Introduction
Glioblastoma (GBM) is the most aggressive subset of
primary brain tumor in adults, and is responsible for ~50% of all
cranial tumors. GBMs are highly infiltrative which results in
difficulty for them to be resected completely (1–3).
Comprehensive therapy including radiotherapy and chemotherapy is
the main approach used for treatment; however, the overall survival
of glioma patients is only 12–14 months post-diagnosis (4).
Allicin (Fig. 1A)
is extracted from freshly crushed garlic (Allium sativum).
The anti-bacterial and antiinflammatory effects of Allicin have
been indicated, and previous studies have demonstrated the
antitumor capacity which may inhibit tumor growth and induce
apoptosis (5–7). Allicin has been demonstrated to have
an inhibitory effect on different kinds of tumors including lung
cancer, colorectal carcinoma, stomach cancer and liver cancer
(8,9). Previous studies have further explored
the effect of Allicin as an anticancer drug. However, the molecular
mechanism underlying the apoptosis effect of Allicin in glioma
remains to be clarified.
Activation of apoptosis signaling pathways may be
involved in the treatment of malignant tumor. Two main
apoptosis-associated signaling pathways have been addressed: The
extrinsic death receptor pathway and the intrinsic mitochondrion
pathway (10). The activation of
Fas binding to its Fas ligand (FasL) could initiate the extrinsic
pathway of apoptosis and serve a key role involved in death
signaling in many cancer types (11). Both signaling pathways contain
mitochondrial membrane and B-cell lymphoma 2 (Bcl-2) family
proteins. Fas-associated protein with death domain (FADD) together
with caspase-8 leads to autoproteolysis, and results in enzymatic
activation of caspase-8 which in turn activates caspase-3, −6 and
−7, resulting in the hydrolysis of cytosolic and substrates.
However, the roles of Allicin in human glioma remain
largely unclear. The present study aimed to investigate the
apoptotic activity of Allicin in human glioma cell lines, and
explore the underlying mechanism. These results provide evidence
that Allicin could inhibit proliferation and induce glioma cell
apoptosis in vitro.
Materials and methods
Cell line and culture
The U251 human glioma cell line was obtained from
the Chinese Academy of Sciences Cell Bank. Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM)/F12 (Hyclone; GE
Healthcare Life Sciences, Chicago, IL, USA), supplemented with 10%
(FBS) at 37°C with 5% CO2. Allicin (purity ≥90%) was
purchased from Shanghai Harvest Pharmaceutical Co., Ltd. (Shanghai,
China).
Cytotoxicity assay
Glioma cells were cultured at 5,000–8,000 cells/well
in 96-well plates overnight. Cells were treated with 15, 30, 60 or
90 µg/ml of Allicin for 20 h. Subsequently, 5 mg/ml MTT
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added for
additional 4 h at 37°C. Optical density (OD) was assessed by using
a TECAN microplate reader at 490 nm.
Cell proliferation assay and colony
formation assay
Cell viability was measured by MTT assay. Cells were
seeded at 5×103 cells/well into a 96-well plate and
incubated with Allicin at various concentrations (15, 30, 60 and 90
µg/ml) for different periods (24, 48 and 72 h). The OD was assessed
by using a TECAN microplate reader at 490 nm. For the colony
formation assay, cells were cultured in a 6 cm dish
(0.5×103 cells/well) and incubated at 37°C. Following
this, different concentrations of Allicin were added for 24 h. The
medium was then removed and cells were cultured for another 12
days. The colonies formed were fixed with 10% formalin for 10 min.
Giemsa staining was used to stain the samples obtained for 30 min
in room temperature and the number of colonies (>50 cells) was
counted by using upright light microscope (Leica DM2000; Leica
Microsystems, GmbH, Wetzlar, Germany).
