protein expression levels in HFD-treated mouse brains
COX-2 and iNOS are important regulators in the
progression of inflammation, whose expression levels are relatively
low in normal tissues (35).
However, when stimulated with endotoxin or inflammatory cytokines,
such as IL-6, IL-1β or TNF-α, their expression levels markedly
increase. In the present study western blot analysis was performed
to determine the effect of a HFD and PSPC treatment on the
expression of COX-2 and iNOS proteins. HFD treatment significantly
upregulated the expression levels of COX-2 and iNOS compared with
Control mice; this elevated expression was significantly reduced in
HFD-fed mice co-treated with PSPC (Fig. 2A and B). No significant differences
were identified between the Control and PSPC groups.
Effects of PSPC treatment on the
expression levels of neuroinflammatory cytokines in HFD-treated
mice
Neuroinflammatory cytokines are important
contributors to the pathogenesis and development of cognitive
impairment, and increased levels of expression have been reported
to disrupt hippocampal synaptic plasticity (36). Therefore, the effects of a HFD and
PSPC treatment on the levels of neuroinflammatory cytokines,
including TNF-α, IL-6, IL-1β and IL-10 in the mouse brain were
determined by western blotting and ELISA. As shown in Fig. 3A and B, mice fed a HFD exhibited a
significant increase in the protein expression levels of the
proinflammatory cytokines IL-6, IL-1β and TNF-α, and a decrease in
the expression of anti-inflammatory cytokine IL-10, compared with
Control mice. However, these effects were significantly reversed by
PSPC treatment in the HFD + PSPC group compared with the HFD group.
Results from ELISA analysis confirmed the effects of HFD and PSPC
treatment on the production of neuroinflammatory cytokines in the
mouse brain (Fig. 3C). A
significant increase in the expression levels of IL-6, IL-1β and
TNF-α was observed, in addition to a marked decrease in the level
of IL-10 in the brain of HFD-fed mice, compared with the Control
group. PSPC markedly decreased the expression levels of IL-6 by
43.93%, IL-1β by 32.25% and TNF-α by 42.42%, and increased the
level of IL-10 by 40.82% in the HFD + PSPC group compared with the
HFD-only group (Fig. 3C). No
significant differences were identified between the PSPC group and
the Control group in either the western blotting or ELISA
analyses.
Effects of PSPC treatment on
HFD-induced mitogen-activated protein kinase (MAPK) and NF-κB
activation in mouse brains
A number of previous studies have demonstrated that
MAPK and NF-κB activation serves a crucial role in the progression
of inflammation (37,38). To further determine the molecular
mechanisms underlying the suppressive effects of PSPC on
neuroinflammation in the mouse brain, the effects of PSPC on the
expression and phosphorylation levels of the MAPK family members
extracellular signal-regulated kinase (ERK), c-Jun N-terminal
kinase (JNK), and p38 were determined by western blot analysis. The
brains of mice fed a HFD had significantly increased expression
levels of phosphorylated (p)-ERK, p-JNK and p-p38 proteins compared
with Control mice (Fig. 4A and B).
However, this increase in p-ERK, p-JNK and p-p38 protein expression
levels in mice challenged with a HFD was significantly reduced by
PSPC treatment (Fig. 4A and
B).
