Introduction
Knee osteoarthritis (KOA) is a chronic and
degenerative disease that threatens global health. With increased
population aging, the incidence of KOA is growing at an alarming
rate (1). Occurring predominantly
in the elderly, it is manifested by pain and articular function
disorder (2) in clinic, cartilage
degeneration, subchondral sclerosis and osteophyte formation in
pathology. Among the recent research outcomes, novel biological
markers have become more promising tools or indicators to assess
the severity and progress of local KOA (3).
The fibroblast-like synoviocyte (FLS) has been
identified to involve in the progression of KOA, it may play a
crucial role via the secretion of various of pro-inflammatory
cytokines, such as IL-1β, IL-18 and TNF-α. These cytokines may
directly participate and induce the local synovial inflammation and
cartilage matrix degradation. IL-1β can activate the expression of
Transient Receptor Proteins (TRPs), which may lead to both thermal
and mechanical hyperalgesia (4–5).
IL-18 has been identified as the trigger of cartilage proteoglycan
degradation. TNF-α may regulate the sensitization of thermal and
mechanical nociceptors through activating the related TRP channels
(6). Current studies toward these
cytokines mostly focus on the inflammation-related cell signal
pathways, yet the clinical treatments still remain unsatisfactory.
Further hypothesis and research are needed to achieve the clinical
goal (7–10).
Cell apoptosis was classically defined as the
autonomous and programmed death controlled by genes in order to
maintain a stable internal environment. Chondrocyte and FLS
apoptosis is an active process involving the activation, expression
and regulation of many genes (11), they may be the main cause of
synovium inflammation or clinical symptoms.
Inflammasomes are intracellular multi-protein
complexes that can be activated by many exogenous and endogenous
factors, such as lipopolysaccharide (LPS), oxidative stress,
potassium efflux, and monosodium urate crystals (12). Inflammasome activation triggers the
maturation and expression of proinflammatory cytokines, such as
IL-1β and IL-18 mentioned above, to initiate innate immune
responses and subsequently induce cellular injury or even death
(13). The inflammasome-induced
cell death has been considered as a non-canonical programmed cell
death which mainly occurs in the inflammatory conditions (14). It is also defined as ‘cell
pyroptosis’. NLRP1 and NLRP3 inflammasomes are the most thoroughly
researched among all types of inflammasomes.
The NLRP inflammasomes consist of NLRP1/3 receptors,
apoptosis-associated Speck-like protein with a caspase-recruitment
domain (ASC) and pro-caspase-1 (caspase-1-induced pyroptosis is an
innate immune effector mechanism against intracellular bacteria).
The NLRP inflammasome is formed after the oligomerization of NLRP
receptors and subsequent recruitment of adaptor ASC and
pro-caspase-1. The activation of NLRP receptors inflammasome
finally triggers the maturation of IL-1β and IL-18 via activated
caspase-1 (15,16). Meanwhile, various of inflammatory
cytokines may also rapidly overflow, such as high mobility group
box proteins (HMGBs). Finally, the activation of NF-κB and other
canonical inflammatory signaling pathways may result in a cascaded
release of inflammatory factors, further promote the rapid progress
of inflammatory response (17,18).
KOA is a common aseptic inflammation disease, cell
apoptosis may participated in the entire pathogenesis of KOA.
However, the programmed cell death should be regulated by certain
signaling pathways without inducing any inflammatory reactions.
Cell pyroptosis is another form of programmed inflammatory cell
death, when cell apoptosis cannot be activated properly and cell
death indeed occurs. In the present study, we investigated the
correlation between the NLRP inflammasomes, OA inflammation and FLS
pyroptosis in patients' tissue and in vitro cells via
molecular biology and histochemical methods (19). Our hypothesis is that NLRP1 and
NLRP3 inflammasomes are crucial mediators of FLS pyroptosis in KOA.
Inhibition of NLRP inflammasome may be exert a protective
effect.
Patients and methods
Patients and synovial samples
Human synovial samples were collected with informed
consent in line with the Declaration of Helsinki (2000 revision).
