Introduction
Acetaminophen (APAP) is widely used clinically as an
antipyretic and analgesic medicine (1–3).
Generally, APAP is considered to be safe when used within the
therapeutic dose range; however, APAP overdose can cause liver and
renal damage (4–6). Because APAP is cheap and readily
available, patients may overdose easily, leading to reports of
self-poisoning in numerous countries (7–9).
Many studies have shown that high doses of APAP can induce cell
death through either the apoptotic or necrotic death pathways
(10–12). Previous studies have demonstrated
that APAP-induced cytotoxicity is related to increased oxidative
stress and glutathione depletion (13–16).
It is well-known that cellular glutathione can convert
H2O2 to H2O via a glutathione
peroxidase reaction to attenuate cellular oxidative stress
(17,18). Therefore, APAP-induced glutathione
depletion may cause the H2O2 level to
increase in APAP-treated cells (3,6,19).
In addition, mitogen activated protein kinase (MAPK) and caspase
signals can be activated in APAP-treated cells (3,20,21).
Because APAP is generally used in a clinical setting, understanding
how to reduce the threshold of APAP-induced cytotoxicity is
important.
Guavas have many functional phytochemicals such as
vitamins, tannins, phenolic compounds, flavonoids, and triterpenoid
acids (22–24). Therefore, many studies indicated
that guavas have anti-inflammatory, anti-microbial, anti-oxidative,
anticancer, and anti-diarrheal functions (25–27).
Many studies have demonstrated that guavas can improve anti-oxidant
functions such as glutathione levels and activities of superoxide
dismutase, catalase, and glutathione peroxidase against oxidative
stress-induced damage (28–31).
Guavas can inhibit arsenic-induced (29), streptozotocin-induced (30,31),
and alloxan-induced (28)
oxidative stress. Furthermore, a recent study suggested that guavas
can inhibit caspase activity to attenuate cell apoptosis in type II
diabetic rats (32). Because
APAP-induced cytotoxicity is related to oxidative stress and
caspase activity (13–16,33,34),
and because guavas can inhibit oxidative stress and caspase
activity, the present study investigated whether guavas can
attenuate APAP-induced renal cytotoxicity.
Guava fruit has antioxidant activities (35–38).
Previous studies have demonstrated that guavas can protect renal
cells against oxidative stress-induced damage (32,39,40).
Therefore, we hypothesized that guava may be a potential diet to
reduce the threshold of APAP-induced renal cytotoxicity. Although
guavas have many antioxidant components including ascorbate,
flavonoids, glutathione, superoxide dismutase, catalase,
peroxidase, ascorbate peroxidase, and glutathione reductase, the
types and quantity of these antioxidant components are different at
different stages of maturation (38,41).
Generally, the ripening stages of guavas are classified as immature
green (IG), mature green (MG), turning fruits (T), ripe (R), and
over-ripe (OR) (41). Many
components and high levels of antioxidant phytochemicals are found
at the MG and T stages (41). This
study showed that antioxidant molecules including glutathione
reductase, total glutathione, and GSH are found predominately at
the MG stage, antioxidant molecules including catalase, POX,
ascorbate, and ascorbate peroxidase are primarily found at the T
stage, and the antioxidant molecule, SOD, is found at the R stage.
However, short antioxidant molecules are found at the IG and OR
stages. In addition, a recent study showed that different guava
cultivars have different antioxidant components and activities
(37). In order to find potential
guava extracts to protect renal cells against APAP-induced renal
damage, the extracts of pearl guava, imperial guava, and red pulp
guava were investigated in this study.
Materials and methods
Materials
Pearl guavas, imperial guavas, and red pulp guavas
were kindly provided from farmer Lin Chao Hsiung (A Fong guava
farm, Tainan, R.O.C.). Luminol and lucigenin were bought from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). An MTT assay kit
was bought from Bio Basic Canada Inc. (Markham, ON, Canada).
Anti-tubulin bought polyclonal antibody from Bioworld (Louis Park,
MN, USA). Anti-caspase-3 and anti-cleaved-caspase-3 rabbit
polyclonal antibodies and horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG secondary antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Western Lightning
Chemiluminescence Reagent Plus was bought from PerkinElmer, Inc.
(Waltham, MA, USA). Fetal bovine serum, DMEM, non-essential amino
acids, L-glutamine, and penicillin/streptomycin were bought from
Gibco-BRL (Invitrogen Life Technologies, Carlsbad, CA, USA).
Cell line and cell culture
Rat renal tubular epithelial cells (NRK-52E) and
human renal tubular epithelial cells (HK-2 cells) were bought from
the Bioresource Collection and Research Center (Shinchu, Taiwan).
Cells were cultured and maintained in DMEM (containing 10% fetal
bovine serum, 2 mM L-glutamine, 100 IU/ml penicillin/streptomycin,
and 0.1 mM non-essential amino acids) at 37°C in a humidified
atmosphere containing 5% CO2.