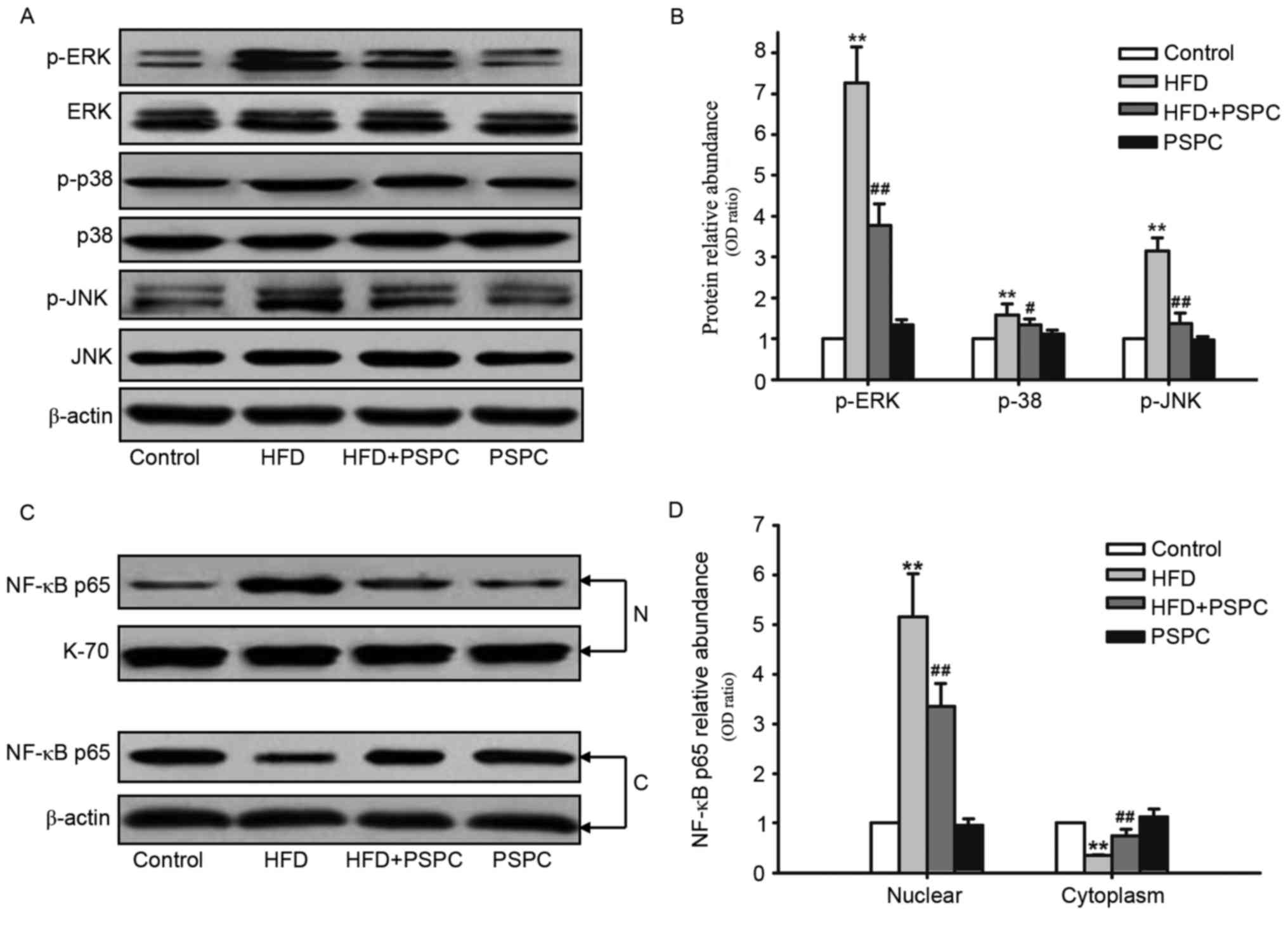 | Figure 4.Effects of PSPC treatment on
HFD-induced MAPK phosphorylation and NF-κB activation in mouse
brains (n=5). (A) Western blots revealing the expression levels of
p-ERK, ERK, p-p38, p38, p-JNK and JNK. (B) Densitometric analysis
of the proteins in (A); expression levels were normalized to
β-actin and expressions in the Control group were set to 1.0. (C)
Western blots revealing the expression levels of NF-κB p65 protein
in nuclear and cytoplasmic fractions in the brain tissues of mice
from each group under the indicated experimental conditions. (D)
Densitometric analysis of the proteins in (C); nuclear and
cytoplasmic expression levels were normalized to β-actin or K-70,
respectively; expressions in the Control group were set to 1.0.
Data are presented as the mean ± standard error of the mean.
**P<0.01 vs. Control group; #P<0.05 and
##P<0.01 vs. HFD group. C, cytoplasm; ERK,
extracellular signal-regulated kinase; HFD, high fat diet; JNK,
c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; N,
nuclear; NF-κB, nuclear factor-κB; p-, phosphorylated; PSPC, purple
sweet potato color. |
NF-κB activation induces its translocation from the
cytoplasm to the nucleus. The present study investigated the
nuclear and cytoplasmic expression levels of NF-κB p65 (Fig. 4C and D). NF-κB p65 levels in the
nuclear fractions were significantly increased in HFD-treated mice
compared with Control mice, whereas the cytoplasmic expression
levels were significantly decreased in HFD-treated mice. PSPC
treatment in mice fed a HFD inhibited the activation of NF-κB, as
demonstrated by the increased cytoplasmic expression of NF-κB p65
and the decreased levels in the nuclear fractions comparing the
HFD-only group. No statistically significant differences were
identified between the PSPC and Control groups for MAPK
phosphorylation or NF-κB activation.