The study protocol was approved by the local Ethics Committee of
Affiliated Hospital of Nanjing University of Traditional Chinese
Medicine (Jiangsu, China; approval no. 2015NL-068-02). The
diagnosis of OA was based on clinical and radiological evidence of
degenerative changes during surgery. Synovial tissues were obtained
under aseptic conditions from 10 OA patients undergoing total knee
replacement surgery. Samples of non-arthritic synovial tissues were
obtained at arthroscopy following trauma/joint derangement.
All the patients in this study were enrolled from
the Department of Orthopedic Surgery, The First Affiliated Hospital
of Nanjing University of Chinese Medicine (Jiangsu, China). Data
from a medical history and physical examination were compiled for
each patient. A total of 50% of the enrolled patients were
receiving non-steroidal anti-inflammatory drugs (NSAIDs) and 50%
were not on medication.
Culture and treatment of FLSs
Synovial tissues were minced into pieces of 2~3
mm2, homogenized in DMEM and then incubated overnight at
37°C with 1 mg/ml type I collagenase and 1 mg/ml type II dispase
(both Sigma-Aldrich, St. Louis, MO, USA). Following cell
dissociation, the samples were filtered through a cell strainer.
After dissociation, fibroblasts were pelleted by centrifugation at
1,000 rpm for 8 min and plated in DMEM supplemented with 10% FCS
(Gibco; Thermo Fisher Scientific, Waltham, MA, USA) and antibiotics
(100 U/ml penicillin, 100 ug/ml streptomycin; Invitrogen, Carlsbad,
CA, USA). Cells were cultured at 37°C in a humidified 95% air and
5% CO2 atmosphere. The cell confluence and morphology
were assessed throughout the experiments by immunohistochemical
analysis. All the functional experiments were conducted using
primary synovial cultured cells from passages 3–6.
FCL were stimulated with LPS (1 ug/ml) in DMEM for 6
h to stimulate the inflammatory reactions, and they were then
challenged with ATP (3 mM) for 1 h to activate the NLRP1 and NLRP3
inflammasome. The FCL exposed to DMEM with same volume of saline
served as controls (20,21).
Small-interfering RNA preparation and
transfection
To inhibit the NLRP1 and NLRP3 expression in the
FLS, commercially available NLRP1, NLRP3 and vehicle control small
interfering RNA (siRNA; Invitrogen) were used. FLSs were
transfected with siRNAs by using Lipofectamine 2000 (Invitrogen)
according to the manufacturer's instructions. siRNA was diluted in
transfection reagent and culture medium, and the cells were
incubated with 20 pmol siRNA for 6 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from synovial tissues and
FLSs by using TRIzol (Invitrogen). The reverse transcription was
performed by using a first strand cDNA synthesis kit (Takara, Otsu,
Japan) according to the manufacturer's instructions. qPCR was
performed using Premix Ex Taq SYBR-Green PCR (Takara) according to
the manufacturer's instructions on an ABI PRISM 7300 (Applied
Biosystems, Foster City, CA, USA). The sequences of the primers
were as follows: caspase-1 forward, 5′-CTACTTCCCTGAATGCTTGGC-3′ and
reverse, 5′-GCTCCTGGGTTTGTCCACTC-3′; NLRP1 forward,
5′-GCATGGGGACCTGACATGGG-3′ and reverse,
5′-GATGCAATCTCAGCACCGGAA-3′; NLRP3 forward,
5′-GCTCATATCATCATTCCCGCT-3′ and reverse,
5′-ACCAGCTACAAAAAGCATGGA-3′; ASC forward,
5′-CCAAATGCTTCCCCCATCCT-3′ and reverse, 5′-GCCCTTTGGTACATGCCTCT-3′;
GSDMD forward, 5′-TCACAACCTTGGGGCATCAG-3′ and reverse,
5′-TCCTTCCTGCAAGCTGGTTC-3′; IL-18 forward,
5′-TGCTGCAGTCTACACAGCTT-3′ and reverse,
5′-TTGTTGCGAGAGGAAGCGAT-3′GADPH; forward,
5′-ACGGGAAGCTTGTCATCAAT-3′ and reverse, 5′-TGGAAGATGGTGATGGGATT-3.
GAPDH was used as internal control.
Western blot analysis
Synovial tissues were dissected, weighed and mixed
with RIPA lysate. Samples were centrifuged at 4°C with 15,000 r/min
for 15 min. The supernatant was removed. Cultured cells were washed
and lysed. Then the protein levels were quantified with a BCA
protein Assay kit (Roche, Basel, Switzerland). The protein samples
were electrophoresed in SDS-PAGE to separate protein bands.