Guava extract preparation
Guava extracts were obtained using a similar method
to a previous study (32). The
extracts from the MG and T stages of the pearl guavas, imperial
guavas, and red pulp guavas were used in this study following
cleaning. The fruits were cut and the seed sections were removed,
before grinding the crude juice by treated with pure juice machine
(National MJ-C85 N) without extra water. The crude juice was
centrifuged at 4,000 × g (Allegra X-15®; Beckman
Coulter, Inc., Brea, CA, USA) for 30 min, following which the
supernatant was collected. The guava extracts were obtained after
the supernatant was filtered with a 0.22 mM filter. The final
concentration of guava extracts was ~2 g/ml (guava weight/final
liquid volume). The guava extracts were stored at −80°C. Guava
extracts (1 and 20 ml) were used in the study because 1 and 20 ml
guava extracts had antioxidant activity and did not cause cell
cytotoxicity.
Cell viability assay
An MTT assay kit was used for cell viability assay
(3,42). Cells were cultured used 96-well
plates (6×103 cells/well). Cell viability was determined
every 24 h. The control and experimental samples were added with an
MTT assay kit and incubated for 3 h at 37°C. The absorbance of the
reactive product was measured at 570 nm (A570) by using a
Multiskan™ FC Microplate Photometer (Molecular Devices,
Sunnyvale, CA, USA). The cell viability (%) was calculated as (A570
experimental group)/(A570 control group) × 100.
H2O2 level
determination
Intracellular H2O2 levels were
determined by using the lucigenin-amplified chemiluminescence
method (6,32). Each sample (200 µl containing 8,000
cells) was treated with luminol solution (100 µl; 0.2 mM/ml) and
incubated for 5 min. The H2O2 levels of these
samples were analyzed by using a chemiluminescence analyzing system
(CLA-FSI; Tohoko Electronic Industrial Co., Ltd., Sendai,
Japan).
SDS PAGE and western blotting
Primary anti-tubulin polyclonal antibody (1:1,000;
cat. no. BS1699) were bought from Bioworld. Primary anti-caspase-3
(1:1,000; cat. no. 9965), anti-cleaved caspase-3 antibody (1:500;
cat. no. 9662) and secondary horseradish peroxidase conjugated goat
anti-rabbit IgG (1:2,000, cat. no. 7074) were bought from Cell
Signaling Technology, Inc. (Beverly, MD, USA). These antibodies
were used for western blotting. The control and experimental cells
(~3×107 cells) were collected and lysed with RIPA buffer
(cat. no. 20–188; EMD Millipore, Billerica, MA, USA). After
centrifugation (16,000 × g; at 4°C) for 20 min, cellular proteins
were obtained from the supernatant layer. The protein concentration
was determined with a protein assay kit (cat. no. 23200; Thermo
Fischer Scientific, Inc.). Equal quantities (40 µg) of protein were
loaded onto a 13.3% SDS gel and separated by SDS electrophoresis,
prior to being transferred onto polyvinylidene difluoride membranes
(EMD Millipore). The membranes were blocked with 5% non-fat milk
solution for 2 h at room temperature and washed with PBS three
times (each time for 5 min). Next, the membranes were treated with
primary antibodies for 2 h at room temperature and washed with PBS
three times at room temperature. The membranes were subsequently
treated with anti-rabbit HRP-conjugated secondary antibodies for 1
h at room temperature. Finally, the membranes were treated with
Western Lightning® Chemiluminescence Plus reagent
(PerkinElmer, Inc.) and immunolabeled proteins were observed using
a Luminescence Image Analysis system (LAS-4000; FujiFilm Electronic
Materials Taiwan, Co., Ltd., Tainan, Taiwan).
Statistical analysis
Data were collected and calculated from four
independent experiments and presented as the mean ± SE. Means were
calculated with the Student's t-test method by using Microsoft
Excel (http://microsoft-excel-2010.updatestar.com/zh-tw)
and Bonferroni correction (SPSS 20.0 statistical software; IBM
SPSS, Armonk, NY, USA). P-value <0.05 was considered to indicate
a statistically significant difference.
Results
High-doses of APAP induces cell
cytotoxicity and increases H2O2 levels
Based on our previous studies (4,6,19), a
therapeutic dose of APAP (0.12 mM) is considered safe, whereas a
high dose of APAP (1.2 mM) is toxic to cells and induces increases
in H2O2 levels. The concentrations of APAP
that induce cytotoxicity were determined in this study. The cell
viability was above 80% in the 0.12 mM APAP-treated group at 24 and
96 h; however, the cell viability was below 40 and 30% in the 1.2
mM APAP treated-group at 72 and 96 h, respectively (Fig. 1A). Our data indicated that the high
dose of APAP is cytotoxic to rat renal tubular epithelial cells
(NRK-52E cells). Next, we determined whether high-dose APAP could
induce H2O2 level increases. Our results
showed that H2O2 levels significantly
increased in the APAP-treated group at 4 and 6 h (Fig. 1B).
