Introduction
Sepsis is considered to be a systemic inflammatory
response syndrome that occurs in response to severe infections,
which includes pathogen invasion, extensive burns, severe trauma
and shock (1). Recently, it has
been demonstrated that sepsis is a multifaceted host response to
infectious pathogens, which may be amplified by endogenous factors
and is associated with a high morbidity and mortality (2). Although substantial developments have
been made in the understanding of the basic pathogenesis of sepsis,
no specific anti-sepsis treatments exist. As such, the management
of patients relies mainly on early recognition allowing correct
therapeutic measures to be conducted rapidly, including the
administration of appropriate antibiotics, source control measures
when necessary, resuscitation with intravenous fluids and
vasoactive drugs when required (3). Research has indicated that admissions
for sepsis are greater than those for myocardial infarction and
stroke in the United States (4).
In-hospital mortality remains high at 25–30% (3); therefore, it is necessary to develop
a novel treatment for sepsis.
Within the hematopoietic system, macrophages are a
one of the cell types that exhibit the highest plasticity, and they
are present in every body tissue, where they trigger, regulate and
affect the development of inflammation. Macrophages are
differentiated into different phenotypes depending on the
microenvironment (5–7). The classical activation phenotype
(M1) and alternative activation phenotype (M2) express different
surface makers, secrete different cytokines and have different
roles in immunoregulation (7–9).
Various studies concerning sepsis have demonstrated that
macrophages are crucial participants in the systemic inflammatory
response syndrome. Once activated, various soluble factors that
directly and indirectly act on blood vessels, the immune system and
other tissues are produced and secreted by macrophages, thus
leading to a series of changes (10). However, excessive production of
cytokines may lead to multiple organ failure and potentially death.
Studies have reported that the development of sepsis is associated
with the overactivation of M1 macrophages; excessive activation of
M1 macrophages leads to the increased release of various
inflammatory factors, including tumor necrosis factor (TNF)-α,
interleukin (IL)-1 and IL-6, subsequently resulting in severe
inflammation (11–13).
Bone marrow mesenchymal stem cells (BMSCs) are a
type of somatic stem cell (14,15)
that are isolated from bone myeloid tissue and exhibit
self-renewal, unlimited proliferation and multipotent
differentiation properties. Furthermore, BMSCs may be induced to
differentiate into osteoblasts, adipocytes and chondrocytes in
vitro. BMSCs have an important role in immunosuppression and
also immunomodulation, in vitro and in vivo, in
innate and adaptive immunity processes (16). A recent study demonstrated that
human mesenchymal stem cells (MSCs) influence the process of
inflammation by immunomodulating the expression of inflammatory
cytokines from a variety of immune cells, including macrophages and
altering the polarization of macrophages (17). The majority of previous studies
have involved co-culture of RAW264.7 macrophage cells with
different types of stem cells in vitro, followed by
observation of the effects of stem cells on the polarization of
macrophages (18,19). However, there is no direct evidence
as to whether stem cells have the ability to influence the resident
peritoneal macrophages in vitro or alter the polarization of
macrophages following injection into animals in vivo.
Therefore, the present study investigated the effect of BMSCs on
macrophage polarization in vitro and in vivo.
Materials and methods
Animals
All rats were maintained in specific-pathogen free
environments at 20–26°C and 40–70% humidity under a 12 h light/dark
cycle with, and free access to food and water. Rats were obtained
from the Experimental Animal Center of the Sun Yat-sen University
(license no. SCXK 2016–0029; Guangzhou, China). The animals were
acclimatized for 3 days prior to initiating the experiment. A total
of 70 male Sprague-Dawley rats weighing 100±20 g (4 weeks) were
used for the isolation of BMSCs and macrophages; 36 male
Sprague-Dawley rats weighing 130±20 g (5 weeks) were used as the
animal model. All experiments were performed following approval by
the Ethics Committee of Guangdong General Hospital, Guangdong
Academy of Medical Sciences (Guangzhou, China; approval no.
GDREC2016254A).
Isolation and culture of rat
BMSCs
BMSCs were obtained using adhesion to cell culture
plastic as previously described (14). A total of 20 Sprague-Dawley rats,
weighing 100±20 g (4 weeks), were euthanized via dislocation of the
cervical vertebra and BMSCs were collected from the femur and tibia
by flushing the shaft with chilled complete medium, which consisted
of Dulbecco's modified Eagle's medium (DMEM, Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Cells were washed with medium three times and centrifuged at 4°C
for 5 min at 192 × g. The pellet was resuspended and cultured in 25
cm2 culture flasks with complete medium at 37°C and 5%
CO2 in air. BMSCs preferentially attached to polystyrene
surfaces, and, after 72 h, non-adherent cells were discarded. Fresh
medium was added and replaced every 2–3 days. When cells reached
80–90% confluence, the adherent cells were trypsinized with 0.25%
trypsin-EDTA (Invitrogen; Thermo Fisher Scientific, Inc.).
Following three cell passages, the expression of cell surface
markers, including cluster of differentiation (CD)29, CD90, CD45
and CD44 (Table I), were analyzed
by flow cytometry, which is described in a later paragraph.
Additionally, cells were induced to differentiate into osteoblasts
and adipocytes, to determine the purity of the BMSC population. For
standard osteogenic differentiation, confluent monolayers of BMSCs
were incubated in medium supplemented with 0.01% dexamethasone,
0.2% ascorbic acid, 1% glutamine and 1% β-glycerol phosphate
(Cyagen Biosciences, Inc., Guangzhou, China). Culture medium was
replaced every 3 days. After 30 days, the culture medium was fixed
with 4% paraformaldehyde for 30 min at room temperature and
incubated with Alizarin Red (Cyagen Biosciences, Inc.) for 30 min
at room temperature. Excess stain was removed by washing four times
with PBS. For standard adipogenic differentiation, confluent
monolayers of BMSCs were incubated in medium supplemented with 1%
glutamine, 0.1% 3-isobutyl-1-methylxanthine, 0.1% rosiglitazone and
0.2% insulin (Cyagen Biosciences, Inc.) with medium changed every
1–3 days. After 30 days, the culture medium was fixed with 4%
paraformaldehyde for 30 min at room temperature and incubated with
Oil Red O for 30 min at room temperature. BMSCs were collected for
experiments in the third passage via trypsinization and
resuspension in PBS to a concentration of 2×106 cells/ml
immediately prior to injection; cells were observed under a light
microscope.
 | Table I.Details of antibodies used for flow
cytometry and western blotting. |
Table I.
Details of antibodies used for flow
cytometry and western blotting.
| Antibody | Source | Dilution | Manufacturer | Catalogue
number |
|---|
| CD29-FITC | Hamster | 1:20 | BD Biosciences | 555005 |
| CD44-FITC | Mouse | 1:20 | BD Biosciences | 550974 |
| CD45-FITC | Mouse | 1:20 | BD Biosciences | 554877 |
| CD90-PE | Mouse | 1:20 | BD Biosciences | 554898 |
| CD11b-APC | Mouse | 1:20 | BD Biosciences | 562102 |
| CD68-Alexa Fluor
488 | Mouse | 1:20 | Bio-Rad
Laboratories, Inc. | MCA341A488 |
| CD11c-Alexa Fluor
647 | Mouse | 1:20 | Bio-Rad
Laboratories, Inc. | MCA1441A647 |
| CD206 | Rabbit | 1:20 | Abcam | ab64693 |
| iNOS | Mouse | 1:1,000 | Abcam | ab49999 |
| Arg-1 | Rabbit | 1:1,000 | Abcam | ab124917 |
| F(ab')2 donkey
anti-rabbit IgG-PE | Donkey | 1:100 | eBioscience; Thermo
Fisher Scientific, Inc. | 12–4739 |
| Anti-rabbit
IgG-HRP | Goat | 1:5,000 | Santa Cruz
Biotechnology, Inc. | sc-2004 |
| Anti-mouse
IgG-HRP | Goat | 1:5,000 | Santa Cruz
Biotechnology, Inc. | sc-2005 |
| β-actin | Mouse | 1:5,000 | Santa Cruz
Biotechnology, Inc. | sc-47778 |
Isolation and culture of rat
peritoneal macrophages
A total of 50 male Sprague-Dawley rats, weighing
100±20 g (4-weeks-old) used for obtaining peritoneal macrophages.