Proteins were transferred from gel onto PVDF membrane, blocked with
5% non-fat dry milk for 2 h. The membrane was incubated with
monoclonal rabbit antibodies specific for human NLRP1, NLRP3, ASC,
caspase-1, IL-18, GSDMD, β-actin and GAPDH (1:1,000; Invitrogen),
overnight at 4°C. On the next day, membranes were incubated with
second antibody (1:5,000; Invitrogen) for 2 h. The bands were
visualized by ECL method (Invitrogen) and the overall gray value of
protein bands (average gray value × gray value area) was quantified
with Photoshop CS5 (Adobe Systems, Inc., San Jose, CA, USA)
software to calculate the target protein relative value (target
protein gray value/internal reference overall gray value).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL assay)
Apoptosis in tissue was assessed using a TUNEL assay
and identified using the in situ cell death detection kit
(Roche) according to the protocol. In brief, sections were
deparaffinized using xylene reagent. The deparaffinized sections
were then incubated with proteinase K (30 g/ml), followed by
treatment with 0.3% hydrogen peroxide for 30 min at room
temperature. Incubation with the TUNEL reaction mixture was
performed in a humidified chamber at 37°C. Converter-POD was added
for signal conversion and incubated in a 3,3-diaminobenzidine
reaction mixture, which served as the chromogen. After dehydration,
the sections were mounted in DPX.
H&E and immunostaining
Both H&E staining and immunostaining were
applied to observe the morphology of FLS. The cells were washed
with PBS and treated with blocking buffer containing 1% BSA and
0.2% Triton X in PBS for 1 h at room temperature. The samples were
then incubated overnight at 4°C with anti-vimentin rabbit
polyclonal antibody (Invitrogen). After washing with PBS, the
secondary antibody was applied, and the signals were visualized
using an ABC kit (Santa Cruz Biotechnology, Dallas, TX, USA).
Flow cytometry
After treatment, the cells were double stained with
Annexin V-fluorescein isothiocyanate and propidium iodide (Annexin
V:FITC apoptosis detection kit; BD Biosciences, Santa Cruz, CA,
USA) according to the instructions. Quantification was then
performed by flow cytometry (Beckman Coulter, Miami, FL, USA).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6.0 Software (San Diego, CA, USA). All data shown in bar
graphs are mean ± SD. Data were analyzed by one-way or two-way
ANOVA with Bonferroni's or Dunnett's post hoc test for comparison
of multiple columns (as appropriate).
Results