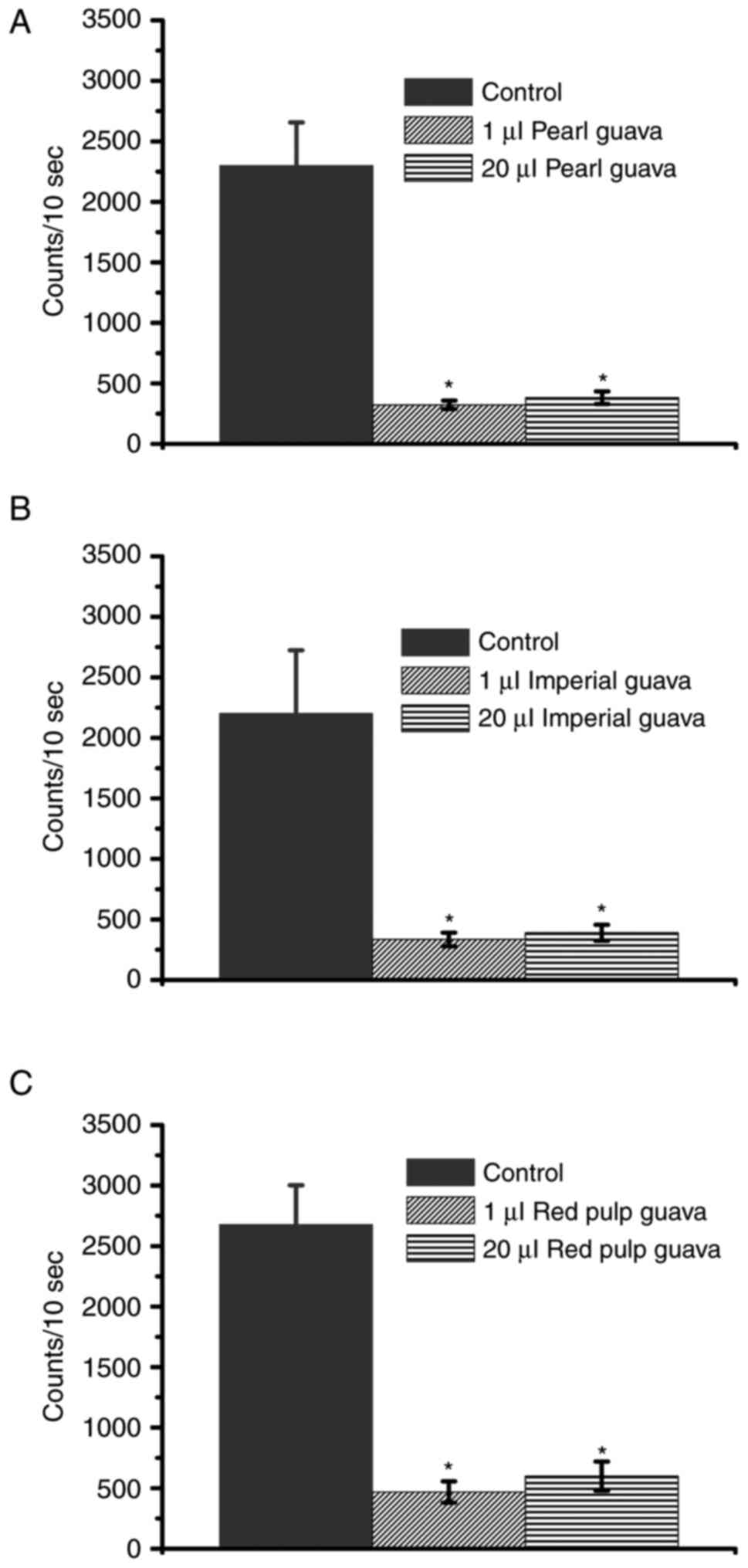
Guava extracts decrease
H2O2 levels and are not toxic to NRK-52E
cells
A previous study showed that guavas may be
classified as IG, MG, T, R, and OR according to their ripeness
(41). In this study, the extracts
from pearl guava, imperial guava, and red pulp guavas in the MG and
T stages were used. As different guava cultivars present different
antioxidant activities (37), we
first determined whether the extracts from three guava cultivars
could decrease H2O2 levels. Our data
suggested that all extracts from the three cultivars could decrease
H2O2 levels, especially at the MG stage
(Fig. 2). We further determined
the cytotoxicity in NRK-52E cells after treatment with the three
guava cultivar extracts. As shown in Fig. 3, cell viability rates were
approximately 100% in pearl guava and imperial guava-treated groups
(Fig. 3A and B), and cell
viability was ~80% in the red pulp guava-treated group (Fig. 3C). Our data suggested that extracts
of the three guava cultivars were not cytotoxic to NRK-52E
cells.