PBS (20 ml) supplemented with 10% FBS was injected into the
peritoneal cavity and the rat abdomen was gently massaged for 5 min
(20). Macrophages were isolated
from the peritoneal cavity by collecting as much peritoneal fluid
as possible, and the collected fluid was centrifuged at 4°C for 5
min at 192 × g to pellet the cells. The pellet was resuspended and
cultured in 6-well plates with complete medium at 37°C and 5%
CO2 in air. After 6 h, non-adherent cells were
discarded. Fresh medium was added and replaced every 1–2 days.
After 2 days, adherent cells were harvested and purified using CD68
and CD11b (Table I) via flow
cytometry, which is described in a later paragraph. These
peritoneal macrophages were collected for culturing with BMSCs
in vitro.
Co-culture of peritoneal macrophages
with BMSCs in vitro
Initially, peritoneal macrophages were incubated in
6-well plates (Corning Inc., Corning, NY, USA) at a concentration
100,000 cells per 2 ml at 37°C. After 12 h, the culture (complete)
medium was removed and either 100,000 BMSCs in fresh medium or
medium alone was added to each well. Macrophages were divided into
four groups according to different treatments: Macrophages cultured
in normal medium for 96 h; macrophages incubated with
lipopolysaccharide (LPS; 100 ng/ml; Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany) for 12 h followed by culture in normal medium
for 84 h; macrophages co-cultured with BMSCs, separated by a
Transwell insert (0.4 µm pore size polyester membrane; Corning,
Inc.), were incubated with LPS (100 ng/ml) for 12 h followed by
culture in normal medium for 84 h; and a direct co-culture of
macrophages and BMSCs attached to the same substrate was incubated
with LPS (100 ng/ml) for 12 h followed by culture in normal medium
for 84 h. All cell incubations were conducted at 37°C and 5%
CO2. Subsequently, cell culture supernatants, protein
and cells were collected for ELISA, western blotting and flow
cytometry analysis, respectively, to investigate the effects of
co-culture on macrophages.
LPS model of sepsis
Male Sprague-Dawley rats, weight 130±20 g
(5-weeks-old) were fed a standard diet and acclimatized in a quiet
room for three days prior to experimentation. Rats were
administered 5 mg/kg LPS dissolved in PBS via tail vein injection
(21). All animals were randomly
divided into three groups according to different treatments: Normal
control group (n=4), LPS-stimulated group (n=16) and BMSC-treated
group (n=16) by the random number table. The normal control group
was injected with 1 ml PBS (n=4), LPS-stimulated group was injected
with 1 ml LPS (5 mg/kg) dissolved in PBS (n=16). The BMSC-treated
group was injected with LPS (5 mg/kg) and after 1 h, injected with
BMSCs (2×106 cells in 1 ml PBS). Animals in the normal
control group were euthanized to harvest their lungs for
hematoxylin-eosin staining after 24 h, while lungs from animals in
other groups were obtained after 6, 12, 24, 48 h. In addition, rat
peritoneal macrophages were obtained by peritoneal injection of
DMEM after LPS stimulation for 24 h in the normal control group,
while the procedure was performed after LPS stimulation for 24 and
48 h in the LPS-stimulated group and BMSC-treated group.
Macrophages were isolated from the peritoneal cavity by collecting
as much peritoneal fluid as possible, and the collected fluid was
centrifuged at 4°C for 5 min at 192 × g to pellet the cells. The
pellet was resuspended and cultured in 6-well plates, with complete
medium, at 37°C and 5% CO2 in air. After 6 h,
non-adherent cells were discarded. Medium was replaced with fresh
medium. After 24 h, the adherent cells were harvested to analyze
the expression of CD68, CD11c and CD206 markers (Table I) by flow cytometry at the Sun
Yat-sen Memorial Hospital of Sun Yat-sen University, as described
in a later paragraph.
Histopathological examination of
lungs
Whole lung samples were fixed in 10% formalin for 24
h at room temperature and were sent to Google Biological Technology
Co., Ltd. (Wuhan, China) for further procedure, where they were
subjected to conventional methods of dehydration,
paraffin-embedding and hematoxylin and eosin (H&E) staining.
All the lung tissues (thickness: 5 µm) were observed with an
inverted fluorescence microscope (TI-S; Nikon Corporation, Tokyo,
Japan).
Flow cytometry analysis of cell
markers in vitro and in vivo
Flow cytometry was used to assess the cell surface
or intracellular marker, CD68, of BMSCs and macrophages, as
described in previous literature (22–26).
Adherent cells (2×105 cells/centrifuge tube) were
lightly trypsinized, then blocked with complete medium for 2 min at
room temperature, harvested and incubated with the specific primary
and secondary antibodies for 30 min at 4°C in the dark. The
following antibodies were used for BMSCs: Anti-CD29, anti-CD44,
anti-CD45 and anti-CD90 (Table I).
Similarly, the following antibodies for macrophages were used:
Anti-CD11b and anti-CD68 (Table
I). The following antibodies were used for macrophages that
were collected from the in vitro co-culture system or in
vivo animal model: Anti-CD206, F(ab')2 donkey anti-rabbit
IgG-phycoerythrin, anti-CD11c and anti-CD68 (Table I). Specifically, cells were fixed
and permeabilized using a FIX & PERM™ Cell Permeabilization kit
(Invitrogen; Thermo Fisher Scientific, Inc.) prior to the addition
of anti-CD68. The recommended isotype controls for each
fluorochrome were used. Following incubation, cells were washed
with PBS and analyzed using a FACSVerse flow cytometer and FACSuite
(BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
For protein extraction in vitro, following
direct or Transwell-separated co-culture of macrophages with BMSCs
for 48 h, including an initial 12 h period of LPS treatment, cells
were washed with ice-cold PBS and lysed in radioimmunoprecipitation
assay buffer (BestBio Science, Shanghai, China) containing a
protease inhibitor cocktail. After 30 min, cell lysates were
collected and centrifuged at 13,400 × g for 20 min at 4°C; the
supernatant was prepared as a protein extract. The supernatant was
measured for protein concentration by a Bicinchoninic Acid Protein
assay kit (Bioworld Technology, Inc., St. Louis Park, MN, USA). A
total of 20 µg protein was separated by 10% SDS-PAGE Bis-Tris gels
and transferred to polyvinylidene fluoride membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) in ice-cold water for 2 h.
The membranes were washed three times with Tris-buffered saline
with Tween-20 (TBST) for 5 min and then blocked with 5% skimmed
milk powder dissolved in TBST for 2 h at room temperature.