Expression of NLRP1 and NLRP3
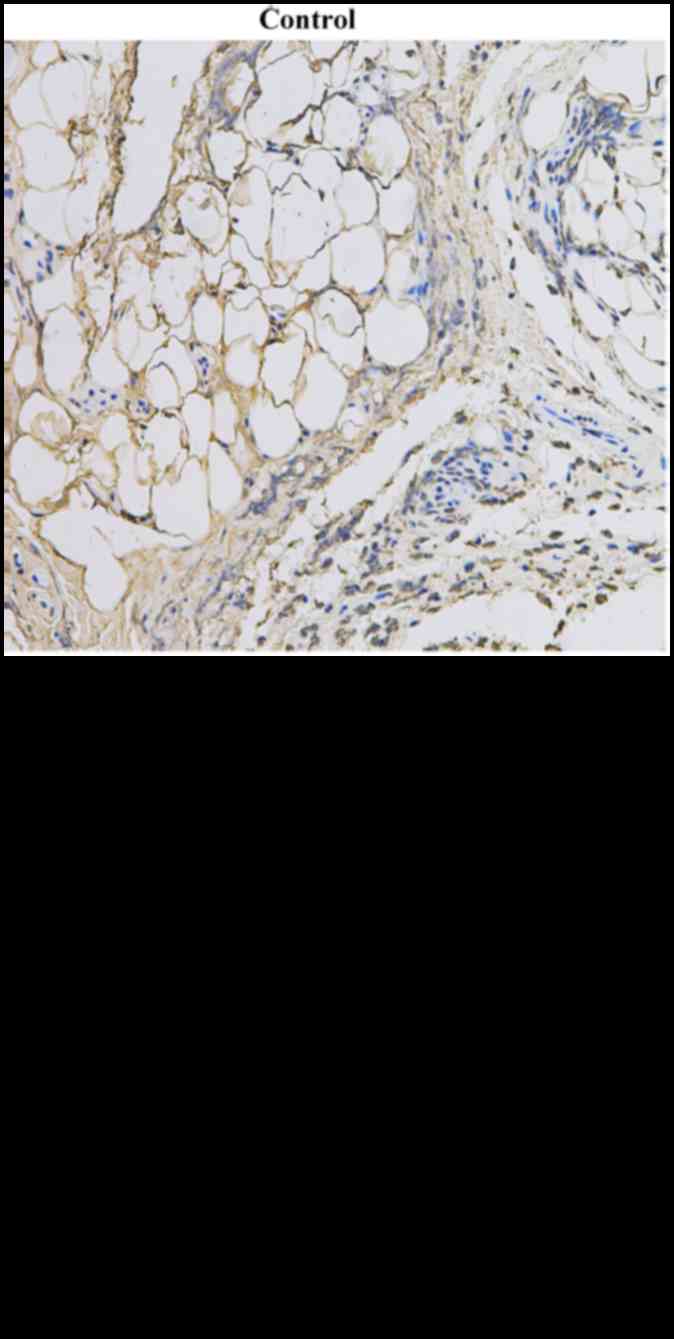
inflammasomes are upregulated in the synovia of KOA patients
As inflammation is an important part of KOA
progression, we examined the protein and mRNA levels of NLRP1 and
NLRP3 in the synovia of KOA patients. Patients with KOA were
included in this study, as shown in Table I. The protein and mRNA expression
of both NLRP1 and NLRP3 inflammasomes (consist of
NLRP1/NLRP3+ASC+caspase-1) were significantly upregulated in the
KOA patient tissues compared with controls (Figs. 1 and 2), TUNEL staining revealed the
TUNEL-positive cells in the KOA region (Fig. 3).
 | Table I.Information and histological diagnoses
of selected patients. |
Table I.
Information and histological diagnoses
of selected patients.
| Number | Sex | Age (years) | KOA duration
(years) | Receiving NSAIDs |
|---|
| 1 | Female | 51 | 6 | Yes |
| 2 | Female | 75 | 15 | Yes |
| 3 | Female | 52 | 2 | No |
| 4 | Male | 65 | 7 | No |
| 5 | Male | 54 | 10 | Yes |
| 6 | Female | 41 | 5 | No |
| 7 | Female | 59 | 7 | Yes |
| 8 | Male | 63 | 8 | No |
| 9 | Male | 77 | 20 | Yes |
| 10 | Female | 65 | 2 | No |
LPS+ATP induced cell programmed death
leads to the activation of NLRP1 and NLRP3 inflammasomes
The FLSs were firstly identified by H&E and
immunostaining (Fig. 4). After the
intervention of LPS and ATP, cell apoptosis was detected by flow
cytometry (Fig. 5). The cell
apoptosis rate in the LPS+ATP group was significantly higher than
the normal group (P<0.01). Meanwhile, the NLRP1 and NLRP3
inflammasome-related proteins were upregulated (Figs. 6 and 7), which means the pyroptosis may take
place during the subsequent LPS+ATP-induced inflammatory
reactions.
NLRP1 siRNA attenuates the LPS+ATP
induced inflammasomes activation in FLS
The silencing effect of the NLRP1 siRNA was also
confirmed by western blotting (Fig. 6A
and B). The normal FLS were used as a normal control. The
LPS-induced cell inflammation and pyroptosis were inhibited by the
NLRP1 siRNA, and the expression of ASC, caspase-1, IL-18 and GSDMD
in LPS+ATP and vehicle scrambled siRNA groups were significantly
higher than the NLRP1 siRNA group (Fig. 6C and D), meanwhile the relative
mRNA expression of IL-18 in NLRP1 siRNA group showed no significant
alternation comparing with LPS+ATP and vehicle scrambled siRNA
groups (Fig. 6E). The relative
mRNA expression of ASC, caspase-1, IL-18 and GSDMD in LPS+ATP and
vehicle scrambled siRNA groups were significantly higher than the
NLRP1 siRNA group (Fig. 6E).
