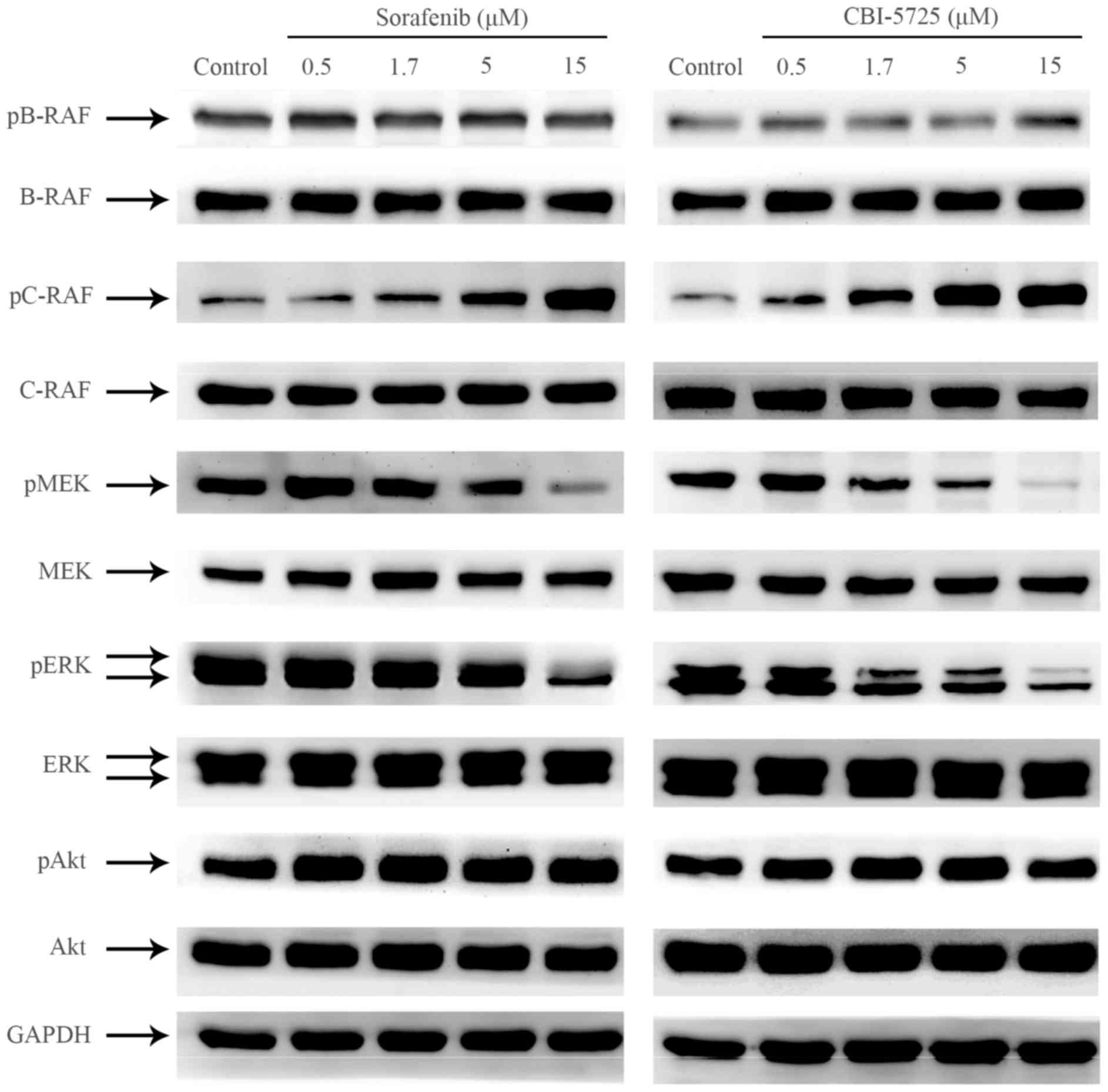
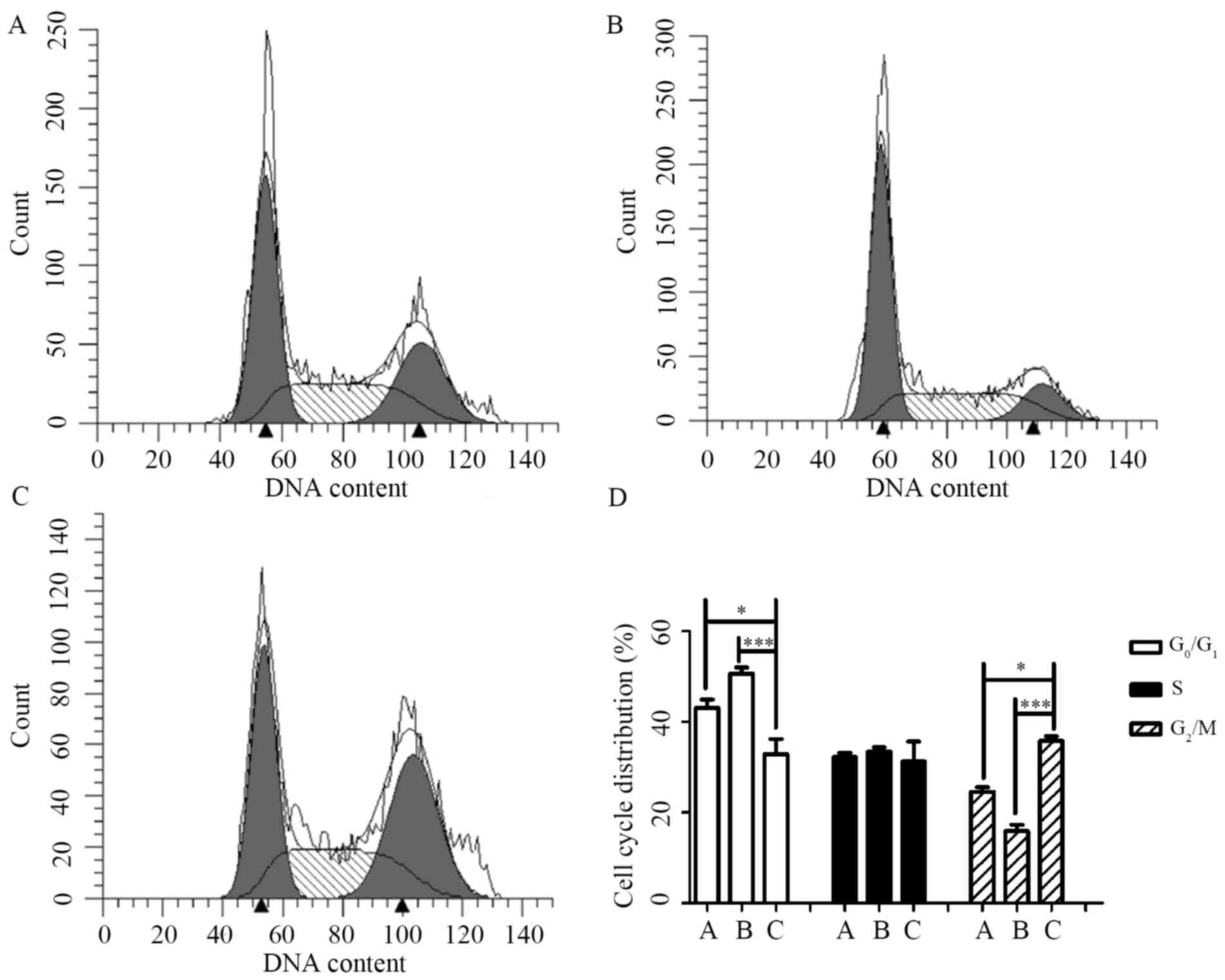
|
1
|
Lamarca A, Mendiola M and Barriuso J:
Hepatocellular carcinoma: Exploring the impact of ethnicity on
molecular biology. Crit Rev Oncol Hematol. 105:65–72. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swamy SG, Kameshwar VH, Shubha PB, Looi
CY, Shanmugam MK, Arfuso F, Dharmarajan A, Sethi G, Shivananju NS
and Bishayee A: Targeting multiple oncogenic pathways for the
treatment of hepatocellular carcinoma. Target Oncol. 12:1–10. 2016.
View Article : Google Scholar
|
|
3
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azumi J, Tsubota T, Sakabe T and Shiota G:
miR-181a induces sorafenib resistance of hepatocellular carinoma
cells through downregulation of RASSF1 expression. Cancer Sci.
107:1256–1262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ezzoukhry Z, Louandre C, Trécherel E,
Godin C, Chauffert B, Dupont S, Diouf M, Barbare JC, Mazière JC and
Galmiche A: EGFR activation is a potential determinant of primary
resistance of hepatocellular carcinoma cells to sorafenib. Int J
Cancer. 131:2961–2969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poulikakos PI, Zhang C, Bollag G, Shokat
KM and Rosen N: RAF inhibitors transactivate RAF dimers and ERK
signalling in cells with wild-type BRAF. Nature. 464:427–430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lackner MR, Wilson TR and Settleman J:
Mechanisms of acquired resistance to targeted cancer therapies.