Membranes were subsequently incubated with primary antibodies
against inducible nitric oxide synthase (iNOS), arginase-1 (Arg-1)
and β-actin for 16 h at 4°C (Table
I). The following horseradish peroxidase (HRP)-conjugated
secondary antibodies were used: Goat anti-rabbit IgG and goat
anti-mouse IgG for 3 h at room temperature (Table I). Protein expression was detected
using Biodlight™ ECL chemiluminescent HRP Substrate (Bioworld
Technology, Inc.) and an ImageQuant LAS 500 imaging system (GE
Healthcare Life Sciences, Buckinghamshire, UK). Blots against
β-actin served as the loading control. The amount of target protein
calculated by gray scanning via ImageJ v1.39 software (National
Institutes of Health Bethesda, MD, USA).
Analysis of cytokine production by
ELISA
In vitro, cell-free supernatants were
collected and centrifuged at 13,400 × g for 30 min at 4°C of each
group at 3, 7, 12, 24, 48, 72 h after LPS stimulation and assayed
for the concentration of TNF-α, transforming growth factor (TGF)-β
and IL-10 with ELISA kits (rat IL-10, DKW12-3100-096; rat TNF-α
DKW12-3720-096 and rat TGF-β, DKW12-3710-096; Dakewe Bioengineering
Co., Ltd., Shenzhen, China). The optical density was measured at
450 nm using a microplate reader (Multiskan GO; Thermo Fisher
Scientific, Inc.) with the correction wavelength set at 620 nm.
Statistical analysis
Data are presented as the mean ± standard error of
the mean from at least three independent experiments. According to
the characteristics of the data, different statistical methods were
used for analysis. The differences between groups were analyzed
with one-way analysis of variance followed by Fisher's least
significant difference or Dunnetts post-hoc tests, and Nemenyi
test, which was performed to analyze the results for the
percentages of CD206 and western blot analysis as a post hoc test
following analysis via the Kruskall-Wallis test. SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA) was used to perform statistical
analysis. GraphPad Prism 5.0 software was used to produce graphs
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of macrophages and
BMSCs
The cell surface antigen phenotype of macrophages
and BMSCs isolated from rats was assessed by flow cytometry.
Macrophages expressed both CD68 (100%) and CD11b (94.07%), while
BMSCs expressed high levels of CD44 (95.15%), CD29 (99.98%) and
CD90 (99.41%), and were considered negative for expression of CD45
(0.63%; data not shown). In addition, staining with Alizarin Red
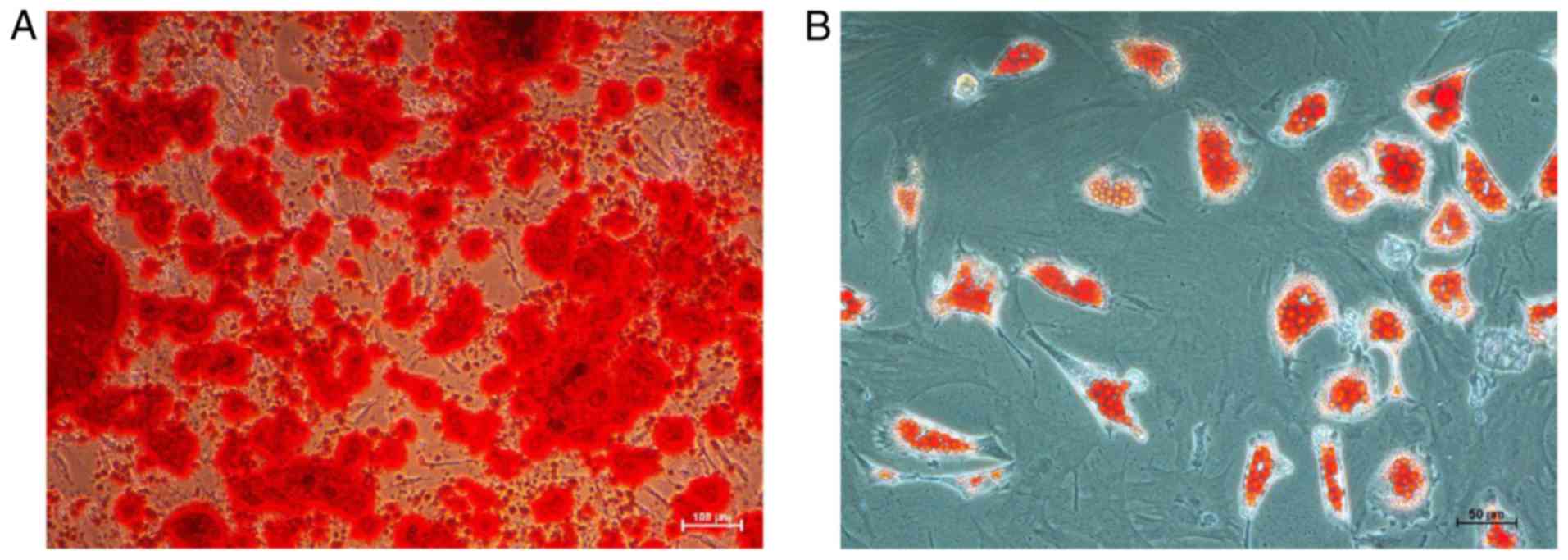
and Oil Red O demonstrated that BMSCs obtained from rats
successfully differentiated into osteogenic and adipogenic
mesenchymal lineages, respectively (Fig. 1).