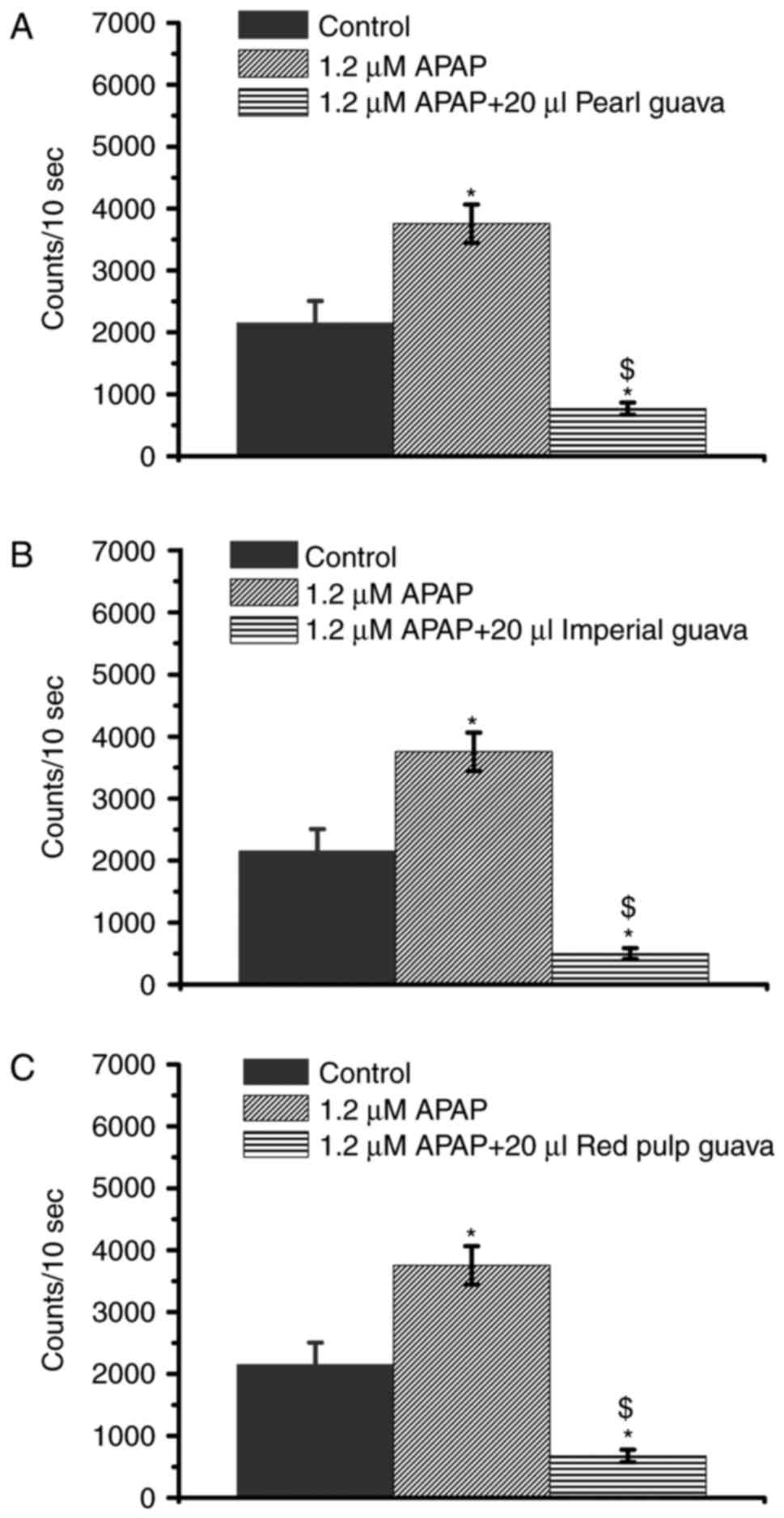
Guava extracts inhibit APAP-induced
H2O2 level increases
High-dose APAP caused H2O2
levels to increase in NRK-52E cells (Fig. 1B) while the guava extracts were
indicated to decrease cellular H2O2 levels
(Fig. 2). Therefore, we decided to
further investigate whether guava could inhibit the increased
H2O2 levels in APAP-treated NRK-52E cells.
Our data showed that all extracts from three guava cultivars (pearl
guava, imperial guava, and red pulp guava) effectively attenuated
the APAP-induced increases in H2O2 level in
the 1.2 mM group (Fig. 4).
Previous studies indicated that increased
H2O2 level is an important factor that
results in APAP cytotoxicity (3,6,19).
As shown in Fig. 4, guava extracts
inhibited APAP-induced H2O2 levels, guava
extracts were supposed to prevent APAP-induced cytotoxicity.
Pearl and imperial guava extracts
inhibit APAP-induced cytotoxicity
Extracts from the three guava cultivars (pearl
guava, imperial guava, and red pulp guava) could attenuate
APAP-arose H2O2 levels (Fig. 4). We further determined whether the
three guava cultivars extracts can inhibit APAP-induced
cytotoxicity. Guava extracts were added to APAP-treated NRK-52E
cells and the cell viability was determined. Following
determination for 96 h, the cell viability was below 50% in the
APAP-treated group at 48 and 96 h, however the cell viability was
~80% in APAP plus guava extract (pearl or imperial guava)-treated
groups (Fig. 5A and B). Therefore,
pearl and imperial guava extracts could inhibit
APAP-induced-cytotoxicity in NRK-52E cells. However, the cell
viabilities of the APAP-treated-group and the APAP plus red pulp
guava extract-treated-group were similar (Fig. 5C). Taken together, our data
indicated that pearl and imperial guava extracts inhibited
APAP-induced-cytotoxicity effectively, while red pulp guava
extracts did not.
Guava extracts inhibit APAP-activated
caspase-3 activity
Previous studies showed that APAP causes cell
cytotoxicity via the caspase-3 signaling death pathway (3,33).
In this study, different concentrations of APAP were used to treat
NRK-52 cells. The caspase-3 activation was observed easily with
APAP-treated for 48 h, therefore, the 48-h incubation time were
showed in Fig. 6. The results
showed that the levels of cleaved caspase-3 levels obviously
increased in the 1.2 mM APAP-treated group, but not in the 0.12 mM
APAP-treated group (Fig. 6).