Western blot analysis
U251 cells were treated with different
concentrations Allicin (30 and 60 µg/ml) for 48 h, and then washed
with ice-cold PBS. The cell lysates were centrifuged at 6,037 × g
for 15 min at 4°C and the total protein was extracted using
radioimmunoprecipitation buffer (Pierce; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with protease inhibitors, then subsequently
separated via 4–10% Tris glycin/SDS-PAGE. A bicinchoninic acid
protein assay kit was used to determine the concentration of
protein. The total quantity of protein loaded onto each lane was 40
µg; separated proteins were electrotransferred to ECL
nitrocellulose membranes (IPFL00010; EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% non-fat milk (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) at room temperature for
1 h and incubated separately with mouse anti-human Fas (1:1,000
dilution, cat. no. 8023S; Cell Signaling Technology, Inc., Danvers,
MA, USA), mouse anti-human FasL (1:1,000 dilution, cat. no. 4273;
Cell Signaling Technology, Inc.), mouse anti-human Bcl-2 (1:500
dilution, cat. no. sc-509; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), rabbit anti-human Bcl-2-associated X protein (Bax; 1:500
dilution, cat. no. sc-526; Santa Cruz Biotechnology, Inc.), rabbit
anti-human caspase-3 (1:1,000 dilution, cat. no. 9665; Cell
Signaling Technology, Inc.) and mouse anti-human β-actin (1:500
dilution, cat. no. 376421; Santa Cruz Biotechnology, Inc.) at 4°C
overnight, and then incubated with a goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:10,000 dilution, cat.
no. ab150077; Abcam, Cambridge, MA, USA) for 1 h. The protein
levels were visualized using an enhanced chemiluminescence kit
(BeyoECL Plus, cat. no. P0018; Beyotime Institute of Biotechnology,
Haimen, China) and determined by a Gel Doc 2000 imaging system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total U251 cell RNA treated with different
concentration of Allicin (0, 30 or 60 µg/ml) were isolated using
TRIzol reagent (Thermo Fisher Scientific, Inc.). PCR was performed
with ABI 7500 Real-Time PCR system (Applied Biosystems, Foster
City, CA, USA). The thermocycling condition were as follows: 95°C
for 30 sec, then followed by 40 cycles at 95°C for 5 sec, 60°C for
30 sec, and 72°C for 15 sec. The PCR primers were as follows: Fas
sense, 5′-TTCTGCCATAAGCCCTGTC-3′ and antisense,
5′-TTGGTGTTGCTGGTGAGT-3′ (amplification fragment 320 bp); FasL
sense, 5′-TTCAGCTCTTCCACCTACAG-3′ and antisense,
5′-ACATTCTCGGTGCCTGTAAC-3′ (amplification fragment 599 bp);
caspase-3 sense, 5′-GACAGACAGTGGAAGCGACTGGAT-3′ and antisense,
5′-GCATGGCACAAAGCGACTGGAT-3′; Bcl-2 sense,
5′-CGCCCTGTGGATGACTGAGTA-3′ and antisense,
5′-GGGCCGTACAGTTCCACAAAG-3′; Bax sense,
5′-CCCTTTTGCTTCAGGGTTTCATCCA-3′ and antisense,
5′-CTTGAGACACTCGCTCAGCTTCTTG-3′; U6 sense,
5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and antisense,
5′-CCAGTGCAGGGTCCGAGGT-3′. Fas/FasL, caspase-3, Bcl-2 and Bax mRNA
expression levels were determined using a SYBR PrimeScript RT-PCR
kit (Takara, Bio, Inc., Otsu, Japan) and normalized to U6 mRNA. The
relative expression levels were analyzed using the
2−ΔΔCq method (12).
Detection of caspase activity
A caspase activity kit (Beyotime Institute of
Biotechnology) was used to detected the activity of caspase-3, −8
and −9. Cells (1×106 cells/well) were added to Allicin
(0, 30 and 60 µg/ml) for 24 h. Data was obtained by measuring the
enzyme labeling meter with the wavelength of 490 nm via a
microplate reader (Infinite F50 Tecan Group, Ltd., Männedorf,
Switzerland). The U251 cells were isolated with caspase assay
buffer (Nanjing Kaiji Materials, Co., Ltd., Nanjing, China) and
then supernatant was collected and centrifuged at 7,546 × g at 4°C
for 10 min. The relative activity of caspases was measuring by an
enzyme-labeling meter with a microplate reader.
Hoechst 33258 staining
Cells were treated with Allicin for 24 h. The media
was then removed and cells were fixed with 4% formaldehyde. Cells
were then stained with 200 µM Hoechst 33258 for 10 min at room
temperature, and the slides were examined under a fluorescence
microscope (Olympus Corporation, Tokyo, Japan).
Cell apoptosis assay
U251 cells were seeded onto a 6-well plate at
5×105 cells/well for 24 h and treated with different
amounts of Allicin (0, 30 and 60 µg/ml) for 48 h. The samples were
collected at a concentration of 1×106 cells/ml with 4°C
PBS and suspended in 400 µl binding buffer. Annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide (PI; BD Pharmingen; BD
Biosciences, Franklin Lakes, NJ, USA) were added into the labeled
tube and cells were incubated for 20 min. The samples were examined
by using flow cytometry (BD Biosciences; Clontech, Palo Alto, CA,
USA).