Co-culture of macrophages with BMSCs
blocks LPS-induced macrophage polarization to the M1 phenotype
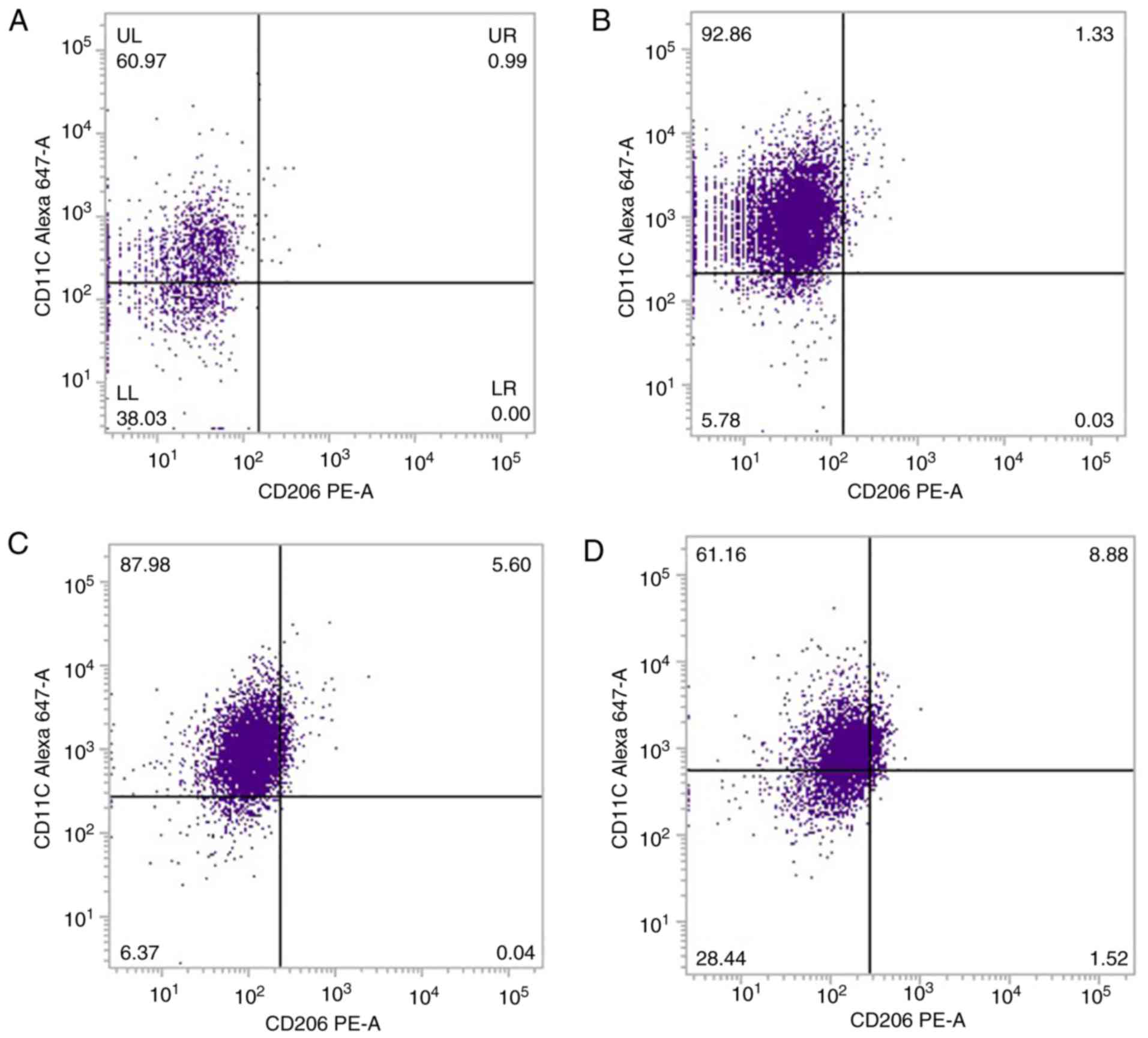
Flow cytometry analysis with a CD68 gating strategy
demonstrated that there was a high expression level of CD11c in
90.00±4.03% of LPS-stimulated peritoneal macrophages, compared with
unstimulated macrophages (Fig. 2A and
B; Table II), indicating that
the majority of macrophages in the LPS-stimulated group were M1
macrophages. Exposure of macrophages to LPS, while being indirectly
co-cultured with BMSCs in a Transwell, resulted in macrophages
acquiring the M1 phenotype, with 86.79±0.69% of the macrophages
expressing a high level of CD11c (Fig.
2C; Table II). However, when
peritoneal macrophages co-cultured directly with BMSCs on the same
substrate were exposed to LPS, a lower level of CD11c expression
was observed among macrophages, with 66.21±3.19% (Fig. 2D; Table II). The expression of CD206 in the
different groups was low and there was no significant difference
among the groups (Fig. 2; Table II).
 | Table II.Percentage of CD11c-positive and
CD206-postive macrophages in the different groups in
vitro. |
Table II.
Percentage of CD11c-positive and
CD206-postive macrophages in the different groups in
vitro.
| Group | CD11c, % | CD206, % |
|---|
| M | 53.48±5.43 | 0.61±0.27 |
| ML |
90.00±4.03a,b | 0.03±0.02 |
| MT | 86.79±0.69a,b | 0.03±0.15 |
| MM | 66.21±3.19 | 0.57±0.31 |
Macrophage co-culture with BMSCs
reduces LPS-induced cytokine release
Levels of TNF-α, IL-10 and TGF-β were quantified by
ELISA in cell culture supernatants. LPS stimulated macrophages to
produce high levels of TNF-α, compared with the control group
(Fig. 3A). No differences in TNF-α
release were observed among LPS-stimulated macrophage alone,
Transwell co-culture with BMSCs and direct co-culture with BMSCs
groups at 3, 7 and 12 h following LPS addition. However, after 24,
48 and 72 h, TNF-α levels were decreased when macrophages were
directly co-cultured with BMSCs, compared with the LPS-stimulated
macrophage alone group (Fig. 3A).
IL-10 levels were increased markedly at 7, 12, 48 and 72 h
following LPS stimulation, compared with the control group, with
levels particularly high when macrophages were co-cultured with
BMSCs (Fig. 3B). TGF-β was induced
in the group that consisted of direct co-culture of macrophages and
BMSCs stimulated by LPS, but not in the Transwell co-culture of
macrophages with BMSCs (Fig. 3C).
These results indicate that co-culture of macrophages with BMSCs
may lead to the inhibition of proinflammatory cytokine production
and stimulation of anti-inflammatory factor production (Fig. 3).
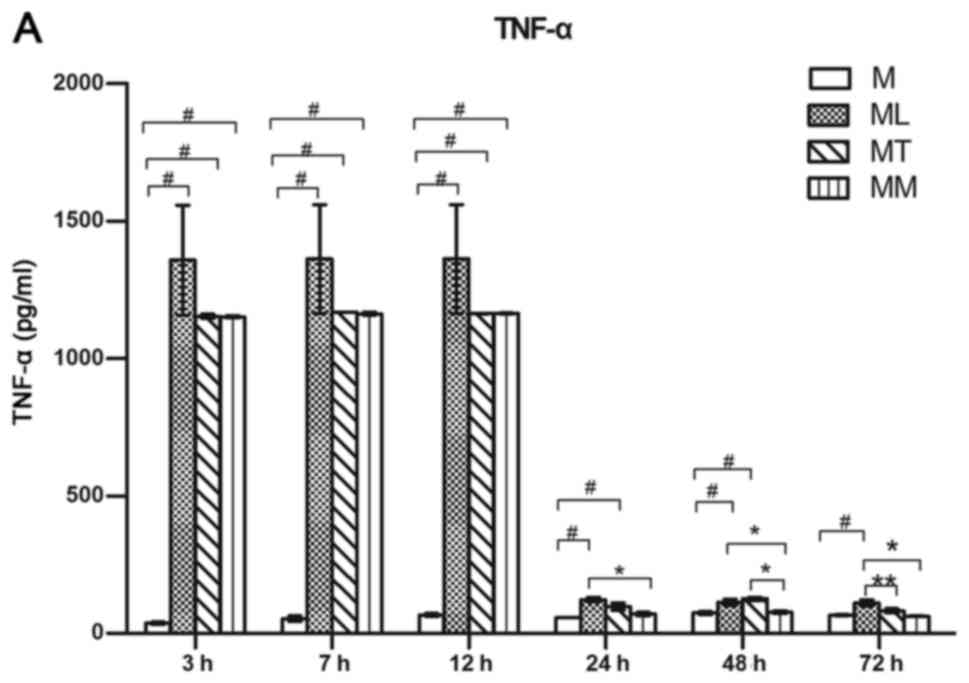 | Figure 3.Secretion levels of TNF-α, IL-10 and
TGF-β in cell culture medium were measured by ELISA. (A) Direct
co-culture of with BMSCs inhibited the production of the
proinflammatory cytokine TNF-α in rat peritoneal macrophages.
Direct co-culture with BMSCs enhanced the production of (B) IL-10
and (C) TGF-β in peritoneal macrophages. Results are presented as
the mean ± standard error of the mean, n=3 per group.