Discussion
An increasing number of studies have demonstrated
that a HFD contributed to the development of obesity and fat
deposition in a variety of tissues and is associated with
hyperlipemia and detrimental effects on the brain (9,11).
HFD-induced chronic inflammation, primarily elicited by glial cells
in the brain, serves a central role in cognition and memory
impairment, as well as in neurodegenerative diseases (10,39).
Neuroinflammation is a complex process that involves a diverse set
of proinflammatory cytokines and reactive oxygen species (ROS),
which may be detrimental to neuronal health and may reduce synaptic
plasticity (12,36). Therefore, novel therapeutic
strategies are required that will improve obesity-associated
metabolic perturbations and neuroinflammation. PSPC belongs to a
class of naturally occurring anthocyanins, which are used to give
food color, and has recently been reported to have a number of
pharmacological activities, including as potent antioxidant,
anti-inflammatory and neuroprotective effects (26–30).
In the present study, the effects of PSPC on HFD-induced
neuroinflammation in the mouse brain was investigated following 20
weeks of daily administration of PSPC (700 mg/kg body weight/day)
via oral gavage.
The results of biochemical analysis revealed that
HFD treatment induced hyperlipemia and significantly increased BWG
and FC in mice, which was consistent with a previous report
(11). However, the HFD-induced
increases in BWG, FC and hyperlipemia were all suppressed by PSPC
co-treatment. In behavioral tests, HFD-treatment resulted in a
marked impairment of mouse behavior and memory function. HFD +
PSPC-treated mice exhibited improved active behavioral responses to
the open field test, and PSPC treatment inhibited HFD-induced
behavioral retrogression, including distance and speed travelled,
and the number of grooming and rearing and leaning events. In
addition, PSPC treatment also restored the HFD-induced memory
impairment to some extent in the step-through tasks.
To characterize the effects of PSPC on
neuroinflammation in the mouse brain, the expression profile of two
mediators of inflammation, iNOS and COX-2, were detected in mouse
brains. Previous studies have implicated iNOS expression in
neuronal damage and death in a number of central nervous
system-associated diseases (40),
and COX-2 expression was reported to be induced by inflammatory
stimuli in the hippocampus (41).
In the present study, western blot analyses revealed that HFD
treatment upregulated the expression levels of iNOS and COX-2
proteins, which were significantly reduced following PSPC
administration. In addition, the expression levels of
neuroinflammatory cytokines IL-6, IL-1β, IL-10 and TNF-α were
determined in mouse brains by western blotting and ELISA analyses,
as these cytokines have a crucial role in the progression of
inflammation. Western blotting and ELISA results demonstrated that
the expression levels of IL-6, IL-1β and TNF-α were elevated, and
the level of IL-10 was decreased in mice fed a HFD, which was
comparable to the results of a study in diet-induced obese animal
models (36). However, these
alterations in the levels of neuroinflammatory cytokines were
attenuated by PSPC treatment, confirming the anti-inflammatory and
neuroprotective effects of PSPC.
To further determine the mechanisms underlying the
PSPC-induced suppression of neuroinflammation in mouse brains, the
present study investigated the effects of PSPC on the expression
levels of MAPK family members ERK, JNK and p38 proteins, as MAPK
activation is known to be involved in the expression of
proinflammatory cytokines (42).
Activated ERK has been demonstrated to have a neurotoxic effect,
JNK was revealed to be involved in cell apoptotic responses and p38
was reported to participate in neuronal responses to various
stressors (43). The results of
the present study revealed that PSPC treatment ameliorated the
HFD-induced phosphorylation/activation of ERK, JNK and p38 MAPKs in
mouse brains. These results are comparable to those from a previous
study that demonstrated that ERK and JNK are involved in the
LPS-induced acute inflammatory response in mouse brain (44). However, they did not detect a
significant difference in p38 expression with LPS-treatment mice,
which is not consistent with the results of the present study.
Based on these results, the MAPK pathway may be involved in
HFD-induced neuroinflammation and PSPC treatment may exert an
anti-inflammatory effect on the mouse brain, at least in part, by
inhibiting the phosphorylation of ERK, JNK and p38 MAPKs.