Statistical analysis
All results were summarized from three independent
experiments. Results are expressed as the mean ± standard error.
Comparisons of each treatment data with control were carried out
for statistical difference by the paired t-test. One way analysis
of variance with followed by a Turkey's post-hoc test was used to
analyze statistical differences between groups by using the
statistical software SPSS 17.0 (SPSS, Inc., Chicago, IL, USA);
graphs were produced using by GraphPad Prism software (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Cytotoxic effects of Allicin on U251
glioma cells
The cytotoxic effect of Allicin (P<0.05; Fig. 1B) on U251 cells was measured by MTT
assay. The results demonstrated that Allicin decreased cell
survival in a dose-dependent manner, indicating its cytotoxicity.
The median IC50 was 41.97 µg/ml; therefore, 30 and 60 µg/ml doses
of Allicin were chosen for the present study.
Allicin induces apoptosis of glioma
cells
In the present study, Annexin V/PI staining was used
to demonstrate the apoptosis-inducing effect of Allicin. The
results demonstrated that the rate of apoptosis increased (30 µg/ml
for 9.1±3.2% and 60 µg/ml for 51.4±3.8%) at higher Allicin
concentrations compared with control cells (3.3±1.5%). Flow
cytometry assay demonstrated that the rate of apoptosis increased
in response to treatment with Allicin in a dose-dependent manner
(P<0.05; Fig. 1C).
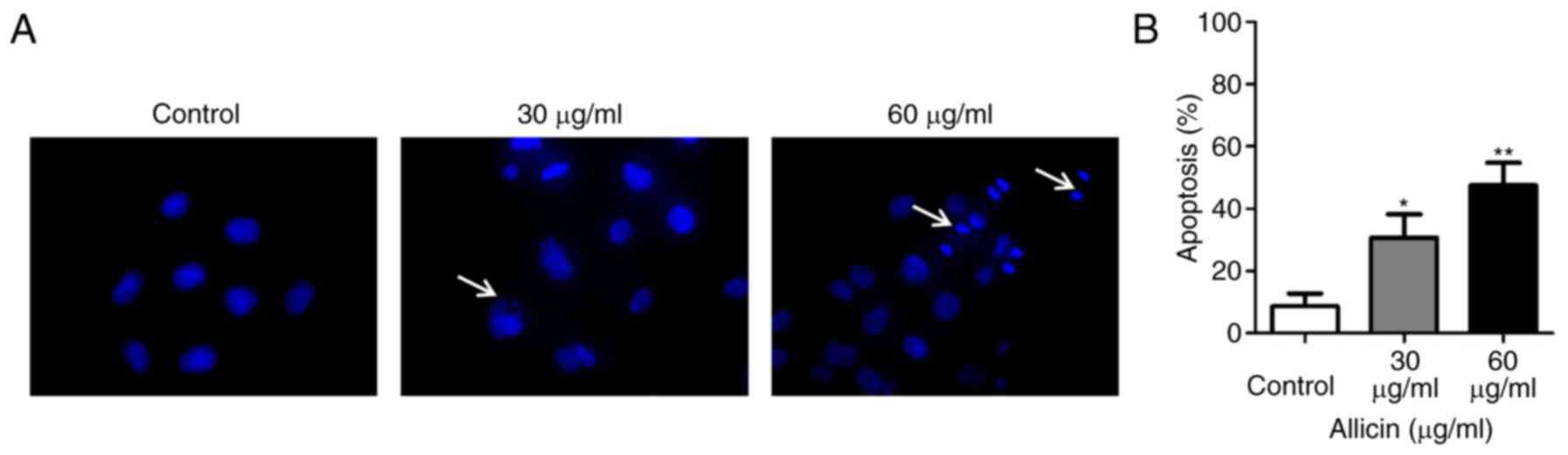
U251 cells were treated with Allicin for 24 h, then
stained with Hoechst 33258. The morphological changes of apoptosis
cells (such as condensation of chromatin and nuclear fragmentation)
was detected under a fluorescence microscope (Fig. 2A). The level of apoptosis was
increased with the dose of Allicin in U251 cells (Fig. 2B). These results demonstrated that
Allicin induced apoptosis in a dose-dependent manner.