#P<0.05 vs. M group, **P<0.05 vs. MT group and
*P<0.05 vs. MM group, as indicated. TNF, tumor necrosis factor;
IL, interleukin; TGF, transforming growth factor; BMSCs, bone
marrow mesenchymal stem cells; M, macrophages cultured in normal
medium for 72 h; ML, macrophages undergoing a 12 h incubation with
LPS (100 ng/ml) followed by culture in normal medium for 60 h; MT,
macrophages co-cultured with BMSCs, separated in a Transwell,
undergoing a 12 h incubation with LPS followed by culture in normal
medium for 60 h; MM, macrophages and BMSCs directly co-cultured,
attached to the same substrate, undergoing a 12 h incubation with
LPS followed by culture in normal medium for 60 h. |
BMSCs reciprocally regulate iNOS and
Arg-1 in activated macrophages
iNOS and Arg-1 are markers of the M1 and M2
phenotypes, respectively. The present study analyzed the protein
expression of iNOS and Arg-1 by western blot analysis in the four
groups in vitro. The results demonstrated that iNOS
expression was increased in macrophages following LPS stimulation,
which was markedly decreased by direct co-culture with BMSCs
(Fig. 4). By contrast, Arg-1 was
markedly induced in macrophages following direct co-culture with
BMSCs, compared with the other three groups. These results
demonstrated that LPS stimulation of macrophages alone induced the
expression of iNOS, while the presence of BMSCs caused macrophages
to polarize towards an M2 phenotype following LPS stimulation
(Fig. 4).
 | Figure 4.Western blot analysis of iNOS and
Arg-1 protein expression following co-culture of peritoneal
macrophages with BMSCs. (A) Representative western blot bands for
iNOS, Arg-1 and β-actin in each group. (B) Densitometric analysis
was performed to quantify protein expression. BMSCs inhibited
LPS-induced induction of iNOS and enhanced Arg-1 expression in
peritoneal macrophages. n=5 per group; *P<0.05 as indicated.
iNOS, inducible nitric oxide synthase; Arg-1, arginase-1; BMSCs,
bone marrow mesenchymal stem cells; LPS, lipopolysaccharide; M,
macrophages cultured in normal medium for 48 h; ML, macrophages
undergoing a 12 h incubation with LPS (100 ng/ml) followed by
culture in normal medium for 36 h; MT, macrophages co-cultured with
BMSCs, separated in a Transwell, undergoing a 12 h incubation with
LPS followed by culture in normal medium for 36 h; MM, macrophages
and BMSCs directly co-cultured, attached to the same substrate,
undergoing a 12 h incubation with LPS followed by culture in normal
medium for 36 h. |
BMSC administration reduces
LPS-induced sepsis in vivo
The effect of BMSCs in the septic rat model induced
by LPS (5 mg/kg) via tail vein injection was also investigated. At
1 h after injection of LPS, rats in the BMSC-treated group were
injected with 1 ml BMSCs (2×106 cells/ml). Subsequently,
lung tissues from LPS-stimulated and BMSC-treated groups were
collected at 6, 12, 24 and 48 h for histopathological examination,
while lung tissues from the normal control group were only
collected at 24 h after injection with LPS. Lung tissues in the
normal control group at 24 h after injection of LPS exhibited
normal and complete alveoli without congestion or inflammatory cell
infiltration. Histological assessment of representative lung
sections from rats with LPS-induced sepsis highlighted a large
degree of inflammatory cell infiltration, serious congestion and
pulmonary interstitial thickening at 12 h after LPS administration.
However, the inflammatory response detected in lung tissues was
decreased by injection of BMSCs, as tissues exhibited relatively
normal alveoli accompanied by a small degree of inflammatory cell
infiltration, and marginal congestion and pulmonary interstitial
thickening. The pathological observations at 12 and 24 h in the
LPS-stimulated and BMSCs-treated groups were similar. At 48 h, the
inflammatory response was marginally improved in the LPS-simulated
group, with inflammatory cell infiltration, congestion, while
pulmonary interstitial thickening was decreased compared with at 12
and 24 h in the LPS-stimulated group. Alterations in the
BMSC-treated group returned to normal levels without congestion or
inflammatory cell infiltration (Fig.
5).
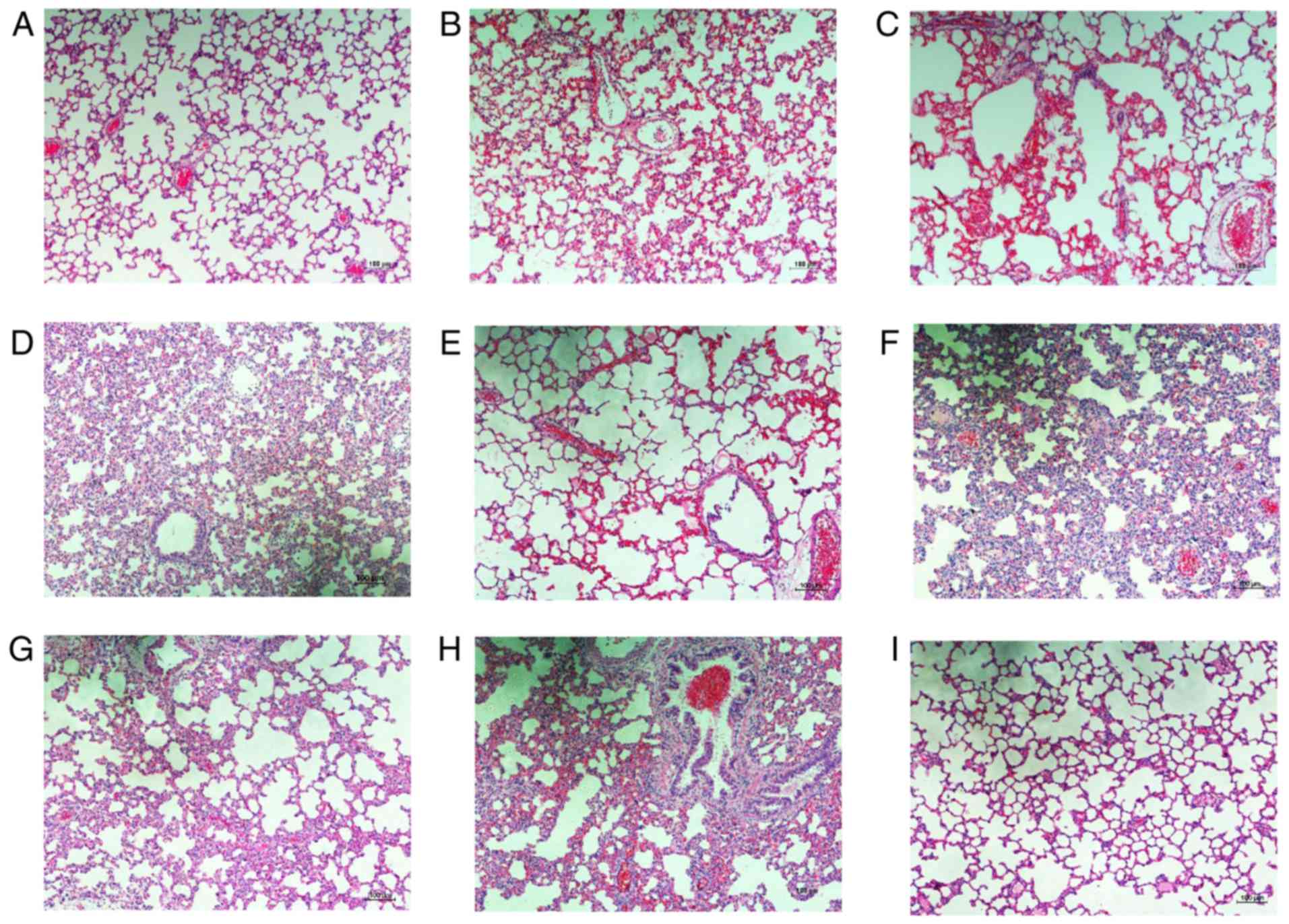 | Figure 5.Representative images of
H&E-stained lung tissue from experimental animals.