NLRP3 siRNA attenuates the LPS+ATP
induced inflammasomes activation in FLS
The silencing effect of the NLRP3 siRNA were
confirmed by western blotting (Fig. 7A
and B). The normal FLS were used as a normal control. The
LPS-induced cell inflammation and pyroptosis were inhibited by the
NLRP3 siRNA, the protein and relative mRNA expression of ASC,
caspase-1 and GSDMD expression in LPS+ATP and vehicle scramble
siRNA group were significantly higher than the NLRP3 siRNA group,
which may reveal the fact that NLRPs are crucial determinant in
LPS-induced FLS pyroptosis (Fig.
7C-E).
Discussion
In the present study, the expression of NLRP1 and
NLRP3 inflammasomes were confirmed to be up-related in the tissue
of KOA patients. Meanwhile, in vitro experiments
demonstrated that after the treatment of LPS+ATP, NLRP1 and NLRP3
siRNAs may lead to a significantly reduction of
inflammasome-related mRNA and protein expression compared with
those with normal FLS. These findings indicated that both NLRP1 and
NLRP3 act as important inducers of FLS pyroptosis.
Although the etiology and pathogenesis of KOA has
not yet been fully defined, the cytokines IL-1β and IL-18 have
received extensive attention to the central pathogenesis of KOA. In
a previous study, Denoble et al (22) found that uric acid can activate
NLRP3 inflammasome, which results in the increase of IL-18 and
IL-1β. The uric acid and IL-18 and IL-1β in synovial fluid were
closely correlated with the severity of KOA, suggesting that
inflammasome signaling pathways may be involved in the development
and progression of KOA.
Activation of NLR inflammasomes and induction of
pyroptosis may require a two-step mechanism. The first step is the
priming step. Pro-inflammatory mediators, such as pro-IL-1β,
NLRP1/3 and caspase family members, are transcriptionally
generated. The second step is the activation step, where the
inflammasomes are assembled and caspase-1 is activated. Active
caspase-1 proteolytically matures pro-IL-1β and proIL-18 and
therefore induces pyroptosis partially through cleavage of
gasdermin D (GSDMD). Unlike caspase-1, caspase-11 is activated
through direct sensing of intracellular LPS in the cytoplasm of
infected cells, and promotes pyroptosis through GSDMD cleavage.
Pyroptosis features rapid plasma membrane rupture, resulting in the
release of intracellular pathogens and pro-inflammatory mediators,
including IL-1β, IL-18 and other inflammatory cytokines.
NAcht leucine-rich-repeat protein 1 (NLRP1, also
known as NALP1), a regulator of the innate immune response
expressed in various cell types. The NLRP1/IL-1β axis has been
reported to be associated with autoimmunity (23). The assembly of the NLRP1
inflammasome and the subsequent activation of caspase-1 cleaves the
inactive IL-1β precursor to the bioactive IL-1β, thus stimulating
downstream inflammatory responses (24). Some clinical evidences also suggest
that NLRP1 polymorphisms are also involved in the predisposition to
rheumatoid arthritis and other autoimmune diseases (25).
As mentioned above, the NLRP3 inflammasome is
receiving increasing attention in the recent researches since it
participates various diseases (26). Multiple mechanisms have been
proposed to activate NLRP3, including bacterial, viral and fungal
agents, K+ efflux, lysosomal destabilization, pore formation in the
plasma membrane, mitochondrial damage, the production of reactive
oxygen species, Ca2+ influx, and cell swelling. In the
progression of KOA, chronic inflammation should be the predominant
pathological change in the synovial tissues. Various exogenous and
endogenous stimuli are recognized by the NLRP3 receptor and trigger
the inflammasome activation (8).
In the recent study, Shi et al (27) revealed that increased LPS and ATP
in joint-space may promote KOA by NLRP3 Inflammasome, they showed
that inhibiting NLRP3 inflammasome may exert protective effect. Our
result supported the same fact that NLRP3 inflammasome is a crucial
part of KOA, especially in the pyroptosis of FLS.