Future Oncol. 8:999–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bagrodia S, Smeal T and Abraham RT:
Mechanisms of intrinsic and acquired resistance to kinase-targeted
therapies. Pigment Cell Melanoma Res. 25:819–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bottsford-Miller JN, Coleman RL and Sood
AK: Resistance and escape from antiangiogenesis therapy: Clinical
implications and future strategies. J Clin Oncol. 30:4026–4034.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moriguchi M, Umemura A and Itoh Y: Current
status and future prospects of chemotherapy for advanced
hepatocellular carcinoma. Clin J Gastroenterol. 9:184–190. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pillet S and von Messling V: Canine
distemper virus selectively inhibits apoptosis progression in
infected immune cells. J Virol. 83:6279–6287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas E, Gopalakrishnan V, Somasagara RR,
Choudhary B and Raghavan SC: Extract of Vernonia condensata,
inhibits tumor progression and improves survival of tumor-allograft
bearing mouse. Sci Rep. 6:232552016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brauchle E, Thude S, Brucker SY and
Schenke-Layland K: Cell death stages in single apoptotic and
necrotic cells monitored by Raman microspectroscopy. Sci Rep.
4:46982014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wlodkowic D, Telford W, Skommer J and
Darzynkiewicz Z: Apoptosis and beyond: Cytometry in studies of
programmed cell death. Methods Cell Biol. 103:55–98. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuo WT, Ho YJ, Kuo SM, Lin FH, Tsai FJ,
Chen YS, Dong GC and Yao CH: Induction of the mitochondria
apoptosis pathway by phytohemagglutinin erythroagglutinating in
human lung cancer cells. Ann Surg Oncol. 18:848–856. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lam CR, Tan MJ, Tan SH, Tang MB, Cheung PC
and Tan NS: TAK1 regulates SCF expression to modulate PKBα activity
that protects keratinocytes from ROS-induced apoptosis. Cell Death
Differ. 18:1120–1129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang LJ, Chen Y, He J, Yi S, Wen L, Zhao
J, Zhang BP and Cui GH: Betulinic acid inhibits autophagic flux and
induces apoptosis in human multiple myeloma cells in vitro. Acta
Pharmacol Sin. 33:1–1548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buscà R, Pouysségur J and Lenormand P:
ERK1 and ERK2 map kinases: Specific roles or functional redundancy.
Front Cell Dev Biol. 4:532016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robinson MJ and Cobb MH: Mitogen-activated
protein kinase pathways. Curr Opin Cell Biol. 9:180–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hoshino R, Chatani Y, Yamori T, Tsuruo T,
Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J and Kohno
M: Constitutive activation of the 41-/43-kDa mitogen-activated
protein kinase signaling pathway in human tumors. Oncogene.
18:813–822. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Q, Wei L, Yang H, Yang W, Yang Q,
Zhang Z, Wu K and Wu J: Bromodomain containing protein represses
the Ras/Raf/MEK/ERK pathway to attenuate human hepatoma cell
proliferation during HCV infection. Cancer Lett. 371:107–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pucci B, Kasten M and Giordano A: Cell
cycle and apoptosis. Neoplasia. 2:291–299. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu H, Che X, Zheng Q, Wu A, Pan K, Shao A,
Wu Q, Zhang J and Hong Y: Caspases: A molecular switch node in the
crosstalk between autophagy and apoptosis. Int J Biol Sci.
10:1072–1083. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duriez PJ and Shah GM: Cleavage of
poly(ADP-ribose) polymerase: A sensitive parameter to study cell
death. Biochem Cell Biol. 75:337–349. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prieto-Domínguez N, Ordóñez R, Fernández
A, García-Palomo A, Muntané J, González-Gallego J and Mauriz JL:
Modulation of autophagy by sorafenib: Effects on treatment
response. Front Pharmacol. 7:1512016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Zhao GD, Shi Z, Qi LL, Zhou LY and
Fu ZX: The Ras/Raf/MEK/ERK signaling pathway and its role in the
occurrence and development of HCC. Oncol Lett. 12:3045–3050. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin T, Lavoie H, Sahmi M, David M, Hilt C,
Hammell A and Therrien M: RAF inhibitors promote RAS-RAF
interaction by allosterically disrupting RAF autoinhibition. Nat
Commun. 8:12112017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holderfield M, Deuker MM, McCormick F and
McMahon M: Targeting RAF kinases for cancer therapy: BRAF-mutated
melanoma and beyond. Nat Rev Cancer. 14:455–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al: Improved survival with vemurafenib in melanoma with BRAF V600E
mutation. N Engl J Med. 364:2507–2516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hauschild A, Grob JJ, Demidov LV, Jouary
T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr,
Kaempgen E, et al: Dabrafenib in BRAF-mutated metastatic melanoma:
A multicentre, open-label, phase 3 randomised controlled trial.