NF-κB is a transcription regulator that serves
important roles in cell proliferation and survival, inflammation
and immunity, and is considered the main signaling pathway that is
involved in the development of inflammatory diseases, including
hepatitis, nerve injury and lung injury (45). NF-κB is composed of two subunits,
p50 and p65, which form a heterodimer that is bound by members of
the inhibitors of κB (IκB) family of inhibitor proteins and is
sequestered in the cytoplasm under resting conditions. When exposed
to various stimuli that activate NF-κB, IκB proteins are
phosphorylated and subsequently ubiquitinated and degraded. The
unbound NF-κB dimers translocate to the nucleus where they bind to
cis-regulatory elements called kB sites in target genes and
initiate transcription (46). In
the nervous system, NF-κB is activated by a number of stimuli,
including ROS, neurotrophic factors and neurotransmitters, and has
been previously reported to function in the control of cell growth,
cell survival, inflammatory response, synaptic plasticity, behavior
and cognition (37). Considering
the potential role of NF-κB in neuroinflammation, the present study
examined the activation of NF-κB in the brain tissues of mice
challenged with HFD and treated with PSPC. The results indicated
that a HFD induced a significant decrease in the cytoplasmic
expression of NF-κB subunit p65 and a significant increase in
nuclear expression, suggesting that HFD may promote NF-κB
activation in mouse brains. This effect was inhibited when mice
were treated with PSPC, which is consistent with the levels of LPS
detected in the serum. LPS is a Gram-negative bacterial endotoxin,
which activates the biosynthesis of proinflammatory cytokines by
binding to toll-like receptor 4 and inducing signaling by
activating NF-κB (47), suggesting
that PSPC may exert anti-neuroinflammatory actions in the mouse
brain by decreasing the levels of LPS and the subsequent inhibition
of NF-κB activation.
To clarify whether PSPC was able to affect mouse
metabolism and physiological processes, the present study compared
the aforementioned characteristics between the experimental group
receiving PSPC alone and the Control group; both were fed a normal
diet. The results demonstrated that there were no significant
differences in the biochemical parameters or in the expression
levels of iNOS, COX-2 and the inflammatory cytokines, or in the
phosphorylation/activation of MAPKs and NF-κB, indicating that PSPC
itself may not alter metabolism and physiological processes in
mice. These results further confirmed that the observed behavior
and biochemical alterations may be due to the ameliorating effect
of PSPC on HFD-induced neuroinflammation in mouse brains. The
present study confirmed the anti-inflammatory and neuroprotective
effects of PSPC.
In conclusion, the present study demonstrated that
PSPC attenuates HFD-induced neuroinflammation, and thereby improves
behavior and memory function impairments in mouse brains by
downregulating the expression of iNOS, COX-2, IL-1β, IL-6 and
TNF-α, and upregulating the expression of IL-10, through the
suppression of ERK, JNK and p38 MAPK phosphorylation and NF-κB
signaling pathway activation. These findings about the
pharmacological efficacy of PSPC may offer a novel therapeutic
approach to ameliorate obesity-associated neuroinflammation.
Acknowledgements
The present study was funded by a Special Grant from
the Key Laboratory of Sichuan Province for Developmental
Regeneration (grant no. SYS10-006).
Glossary
Abbreviations
Abbreviations:
|
BWG
|
body weight gain
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
FC
|
fat content
|
|
HFD
|
high-fat diet
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
PSPC
|
purple sweet potato color
|
References
|
1
|
Meissburger B, Ukropec J, Roeder E, Beaton
N, Geiger M, Teupser D, Civan B, Langhans W, Nawroth PP,
Gasperikova D, et al: Adipogenesis and insulin sensitivity in
obesity are regulated by retinoid-related orphan receptor gamma.
EMBO Mol Med. 3:637–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trayhurn P and Beattie JH: Physiological
role of adipose tissue: White adipose tissue as an endocrine and
secretory organ. Proc Nutr Soc. 60:pp. 329–339. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Proença AR, Sertié RA, Oliveira AC,
Campaña AB, Caminhotto RO, Chimin P and Lima FB: New concepts in
white adipose tissue physiology. Braz J Med Biol Res. 47:192–205.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smitka K and Marešová D: Adipose tissue as
an endocrine organ: An update on pro-inflammatory and
anti-inflammatory microenvironment. Prague Med Rep. 116:87–111.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wellen KE and Hotamisligil GS:
Obesity-induced inflammatory changes in adipose tissue. J Clin
Invest. 112:1785–1788. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Golay A and Bobbioni E: The role of
dietary fat in obesity. Int J Obes Relat Metab Disord. 21 Suppl
3:S2–S11. 1997.PubMed/NCBI
|
|
7
|
Ginsberg HN, Zhang YL and Hernandez-Ono A:
Metabolic syndrome: Focus on dyslipidemia. Obesity (Silver Spring).