Therefore, high-dose APAP induced the caspase-3 signaling pathway
while low-dose APAP did not. In addition, compared with the 1.2 mM
APAP-treated group, cleaved caspase-3 levels obviously decreased in
the 1 2 mM APAP plus guava extract-treated group (Fig. 6). The data suggested that guava
extracts could inhibit high-dose APAP-activated caspase-3
signals.
Guava extracts inhibit APAP-induced
cytotoxicity in HK-2 cells
NRK-52E cells are rat renal tubular epithelial
cells. As shown in Fig. 5, guava
extracts were able to inhibit APAP-induced cytotoxicity in NRK-52E
cells. HK-2 cells are human renal tubular epithelial cells. In
order to determine whether guava extracts had similar anti-APAP
effects on HK2 cells, both 48- and 96-h points were used to
determine the cell viability of HK-2 cells. As shown in Fig. 7, the cell viability rate was below
50% at 96 h in the APAP-treated group, while the cell viability
rates were approximately 100% in the APAP plus pearl guava-treated
group (Fig. 7A) and the APAP plus
imperial guava-treated group (Fig.
7B). Furthermore, similar to the data presented Fig. 5C, our study showed that the pulp
guava extracts did not effectively inhibit
APAP-induced-cytotoxicity in HK-2 cells (data not shown). Taken
together, these data (Figs. 5 and
7) indicated that pearl and
imperial guava extracts inhibited APAP-induced cytotoxicity
effectively in NRK-52 and HK-2 renal tubular cells.
Discussion
Previous studies showed that high-dose APAP causes
kidney and liver damage related to ROS increases, especially
increases in H2O2 levels (3,6,19).
Similar to these studies, our data demonstrated that high-dose APAP
could decrease the cell viability rate in renal tubular cells and
arise H2O2 increased level. In addition, our
data showed that increased H2O2 levels were
found at 4 and 6 h after high-dose APAP treatment, while cell
viability rates obviously decreased after high-dose APAP treatment
for 72 h. Cleaved caspase-3 was observed following high-dose APAP
treatment for 48 h. These data suggested that the increased
H2O2 level was upstream of APAP-induced
cytotoxicity in renal tubular cells, and caspase-3 activation was
downstream of APAP-induced cytotoxicity in renal tubular cells.
Previous studies have demonstrated that cytochrome
P450 enzymes can regulate cell proliferation (43,44).
APAP interacts with the active sites of cytochrome P450 enzymes
related to APAP-induced cytotoxicity (45,46).
Many studies have shown that APAP-induced cytotoxicity requires
bioactivation by cytochrome P450 enzymes (47,48),
and APAP can induce the apoptotic death pathway by increasing
cytochrome P450 activity (34). On
the other hand, a study has shown that guava juice can inhibit
cytochrome P450 enzyme activities (49). The results of the present study
demonstrated that guava extracts can attenuate APAP-induced
cytotoxicity. These studies indicated that the protective effects
of guava extracts against APAP-induced cytotoxicity may be via
cytochrome P450 signals. However, a recent study showed that
APAP-induced cytotoxicity may occur either in a cytochrome
P450-dependent manner or independent manner (47). Whether cytochrome P450-independent
signals are involved in the effects of guava extracts against
APAP-induced cytotoxicity remain to be investigated.
There are many different guava cultivars planted
worldwide. Different guava cultivars have different components and
quantity of phytochemicals; therefore, antioxidant activities may
differ in different guava cultivars (37). In this study, pearl guava, imperial
guava, and red pulp guava were investigated. Although their
phytochemical components and antioxidant activities have not
previously been elucidated, our study indicated that the three
guava cultivars had a similar activity against
H2O2 levels. Guavas are either classified as
immature green (IG), mature green (MG), turning fruits (T), ripe
(R), and over-ripe (OR). Generally, most antioxidant phytochemicals
exist in the MG and T stages; however, short antioxidant molecules
were found in the IG, R, and OR stages (41). Therefore, guava extracts from the
MG and T stages were choice against APAP-induced cytotoxicity and
exhibited anti-H2O2 activities in this study.
Guavas in the IG stage are immature and bitter-tasting, deeming
their extracts unsuitable for drinking. Therefore, guava extracts
from the IG stage were not used in our study. Guava extracts from
T, R, and OR stages had been pre-tested and it was shown that these
extracts did not exhibit any anti-H2O2
activities. This study suggested that guava extracts exhibited
anti-H2O2 activities relating to each
different guava stage, as different stages have different levels of
antioxidant molecules.
In our primary studies, we also found that the
extracts from the MG stage guava had a better activity against
H2O2 levels than the T stage guava extract
(data not shown). A previous study showed that high glutathione
levels and high glutathione reductase activity were found at the MG
stage, while high ascorbate and catalase activities were found at
the T stage (41). These studies
indicated that distinct antioxidant molecules exhibited different
anti-H2O2 activities. In addition,
glutathione is a major cellular factor to convert
H2O2 to H2O (17,18).
We considered high GSH levels and glutathione reductase activity to
be important factors related to MG stage-expressed
anti-H2O activity. In addition, N-acetylcysteine
(NAC), a precursor of glutathione, is common clinical drug to treat
acute APAP-induced intoxication (50–52).
Therefore, we determined that the MG stage of guava fruits can
decrease APAP-induced cytotoxicity.
As shown in Figs.