Lancet. 380:358–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao Z, Torres NM, Tao A, Gao Y, Luo L, Li
Q, de Stanchina E, Abdel-Wahab O, Solit DB, Poulikakos PI and Rosen
N: BRAF mutants evade ERK-dependent feedback by different
mechanisms that determine their sensitivity to pharmacologic
inhibition. Cancer Cell. 28:370–383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hatzivassiliou G, Song K, Yen I,
Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor
SL, Vigers G, et al: RAF inhibitors prime wild-type RAF to activate
the MAPK pathway and enhance growth. Nature. 464:431–435. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heidorn SJ, Milagre C, Whittaker S, Nourry
A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer
CJ, Pritchard C and Marais R: Kinase-dead BRAF and oncogenic RAS
cooperate to drive tumor progression through CRAF. Cell.
140:209–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prahallad A, Sun C, Huang S, Di
Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A
and Bernards R: Unresponsiveness of colon cancer to BRAF(V600E)
inhibition through feedback activation of EGFR. Nature.
483:100–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cox AD and Der CJ: The raf inhibitor
paradox: Unexpected consequences of targeted drugs. Cancer Cell.
17:221–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peng SB, Henry JR, Kaufman MD, Lu WP,
Smith BD, Vogeti S, Rutkoski TJ, Wise S, Chun L, Zhang Y, et al:
Inhibition of RAF isoforms and active dimers by LY3009120 leads to
anti-tumor activities in RAS or BRAF mutant cancers. Cancer Cell.
28:384–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nakamura A, Arita T, Tsuchiya S, Donelan
J, Chouitar J, Carideo E, Galvin K, Okaniwa M, Ishikawa T and
Yoshida S: Antitumor activity of the selective pan-RAF inhibitor
TAK-632 in BRAF inhibitor-resistant melanoma. Cancer Res.
73:7043–7055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Girotti MR, Lopes F, Preece N,
Niculescu-Duvaz D, Zambon A, Davies L, Whittaker S, Saturno G,
Viros A, Pedersen M, et al: Paradox-breaking RAF inhibitors that
also target SRC are effective in drug-resistant braf mutant
melanoma. Cancer Cell. 31:4662017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Knievel J, Schulz WA, Greife A, Hader C,
Lübke T, Schmitz I, Albers P and Niegisch G: Multiple mechanisms
mediate resistance to sorafenib in urothelial cancer. Int J Mol
Sci. 15:20500–20517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weber GF, Gaertner FC, Erl W, Janssen KP,
Blechert B, Holzmann B, Weighardt H and Essler M: IL-22-mediated
tumor growth reduction correlates with inhibition of ERK1/2 and AKT
phosphorylation and induction of cell cycle arrest in the G2-M
phase. J Immunol. 177:8266–8272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera
S and Ikejima T: Oridonin induces G2/M arrest and apoptosis via
activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK
survival pathway in murine fibrosarcoma L929 cells. Arch Biochem
Biophys. 490:70–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nicholson DW, Ali A, Thornberry NA,
Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle
M, Lazebnik YA, et al: Identification and inhibition of the
ICE/CED-3 protease necessary for mammalian apoptosis. Nature.
376:37–43. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tewari M, Quan LT, O'Rourke K, Desnoyers
S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS and Dixit VM:
Yama/CPP32 beta, a mammalian homolog of CED-3, is a
CrmA-inhibitable protease that cleaves the death substrate
poly(ADP-ribose) polymerase. Cell. 81:801–809. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Le RY, Kirkland JB and Shah GM: Cellular
responses to DNA damage in the absence of Poly(ADP-ribose)
polymerase. Biochem Biophys Res Commun. 245:1–10. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vizetto-Duarte C, Custodio L, Gangadhar
KN, Lago JH, Dias C, Matos AM, Neng N, Nogueira JM, Barreira L,
Albericio F, et al: Isololiolide, a carotenoid metabolite isolated
from the brown alga Cystoseira tamariscifolia, is cytotoxic and
able to induce apoptosis in hepatocarcinoma cells through caspase-3
activation, decreased Bcl-2 levels, increased p53 expression and
PARP cleavage. Phytomedicine. 23:550–557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Oliver FJ, de la Rubia G, Rolli V,
Ruiz-Ruiz MC, de Murcia G and Murcia JM: Importance of
poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson
from an uncleavable mutant. J Biol Chem. 273:33533–33955. 1998.
View Article : Google Scholar : PubMed/NCBI
|