Allicin inhibits proliferation in U251
glioma cells
Colony formation was used to confirm the
proliferation ability by treating with Allicin in U251 glioma
cells, which indicated that the inhibition ability of Allicin was
irreversible (Fig. 3A). MTT assay
was used to detect the effect of Allicin on the growth of glioma
cells. Allicin was demonstrated inhibit the viability of glioma
cell in a dose- and time-dependent manner (Fig. 3B; P<0.05).
Allicin increases Fas and FasL
expression in glioma cells
Fas (CD95/APO-1) induces apoptosis by binding to a
member of the tumor necrosis factor receptor (TNFR) family (FasL),
which is a cell surface receptor protein (13). RT-qPCR was used to measure the
expression of Fas and FasL mRNA. The data demonstrated that after
treating with different amounts of Alllicin, Fas, FasL and Bax mRNA
expression levels were significantly increased, while Bcl-2
expression levels were decreased, in a dose-dependent manner
(P<0.05, Fig. 4A). These
results were reflected at the protein level (P<0.05; Fig. 4B and C). These results demonstrated
that Allicin induces apoptosis a dose-dependent manner.
Allicin increases the activity of
caspases
After treating cells with various amounts of Allicin
for 24 h, caspase-3, −8 and −9 enzyme activities were upregulated
(P<0.01; Fig. 4D). Therefore,
apoptosis signaling pathways which were activated by caspases may
be involved in the antitumor effects of Allicin.
Discussion
GBM is the most common and aggressive type of brain
cancer, has an unfavorable prognosis and treatment still remains
great challenge. The majority of chemotherapy options are not
recommended because of various severe side effects, and the median
survival time of the patients is extremely poor (14).
Garlic is the bulb of Allium plants, and Allicin
(C6H10S3) is the main flavor
compound. A previous study reported that Allicin supresses the
growth of certain cancers, and may possess anticancer activity
in vitro and in vivo (15). Allicin may induce cell apoptosis,
cell cycle arrest and inhibit proliferation, but the underlying
mechanisms remain unknown. A previous study demonstrated that
Allicin can mediate the apoptosis of cancer cells by activating
caspase-3, −8 and −9 (16).
Allicin could activate autophagy of human liver cancer cells and
induce cell death through apoptosis (7,17).
Apoptosis involves a series of biochemical processes
of cell death controlled by several signaling pathways, such as the
caspase and mitochondrial pathways. There are two main apoptotic
pathways involved: The receptor-mediated pathway and the
mitochondrial pathway, which is known as extrinsic and intrinsic.
Allicin induces apoptosis by activating the extrinsic and intrinsic
apoptosis pathways in gastric cancer cells (18).
The extrinsic pathway is initiated by stimulating
the ligand of the death receptors on the cell surface, such as
TNFR, Fas and FasL. Fas is a transmembrane protein binding to FasL,
then trimerization and recruitment of FADD proteins occurs by
activating caspase-8 and −10 (19). Caspase-8 regulates the expression
of pro-caspase-3, −6, or −7. Caspase-3 serves an important role in
cell death. In the present study, following Allicin treatment, the
level of Fas and FasL increased in glioma cells, which has also
been demonstrated in gastric cancer cells (20).
This leads to the activation of Bax, which belong to
the Bcl-2 family, which leads to the release of cytochrome c
(21). It has been reported that
mitochondria are involved in inducing the intrinsic pathway, which
is initiated via the release of signaling factors from the
mitochondria. Cytochrome c is the first to be released into
the cytosol, and interacts with pro-caspase-9, which could regulate
caspase-3 or −7 (22).
The Bcl-2 family serves a pivotal a role in the
intrinsic apoptosis pathway, which could maintain cell viability by
inhibiting the capacity of Bax and impeding the release of
cytochrome c (23). The
results of the present study demonstrated that Allicin may inhibit
the proliferation of U251 cells in vitro by suppressing
Bcl-2 and inducing the expression of Bax.
In conclusion, the results of the present study
indicated that Allicin can effectively inhibit proliferation and
induce apoptosis in U251 glioma cells in vitro. It revealed
that Allicin treatment increases the activation of both intrinsic
and extrinsic apoptosis signaling pathways in U251 cells. Future
studies should evaluate Allicin as a novel antitumor agent in
treating glioma.
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bush NA, Chang SM and Berger MS: Current
and future strategies for treatment of glioma. Neurosurg Rev.