14 Suppl 1:41S–49S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seidell JC: Dietary fat and obesity: An
epidemiologic perspective. Am J Clin Nutr. 67 3 Suppl:546S–550S.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freeman LR, Haley-Zitlin V, Stevens C and
Granholm AC: Diet-induced effects on neuronal and glial elements in
the middle-aged rat hippocampus. Nutr Neurosci. 14:32–44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thirumangalakudi L, Prakasam A, Zhang R,
Bimonte-Nelson H, Sambamurti K, Kindy MS and Bhat NR: High
cholesterol-induced neuroinflammation and amyloid precursor protein
processing correlate with loss of working memory in mice. J
Neurochem. 106:475–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang DH, Heo RW, Yi CO, Kim H, Choi CH and
Roh GS: High-fat diet-induced obesity exacerbates kainic
acid-induced hippocampal cell death. BMC Neurosci. 16:722015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nerurkar PV, Johns LM, Buesa LM, Kipyakwai
G, Volper E, Sato R, Shah P, Feher D, Williams PG and Nerurkar VR:
Momordica charantia (bitter melon) attenuates high-fat
diet-associated oxidative stress and neuroinflammation. J
Neuroinflammation. 8:642011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Orr CF, Rowe DB and Halliday GM: An
inflammatory review of Parkinson's disease. Prog Neurobiol.
68:325–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian L, Flood PM and Hong JS:
Neuroinflammation is a key player in Parkinson's disease and a
prime target for therapy. J Neural Transm (Vienna). 117:971–979.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee YJ, Han SB, Nam SY, Oh KW and Hong JT:
Inflammation and Alzhe-imer's disease. Arch Pharm Res.
33:1539–1556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simmons RK, Alberti KG, Gale EA, Colagiuri
S, Tuomilehto J, Qiao Q, Ramachandran A, Tajima N, Brajkovich
Mirchov I, Ben-Nakhi A, et al: The metabolic syndrome: Useful
concept or clinical tool? Report of a WHO Expert Consultation.
Diabetologia. 53:600–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Palacios N, Gao X, McCullough ML, Jacobs
EJ, Patel AV, Mayo T, Schwarzschild MA and Ascherio A: Obesity,
diabetes, and risk of Parkinson's disease. Mov Disord.
26:2253–2259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iwashina T: Contribution to flower colors
of flavonoids including anthocyanins: A review. Nat Prod Commun.
10:529–544. 2015.PubMed/NCBI
|
|
19
|
He J and Giusti MM: Anthocyanins: Natural
colorants with health-promoting properties. Annu Rev Food Sci
Technol. 1:163–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang WY, Liu YM, Wang J, Wang XN and Li
CY: Anti-inflammatory effect of the blueberry anthocyanins
malvidin-3-glucoside and malvidin-3-galactoside in endothelial
cells. Molecules. 19:12827–12841. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pool-Zobel BL, Bub A, Schröder N and
Rechkemmer G: Anthocyanins are potent antioxidants in model systems
but do not reduce endogenous oxidative DNA damage in human colon
cells. Eur J Nutr. 38:227–234. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang HG, Jeong SH and Cho JH:
Antimutagenic and anticarcinogenic effect of methanol extracts of
sweetpotato (Ipomea batata) leaves. Toxicol Res. 26:29–35. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mano H, Ogasawara F, Sato K, Higo H and
Minobe Y: Isolation of a regulatory gene of anthocyanin
biosynthesis in tuberous roots of purple-fleshed sweet potato.