Representative images of lung tissue stained with H&E are
presented for (A) control group at 24 h, (B) LPS-stimulated group
at 6 h, (C) BMSC-treated group at 6 h, (D) LPS-stimulated group at
12 h, (E) BMSC-treated group at 12 h, (F) LPS-stimulated group at
24 h, (G) BMSC-treated group at 24 h, (H) LPS-stimulated group at
48 h and (I) BMSC-treated group at 48 h. Scale bar, 100 µm.
Magnification, ×100, n=4 per group. H&E, hematoxylin and eosin;
LPS, lipopolysaccharide; BMSC, bone marrow mesenchymal stem
cells. |
Flow cytometry analysis of cell
markers in LPS-induced sepsis in rats
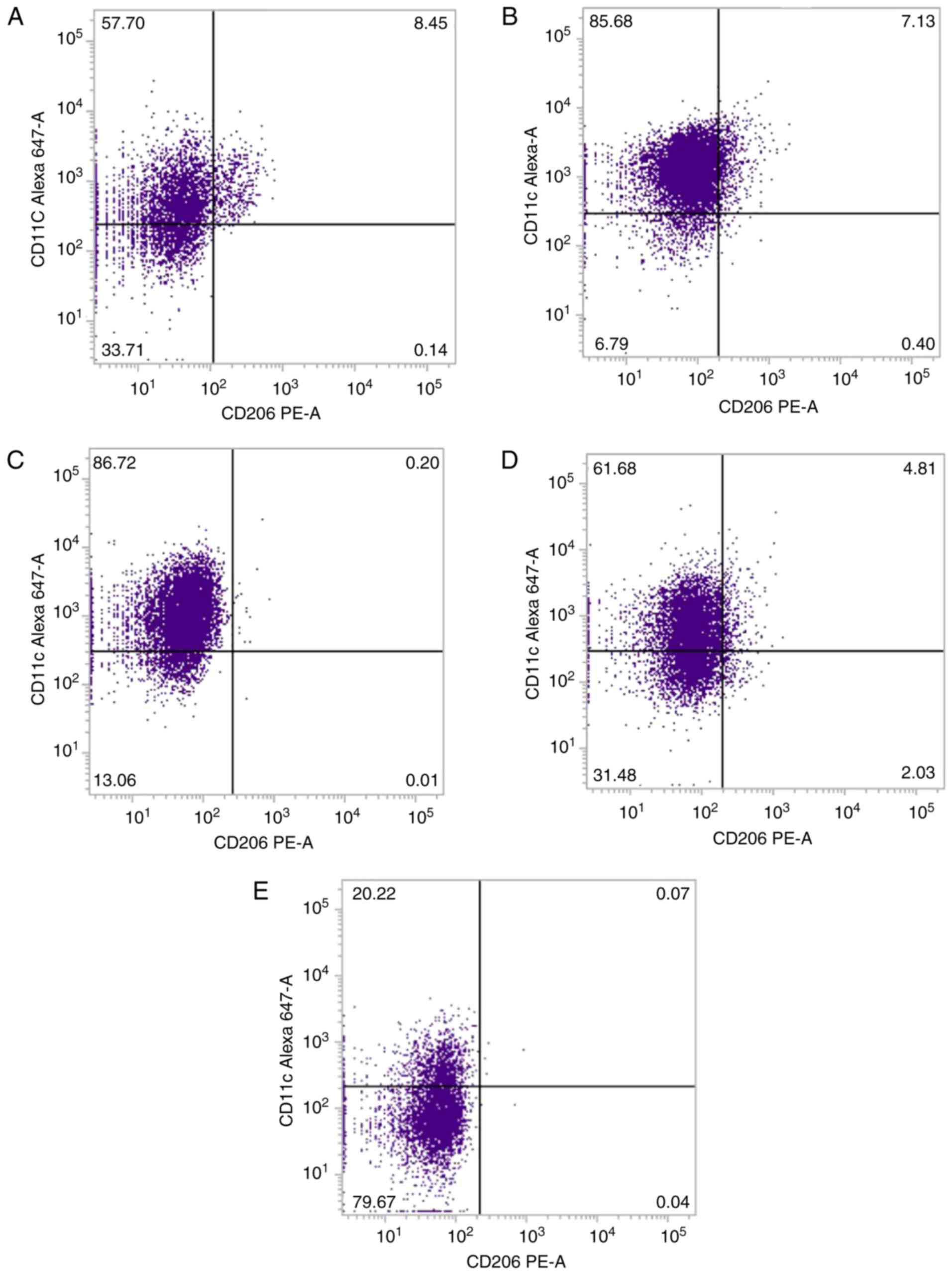
In order to confirm our hypothesis that BMSCs may
enhance the anti-inflammatory phenotype and inhibit the
proinflammatory phenotype of macrophages, the present study
investigated CD11c and CD206 expression on the surface of
macrophages isolated from septic rats. The ratio of macrophages
that expressed CD11c in the LPS-stimulated group was similar to the
ratio in the BMSC-treated group at 24 h after LPS injection
(P>0.05; Table III; Fig. 6), while it was significantly higher
compared with the normal control group (P<0.01; Table III; Fig. 6). Compared with the normal control
group, the ratio of CD11c-positive macrophages was marginally
increased in the LPS-stimulated group at 48 h after LPS injection
(P>0.05; Table III; Fig. 6), while it was significantly higher
compared with the BMSC-treated group at 48 h after LPS injury
(P<0.01; Table III; Fig. 6). The ratio of CD206-positive
macrophages was similar in each group, with no significant
differences among the groups (Table
III; Fig. 6).
 | Table III.Percentage of CD11c-positive and
CD206-positive macrophages in the different groups in
vivo. |
Table III.
Percentage of CD11c-positive and
CD206-positive macrophages in the different groups in
vivo.
| Group | CD11c, % | CD206, % |
|---|
| Normal control
group | 50.90±4.94 | 0.22±0.12 |
| LPS, 24 h |
87.43±2.24a | 0.11±0.10 |
| LPS+BMSCs, 24
h | 86.82±3.97a | 0.03±0.02 |
| LPS, 48 h |
61.40±5.57b | 1.83±0.28 |
| LPS+BMSCs, 48
h | 23.07±4.64a | 0.61±0.56 |
Discussion
The pathophysiology of sepsis involves complex
interactions between microbial pathogens and the host immune system
to regulate inflammation and coagulation (27). The immune and neurohumoral systems
closely control inflammation under normal circumstances and
eliminate pathogen invasion. When microorganisms or microbial
products invade the body and macrophages are polarized into the M1
phenotype, excessive levels of proinflammatory factors, including
TNF-α, IL-1β and IL-6, are produced, which results in a cascade of
inflammatory responses and leads to systemic inflammation, severe
sepsis and potentially septic shock (28–31).
Therefore, it is important that macrophage phenotype is regulated
and the excessive activation of M1 macrophages is avoided in
sepsis. Due to their immunoregulatory effects, BMSCs have wide
application potential in tissue engineering. BMSCs have been
reported to regulate immune cells, including mononuclear
macrophages, and control excessive inflammatory reactions (32).