4, 5 and 7, both extracts obtained from pearl guava
and imperial guava could attenuate APAP-induced increases to
H2O2 levels and inhibit APAP-induced
cytotoxicity in renal tubular cells effectively. However, extracts
obtained from red pulp guava also attenuated APAP-induced increases
to H2O2 levels but did not inhibit
APAP-induced cytotoxicity. The results indicated that
H2O2 level increases was one of the possible
factors resulting in APAP-induced cytotoxicity; other factors
leading to APAP-induced cytotoxicity should be investigated in
future studies. In addition, different phytochemicals may be
present in different guava cultivars (37). Whether different phytochemicals
exist in pearl guava, imperial guava, and red pulp guava influence
APAP-induced cytotoxicity remains unclear.
Proliferating cell nuclear antigen (PCNA) is a
proliferating maker found abundantly in proliferating cells such as
tumor cells, stem cells and regenerating liver (53,54).
However, PCNA levels were not abundant in renal epithelial cells
(55). Up to now, whether APAP or
guava can alter PCNA expression in renal tubular cells remained
unclear. Today our primary date indicated PCNA levels were very few
in NRK-52E cells and were not obvious difference in control,
APAP-treated and APAP plus pearl guava-treated group (data not
show). Our studies suggested APAP-induced cytotoxicity in NRK-52
cells was not related to PCNA and guava extracts
inhibited-APAP-induced cytotoxicity was also not associated with
PCNA. In conclusion, this study suggests that extracts of pearl
guava and imperial guava could inhibit APAP-induced cytotoxicity in
renal tubular cells.
Acknowledgements
This study was supported by funding from Taipei Tzu
Chi Hospital, Taiwan (TCRD-TPE-106-35; TCRD-TPE-106-36;
TCRD-TPE-104-34; TCRD-TPE-105-20; TCRD-TPE-105-02) and from the
Ministry of Science and Technology, Taiwan (MOST103
2320-B-039-052-MY3; MOST105-2321-B-039-002), and the Ministry of
Health and Welfare, Taiwan (MOHW106-TDU-B-212-144003). Guavas were
kindly provided by Mr. Chao-Hsiung Lin from a fong guava farm,
Houbi, Tainan, Taiwan.
References
|
1
|
Bertolini A, Ferrari A, Ottani A, Guerzoni
S, Tacchi R and Leone S: Paracetamol: New vistas of an old drug.
CNS Drug Rev. 12:250–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klotz U: Paracetamol (acetaminophen) - a
popular and widely used nonopioid analgesic. Arzneimittelforschung.
62:355–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yiang GT, Yu YL, Lin KT, Chen JN, Chang WJ
and Wei CW: Acetaminophen induces JNK/p38 signaling and activates
the caspase-9-3-dependent cell death pathway in human mesenchymal
stem cells. Int J Mol Med. 36:485–492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bauer M, Babel B, Giesen H and Patzelt D:
Fulminant liver failure in a young child following repeated
acetaminophen overdosing. J Forensic Sci. 44:1299–1303. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Young RJ: Dextropropoxyphene overdosage.
Pharmacological considerations and clinical management. Drugs.
26:70–79. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu YL, Yiang GT, Chou PL, Tseng HH, Wu TK,
Hung YT, Lin PS, Lin SY, Liu HC, Chang WJ and Wei CW: Dual role of
acetaminophen in promoting hepatoma cell apoptosis and kidney
fibroblast proliferation. Mol Med Rep. 9:2077–2084. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hawton K, Bergen H, Simkin S, Arensman E,
Corcoran P, Cooper J, Waters K, Gunnell D and Kapur N: Impact of
different pack sizes of paracetamol in the United Kingdom and
Ireland on intentional overdoses: A comparative study. BMC Public
Health. 11:4602011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hawton K, Townsend E, Deeks J, Appleby L,
Gunnell D, Bennewith O and Cooper J: Effects of legislation
restricting pack sizes of paracetamol and salicylate on self
poisoning in the United Kingdom: Before and after study. BMJ.
322:1203–1207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Daly FF, Fountain JS, Murray L, Graudins A
and Buckley NA; Panel of Australian and New Zealand clinical
toxicologists, : Guidelines for the management of paracetamol
poisoning in Australia and New Zealand-explanation and elaboration.
A consensus statement from clinical toxicologists consulting to the
Australasian poisons information centres. Med J Aust. 188:296–301.
2008.PubMed/NCBI
|
|
10
|
Guo C, Xie G, Su M, Wu X, Lu X, Wu K and
Wei C: Characterization of acetaminophen-induced cytotoxicity in
target tissues. Am J Transl Res. 8:4440–4445. 2016.PubMed/NCBI
|
|
11
|
Murad HA, Habib H, Kamel Y, Alsayed S,
Shakweer M and Elshal M: Thearubigins protect against
acetaminophen-induced hepatic and renal injury in mice:
Biochemical, histopathological, immunohistochemical, and flow
cytometry study. Drug Chem Toxicol. 39:190–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramachandran A, McGill MR, Xie Y, Ni HM,
Ding WX and Jaeschke H: Receptor interacting protein kinase 3 is a
critical early mediator of acetaminophen-induced hepatocyte
necrosis in mice. Hepatology. 58:2099–2108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inkielewicz-Stępniak I and Knap N: Effect
of exposure to fluoride and acetaminophen on oxidative/nitrosative
status of liver and kidney in male and female rats. Pharmacol Rep.