In the present study, IL-18 mRNA expression showed
no significant alternation after the treatment of NLRP1 siRNA. The
possible reason should be that IL-18 is not mediated by NLRP1
inflammasome, the IL-18 protein expression is regulated by some
other inflammatory signal pathways. Besides, more in vivo
experiments should be undertaken in the future, in order to
approach more molecular mechanisms in the treatment of KOA
inflammation.
Inflammasomes are intracellular pattern recognition
receptors (PRRs) that play an important regulatory role in
stimulating and regulating immune and inflammatory responses.
Constantly updated data show that inflammasome/caspase-l/IL-1β
inflammatory pathways involved in the occurrence and development of
a variety of inflammatory diseases. Based on the data obtained from
patients and in vitro cells, we concluded that both NLRP1
and NLRP3 inflammasomes are highly involved in the FLS inflammation
and pyroptosis. Moreover, inhibition of NLRP1 and NLRP3 led to a
remarkable reduction of pyroptosis-related cytokines. But
currently, further study is required to determine the effect of
NLRP1 and NLRP3 inflammasome inhibitors/blockers in vivo.
Our further clinical study will focus on the application of
inflammasome/caspase-l/IL-1β pathway inhibitors in the treatment of
KOA. Its mechanism will provide new ideas and means for
understanding and treating KOA-related inflammatory diseases.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81573993).
Glossary
Abbreviations
Abbreviations:
|
KOA
|
knee osteoarthritis
|
|
FLS
|
fibroblast-like synoviocytes
|
|
NLRP
|
nod-like receptor protein
|
|
LPS
|
lipopolysaccharide
|
|
ATP
|
adenosine triphosphate
|
|
ASC
|
apoptosis-associated speck-like
protein with a caspase-recruitment domain
|
|
GSDMD
|
gasdermin D
|
|
siRNAs
|
small interfering RNAs
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
SDS-PAGE
|
sodium dodecyl sulphate polyacrylamide
gel electrophoresis
|
References
|
1
|
Higgs R: Osteoarthritis: Concentrated
efforts to detect early OA. Nat Rev Rheumatol. 6:6162010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okubo M and Okada Y: Destruction of the
articular cartilage in osteoarthritis. Clin Calcium. 23:1705–1713.
2013.(In Japanese). PubMed/NCBI
|
|
3
|
Wright AA, Cook C and Abbott JH: Variables
associated with the progression of hip osteoarthritis: A systematic
review. Arthritis Rheum. 61:925–936. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kochukov MY, McNearney TA, Yin H, Zhang L,
Ma F, Ponomareva L, Abshire S and Westlund KN: Tumor necrosis
factor-alpha (TNF-alpha) enhances functional thermal and chemical
responses of TRP cation channels in human synoviocytes. Mol Pain.
5:492009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernandes ES, Russell FA, Spina D,
McDougall JJ, Graepel R, Gentry C, Staniland AA, Mountford DM,
Keeble JE, Malcangio M, et al: A distinct role for transient
receptor potential ankyrin 1, in addition to transient receptor
potential vanilloid 1, in tumor necrosis factor α-induced
inflammatory hyperalgesia and Freund's complete adjuvant-induced
monarthritis. Arthritis Rheum. 63:819–829. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El Karim I, McCrudden MT, Linden GJ,
Abdullah H, Curtis TM, McGahon M, About I, Irwin C and Lundy FT:
TNF-α-induced p38MAPK activation regulates TRPA1 and TRPV4 activity
in odontoblast-like cells. Am J Pathol. 185:2994–3002. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jotanovic Z, Mihelic R, Sestan B and
Dembic Z: Role of interleukin-1 inhibitors in osteoarthritis: An
evidence-based review. Drugs Aging. 29:343–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loreto C, Musumeci G and Leonardi R:
Chondrocyte-like apoptosis in temporomandibular joint disc internal
derangement as a repair-limiting mechanism. An in vivo study.