The current study investigated the effects of BMSCs
on the polarization of macrophages in vitro and in
vivo. Identification of BMSCs by flow cytometry demonstrated
that the percentage of cells expressing BMSC surface marker
characteristics was consistent with the definition of BMSCs
provided by the International Society of Cellular Therapy (22,23).
Furthermore, the results of the present study also confirmed the
BMSCs were capable of differentiating into osteoblast and adipocyte
phenotypes, indicating that BMSCs have the capacity to
differentiate into mesenchymal lineages under the appropriate
conditions in vitro. In the macrophage system of the present
study, >90% of cells expressed the macrophage markers CD11b and
CD68. These results indicated that the purity of BMSCs and
macrophages in the current study complied with the essential
requirements for our experiments. According to previous literature,
macrophages express a variety of markers. CD11c and CD206 are
specific and widely used markers of M1 and M2 macrophages,
respectively, and these two surface molecules were selected in the
present study to represent M1 and M2 macrophages (6).
In the present study, the in vitro co-culture
experiments demonstrated that macrophages stimulated by LPS
expressed higher levels of CD11c and iNOS, in addition to increased
secretion of the proinflammatory cytokine TNF-α. However, these
observations were reversed following direct co-culture of
macrophages with BMSCs, and increased levels of Arg-1, IL-10 and
TGF-β were also reported in the direct co-culture group compared
with LPS stimulation of macrophages alone. Liu et al
(11) reported that TNF-α release
was the standard response of LPS-stimulated macrophages and has a
central role in death caused by endotoxemic shock. The results of
the current study demonstrated that LPS stimulation of macrophages
resulted in a rapid increase in the levels of TNF-α, with a
marginal repression by BMSCs as early as 3, 7 and 12 h after LPS
stimulation. However, after 24, 48 and 72 h, direct co-culture of
macrophages with BMSCs led to significant reductions in the TNF-α
levels, compared with the stimulation of macrophages alone with
LPS. In addition, at the majority of time-points, the levels of
IL-10 and TGF-β peaked in the group of macrophages that were
directly co-cultured with BMSCs. These results illustrated that LPS
promoted the ratio of M1-polarized macrophages and increased the
secretion of proinflammatory cytokines; however, the presence of
BMSCs inhibited such alterations and increased the differentiation
of M2 macrophages when co-cultured with macrophages directly. This
indicates that the interaction between BMSCs and macrophages may be
due to cell-to-cell contact, rather than paracrine cytokines, which
differs from previous reports (16,33–35).
We hypothesize that this may due to the fact that, after LPS
stimulation for 12 h, the original medium was replaced by the
normal complete medium, and cytokines in supernatants were
subsequently replaced, which may cause the paracrine effect to be
less obvious, thus the effect of direct cell-cell contact effect in
the experiment played a major role relatively. Western blot
analysis and ELISA results demonstrated that macrophages in the
BMSC treatment group expressed M2 markers, which was inconsistent
with the results of flow cytometry. A number of factors may explain
these inconsistencies. Firstly, the polarization of macrophages was
a continuous process, with the M1 and M2 phenotypes being two
extreme examples. At any stage of the polarization of macrophages
towards M1 or M2 phenotypes, there may be a process of secretion of
special proteins and cytokines by macrophages during polarization.
LPS activated macrophages strongly and an M1 phenotype was induced.
However, surface markers for M2 were not detected in the present
research. In addition, the present study employed different
compared with other studies. The RAW264.7 cell line has been
employed in other reports (18,19),
which may not reflect the real in vivo situation as they
have been previously modified. In the current study, the peritoneal
macrophages used differ from RAW264.7 cells as they cannot be
induced to polarize towards the M2 phenotype, and the use of
peritoneal macrophages in the present study primarily reflected
alterations in the physiological functions of macrophages in
response to external stimuli. Furthermore, different types of
antibodies were employed in the current study; our experimental
team searched a number of antibody manufacturers and did not locate
a direct labelled antibody for CD206, which was the most suitable
type for the experiment, and an indirect antibody was employed to
mark CD206, which meant the efficiency of labeling was lower, while
direct labeling was used to detect the expression of other cell
surface markers.
The histopathology determined by H&E staining of
lungs from septic rats demonstrated that, 12 h after LPS injection,
the inflammatory response in lung tissues was increased, which was
confirmed by inflammatory cell infiltration, thickened lung
interstitium and decreased alveolar space, indicating that lungs
suffered from sepsis. However, injection of BMSCs alleviated the
pathological changes. This was similar to previous findings that
stem cells reduced inflammation in various organs (18,36,37).
In the present study, flow cytometry results demonstrated that LPS
induced macrophages to differentiate into M1 macrophages after 24
h, and the polarization was not be reversed by BMSCs at the early
stage. However, by 48 h, the expression of CD11c returned to a
normal level in the LPS-stimulated group, while rats injected with
BMSCs exhibited significantly reduced polarization of macrophages
into the M1 phenotype, compared with the LPS-stimulated group.
However, BMSC injection did not promote the polarization of
macrophages into the M2 phenotype when examined at 24 or 48 h.
These results indicate that BMSCs may decrease the inflammatory
response in the lung tissues and inhibit the polarization of
peritoneal macrophages into an M1 phenotype. The above results were
consistent with previous that investigate whether the injection of
BMSCs in animals may reduce the inflammatory response in various
diseases (18,24,27,37,38).
Investigating the effect of BMSCs on alterations in
macrophage polarization in vivo and in vitro may
provide the basis for the future application of BMSCs in the
treatment of different diseases. Due to the complexity of the
immune system, the mechanism of BMSCs in in vivo treatment
are associated with more complex regulatory networks and specific
factors than in vitro, and its mechanism for initiation and
termination remain unclear. However, stem cells are associated with
their own limitations and safety issues. The strict requirements
for donor age and the insufficient systematic delivery of MSCs to
target tissues limits the use of stem cells (39). In the present study, animal model
results indicated that BMSCs may have great potential, however, rat
studies may not be identical with humans. Further studies
overcoming the application of MSCs are required.
In conclusion, the results of the present study
demonstrated that BMSCs co-cultured directly with macrophages
prevented LPS-stimulated macrophages from differentiating into an
M1 phenotype, as determined by the reduced expression of CD11c in
peritoneal macrophages and the reduced levels of inflammatory
cytokines released. Furthermore, in vivo results indicated
that BMSCs exhibited therapeutic effects on experimental sepsis by
reducing the pathological inflammatory response in the lung
tissues.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81271329 and
81471237) and the Medical Scientific Research Foundation of
Guangdong Province of China (grant no. A2016108). The authors
gratefully acknowledge Dr Stanley Lin (Shantou University, Shantou,
China) for his assistance with the English drafting of the
manuscript.
References
|
1
|
Annane D, Bellissant E and Cavaillon JM:
Septic shock. Lancet. 365:63–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). Jama.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen J, Vincent JL, Adhikari NK, Machado
FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S,
et al: Sepsis: A roadmap for future research. Lancet Infect Dis.
15:581–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seymour CW, Rea TD, Kahn JM, Walkey AJ,
Yealy DM and Angus DC: Severe sepsis in pre-hospital emergency
care: Analysis of incidence, care and outcome. Am J Respir Crit
Care Med. 186:1264–1271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang X, Goncalves R and Mosser DM: The
isolation and characterization of murine macrophages. Curr Protoc
Immunol. 14Unit 14.1. 2008. View Article : Google Scholar
|
|
7
|
Ying W, Cheruku PS, Bazer FW, Safe SH and
Zhou B: Investigation of macrophage polarization using bone marrow
derived macrophages. J Vis Exp. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ivashkiv LB: Epigenetic regulation of
macrophage polarization and function. Trends Immunol. 34:216–223.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ka MB, Daumas A, Textoris J and Mege JL:
Phenotypic diversity and emerging new tools to study macrophage
activation in bacterial infectious diseases. Front Immunol.