64:902–911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slitt AM, Dominick PK, Roberts JC and
Cohen SD: Effect of ribose cysteine pretreatment on hepatic and
renal acetaminophen metabolite formation and glutathione depletion.
Basic Clin Pharmacol Toxicol. 96:487–494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McGill MR, Kennon-McGill S, Durham D and
Jaeschke H: Hearing, reactive metabolite formation, and oxidative
stress in cochleae after a single acute overdose of acetaminophen:
An in vivo study. Toxicol Mech Methods. 26:104–111. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galal RM, Zaki HF, Seif El-Nasr MM and
Agha AM: Potential protective effect of honey against
paracetamol-induced hepatotoxicity. Arch Iran Med. 15:674–680.
2012.PubMed/NCBI
|
|
17
|
Wołonciej M, Milewska E and
Roszkowska-Jakimiec W: Trace elements as an activator of
antioxidant enzymes. Postepy Hig Med Dosw (Online). 70:1483–1498.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kefer JC, Agarwal A and Sabanegh E: Role
of antioxidants in the treatment of male infertility. Int J Urol.
16:449–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lores Arnaiz S, Llesuy S, Cutrín JC and
Boveris A: Oxidative stress by acute acetaminophen administration
in mouse liver. Free Radic Biol Med. 19:303–310. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji L, Jiang P, Lu B, Sheng Y, Wang X and
Wang Z: Chlorogenic acid, a dietary polyphenol, protects
acetaminophen-induced liver injury and its mechanism. J Nutr
Biochem. 24:1911–1919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang AY, Lian LH, Jiang YZ, Wu YL and Nan
JX: Gentiana manshurica Kitagawa prevents acetaminophen-induced
acute hepatic injury in mice via inhibiting JNK/ERK MAPK pathway.
World J Gastroenterol. 16:384–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ravi K and Divyashree P: Psidium guajava:
A review on its potential as an adjunct in treating periodontal
disease. Pharmacogn Rev. 8:96–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morais-Braga MFB, Sales DL, Carneiro JNP,
Machado AJT, Dos Santos ATL, de Freitas MA, Martins GMAB, Leite NF,
de Matos YMLS, Tintino SR, et al: Psidium guajava L. and Psidium
brownianum Mart ex DC.: Chemical composition and anti-Candida
effect in association with fluconazole. Microb Pathog. 95:200–207.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shao M, Wang Y, Huang XJ, Fan CL, Zhang
QW, Zhang XQ and Ye WC: Four new triterpenoids from the leaves of
Psidium guajava. J Asian Nat Prod Res. 14:348–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin HC and Lin JY: Immune cell-conditioned
media suppress prostate cancer PC-3 cell growth correlating with
decreased proinflammatory/anti-inflammatory cytokine ratios in the
media using 5 selected crude polysaccharides. Integr Cancer Ther.
15:NP13–NP25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ashraf A, Sarfraz RA, Rashid MA, Mahmood
A, Shahid M and Noor N: Chemical composition, antioxidant,
antitumor, anticancer and cytotoxic effects of Psidium guajava leaf
extracts. Pharm Biol. 54:1971–1981. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin J, Puckree T and Mvelase TP:
Anti-diarrhoeal evaluation of some medicinal plants used by Zulu
traditional healers. J Ethnopharmacol. 79:53–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Obode O, Okafor O, Erukainure O, Ajayi A,
Suberu Y, Ogunji A, Okporua T, Oluwole O, Ozumba A and Elemo G:
Protective effect of some selected fruit blends on testicular
toxicity in alloxan-induced diabetic rats. J Complement Integr Med.
12:137–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tandon N, Roy M, Roy S and Gupta N:
Protective effect of Psidium guajava in arsenic-induced oxidative
stress and cytological damage in rats. Toxicol Int. 19:245–249.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang CS, Yin MC and Chiu LC:
Antihyperglycemic and antioxidative potential of Psidium guajava
fruit in streptozotocin-induced diabetic rats. Food Chem Toxicol.
49:2189–2195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soman S, Rauf AA, Indira M and
Rajamanickam C: Antioxidant and antiglycative potential of ethyl
acetate fraction of Psidium guajava leaf extract in
streptozotocin-induced diabetic rats. Plant Foods Hum Nutr.
65:386–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin CF, Kuo YT, Chen TY and Chien CT:
Quercetin-rich guava (Psidium guajava) juice in combination with
trehalose reduces autophagy, apoptosis and pyroptosis formation in
the kidney and pancreas of type II diabetic rats. Molecules.
21:3342016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baek HJ, Lee YM, Kim TH, Kim JY, Park EJ,
Iwabuchi K, Mishra L and Kim SS: Caspase-3/7-mediated cleavage of
β2-spectrin is required for acetaminophen-induced liver damage. Int
J Biol Sci. 12:172–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Posadas I, Santos P and Ceña V:
Acetaminophen induces human neuroblastoma cell death through NFKB
activation. PLoS One. 7:e501602012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu L, Lu W and Zhou X: Phenolic compounds
and in vitro antibacterial and antioxidant activities of three
tropic fruits: Persimmon, Guava, and Sweetsop. Biomed Res Int.