14:1–14. 2017. View Article : Google Scholar
|
|
4
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou H, Qu Z, Mossine VV, Nknolise DL, Li
J, Chen Z, Cheng J, Greenlief CM, Mawhinney TP, Brown PN, et al:
Proteomic analysis of the effects of aged garlic extract and its
FruArg component on lipopolysaccharide-induced neuroinflammatory
response in microglial cells. PLoS One. 9:e1135312014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lissiman E, Bhasale AL and Cohen M: Garlic
for the common cold. Cochrane Database Syst Rev: CD006206. 2009.
View Article : Google Scholar
|
|
7
|
Chu YL, Ho CT, Chung JG, Rajasekaran R and
Sheen LY: Allicin induces p53-mediated autophagy in HepG2 human
liver cancer cells. J Agric Food Chem. 60:8363–8371. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chu YL, Ho CT, Chung JG, Raghu R, Lo YC
and Sheen LY: Allicin induces anti-human liver cancer cells through
the p53 gene modulating apoptosis and autophagy. J Agric Food Chem.
61:9839–9848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou X, Liang J, Sun J, Hu X, Lei L, Wu D
and Liu L: Allicin sensitizes hepatocellular cancer cells to
anti-tumor activity of 5-fluorouracil through ROS-mediated
mitochondrial pathway. J Pharmacol Sci. 131:233–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Zheng Z, Yu W, Lin H, Cui B and
Cao F: Polymorphisms of the FAS and FASL genes and risk of breast
cancer. Oncol Lett. 3:625–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lapinski TW, Jaroszewicz J and
Wiercinska-Drapalo A: Concentrations of soluble Fas and soluble Fas
ligand as indicators of programmed cell death among patients
coinfected with human immunodeficiency virus and hepatitis C virus.
Viral Immunol. 19:570–575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shea A, Harish V, Afzal Z, Chijioke J,
Kedir H, Dusmatova S, Roy A, Ramalinga M, Harris B, Blancato J, et
al: MicroRNAs in glioblastoma multiforme pathogenesis and
therapeutics. Cancer Med. 5:1917–1946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dvořáková M, Weingartová I, Nevoral J,
Němeček D and Krejčová T: Garlic sulfur compounds suppress
cancerogenesis and oxidative stress: A review. Scientia
Agriculturae Bohemica. 46:65–72. 2015. View Article : Google Scholar
|
|
16
|
Wang W, Du Z, Nimiya Y, Sukamtoh E, Kim D
and Zhang G: Allicin inhibits lymphangiogenesis through suppressing
activation of vascular endothelial growth factor (VEGF) receptor. J
Nutr Biochem. 29:83–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oommen S, Anto RJ, Srinivas G and
Karunagaran D: Allicin (from garlic) induces caspase-mediated
apoptosis in cancer cells. Eur J Pharmacol. 485:97–103. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hebert DN, Lamriben L, Powers ET and Kelly
JW: The intrinsic and extrinsic effects of N-linked glycans on
glycoproteostasis. Nat Chem Biol. 10:902–910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li CL, Chang L, Guo L, Zhao D, Liu HB,
Wang QS, Zhang P, Du WZ, Liu X, Zhang HT, et al: β-Elemene induces
caspase-dependent apoptosis in human glioma cells in vitro through
the upregulation of Bax and Fas/FasL and downregulation of Bcl-2.
Asian Pac J Cancer Prev. 15:10407–10412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Ha M, Gong Y, Xu Y, Dong N and
Yuan Y: Allicin induces apoptosis in gastric cancer cells through
activation of both extrinsic and intrinsic pathways. Oncol Rep.
24:1585–1592. 2010.PubMed/NCBI
|
|
21
|
Liu Z, Ding Y, Ye N, Wild C, Chen H and
Zhou J: Direct activation of Bax protein for cancer therapy. Med
Res Rev. 36:313–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang X and Wang X: Cytochrome c promotes
caspase-9 activation by inducing nucleotide binding to Apaf-1. J
Biol Chem. 275:31199–31203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du X, Zhao YP, Zhang TP, Zhou L, Chen G,
Wang TX, You L and Shu H: Alteration of the intrinsic apoptosis
pathway is involved in Notch-induced chemoresistance to gemcitabine
in pancreatic cancer. Arch Med Res. 45:15–20. 2014. View Article : Google Scholar : PubMed/NCBI
|