5:5002014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
López-Bojórquez LN, Dehesa AZ and
Reyes-Terán G: Molecular mechanisms involved in the pathogenesis of
septic shock. Arch Med Res. 35:465–479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu LL, Gong LK, Wang H, Xiao Y, Wu XF,
Zhang YH, Xue X, Qi XM and Ren J: Baicalin inhibits macrophage
activation by lipopolysaccharide and protects mice from endotoxin
shock. Biochem Pharmacol. 75:914–922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simovart HE, Põldoja E, Kokk K, Tapfer H,
Liigant A, Talvik R and Roosaar P: Changes of activated macrophages
and apoptotic cell count in the organs of rats during experimental
sepsis. Medicina (Kaunas). 39:932–939. 2003.PubMed/NCBI
|
|
13
|
Prockop DJ and Oh JY: Mesenchymal
stem/stromal cells (MSCs): Role as guardians of inflammation. Mol
Ther. 20:14–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soleimani M and Nadri S: A protocol for
isolation and culture of mesenchymal stem cells from mouse bone
marrow. Nat Protoc. 4:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB,
Choi SJ, Kim SW, Yang YS, Oh W and Chang JW: Comparative analysis
of human mesenchymal stem cells from bone marrow, adipose tissue,
and umbilical cord blood as sources of cell therapy. Int J Mol Sci.
14:17986–18001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang QZ, Su WR, Shi SH, Wilder-Smith P,
Xiang AP, Wong A, Nguyen AL, Kwon CW and Le AD: Human
gingiva-derived mesenchymal stem cells elicit polarization of m2
macrophages and enhance cutaneous wound healing. Stem Cells.
28:1856–1868. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wise AF, Williams TM, Kiewiet MB, Payne
NL, Siatskas C, Samuel CS and Ricardo SD: Human mesenchymal stem
cells alter macrophage phenotype and promote regeneration via
homing to the kidney following ischemia-reperfusion injury. Am J
Physiol Renal Physiol. 306:F1222–F1235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geng Y, Zhang L, Fu B, Zhang J, Hong Q, Hu
J, Li D, Luo C, Cui S, Zhu F and Chen X: Mesenchymal stem cells
ameliorate rhabdomyolysis-induced acute kidney injury via the
activation of M2 macrophages. Stem Cell Res Ther. 5:802014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao S, Mao F, Zhang B, Zhang L, Zhang X,
Wang M, Yan Y, Yang T, Zhang J, Zhu W, et al: Mouse bone
marrow-derived mesenchymal stem cells induce macrophage M2
polarization through the nuclear factor-κB and signal transducer
and activator of transcription 3 pathways. Exp Biol Med (Maywood).
239:366–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ray A and Dittel BN: Isolation of mouse
peritoneal cavity cells. J Vis Exp. 28:pii: 14882010.
|
|
21
|
Chai X, Guo Y, Jiang M, Hu B, Li Z, Fan J,
Deng M, Billiar TR, Kucera HR, Gaikwad NW, et al: Oestrogen
sulfotransferase ablation sensitizes mice to sepsis. Nat Commun.
6:79792015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng G, Ge M, Qiu G, Shu Q and Xu J:
Mesenchymal stromal cells affect disease outcomes via macrophage
polarization. Stem Cells Int. 2015:9894732015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim J and Hematti P: Mesenchymal stem
cell-educated macrophages: A novel type of alternatively activated
macrophages. Exp Hematol. 37:1445–1453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JW, Gupta N, Serikov V and Matthay MA:
Potential application of mesenchymal stem cells in acute lung
injury. Expert Opin Biol Ther. 9:1259–1270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murray PJ and Wynn TA: Obstacles and
opportunities for understanding macrophage polarization. J Leukoc
Biol. 89:557–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abumaree MH, Al Jumah MA, Kalionis B,
Jawdat D, Al Khaldi A, Abomaray FM, Fatani AS, Chamley LW and Knawy
BA: Human placental mesenchymal stem cells (pMSCs) play a role as
immune suppressive cells by shifting macrophage differentiation
from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell
Rev. 9:620–641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mei SH, Haitsma JJ, Dos Santos CC, Deng Y,
Lai PF, Slutsky AS, Liles WC and Stewart DJ: Mesenchymal stem cells
reduce inflammation while enhancing bacterial clearance and
improving survival in sepsis. Am J Respir Crit Care Med.
182:1047–1057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zenker S, Panteleev-Ivlev J, Wirtz S,
Kishimoto T, Waldner MJ, Ksionda O, Tybulewicz VLJ, Neurath MF and
Atreya I: A key regulatory role for Vav1 in controlling
lipopolysaccharide endotoxemia via macrophage-derived IL-6. J
Immunol. 192:2830–2836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boomer JS, To K, Chang KC, Takasu O,
Osborne DF, Walton AH, Bricker TL, Jarman SD II, Kreisel D,
Krupnick AS, et al: Immunosuppression in patients who die of sepsis
and multiple organ failure. Jama. 306:2594–2605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang SH, Hou YC, Chang WC, Hsiu SL, Lee
Chao PD and Chiang BL: Morin sulfates/glucuronides exert
anti-inflammatory activity on activated macrophages and decreased
the incidence of septic shock. Life Sci. 74:743–756. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ayala A, Elphick GF, Kim YS, Huang X,
Carreira-Rosario A, Santos SC, Shubin NJ, Chen Y, Reichner J and
Chung CS: Sepsis-induced potentiation of peritoneal macrophage
migration is mitigated by programmed cell death receptor-1 gene
deficiency. J Innate Immun. 6:325–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oh JY, Ko JH, Lee HJ, Yu JM, Choi H, Kim
MK, Wee WR and Prockop DJ: Mesenchymal stem/stromal cells inhibit
the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen
species. Stem Cells. 32:1553–1563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gotts JE and Matthay MA: Mesenchymal stem
cells and acute lung injury. Crit Care Clin. 27:719–733. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao G, Miao H, Li X, Chen S, Hu Y, Wang Z
and Hou Y: TGF-beta3-induced miR-494 inhibits macrophage
polarization via suppressing PGE2 secretion in mesenchymal stem
cells. FEBS Lett. 590:1602–1613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luz-Crawford P, Djouad F, Toupet K, Bony
C, Franquesa M, Hoogduijn MJ, Jorgensen C and Noël D: Mesenchymal
stem cell-derived interleukin 1 receptor antagonist promotes
macrophage polarization and inhibits B cell differentiation. Stem
Cells. 34:483–492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nemeth K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E (2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q,
Monsel A, Qu JM, Matthay MA and Lee JW: Human mesenchymal stem cell
microvesicles for treatment of Escherichia coli endotoxin-induced
acute lung injury in mice. Stem Cells. 32:116–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weil BR, Herrmann JL, Abarbanell AM,
Manukyan MC, Poynter JA and Meldrum DR: Intravenous infusion of
mesenchymal stem cells is associated with improved myocardial
function during endotoxemia. Shock. 36:235–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bernardo ME and Fibbe WE: Mesenchymal
stromal cells: Sensors and switchers of inflammation. Cell Stem
Cell. 13:392–402. 2013. View Article : Google Scholar : PubMed/NCBI
|