Plant Physiol. 143:1252–1268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu F, Luo J, Yao S, Ma L and Kong L:
Preparative isolation and purification of anthocyanins from purple
sweet potato by high-speed counter-current chromatography. J Sep
Sci. 32:2146–2151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harada K, Kano M, Takayanagi T, Yamakawa O
and Ishikawa F: Absorption of acylated anthocyanins in rats and
humans after ingesting an extract of Ipomoea batatas purple sweet
potato tuber. Biosci Biotechnol Biochem. 68:1500–1507. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM,
Shan Q and Hu B: Purple sweet potato color attenuates oxidative
stress and inflammatory response induced by d-galactose in mouse
liver. Food Chem Toxicol. 47:496–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shan Q, Lu J, Zheng Y, Li J, Zhou Z, Hu B,
Zhang Z, Fan S, Mao Z, Wang YJ and Ma D: Purple sweet potato color
ameliorates cognition deficits and attenuates oxidative damage and
inflammation in aging mouse brain induced by d-galactose. J Biomed
Biotechnol. 2009:5647372009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hwang YP, Choi JH, Yun HJ, Han EH, Kim HG,
Kim JY, Park BH, Khanal T, Choi JM, Chung YC and Jeong HG:
Anthocyanins from purple sweet potato attenuate
dimethylnitrosamine-induced liver injury in rats by inducing
Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS
expression. Food Chem Toxicol. 49:93–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang ZF, Lu J, Zheng YL, Wu DM, Hu B,
Shan Q, Cheng W, Li MQ and Sun YY: Purple sweet potato color
attenuates hepatic insulin resistance via blocking oxidative stress
and endoplasmic reticulum stress in high-fat-diet-treated mice. J
Nutr Biochem. 24:1008–1018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shan Q, Zheng Y, Lu J, Zhang Z, Wu D, Fan
S, Hu B, Cai X, Cai H, Liu P and Liu F: Purple sweet potato color
ameliorates kidney damage via inhibiting oxidative stress mediated
NLRP3 inflammasome activation in high fat diet mice. Food Chem
Toxicol. 69:339–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hwang YP, Choi JH, Han EH, Kim HG, Wee JH,
Jung KO, Jung KH, Kwon KI, Jeong TC, Chung YC and Jeong HG: Purple
sweet potato anthocyanins attenuate hepatic lipid accumulation
through activating adenosine monophosphate-activated protein kinase
in human HepG2 cells and obese mice. Nutr Res. 31:896–906. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun C, Fan S, Wang X, Lu J, Zhang Z, Wu D,
Shan Q and Zheng Y: Purple sweet potato color inhibits endothelial
premature senescence by blocking the NLRP3 inflammasome. J Nutr
Biochem. 26:1029–1040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McGrath JC, Drummond GB, McLachlan EM,
Kilkenny C and Wainwright CL: Guidelines for reporting experiments
involving animals: The ARRIVE guidelines. Br J Pharmacol.
160:1573–1576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Castellano C, Introini-Collison IB and
McGaugh JL: Interaction of beta-endorphin and GABAergic drugs in
the regulation of memory storage. Behav Neural Biol. 60:123–128.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jain NK, Ishikawa TO, Spigelman I and
Herschman HR: COX-2 expression and function in the hyperalgesic
response to paw inflammation in mice. Prostaglandins Leukot Essent
Fatty Acids. 79:183–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang D, Liu L, Yan J, Wu W, Zhu X and Wang
Y: Cardiotrophin-1 (CT-1) improves high fat diet-induced cognitive
deficits in mice. Neurochem Res. 40:843–853. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mémet S: NF-kappaB functions in the
nervous system: From development to disease. Biochem Pharmacol.
72:1180–1195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krause DL and Muller N: Neuroinflammation,
microglia and implications for anti-inflammatory treatment in
Alzheimer's disease. Int J Alzheimers Dis. 2010:pii:
7328062010.
|
|
40
|
Giri S, Jatana M, Rattan R, Won JS, Singh
I and Singh AK: Galactosylsphingosine (psychosine)-induced
expression of cytokine-mediated inducible nitric oxide synthases
via AP-1 and C/EBP: Implications for Krabbe disease. FASEB J.
16:661–672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marcheselli VL and Bazan NG: Sustained
induction of prostaglandin endoperoxide synthase-2 by seizures in
hippocampus. Inhibition by a platelet-activating factor antagonist.
J Biol Chem. 271:24794–24799. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Koistinaho M and Koistinaho J: Role of p38
and p44/42 mitogen-activated protein kinases in microglia. Glia.
40:175–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang YJ, Zheng YL, Lu J, Chen GQ, Wang XH,
Feng J, Ruan J, Sun X, Li CX and Sun QJ: Purple sweet potato color
suppresses lipopolysaccharide-induced acute inflammatory response
in mouse brain. Neurochem Int. 56:424–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wi SM, Moon G, Kim J, Kim ST, Shim JH,
Chun E and Lee KY: TAK1-ECSIT-TRAF6 complex plays a key role in the
TLR4 signal to activate NF-κB. J Biol Chem. 289:35205–35214. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim KA, Lee IA, Gu W, Hyam SR and Kim DH:
β-Sitosterol attenuates high-fat diet-induced intestinal
inflammation in mice by inhibiting the binding of
lipopolysaccharide to toll-like receptor 4 in the NF-κB pathway.
Mol Nutr Food Res. 58:963–972. 2014. View Article : Google Scholar : PubMed/NCBI
|