Histol Histopathol. 24:293–298. 2009.PubMed/NCBI
|
|
9
|
Musumeci G, Castrogiovanni P, Loreto C,
Castorina S, Pichler K and Weinberg AM: Post-traumatic caspase-3
expression in the adjacent areas of growth plate injury site: A
morphological study. Int J Mol Sci. 14:15767–15784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Puzzo D, Loreto C, Giunta S, Musumeci G,
Frsaca G, Podda MV, Arancio O and Palmeri A: Effect of
phosphodiesterase-5 inhibition on apoptosis and beta amyloid load
in aged mice. Neurobiol Aging. 35:520–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Musumeci G, Cstrogiovanni P, Travato FM,
Weinberg AM, Al-Wasiyah MK, Algahtani MH and Mobasheri A:
Biomarkers of chondrocyte apoptosis and autophagy in
osteoarthritis. Int J Mol Sci. 16:20560–20575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tschopp J and Schroder K: NLRP3
inflammasome activation: The convergence of multiple signaling
pathways on ROS production? Nat Rev Immunol. 10:210–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the Nomenclature Committee on Cell
Death 2012. Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhuang Y, Ding G, Zhao M, Bai M, Yang L,
Ni J, Wang R, Jia Z, Huang S and Zhang A: NLRP3 inflammasome
mediates albumin-induced renal tubular injury through impaired
mitochondrial function. J Biol Chem. 289:25101–25111. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fann DY, Lim YA, Cheng YL, Lok KZ,
Chunduri P, Baik SH, Drummond GR, Dheen ST, Sobey CG, Jo DG, et al:
Evidence that NF-κB and MAPK signaling promotes NLRP inflammasome
activation in neurons following ischemic stroke. Mol Neurobiol. Jan
14–2017.(Epub ahead of print). PubMed/NCBI
|
|
17
|
Li-Jessen NYK, Powell M, Choi AJ, Lee BJ
and Thibeault SL: Cellular source and proinflammatory roles of
high-mobility group box 1 in surgically injured rat vocal folds.
Laryngoscope. 127:E193–E200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Guo Y, Wang C and Yu H, Yu X and
Yu H: MicroRNA-142-3p inhibits chondrocyte apoptosis and
inflammation in osteoarthritis by targeting HMGB1. Inflammation.
39:1718–1728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Musumeci G, Castrogiovanni P, Mazzone V,
Szychlinska MA, Castorina S and Loreto C: Histochemistry as a
unique approach for investigating normal and osteoarthritic
cartilage. Eur J Histochem. 58:23712014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tong S, Liu J and Zhang C: Platelet-rich
plasma inhibits inflammatory factors and represses rheumatoid
fibroblast-like synoviocytes in rheumatoid arthritis. Clin Exp Med.
17:441–449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Tao A, Lan T, Cepinskas G, Kao R,
Martin CM and Rui T: Carbon monoxide releasing molecule-3 improves
myocardial function in mice with sepsis by inhibiting NLRP3
inflammasome activation in cardiac fibroblasts. Basic Res Cardiol.
112:162017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Denoble AE, Huffman KM, Stabler TV, Kelly
SJ, Hershfield MS, McDeniel GE, Coleman RE and Kraus VB: Uric acid
is a danger signal of increasing risk for osteoarthritis through
inflammasome activation. Proc Natl Acad Sci USA. 108:pp. 2088–2093.
2011; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levandowski CB, Mailloux CM, Ferrara TM,
Gowan K, Ben S, Jin Y, McFann KK, Holland PJ, Fain PR, Dinarello CA
and Spritz RA: NLRP1 haplotypes associated with vitiligo and
auto-immunity increase IL-1β processing via the NLRP1 inflammasome.
Proc Natl Acad Sci USA. 110:pp. 2952–2956. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van de Veerdonk FL, Netea MG, Dinarello CA
and Joosten LA: Inflammasome activation and IL-1β and IL-18
processing during infection. Trends Immunol. 32:110–116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sui J, Li H, Fang Y, Liu Y, Li M, Zhong B,
Yang F, Zou Q and Wu Y: NLRP1 gene polymorphism influences gene
transcription and is a risk factor for rheumatoid arthritis in han
chinese. Arthritis Rheum. 64:647–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Strowig T, Henao-Mejia J, Elinav E and
Flavell R: Inflammasomes in health and disease. Nature.
481:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi J, Zhao W, Ying H, Zhang Y, Du J, Chen
S, Li J and Shen B: Estradiol inhibits NLRP3 inflammasome in
fibroblast-like synoviocytes activated by lipopolysaccharide and
adenosine triphosphate. Int J Rheum Dis. Nov 3–2017.(Epub ahead of
print). View Article : Google Scholar :
|