2016:42874612016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cuadrado-Silva CT, Pozo-Bayón MÁ and
Osorio C: Targeted metabolomic analysis of polyphenols with
antioxidant activity in sour guava (Psidium friedrichsthalianum
Nied.) fruit. Molecules. 22:E112016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Flores G, Wu SB, Negrin A and Kennelly EJ:
Chemical composition and antioxidant activity of seven cultivars of
guava (Psidium guajava) fruits. Food Chem. 170:327–335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Araújo HM, Rodrigues FF, Costa WD, Nonato
Cde F, Rodrigues FF, Boligon AA, Athayde ML and Costa JG: Chemical
profile and antioxidant capacity verification of Psidium guajava
(Myrtaceae) fruits at different stages of maturation. EXCLI J.
14:1020–1030. 2015.PubMed/NCBI
|
|
39
|
Arrey Tarkang P, Nwachiban Atchan AP,
Kuiate JR, Okalebo FA, Guantai AN and Agbor GA: Antioxidant
potential of a polyherbal antimalarial as an indicator of its
therapeutic value. Adv Pharmacol Sci. 2013:6784582013.PubMed/NCBI
|
|
40
|
Lin CY and Yin MC: Renal protective
effects of extracts from guava fruit (Psidium guajava L.) in
diabetic mice. Plant Foods Hum Nutr. 67:303–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mondal K, Malhotra SP, Jain V and Singh R:
Oxidative stress and antioxidant systems in Guava (Psidium guajava
L.) fruits during ripening. Physiol Mol Biol Plants. 15:327–334.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yiang GT, Chen JN, Lin PS, Liu HC, Chen SY
and Wei CW: Combined treatment with vitamin E and gefitinib has
synergistic effects to inhibit TGF-β1-induced renal fibroblast
proliferation. Mol Med Rep. 13:5372–5378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kwon YJ, Baek HS, Ye DJ, Shin S, Kim D and
Chun YJ: CYP1B1 enhances cell proliferation and metastasis through
induction of EMT and activation of Wnt/β-catenin signaling via Sp1
upregulation. PLoS One. 11:e01515982016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Imig JD and Khan MA: Cytochrome P450 and
lipoxygenase metabolites on renal function. Compr Physiol.
6:423–441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Johnson BP, Walisser JA, Liu Y, Shen AL,
McDearmon EL, Moran SM, McIntosh BE, Vollrath AL, Schook AC,
Takahashi JS and Bradfield CA: Hepatocyte circadian clock controls
acetaminophen bioactivation through NADPH-cytochrome P450
oxidoreductase. Proc Natl Acad Sci USA. 111:pp. 18757–18762. 2014;
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang Y, Wong SE and Lightstone FC:
Understanding a substrate's product regioselectivity in a family of
enzymes: A case study of acetaminophen binding in cytochrome P450s.
PLoS One. 9:e870582014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miyakawa K, Albee R, Letzig LG, Lehner AF,
Scott MA, Buchweitz JP, James LP, Ganey PE and Roth RA: A
cytochrome P450-independent mechanism of acetaminophen-induced
injury in cultured mouse hepatocytes. J Pharmacol Exp Ther.
354:230–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McGill MR and Jaeschke H: Metabolism and
disposition of acetaminophen: Recent advances in relation to
hepatotoxicity and diagnosis. Pharm Res. 30:2174–2187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chatuphonprasert W and Jarukamjorn K:
Impact of six fruits-banana, guava, mangosteen, pineapple, ripe
mango and ripe papaya-on murine hepatic cytochrome P450 activities.
J Appl Toxicol. 32:994–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
James LP, Letzig L, Simpson PM, Capparelli
E, Roberts DW, Hinson JA, Davern TJ and Lee WM: Pharmacokinetics of
acetaminophen-protein adducts in adults with acetaminophen overdose
and acute liver failure. Drug Metab Dispos. 37:1779–1784. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vargha R, Mostafa G, Burda G, Hermon M,
Trittenwein G and Gole J: Treatment with N-acetylcystein and total
plasma exchange for extracorporeal liver support in children with
paracetamol intoxication. Klin Padiatr. 226:84–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Prescott LF, Park J, Ballantyne A,
Adriaenssens P and Proudfoot AT: Treatment of paracetamol
(acetaminophen) poisoning with N-acetylcysteine. Lancet. 2:432–434.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang Y, Fan X, Wang Y, Chen P, Zeng H,
Tan H, Gonzalez FJ, Huang M and Bi H: Schisandrol B protects
against acetaminophen-induced hepatotoxicity by inhibition of
CYP-mediated bioactivation and regulation of liver regeneration.
Toxicol Sci. 143:107–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yi BR, Kim SU and Choi KC: Synergistic
effect of therapeutic stem cells expressing cytosine deaminase and
interferon-beta via apoptotic pathway in the metastatic mouse model
of breast cancer. Oncotarget. 7:5985–5999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tang J, Yan Y, Zhao TC, Gong R, Bayliss G,
Yan H and Zhuang S: Class I HDAC activity is required for renal
protection and regeneration after acute kidney injury. Am J Physiol
Renal Physiol. 307:F303–F316. 2014. View Article : Google Scholar : PubMed/NCBI
|